Abstract
The major chemical components of four essential oils (EOs) extracted from dry leaves of Citrus limonum, Cymbopogon citratus, Litsea cubeba, and Muristica fragrans were analyzed with gas chromatograph-mass spectrometer and their fumigant, contact, and repellent activities against 10th instar and adults of Tenebrio molitor were also assayed. The results indicated that the major constituents of C. limonum and Cy. citrates were D-limonene (38.22%) and 3,7-dimethyl-6-octenal (26.21%), while which of L. cubeba and M. fragrans were (E)-3, 7-dimethyl-2, 6-octadienal (49.78%) and (E)-cinnamaldehyde (79.31%), respectively. Contact activities of L. cubeba and C. limonum with LC50 values of 21.2 and 13.9 µg/cm2 at 48 h and repellence activities (>89.0% repellence indexes) (P < 0.05) at 12 h on 10th instar were better than those of the other two EOs. Nevertheless, the fumigation activities of L. cubeba on 10th instar and adults (LC50 = 2.7, 3.7 μl/liter) were stronger than those of C. limonum (LC50 = 10.9, 12.0 μl/liter) at 96 h and significant (not overlapping confidence intervals). The EOs of L. cubeba and C. limonum have clearly elongated the growth and development of larvae, egg, and slightly shorten pupae and adults of T. molitor compared with the control. The mainly active ingredients of L. cubeba and C. limonum, including D-limonene and β-pinene, were demonstrated to coinhibit the actives of AChE and enhance the toxicities on 10th instar of T. molitor. These results indicate that the EOs of L. cubeba and C. limonum could have great potential as botanical insecticides against T. molitor.
Keywords: Tenebrio molitor, fumigant toxicity, growth, development, AChE activity
Tenebrio molitor L. (Coleoptera: Tenebrionidae) is one of the mainly stored product pests of Juncus effuses L. (Poales: Juncaceae), which is being widely cultivated in southwest China and is acted as an important straw mat for summer sleeping, such as tatamis (Li et al. 2009, Wang et al. 2014). Some means, including hot treatment, sun treatment, and fumigation with some chemicals, have been attempted to control them. However, many negative consequences (pest resistance, residual toxicities, environmental pollution, and so on) have limited the application of chemical control (Wang et al. 2010). Plant-derived natural chemicals, known as secondary metabolites, are effective in their roles as possible alternatives to synthetic chemical insecticides, and many of them have displayed numerous pesticidal biological activities (Ngassoum et al. 2007, Rossi et al. 2009, Nesci et al. 2011). Recently, the plant oils characterized by a strong volatile and lower density than water, which embraced some insecticidal activities, have become a hotspot in pesticide research as possible alternatives to synthetic chemical insecticides (Copping and Menn 2000, Stefanazzi et al. 2011). There are some early reports that essential oils (EOs) have the potential as insecticide to control some insects or mites. Oyedele et al. (2002) reported that the ointment and cream formulations of lemongrass oil displayed the good repellency active on Ae. aegypti L., and 1% solution (v/v) and 15% cream (v/w) and ointment preparations of the oil exhibited ≥50% repellency lasting 2–3 h. Liu et al. (2007) also reported the EOs extracted from 30 Chinese medicinal herbs, including Artemisia argyi, Dictamnus dasycarpus, Evodia rutaecarpa, Litsea cubeba, Narcissus tazetta var. chinensis, and so on, have exhibited insecticidal or feeding-deterrent activities against two stored-grain insects Sitophilus zeamais and Tribolium castaneum. Williamson et al. (2007) asserted that lemon oil showed good insecticidal active on house dust mites as control agency. Hanifah et al. (2011) researched the acaricidal activity of Cymbopogon citratus EO on house dust mites and found that it was stronger (over 91% topical and contact mortalities with 50% diluted EO) than that of neem (only 40.3% topical mortalities and 15.7% contact mortalities) on the Dermatophagoides pteronyssinus and Dermatophagoides farinae dealt with the same concentration and exposure time. Phasomkusolsil and Soonwera (2011) alleged that the EO of Cy. citratus showed the repellency against An. dirus with ED50 at <0.068 mg/cm2. Furthermore, the Cy. citratus gave strong effective dose (ED50) values at <0.003 mg/cm2 when tested against Cx. quinquefasciatus. Yang et al. (2014) also declared that the EO of L. cubeba possessed strong contact toxicity against the cigarette beetle Lasioderma serricorne adults and the booklouse Liposcelis bostrychophila, with LD50 values of 27.33 μg per adult and 71.56 μg/cm2, respectively, and also showed strong fumigant toxicity against the two stored product insects with LC50 values of 22.97 and 0.73 mg/liter, respectively.
Therefore, the objective of this study was to detect on the main ingredients of EOs extracted from Citrus limonum (Rutales: Rutaceae), Cy. citratus (Ranales: Lauraceae), L. cubeba (Ranales: Lauraceae), and Muristica fragrans (Magnoliales: Myristicaceae) and assayed the fumigant, contact, repellent activities, and growth and development, and the effects on AChE activities on adults and 10th instar of T. molitor to ensure the potential of the tested EOs as effective alternative insecticides of synthetic insecticides against the beetle occurring storage.
Materials and Methods
Insect
T. molitor was obtained from laboratory cultures and maintained in darkness in incubators at 27 ± 2°C and 60 ± 5% relative humidity (RH). Larvae and adults were all reared with a mix of wheat bran, maize powder, and peanut cake at a 7:0.5:1 weight proportion with 15% water. Successively rose three generations, and the 10th instar and adult eclosion 2 d were employed in all experiments.
EO Extraction and Mainly Ingredient Analysis
Our team collected the leaves of C. limonum, Cy. citratus, L. cubeba, and M. fragrans from a Chinese herbal medicine planting bases of Lushan County, Ya'an city, China, located at 30° 03′ 42′′N, 103° 01′35′′ E during the summer season of 2013 and extracted the EOs from leaves using a modified Clevenger apparatus (Beijing Shi Ji Hui Yuan Technology Co., LTD) for 3–4 h, dried over anhydrous sodium sulfate and refrigerated at 4°C (Mahnaz et al. 2012).
The oils were analyzed by gas chromatography-mass spectrometry (GC-MS) with the some modification methods described by Wang et al. (2014). The GC oven temperature was kept at 50°C for 2 min, programmed at 5°C min−1 ramps to 240°C and then held about 10 min. The temperature of injector and detector was 250°C. He was carrier gas (1 ml min−1, split ratio 1:50) and the samples and n-alkanes (consecutive C8-C40, bought from AccuStandard, Inc. www. accustandard. com) were diluted in acetone (injection of 2 µl). Mass spectra were recorded at 70 eV, and the mass range was m/z 30-600 amu. The compounds were identified by comparing the retention indices (Kovats indices) with their mass spectra stored in the MS database (NIST98 MS DATA) (Haouas et al. 2012) and the relative percentage amounts were according to the GC peak areas.
Contact Activity
The concentration of 60 µg/cm2 for each EO diluted by acetone on the Whatman No. 1 filter papers (9 cm in diameter) were prepared and 1 ml acetone was acted as the blank control. After evaporating solvent about 10 min at 25°C, filter paper filled with EOs or not was then placed inside a glass Petri dish with 30 10th-instar T. molitor, coating Teflon on inner wall in case of escaping. Quickly covered Petri dish and cultivated in an incubator at 27 ± 2°C and 60 ± 5% RH and in darkness. Mortality and corrected mortality were calculated at 24 and 48 h after treatment. Contact toxicities of L. cubeba and C. limonum EOs were assayed with a series of concentrations of 5, 10, 20, 40, 80, 160, and 0 (control) µg/cm2 with the same method (Zhao et al. 2012, Wang et al. 2014). Each treatment was triplications and the LC50 and LC95 (lethal concentration) values, and their 95% confidence intervals (CIs) at 24 and 48 h after treatments were calculated with POLO2.0 (Leora Software, www.leorasoftware.com), respectively.
Repellent Activity
The experimental method was as described by Wang et al. (2006, 2014) with some modifications. Whatman no. 1 filter papers (diameter 12.5 cm) were cut in half and each EO (including 300, 600, and 900 µg/cm2, prepared by dissolving different volumes EO into 1 ml acetone) or only acetone (as the control) was applied to half a filter-paper disc for each treatment. The treatments were air dried at about 26°C for 12 min to evaporate the solvent completely and then pasted the treated and untreated halves together on the opposites. Finally, put it into Petri dishes’ bottom (diameter 12.5 cm) and coated Teflon on inner wall to stop escaping. Thirty 10th instars were released separately at the center of each filter paper disc. The dishes were then covered and transferred into an incubator. Triplications were held for each concentration. After 12, 24, and 48 h, the number of larvae present at each amount of treated or control halves was counted. The distribution coefficient was calculated with the following formula.
The C value is behalf of the number of larvae on control half and T is the number of larvae on treated half. Positive values stood for repellency and negative values expressed attractancy.
Fumigant Activity
The fumigant activities of EOs on 10th instar, and adults of T. molitor were conducted with sealing jar (Deng et al. 2004, Wang et al. 2014). Whatman No.1 filter papers were made into filter paper strips (1.5 cm by 6 cm) and holed with a line adhesive at the sealing of plastic film to avoid contact with the 500 ml vial bottom. Thirty 10th-instar larvae were transferred into the jar, followed by adding 10 µl tested EOs in filter paper strips (concentration of 20 μl/liter) and quickly covered the vial with sealing of plastic film. Triplications were set, and treated vials were kept at 27 ± 2°C and 60 ± 5% RH. Mortalities and adjusted mortalities after 48 and 96 h were calculated by using Abbott’s (1925) formula. The toxicities of the two stronger activities EOs on larvae and adults were assayed with the series of concentrations of 2, 4, 8, 16, 32, and 0(control) µl/liter using the same method over mentioned. The toxicities of EOs of LC50, LC95 values, and their 95% CI at 48 and 96 h were calculated as the description of contact activity.
Growth and Development
Thirty sixth instar were transferred into a plastic case with length by width by height (250 mm by 100 mm by 50 mm), and the two stronger active EOs, including L. cubeba and C. limonum, were added in feed with the concentration of 2 µl/liter until 13th-instar larvae. The growth and development of EOs on larva, pupae, adult, and egg of T. molitor were counted. Three repetitions were set for each treatment.
Fumigant Toxicity of Mainly Active Ingredients
The fumigant toxicity of mainly active ingredient, including D-limonene and β-pinene of C. limonum and L. cubeba, which were all purchased from Aladdin reagent (Shanghai) co. Ltd. (http://www.aladdin-e.com/), were assayed on 10th-instar T. molitor with method 2.5. The concentrations of all tested single or mix of compounds (v/v = 1:1) were set for 2, 4, 8, 16, 32, and 0 (control) µl/liter. The toxicities of EOs of LC50, LC95 values, and their 95% CI after 48–96 h were calculated as the description of contact activity.
AChE Activity Assay
When 15 10th-instar larvae were fumigated with the mixture of D-limonene plus β-pinene, sole D-limonene, or β-pinene (with the concentrations of LC50 at 48 h, respectively) at 12, 24, 36, 48, 60, 72, and 96 h after treatment, the tested insect were frozen with liquid nitrogen and homogenized in 10 ml of 0.1 M ice-cold phosphate buffer (pH 7.0) using a mortar. Homogenates were centrifuged at 7,000 rpm for 15 min at 0°C, and the supernatants were used for the enzyme source and acetylcholine bromide as substrate. Enzyme aliquots (50 μl) and 100 μl 5,5’-Dithiobis-(2-nitrobenzoic acid), DTNB (0.01) were added to 0.1 M phosphate buffer (pH 8.0, 2.8 ml) and incubated at 37°C about 15 min. Acetylcholine bromide (30 μl) was added into the system to react at 37°C for 10 min. The inhibitions of AChE activities treated with compounds were displayed with changes of absorbance at 412 nm with UV 2000-Spectrophotometer (Unic [Shang Hai] Instruments Incorporated), and all the experiments were set for triplicate (Yeom et al. 2012, Wang et al. 2014). Inhibition percentage of AChE activity was obtained with the following formula:
where ODB is the optical density of blank enzyme and ODT is the optical density of treatment.
Statistical Analyses
In this article, the fumigant, contact, repellent activities on test insects, growths, and developments of larvae, pupae, adult, and egg of T. molitor were compared using analysis of variance (Duncan's test for multiple – comparison, P < 0.05) with SPSS v.17.0 software package (IBM, www.ibm.com) in Microsoft Windows 7 operating system (www.microsoft.com). And the figure of growth and development of larvae, pupae, adult, egg, and total of T. molitor was drawn by SigmaPlot v.10.0 software (www.sigmaplot.com).
Results
The Main Ingredients of Test EOs
Based on the GC-MS data (Table 1), the main ingredients of C. limonum contained D-limonene (38.22%) and β-pinene (19.74%), and C. citrates were mainly constituted with 3,7-dimethyl-6-octenal (26.21%) and 2,6-octadien-1-ol, 3,7-dimethyl (20.42%). L. cubeba mainly contained D-limonene (20.22%) and (E)-3, 7-dimethyl-, 2, 6-octadienal (49.78%), and M. fragrans composed of methyl salicylate (6.79%) and (E)-cinnamaldehyde (79.31%).
Table 1.
Chemical constituents and yields (in %) of EOs extracted from C. limonum, Cy. citratus, L. cubeba, and M. fragrans
| EO | Compound | Content (%) | RT (min) | Retention index |
|---|---|---|---|---|
| C. limonum | α-phellandrene | 1.54 | 4.59 | 1,001.4 |
| α-pinene | 4.47 | 4.74 | 1,003.7 | |
| β-pinene | 19.74 | 5.75 | 1,019.5 | |
| D-limonene | 38.22 | 6.56 | 1,032.1 | |
| 1-methyl-4-(1-methylethyl)-1,4-cyclohexadiene | 13.08 | 7.18 | 1,041.8 | |
| (Z)-3,7-dimethyl-,2, 6-octadienal | 2.6 | 10.45 | 1,092.9 | |
| (E)- 3,7-dimethyl-, 2,6-octadienal | 4.04 | 10.99 | 1,201.2 | |
| Other compounds | 16.31 | — | — | |
| Cy. citratus | D-limonene | 5.18 | 13.28 | 1,235.0 |
| 3,7-dimethyl-6-octenal | 26.21 | 17.7 | 1,400.2 | |
| (R)-3,7-dimethyl-,6-octen-1-ol | 15.34 | 19.96 | 1,437.4 | |
| 2,6-octadien-1-ol, 3,7-dimethyl | 20.42 | 20.94 | 1,453.5 | |
| (E)-2,6-octadien-1-ol, 3,7-dimethyl-acetate | 5.01 | 24.05 | 1,605.1 | |
| (1S-cis)-2,3,5,6,8α-hexahydro-4,7-dimethyl-1-(1-methyle thyl)-naphthalene | 4.28 | 27.6 | 1,670.3 | |
| 4-ethenyl-α,α,4- trimethyl-3- (1-methylethenyl)- [1R-(11α,3α,4β)]-cyclohexanemethanol | 5.94 | 28.49 | 1,686.6 | |
| Other compounds | 17.62 | — | — | |
| L. cubeba | 1R-α-pinene | 3.04 | 6.5 | 1,031.2 |
| 4-methylene-1-(1-methylethyl)-bicyclo[3,1,0] hexane | 4.11 | 7.85 | 1,052.3 | |
| 6-methyl-5-hepten-2-one | 2.88 | 8.4 | 1,060.9 | |
| D-limonene | 20.22 | 10.21 | 1,089.1 | |
| (Z)-3,7- dimethyl-2,6-octadienal | 10.57 | 19.79 | 1,434.6 | |
| (E)-3,7-dimethyl-,2,6-octadienal | 49.78 | 21.88 | 1,468.9 | |
| Caryophyllene | 3.37 | 26.7 | 1,653.8 | |
| Other compounds | 6.03 | — | — | |
| M. fragrans | benzaldehyde | 0.70 | 5.76 | 1,019.6 |
| methyl salicylate | 6.79 | 11.95 | 1,215.4 | |
| 3-phenyl-2-propenal | 0.18 | 12.56 | 1,224.4 | |
| (E)-cinnamaldehyde | 79.31 | 14.35 | 1,293.1 | |
| 3-allyl-6-methoxyphenol | 3.93 | 16.45 | 1,281.8 | |
| 3-phenyl-2-propenoic acid | 1.08 | 18.68 | 1,416.3 | |
| 7-methyl-1-naphthol | 0.37 | 21.36 | 1,460.4 | |
| Other compounds | 7.64 | — | — |
RT, retention time.
Contact Activity
Good contact activities of EOs on 10th-instar T. molitor are listed in Table 2. The activity of L. cubeba was the highest, followed by C. limonum. Nevertheless, the contact activities of the other two EOs were not perfect with occurring <50% adjusted mortalities during the experimental period.
Table 2.
The contact activity of EOs on 10th-instar T. molitor
| EO | Number of insects tested | Adjusted mortality of T. molitor (%) (± SE) |
|
|---|---|---|---|
| 24 h | 48 h | ||
| C. limonum | 90 | 46.7 ± 3.9 ba | 65.6 ± 4.0 ba |
| Cy. citratus | 90 | 22.4 ± 2.9 c | 46.7 ± 3.9 c |
| L. cubeba | 90 | 66.7 ± 1.9 a | 88.9 ± 2.9 a |
| M. fragrans | 90 | 32.2 ± 2.2 c | 47.8 ± 2.8 c |
| Control | 90 | 0 d | 0 d |
| F4,10 | — | 96.5 | 110.9 |
| P | — | <0.0001 | <0.0001 |
a Means within a column followed by the different letter are significant (P < 0.05) as determined by Duncan’s test.
The toxicity of EOs of L. cubeba on 10th instar of T. molitor was recorded with LC50 value (19.6 µg/cm2) at 24 h after treatment and was not equitoxic with that of C. limonum (42.2 µg/cm2) (not overlapping CIs), whereas the toxicity of C. limonum (21.2 µg/cm2) was quickly promoted and equitoxic with that of L. cubeba (13.9 µg/cm2) posttreatment 48 h (overlapping CIs) (Table 3). No test insect mortality was observed in the control.
Table 3.
The contact toxicity of EOs on 10th instar of T. molitor
| EO | Treatment time (h) | Number of insects tested | LC50 (µg/cm2) 95% CIa | LC95 (µg/cm2) 95% CIa | Slope ± SE | Chi-square (df) | P* |
|---|---|---|---|---|---|---|---|
| L. cubeba | 24 | 450 | 19.6 | 196.8 | 1.64 ± 0.13 | 3.48 (4) | < 0.05 |
| (16.4–23.2) | (141.4–305.7) | ||||||
| 48 | 450 | 13.9 | 196.2 | 1.43 ± 0.13 | 6.67 (4) | < 0.05 | |
| (8.8–19.7) | (105.0–623.1) | ||||||
| C. limonum | 24 | 450 | 42.2 | 583.2 | 1.44 ± 0.13 | 0.84 (4) | < 0.01 |
| (34.9–51.7) | (368.8–1100.0) | ||||||
| 48 | 450 | 21.2 | 285.5 | 1.46 ± 0.12 | 1.20 (4) | < 0.01 | |
| (17.4–25.5) | (192.9–488.3) | ||||||
| Control | — | 90 | — | — | — | — | — |
a LC50 or LC95 values are considered significantly different when the 95% CI do not overlap.
*Goodness-of-fit test is significant at P < 0.05.
Repellent Activity
The repellent activities of L. cubeba and C. limonum were stronger than those of the other two EOs, which were all almost <50% repellence indexes on 10th instar of T. molitor. The effects of all treatments were weaker and weaker with lower concentration and elongation of experiment (Table 4).
Table 4.
Repellent activity of EOs on 10th-instar T. molitor
| EO | Repellence index after 12 h treatment (%) (±SE) |
Repellence index after 24 h treatment (%) (± SE) |
Repellence index after 48 h treatment (%) (± SE) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 300a | 600 | 900 | 300 | 600 | 900 | 300 | 600 | 900 | |
| C. limonum | 71.9 ± 0.1 bb | 77.7 ± 4.0 b | 89.9 ± 1.9 a | 62.2 ± 3.0 b | 68.3 ± 4.2 b | 83.3 ± 1.9 a | 53.7 ± 2.2 a | 63.9 ± 3.6 b | 75.0 ± 2.9 a |
| Cy. citratus | 41.8 ± 2.9 c | 51.4 ± 2.9 c | 63.1 ± 3.9 b | 35.1 ± 4.5 c | 44.5 ± 2.2 c | 55.0 ± 3.5 b | 28.4 ± 4.5 b | 37.1 ± 2.0 c | 45.7 ± 2.8 b |
| L. cubeba | 83.1 ± 1.8 a | 90.0 ± 1.9 a | 95.1 ± 1.0 a | 73.5 ± 2.1 a | 80.6 ± 0.6 a | 88.5 ± 1.0 a | 59.4 ± 5.3 a | 71.2 ± 1.1 a | 77.3 ± 3.7 a |
| M. fragrans | 37.7 ± 1.2 c | 51.7 ± 2.5 c | 59.1 ± 3.0 b | 31.6 ± 1.0 c | 41.8 ± 1.8 c | 47.6 ± 2.8 c | 24.3 ± 3.0 b | 33.7 ± 2.3 c | 41.33 ± 1.3 b |
| Control | 0 d | 0 d | 0 c | 0 d | 0 d | 0 d | 0 c | 0 d | 0 c |
| F4,10 | 110.9 | 173.5 | 252.0 | 120.4 | 183.2 | 255.8 | 46.5 | 171.0 | 156.2 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
a µg/cm2 filter paper.
b Means within a column followed by the different letter differ significantly (P < 0.05) according to analysis of variance (ANOVA). The number of insects tested for each treatment was 90.
Fumigant Activity
The fumigant activity of L. cubeba on the 10th instar of T. molitor was the strongest from 48 to 96 h after treatment, followed by C. limonum, M. fragrans, and Cy. citratus. No test insect mortality was observed in the control (Table 5).
Table 5.
Fumigant activity of EOs on 10th instar of T. molitor
| EO | Number of insects tested | Adjusted mortality of T. molitor (%) (± SE) |
|
|---|---|---|---|
| 48 h | 98 h | ||
| C. limonum | 90 | 55.6 ± 2.2 ba | 73.3 ± 1.9 ba |
| Cy. citratus | 90 | 30.0 ± 1.9 c | 53.3 ± 1.8 c |
| L. cubeba | 90 | 75.6 ± 2.9 a | 92.2 ± 1.1 a |
| M. fragrans | 90 | 32.2 ± 2.2 c | 58.9 ± 2.9 c |
| Control | 90 | 0 d | 0 d |
| F4,10 | — | 183.3 | 344.5 |
| P | — | <0.0001 | < 0.0001 |
a Means within a column followed by the different letter are significant (P < 0.05) as determined by Duncan’s test.
The toxicities of L. cubeba on 10th instar and adults of T. molitor were significant stronger than those of C. limonum (not overlapping CIs). Meanwhile, the toxicities of L. cubeba on 10th instar (2.7 μl/liter) and adults (3.7 μl/liter) were almost equitoxic (overlapping CIs) at 96 h after treatment, respectively (Tables 6 and 7). No test insect mortality was observed in the control.
Table 6.
The fumigant toxicity of EOs on 10th instar of T. molitor
| EO | Treatment time (h) | Number of insects tested | LC50 (µl/liter) 95% CIa | LC95 (µl/liter) 95% CIa | Slope ± SE | Chi-square (df) | P* |
|---|---|---|---|---|---|---|---|
| L. cubeba | 48 | 450 | 4.5 (3.7–5.3) | 50.0 (35.0–80.6) | 1.57 ± 0.13 | 1.94 (4) | < 0.05 |
| 96 | 450 | 2.7 (2.3–3.2) | 22.2 (16.7–32.3) | 1.80 ± 0.15 | 3.05 (4) | < 0.05 | |
| C. limonum | 48 | 450 | 29.2 (21.1–46.5) | 781.3 (325.3–3,110.4) | 1.15 ± 0.14 | 0.51 (4) | <0.01 |
| 96 | 450 | 10.9 (8.8–13.9) | 209.2 (117.6–479.3) | 1.28 ± 0.12 | 0.84 (4) | < 0.05 | |
| Control | — | 90 | — | — | — | — | — |
a LC50 or LC95 values are considered significantly different when the 95% CI do not overlap.
*Goodness-of-fit test is significant at P < 0.05.
Table 7.
The fumigant toxicity of EOs on adults of T. molitor
| EO | Treatment time(h) | Number of insects tested | LC50 (µl/liter) 95% CIa | LC95 (µl/liter) 95% CIa | Slope ± SE | Chi-square (df) | P* |
|---|---|---|---|---|---|---|---|
| L. cubeba | 48 | 450 | 5.3 (4.4–6.3) | 59.3 (41.1–97.1) | 1.57 ± 0.13 | 1.44 (4) | < 0.05 |
| 96 | 450 | 3.7 (3.0–4.5) | 47.6 (32.8–78.9) | 1.48 ± 0.13 | 1.70 (4) | < 0.05 | |
| C. limonum | 48 | 540 | 25.3 (18.7–38.5) | 636.0 (280.1–2,263.8) | 1.17 ± 0.13 | 0.41 (4) | < 0.01 |
| 96 | 450 | 12.0 (9.5–15.9) | 289.0 (149.7–764.0) | 1.19 ± 0.12 | 1.21 (4) | < 0.05 | |
| Control | — | 90 | — | — | — | — | — |
a LC50 or LC95 values are considered significantly different when the 95% CI do not overlap.
*Goodness–of–fit test is significant at P < 0.05.
Growth and Development
The results indicated that the growth and development of larvae of T. molitor treated with EOs were clearly elongated with 52.9 and 52.3 d for L. cubeba and C. limonum, respectively, and significant with that of control (39.6 d, P < 0.05). On the contrary, the growth and development of pupae of T. molitor treated with EOs (with 6.2 d for L. cubeba and 6.3 d for C. limonum) were shorter than that of control with 7.4 d (P < 0.05). The developmental stages of adults dealt with EOs or not were not different, which of the control, L. cubeba, and C. limonum were 43.5, 39.8, and 41.6 d, respectively (P > 0.05). However, the developmental stages of eggs dealt with L. cubeba (6.3 d) were longer than those of the control (5.2 d) but not significant with C. limonum (6.0 d) (P > 0.05). A generation of growths and developments treated with EOs (105.3 d for L. cubeba and 106.2 d for C. limonum, respectively) were significant with that of the control 95.7 d (P < 0.05) (Fig. 1).
Fig. 1.
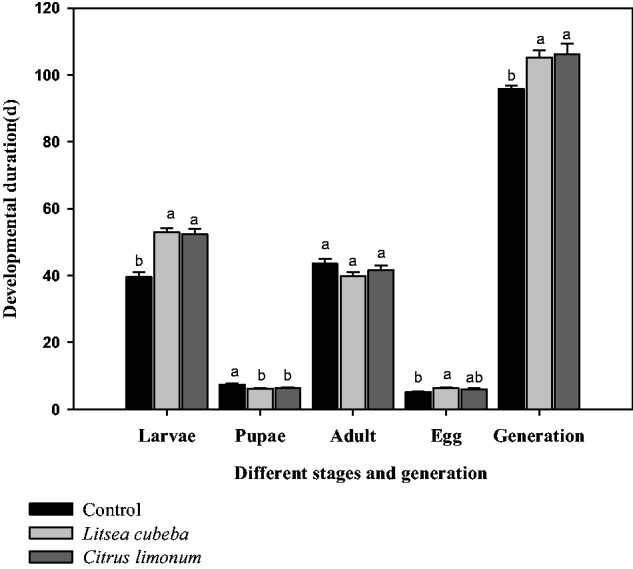
Effects of EOs on growth and development of larvae, pupae, adult, and egg of T. molitor. Note: number of insects tested for each treatment is 90 and the vertical bars represent the standard error of means for three replicates values. The growth and development of larvae, pupae, adult, and egg of T. molitor were the avenue of three replications ± SE. Means followed by the same letters do not differ significantly (P > 0.05) in the analysis of variance (ANOVA) test. F2, 6 = 26.15 and P < 0.001 for larvae; F2, 6 = 7.24 and P = 0.03 < 0.05 for pupae; F2, 6 = 1.09 and P = 0.24, > 0.05 for adult; F2, 6 = 3.40 and P = 0.10, > 0.05 for egg; F2, 6 = 6.76 and P = 0.03, <0.05 for total growth and development treated with EOs or not.
Fumigant Toxicities of Mainly Active Ingredients
The toxicity of the mixture of D-Limonene plus β-pinene on 10th instar of T. molitor was the strongest at 48 h after treatment and significant with the other two sole compounds (not overlapping CIs), followed by the D-Limonene and significant with β-pinene (not overlapping CIs). The toxicities of D-Limonene on 10th instar of T. molitor were quickly enhanced and seemed to be equitoxic with that of mix of D-Limonene plus β-pinene (2.0 μl/liter) 96-h posttreatment (overlapping CIs) (Table 8). No test insect mortality was found in the control.
Table 8.
The fumigant toxicity of main ingredients of EOs on 10th instar of T. molitor
| Treatment | Treatment Time (h) | Number of insects tested | LC50 (µl/liter) 95% CIa | LC95 (µl/liter) 95% CIa | Slope ± SE | Chi-square (df) | P* |
|---|---|---|---|---|---|---|---|
| D-Limonene plus β-pinene | 48 | 450 | 2.9 (2.4–3.5) | 48.0 (30.2–92.5) | 2.42 ± 0.22 | 6.69 (4) | < 0.05 |
| 96 | 450 | 2.0 (1.6–2.4) | 30.7 (20.0–56.1) | 2.48 ± 0.23 | 2.84 (4) | < 0.05 | |
| D-limonene | 48 | 450 | 4.3 (3.6–5.2) | 60.1 (39.8–106.6) | 2.57 ± 0.23 | 3.65 (4) | < 0.05 |
| 96 | 450 | 3.0 (2.0–4.1) | 36.2 (19.7–112.0) | 2.72 ± 0.25 | 6.59 (4) | < 0.05 | |
| β-pinene | 48 | 450 | 10.1 (8.3–12.5) | 168.9 (100.4–356.0) | 2.41 ± 0.23 | 1.82 (4) | < 0.05 |
| 96 | 450 | 6.5 (5.4–7.8) | 94.3 (60.4–175.8) | 2.53 ± 0.23 | 0.83 (4) | < 0.05 | |
| Control | — | 90 | — | — | — | — | — |
aLC50 or LC95 values are considered significantly different when the 95% CI do not overlap.
*Goodness-of-fit test is significant at P < 0.05.
Inhibitions of Mainly Active Ingredient of EOs on AChE Activity
The mix of D-limonene plus β-pinene displayed the strongest inhibition on AChE activity in 10th instar of T. molitor and was very significant with other treatments from 12 h to 96 h (P < 0.01), which of AChE-inhibition rate was<50% before 48 h; however, the effects of D-limonene on AChE activities were clearly improved and significant with those of β-pinene from 60 to 96 h (P < 0.01) (Fig. 2).
Fig. 2.
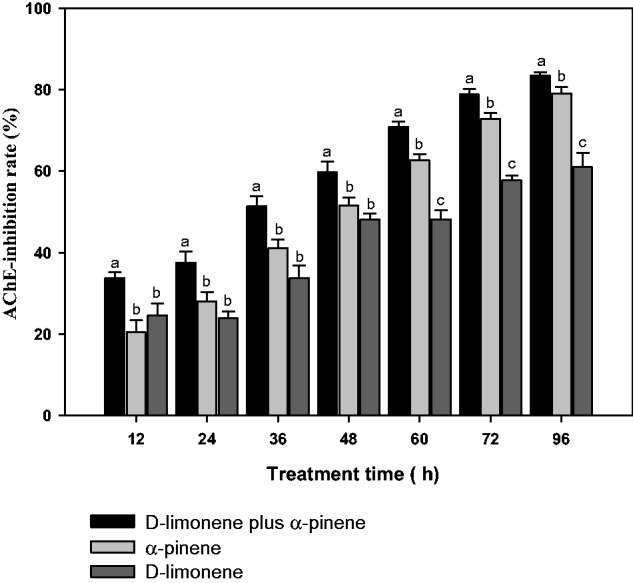
The time course of the mainly active ingredients of EOs on AChE activity of 10th instar T. molitor. Note: number of insects tested for each treatment is 90 and the vertical bars represent the standard error of means for three replicates values. The growth and development of larvae, pupae, adult and egg of T. molitor were the avenue of three replications ± SE. Means followed by the same letters do not differ significantly (P > 0.05) in the analysis of variance (ANOVA) test. The F2,6 values were 6.70, 9.26, 11.75, 8.66, 41.80, 62.55, and 27.14 were at 12, 24, 36, 48, 60, 72, and 96 h, respectively, and all P < 0.01.
Discussion
EO is considered to be an alternative means of controlling many pests (Wang et al. 2010). There were some early reports on the insecticidal activities of EOs from C. limonum (Ponce et al. 2004, Moreira et al. 2005) and L. cubeba (Jiang et al. 2009, Seo et al. 2009) on some storage insects. The results of this study showed that tested EOs had strong insecticidal activities on adult and/or nymphal stages of T. molitor. The EOs of L. cubeba and C. limonum have also displayed strong insecticidal toxicities on larvae than adults of T. molitor, which were consistent with some early researches (Pavela 2008, Jiang et al. 2009, Seo et al. 2009). Our data also demonstrated that EOs of L. cubeba and C. limonum possessed strong contact and repellent activities on T. molitor.
There were some previously researches reported that effects of plant EOs are related to their chemical ingredients (Ngassoum et al. 2007, Ko et al. 2009), including pulegone, linalool, eugenol, thymol, cymol, methyl chavicol, and so on, which were known to be poisonous to many insects (Park et al. 2006b, Thongdon and Inprakhon, 2009). The ingredients of tested EOs in this study were included in following chemical compositions: α- pinene, β- pinene, D-limonene, (E)-3, 7-dimethyl-, 2, 6-octadienal, and so on, which have been reported to be toxic to some insects (Park et al. 2006a, Zapata and Smagghe 2010). We also found an interesting phenomenon that the main ingredient, D-limonene from C. limonum collected during the 2012 autumn from Wenjiang district reached 64.53% (Wang et al. 2014). However, the content of limonene from C. limonum collected in the Lushan County, Ya'an City, in this article was only 38.22%, which could be explained that the ingredients of same plant at different places and seasons could metabolism different compounds, yields of EOs and displayed distinct activities (Hussain et al. 2010). We found that the growth and development of larvae of T. molitor were elongated and pupae were slightly shortened compared with those of the control. The phenomenon was partly consistent with the early results. Qin et al. (2010) reported that the EO from the leaves of Piper sarmentosum could markedly prolong the developmental duration of Brontispa longissima in different instars, which of the control was 25.7d, whereas the P. sarmentosum EOs treatment from 100 mg/liter to 2,000 mg/liter were elongated from 27.69 d to 40.26 d. Meanwhile, a generation of the control was only 43.34 d, but which of the tested EOs were prolonged from 48.06 d to 73.58 d, respectively.
Another stirring result that the mix of D-limonene and β-pinene have been synergy to improve the toxicities and AChE-inhibition on 10th instar of T. molitor compared with single D-limonene and β-pinene have been demonstrated (Table 8 and Fig. 2). The results were supported with some previous reports (Feng et al. 1995, Maurya et al. 2012). So the effects of multi-ingredients of EOs could interact and enhance the ability of inhibitions on the targets, such as AChE, mixed-functional oxidase, carboxylesterase, and glutathione S-transferase, and so on and result in the better efficacies on the pests.
In general, the EOs extracted from C. limonum and L. cubeba have potent fumigant, content, and repellent activities and might be used for effective managements of T. molitor occurred in J. effuses. Our conclusions were necessarily tenuous, and further studies were required since the information about the safe of EOs resided in J. effuses were not clearly even though with heat-treatment. However, the above results have provided a foundation for subsequent efforts for exploiting the safe, environment friendly agency to effectively manage the insect.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities Insecticidal ingredients and actives of Essential Oils on several insects. (2011JS080).
References Cited
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Copping L. G., Menn J. J. 2000. Biopesticides: a review of their action, applications and efficacy. Pest Manage. Sci. 56: 651–676. [Google Scholar]
- Deng Y., Wang J., Ju Y., Zhang H. 2004. Comparison of fumigation activities of 9 kinds of essential oils against the adults of maize weevil, Sitophilus zeamaise Motschulsky (Coleoptera:Curculionidae). Chin. J. Pestic. Sci. 6: 85–88. [Google Scholar]
- Feng R. Y., Chen W. K., Isman M. B. 1995. Synergism of malathion and inhibition of midgut esterase activities by an extract from Melia toosendan (Meliaceae). Pestic. Biochem. Physiol. 53: 34–41. [Google Scholar]
- Hanifah A. L., Awang S. H., Ming H. T., Abidin S. Z., Omar M. H. 2011. Acaricidal activity of Cymbopogon citratus and Azadirachta indica against house dust mites. Asian Pac. J. Trop. Biomed. 1:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouas D., Cioni P. L., Halima-Kamel M. B., Flamini G., Mohamed H. B. 2012. Chemical composition and bioactivities of three Chrysanthemum essential oils against Tribolium confusum (du Val) (Coleoptera: Tenebrionidae). J. Pestic. Sci. 3: 367–379. [Google Scholar]
- Hussain A. I., Anwar F., Nigam P. S., Ashraf M., Gilani A. H. 2010. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 90: 1827–1836. [DOI] [PubMed] [Google Scholar]
- Jiang Z. L., Akhtar Y., Bradbury R., Zhang X., Isman M. B. 2009. Comparative toxicity of essential oils of Litsea pungens and Litsea cubeba and blends of their major constituents against the Cabbage Looper, Trichoplusia ni. J. Agric. Food Chem. 57: 4833–4837. [DOI] [PubMed] [Google Scholar]
- Ko W., Juntarajumnong K., Chandrapatya A. 2009. Fumigant and contact toxicities of Litsea cubeba (Lour.) Persoon against Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). Kasetsart J. Nat. Sci. 43: 56–63. [Google Scholar]
- Li X. Y., Qian Y., Wang X. G., Chen J., Song Y. 2009. Preliminary study on the community structure of storage insects of Juncus effuses L. products in Wujiang area. J. Anhui Agric. Sci. 37: 17545–17546, 17548. [Google Scholar]
- Liu Z. L., Goh S. H., Ho S. H. 2007. Screening of Chinese medicinal herbs for bioactivity against Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J. Stored Prod. Res. 43: 290–296. [Google Scholar]
- Mahnaz K., Alireza F., Hassan V., Mahdi S., Reza M. A., Abbas H. 2012. Larvicidal activity of essential oil and methanol extract of Nepeta menthoides against malaria vector Anopheles stephensi. Asian Pac. J. Trop. Med. 5: 962–965. [DOI] [PubMed] [Google Scholar]
- Maurya P., Sharma P., Mohan L., Verma M. M., Srivastava C. N. 2012. Larvicidal efficacy of Ocimum basilicum extracts and its synergistic effect with neonicotinoid in the management of Anopheles stephensi. Asian Pac. J. Trop. Dis. 2: 110–116. [Google Scholar]
- Moreira M. R., Ponce A. G., Valle Del C. E., Roura S. I. 2005. Inhibitory parameters of essential oils to reduce a foodborne pathogen. Lwt-Food Sci. Technol. 38: 565–570. [Google Scholar]
- Nesci A., Montemarani A., Passone M. A., Etcheverry M. 2011. Insecticidal activity of synthetic antioxidants, natural phytochemicals, and essential oils against an Aspergillus section Flavi vector (Oryzaephilus surinamensis L.) in microcosm. J. Pest Sci. 84: 107–115. [Google Scholar]
- Ngassoum M. B., Tinkeu L.S.N., Ngatanko I., Tapondjou L. A., Lognay G., Malaisse F., Hance T. 2007. Chemical composition, insecticidal effect and repellent activity of essential oils of three aromatic plants, alone and in combination, towards Sitophilus oryzae L. (Coleoptera: Curculionidae). Nat. Prod. Commun. 2: 1229–1232. [Google Scholar]
- Oyedele A. O., Gbolade A. A., Sosan M. B., Adewoyin F. B., Soyelu O. L., Orafidiya O. O. 2002. Formulation of an effective mosquito-repellent topical product from lemongrass oil. Phytomedicine 9: 259–262. [DOI] [PubMed] [Google Scholar]
- Park I. K., Choi K. S., Kim D. H., Choi I. H., Kim L. S., Bak W. C., Choi J. W., Shin S. C. 2006a. Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae). Pest Manage. Sci. 62: 723–728. [DOI] [PubMed] [Google Scholar]
- Park I. K., Choi K. S., Kim D. H., Choi I. H., Kim L. S., Bak W. C., Choi J. W., Shin S. C. 2006b. Fumigant activity of plant essential oils and components from Schizonepeta tenuifolia against Lycoriella ingenua (Diptera: Sciaridae). J. Econ. Entomol. 99: 1717–1721. [DOI] [PubMed] [Google Scholar]
- Pavela R. 2008. Insecticidal properties of several essential oils on the house fly (Musca domestica L.). Phytother. Res. 22: 274–278. [DOI] [PubMed] [Google Scholar]
- Phasomkusolsil S., Soonwera M. 2011. Comparative mosquito repellency of essential oils against Aedes aegypti (Linn.), Anopheles dirus (Peyton and Harrison) and Culex quinquefasciatus (Say). Asian Pac. J. Trop. Biomed. 1:113–118. [PubMed] [Google Scholar]
- Ponce A. G., Valle Del C. E., Roura S. I. 2004. Natural essential oils as reducing agents of peroxidase activity in leafy vegetables. Lebensmittel-Wissenschaft Und-Technologie-Food Sci. Technol. 37: 199–204. [Google Scholar]
- Qin W. Q., Huang S. C., Li C. X., Chen S. T., Peng Z. Q. 2010. Biological activity of the essential oil from the leaves of Piper sarmentosum Roxb. (Piperaceae) and its chemical constituents on Brontispa longissima (Gestro) (Coleoptera: Hispidae). Pestic. Biochem. Physiol. 96: 132–139. [Google Scholar]
- Rossi E., Cosimi S., Cioni P. L., Canale A. 2009. Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: Evaluation of repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). J. Stored Prod. Res. 45: 125–132. [Google Scholar]
- Seo S. M., Kim J., Lee S. G., Shin C. H., Shin S. C., Park I. K. 2009. Fumigant antitermitic activity of plant essential oils and components from Ajowan (Trachyspermum ammi), Allspice (Pimenta dioica), Caraway (Carum carvi), Dill (Anethum graveolens), Geranium (Pelargonium graveolens), and Litsea (Litsea cubeba) oils against Japanese Termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 57: 6596–6602. [DOI] [PubMed] [Google Scholar]
- Stefanazzi N., Stadler T., Ferrero A. 2011. Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored-grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manage. Sci. 67: 639–646. [DOI] [PubMed] [Google Scholar]
- Thongdon J., Inprakhon P. 2009. Composition and biological activities of essential oils from Limnophila geoffrayi Bonati. World J. Microbiol. Biotechnol. 25: 1313–1320. [Google Scholar]
- Wang J., Zhu F., Zhou X. M., Niu C. Y., Lei C. L. 2006. Repellent and fumigant activity of essential oil from Artemisia vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 42: 339–347. [Google Scholar]
- Wang X. G., Wei X. Y., Tian Y. Q., Shen L. T., Xu H. H. 2010. Antifungal flavonoids from Ficus sarmentosa var. henryi (King) Corner. Scientia Agricultura Sinica 9: 690–694. [Google Scholar]
- Wang X. G., Li Q., Shen L. T., Jiang S. R., Yang Q. F., Li Q., Yang J. Z., Jiang C. X. 2014. Fumigant, contact and repellent activities of essential oils against darkling beetle, Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Insect Sci. 14: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E. M., Priestley C. M., Burgess I. F. 2007. An investigation and comparison of the bioactivity of selected essential oils on human lice and house dust mites. Fitoterapia 78: 521–525. [DOI] [PubMed] [Google Scholar]
- Yang K., Wang C. F., You C. X., Geng Z. F., Sun R. Q., Guo S. S., Du S. S., Liu Z. L., Deng Z. W. 2014. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia Pac. Entomol. 17: 459–466. [Google Scholar]
- Yeom H. J., Kang J. S., Kim G. H., Park I. K. 2012. Insecticidal and acetylcholine esterase inhibition activity of apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). J. Agric. Food Chem. 60: 7194–7203. [DOI] [PubMed] [Google Scholar]
- Zapata N., Smagghe G. 2010. Repellency and toxicity of essential oils from the leaves and bark of Laurelia sempervirens and Drimys winteri against Tribolium castaneum. Ind. Crops Prod. 32: 405–410. [Google Scholar]
- Zhao N. N., Zhou L. G., Liu Z. L., Du S. S., Deng Z. W. 2012. Evaluation of the toxicity of the essential oils of some common Chinese spices against Liposcelis bostrychophila. Food Control 26: 486–490. [Google Scholar]


