Abstract
The insect growth regulator novaluron (Rimon 10 EC, Makhteshim-Agan Ltd, Israel) is used against many field pests on corn, vegetables, orchards, forests, and cotton plantations. Previously, we studied various effects of novaluron on stored grain pests. Termination in Tribolium castaneum (Herbst) eggs hatching after treating adults with novaluron and following restoration after adult transfer to untreated media was observed. The objective of this study was to investigate the restoration of T. castaneum egg hatch following transfer of adults from treated media to untreated favorable and unfavorable media. The time needed for hatching restoration of 50% of eggs laid by adults transferred from novaluron (1 ppm) treated flour to untreated flour (RT50) was 2.7 d. RT50 for those transferred to untreated wheat grain was 4.1 d. RT90 in flour was 3.6 d, in grain—6.1 d. Varieties of RTs in grain and in flour with nonoverlapping confidence intervals indicate that RTs were significantly different. Delay of eggs hatching restoration for adults transferred from treated flour to unfavorable media (Petri dishes with limited amount of flour, lying of eggs not detected) was observed. RT50 in flour was 2.1 d and RT90—3.1 d, while RT50 in the unfavorable media was 3.4 d and RT90 6.5 d. Delayed effect of egg hatching restoration after adult transfer to unfavorable media provides evidence of the significant role of insect physiological state in novaluron excretion and (or) degradation by T. castaneum females.
Keywords: chitin synthesis inhibitor, egg hatching restoration, novaluron, Tribolium castaneum
Red flour beetle, Tribolium castaneum (Herbst) is a widespread stored grain insect. It damages flour and bakery, bran, cereals, and dried fruit, while whole dry grain stays almost untouched. The beetle has a stable carbolic smell, which passes from the contaminating grain to the flour. Red flour beetle is originally much more pesticide resistant than other stored product insects, and this resistance can rapidly increase more than 10-fold. Its short development cycle and easiness of laboratory cultures maintaining on a simple medium has made this species a popular choice as a model organism for studying pesticide effects (Zettler and Cuperusi 1990, Assie et al. 2007, Wijayaratne et al. 2012).
One of the main demands for insecticides is their influence only on insect metabolic processes. Development disruption insecticides form the insect growth regulators (IGRs) group. Chitin synthesis inhibitors (CSI) belong to the IGRs group. CSIs are insecticides (usually benzoylphenyl urea based substances) that inhibit chitin production. One of them is novaluron. This insecticide (Rimon 10 EC, Makhteshim-Agan Ltd, Israel) is used against many field pests to protect corn, vegetables, orchards, forests, and cotton plantations. Novaluron activity is based on larvicidal effect. Various effects of novaluron on stored grain pests were studied (Kostyukovsky et al. 2003, Arthur and Fontenot 2012). The results showed significant decrease in rice weevil Sitophilus oryzae L. population, even though the pest develops inside the seed and larva has no direct contact with the pesticide (Kostyukovsky et al. 2003). The same results were observed for other CSIs (McGregor and Kramer 1976; Desmarchelier and Allen 1992; Elek and Longstaff 1994; Oberlander et al.1997; Elek 1998a,b).
We found that exposure of T. castaneum adult to treated flour may serve as a good model for our understanding of CSIs effect on internal feeders. Termination in eggs hatching after treating adults with novaluron and restoration after adult transfer to untreated media was shown. There is the correlation between duration of adults contact with treated media and novaluron concentration with hatching restoration. This most probably resulted from direct contact penetration of novaluron into the insect, and then into the eggs. The eggs hatching in T. castaneum was significantly reduced even at 0.3-ppm novaluron concentration. (Kostyukovsky and Trostanetsky 2006, Trostanetsky and Kostyukovsky 2008). The same results were observed for other CSIs, such as triflumuron (Yasir et al. 2012) and lufenuron (Mishra et al. 2013). Restoration of hatching eggs after the termination of CSIs contact with adults was noted (Desmarchelier and Allen 1992, Kostyukovsky and Trostanetsky 2006, Alyokhin et al. 2009). However, more detailed studies have not been conducted. The objective of this study was to investigate the restoration of egg hatching of T. castaneum following transfer of adults from treated media to untreated favorable and unfavorable media.
Material and Methods
Insects
T. castaneum culture had been reared under laboratory conditions for many years without any contact with insecticides. It was reared on wheat flour and ground grain. The insects were maintained in 0.8-liter glass jars at 30 ± 0.5°С and 65 ± 5% r.h. in dark.
Chemical
Novaluron, 1-[3-chloro-4-(1,1,2-trifluoro-2-trifluoromethoxyethoxy)phenyl]-3-(2,6-difluorobenzoyl) urea, supplied by Makhteshim-Agan Ltd as Rimon 10 EC, was used for the experiments.
Treatment
Thirty milliliter of 16.7-ppm novaluron (Rimon 10 EC) in acetone solution were fractionally introduced into 500 g of wheat flour and were thoroughly mixed. The container was then washed with 10 ml of acetone, afterwards poured into the flour as well. Final novaluron concentration in the flour was 1 ppm. The wheat flour with acetone alone was used as a control. The treated culture was ventilated to evaporate all the acetone from the samples. Approximately 2,000 adults of T. castaneum were transferred into 0.8-liter glass jars with 300-g novaluron-treated or -untreated (control) flour. The jars were covered with airpenetrable paper and stored in dark at 30 ± 0.5°С and 65 ± 5% r.h. Two wks later, adults were transferred to a various media, without novaluron.
Effect of the Medium on Eggs Hatching Restoration
The first experiment was conducted to compare the eggs hatching restoration in wheat flour and wheat grain medium after transfer of adults from novaluron-treated flour. Insects from jars with treated (experiment) and untreated (control) flour were divided into two groups and transferred into jars with untreated flour (experiment and control) or untreated grain (experiment and control).
Every day 60 unsexed adults T. castaneum were taken from each of the four jars and transferred to 70-ml jars with 30 g of flour, 20 adults a jar. The 0- to 24-h-old eggs laid were separated from the flour by 70-mesh sieve. Fifty eggs from each replicate were placed in especially designed plastic slides with individual cell for each egg and clip-on with glass cover and maintained in dark at 30 ± 0.5°С and 65 ± 5% r.h. The egg hatch was counted under a binocular microscope.
The second experiment was designed to prove the hypothesis that unfavorable conditions may delay the secretion of novaluron from the insect organism. For this experiment insects from jars with treated (experiment) and untreated (control) flour were divided into two groups, as it was done in the first experiment, and transferred into two jars with untreated flour (experiment and control) and Petri dishes (20 T. castaneum per dish) containing 0.1 mg of flour each (50 dishes for the experiment and 50 for control). In the preliminary experiment, it was found that T. castaneum do not lay eggs under the conditions created in the dishes. Every day 60 T. castaneum were collected from the jars and transferred to flour medium as described earlier. Insects from six Petri dishes (three for experiment and three for control) were also transferred into jars with flour. The quantity of daily eggs laid after transferring the insects from the dishes to the flour was decreased during the experiment. Thus, T. castaneum adults from two dishes (40 adults) were transferred into each jar from the seventh day. The 0- to 24-h-old eggs separation and hatching percentage calculation was done as described earlier. Both experiments were conducted in three replicates.
Statistical Analysis
All results were corrected with Abbott’s (1925) formula, which takes into account the egg hatching in parallel control assays. The regression analyses were done using Excel. POLO-PC probit analysis program (Le Ora Software 1987) was used for RT50 and RT90 (the time needed for eggs hatching restoration up to 50 and 90%, respectively, after the insects exposure to novaluron) with corresponding 95% confidence interval (CI) and slope with the standard error calculation.
Results
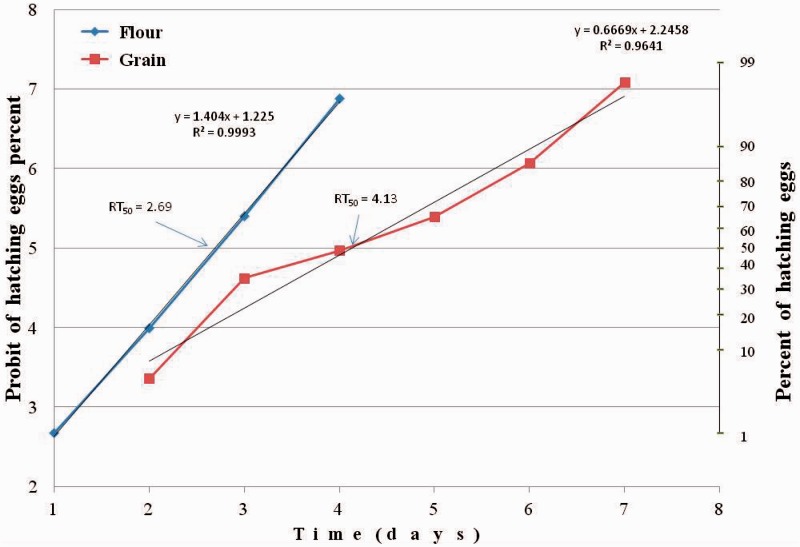
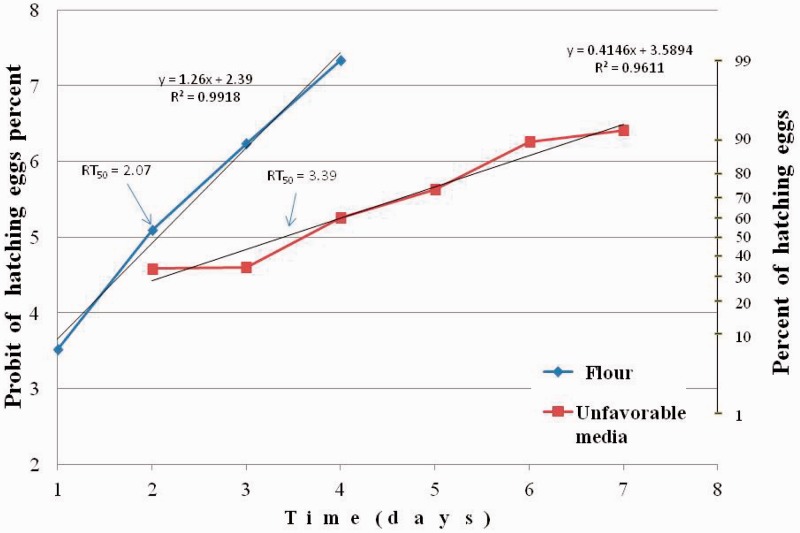
Eggs hatching percentage in the control was 92.6 ± 0.7%. We should note that for the control insects, transferred from flour to flour this percent was higher than for those transferred from grain or Petri dishes to the flour. In the first experiment, we compared the restoration time of normal eggs hatching after transferred T. castaneum adults to different novaluron-free medium (flour and grain). The time needed to restore eggs hatchability was significantly different on these media (ANOVA: F = 4.67; df = 1,16; P = 0.046). The time needed for hatching restoration of 50% eggs laid by adults transferred from novaluron (1 ppm) treated flour to untreated flour (RT50) was 2.7 d (95% CI: 2.6–2.8; slope 1.40 ± 0.02). The time of 50% restoration for those transferred to untreated grain was 4.1 d (CI: 3.5–4.6; slope 0.67 ± 0.06). RT90 in flour—3.6 d (CI: 3.4–3.8; slope 1.40 ± 0.02), in grain—6.1 d (CI: 5.5–7.3; slope 0.67 ± 0.06) (Fig. 1). Similar results were obtained in the second experiment. The same restoration rate for T. castaneum transferred to flour and the delay of eggs hatching restoration for adults transferred to unfavorable media (Petri dishes with limited amount of flour, lying of eggs not detected) were observed. The time needed to restore eggs hatchability was significantly different on these media. (ANOVA: F = 17.63; df = 1,16; P = 0.00068). For the latter, the restoration up to the control level was the same as for those transferred to grain in the first experiment: RT50—3.4 d (CI: 2.8–3.8), RT90 6.5 d (CI: 5.8–7.6); slope 0.41 ± 0.04. RT50 on flour was 2.1 d (CI: 2.0–2.2) and RT90—3.1 d (CI: 2.9–3.3); slope 1.26 ± 0.08 (Fig. 2).
Fig. 1.
Restoration of egg hatch of T. castaneum following transfer from treated with novaluron flour to untreated flour or untreated grain.
Fig. 2.
Restoration of egg hatch of T. castaneum following transfer from treated with novaluron flour to untreated flour or unfavorable media. Limited amount of flour in Petri dishes was used as unfavorable media (lying of eggs not detected).
Discussion
According to the accepted hypothesis, novaluron, as all the benzoylphenyl urea based CSIs, affects the eggs hatching by transmission into the egg during its formation in the insect organism. This is the reason for low laid eggs hatching directly after transferring T. castaneum on insecticide free medium (Desmarchelier and Allen 1992; Elek 1998a,b; Kostyukovsky and Trostanetsky 2006; Alyokhin et al. 2008; Trostanetsky and Kostyukovsky 2008, Kim et al. 2011).
In this study, the restoration of T. castaneum egg hatch after transfer of adults from treated media to untreated media was investigated. Flour and grain were used in this experiment as a media. It should be noted that the use of insecticides in flour has no practical importance. However, flour may serve as a good model for understanding of CSI’s transovarial effect on T. castaneum and other stored product insects. The received data is presented on probit-time correlation scale. Probit analysis was used before to describe the correlation between mortality and time of contact with the insecticide. It was described and recognized as appropriate (Preisler and Robertson 1989, Throne et al. 1995). On the other hand, we did not find any mentioning in relevant literature of the use of this analysis to describe the correlation between the time and eggs hatching restoration percentage after the end of insecticide effect. However, our data perfectly correspond with this correlation. The excretion and (or) degradation rate of an insecticide in a living organism is described as an exponential correlation (Gazit et al. 1989, Stamm et al. 2013); therefore, we used a linear axis scale for a while.
According to our current data, eggs hatching restoration time in T. castaneum after 2 wk of exposure to 1-ppm novaluron medium was 4 d after transferring to untreated flour. Earlier we showed the correlation between the restoration time and novaluron concentration in the treated medium. After 2 wk of exposure to medium with 100-ppm novaluron the restoration time for T. castaneum was more than 3 wk. (Kostyukovsky and Trostanetsky 2006). In this article, we demonstrated that the egg hatching restoration in adults transferred to grain medium was slower than in flour medium. This effect may be caused by unfavorable conditions for insects in the grain medium. We conducted an additional experiment to verify our hypothesis. After 2-wk exposure to novaluron-treated flour T. castaneum adults were transferred to Petri dishes with minimal amount of flour, which allows preventing mortality of T. castaneum adult, but no eggs were laid. There was a delay in egg hatching restoration in comparison to the control but after 7 d the egg hatching returned to normal. Delayed effect of egg hatching restoration after adult transfer to unfavorable media may be explaining significant role of eggs in novaluron excretion. The literature data do not support this conclusion. CSIs can be excreted into eggs but feces play a more significant role (Ivie and Wright 1978, Medina et al. 2002). After topical application of diflubenzuron on lacewing Chrysoperla carnea (Stephens), 20% of the CSI were excreted into the feces while only 1% was excreted into eggs. Diflubenzuron treated Musca domestica L. and Stomoxys calcitrans L. flies also showed low level of the CSI in eggs (<1%).
It is known that various environmental factors, including commodity characteristics of grain affect the sensitivity of insects to insecticides. It may be explained by physiological state of insects (Athanassiou et al. 2011; Kavallieratos et al. 2012, Subramanyam et al. 2012). Alyokhin et al. (2009) investigated dependency of novaluron effects on physiological state of the beetles. It was shown, that time of hatching restoration of eggs Colorado potato beetle Leptinotarsa decemlineata (Say) after transfer from novaluron-treated foliages to -untreated foliages was 4 d in young beetles and 3 d in old. We suggest that in our experiments physiological state of insect plays the main role in delayed effect of egg hatching restoration after adult transfer to unfavorable media.
References Cited
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Alyokhin A., Sewell G., Choban R. 2008. Reduced viability of Colorado potato beetle, Leptinotarsa decemlineata, eggs exposed to novaluron. Pest Manag. Sci. 64: 94–99. [DOI] [PubMed] [Google Scholar]
- Alyokhin A., Guillemette R., Choban R. 2009. Stimulatory and suppressive effects of novaluron on the Colorado potato beetle reproduction. J. Econ. Entomol. 102: 2078–2083. [DOI] [PubMed] [Google Scholar]
- Arthur F. H., Fontenot E. A. 2012. Residual activity of methoprene and novaluron as surface treatments to manage the flour beetles, Tribolium castaneum and Tribolium confusum. J. Insect Sci. 12: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assie L. K., Francis F., Gengler N., Haubruge E. 2007. Response and genetic analysis of malathion-specific resistant Tribolium castaneum (Herbst) in relation to population density. J. Stored Prod. Res. 43: 33–44. [Google Scholar]
- Athanassiou C. G., Arthur F. H., Throne J. E. 2011. Efficacy of layer treatment with methoprene for control of Rhyzopertha dominica (Coleoptera: Bostrychidae) on wheat, rice and maize. Pest Manag. Sci. 67: 380–384. [DOI] [PubMed] [Google Scholar]
- Desmarchelier J. M., Allen S. E. 1992. Diflubenzuron as a grain protectant for control of Sitophilus species. J. Stored Prod. Res. 28: 283–287. [Google Scholar]
- Elek J. A. 1998a. Interaction of treatment of both adult and immature Coleoptera with a chitin synthesis inhibitor affects mortality and development time of their progeny. Entomol. Exp. Appl. 89: 125–136. [Google Scholar]
- Elek J. A. 1998b. Treatment of adult Coleoptera with a chitin synthesis inhibitor affects mortality and development time of their progeny. Entomol. Exp. Appl. 89: 31–39. [Google Scholar]
- Elek J. A., Longstaff B. C. 1994. Effect of chitin-synthesis inhibitors on stored product beetles. Pestic. Sci. 40: 225–230. [Google Scholar]
- Gazit Y., Ishaaya I., Perry A. S. 1989. Detoxification and synergism of diflubenzuron and chlorfluazuron in the red flour beetle Tribolium castaneum. Pestic. Biochem. Physiol. 34: 103–110. [Google Scholar]
- Ivie G. W., Wright J. E. 1978. Fate of diflubenzuron in the stable fly and house fly. J. Agr. Food Chem. 26: 90–94. [DOI] [PubMed] [Google Scholar]
- Kavallieratos N. G., Athanassiou C. G., Vayias B. J., Tomanovic Z. 2012. Efficacy of insect growth regulators as grain protectants against two stored-product pests in wheat and maize. J. Food Protect. 75: 942–950. [DOI] [PubMed] [Google Scholar]
- Kim S. H. S., Wise J. C., Gökçe A., Whalon M. E. 2011. Novaluron causes reduced egg hatch after treating adult codling moths, Cydia pomonella: support for transovarial transfer. J. Insect Sci. 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyukovsky M., Trostanetsky A. 2006. The effect of a new chitin synthesis inhibitor, novaluron, on various developmental stages of Tribolium castaneum (Herbst). J. Stored Prod. Res. 42: 136–148. [Google Scholar]
- Kostyukovsky M., Trostanetsky A., Carmi Y., Frandji H., Schneider R. 2003. Biological activity of Novaluron, a new chitin-synthesis inhibitor, on the major stored product insect pests, pp. 583–587. In Credland P. F., Armitage D. M., Bell C. H., Cogan P. M., Highley E. (eds.), Advances in stored product protection. Proceedings, 8th International Working Conference on Stored Product Protection, 22–26 July 2002, CAB International, York, UK. [Google Scholar]
- Le Ora Software. 1987. POLO-PC. A user’s guide to probit or logit analysis. Berkeley, CA. [Google Scholar]
- McGregor H. E., Kramer K. J. 1976. Activity of Dimilin (TH 6040) against Coleoptera in stored wheat and corn. J. Econ. Entomol. 69: 479–480. [Google Scholar]
- Medina P., Smagghe G., Budia F., Estal P., Tirry L., Viñuela E. 2002. Significance of penetration, excretion, and transovarial uptake to toxicity of three insect growth regulators in predatory lacewing adults. Arch. Insect Biochem. Physiol. 51: 91–101. [DOI] [PubMed] [Google Scholar]
- Mishra P. B., Salokhe S. G., Deshpande S. G. 2013. Biological and biochemical effects of lufenuron (IgR) on growth, development and reproductive performance of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) (Adults). Res. J. Pharm. Biol. Chem. Sci. 4: 802–810. [Google Scholar]
- Oberlander H., Silhacek D. L., Shaaya E., Ishaaya I. 1997. Current status and future perspectives of the use of insect growth regulators for the control of stored product pests. J. Stored Prod. Res. 33: 1–6. [Google Scholar]
- Preisler H. K., Robertson J. L. 1989. Analysis of time-dose-mortality data. J. Econ. Entomol. 82: 1534–1542. [Google Scholar]
- Stamm M. D., Heng-Moss T. M., Baxendale F. P., Siegfried B. D., Gaussoin R. E., Snow D. D., Cassada D. A. 2013. Effect of distribution and concentration of topically applied neonicotinoid insecticides in buffalograss, Buchloe dactyloides, leaf tissues on the differential mortality of Blissus occiduus under field conditions. Pest Manag. Sci. 69: 285–291. [DOI] [PubMed] [Google Scholar]
- Subramanyam B., Hartzer M., Boina D. R. 2012. Performance of pre-commercial release formulations of spinosad against five stored-product insect species on four stored commodities. (Special issue: recent advances in stored product protection.) J. Pest Sci. 85: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throne J. E., Weaver D. K., Chew V., Baker J. 1995. Probit analysis of correlated date: multiple observations over time at one concentration. J. Econ. Entomol. 88: 1510–1512. [Google Scholar]
- Trostanetsky A., Kostyukovsky M. 2008. Transovarial activity of the chitin synthesis inhibitor novaluron on egg hatch and subsequent development of larvae of Tribolium castaneum. Phytoparasitica 36: 38–41. [Google Scholar]
- Wijayaratne L. K. W., Fields P. G., Arthur F. H. 2012. Effect of methoprene on the progeny production of Tribolium castaneum (Coleoptera: Tenebrionidae). Pest Manag. Sci. 68: 217–224. [DOI] [PubMed] [Google Scholar]
- Yasir M., Sagheer M., Hasan M. U., Abbas S. K., Ahmad S. 2012. Bioactivity of a chitin synthesis inhibitor, triflumuron, against red flour beetle, Tribolium Castaneum (Herbst) (Coleoptera:Tenebrionidae). Sarhad J. Agric. 28: 603–609. [Google Scholar]
- Zettler J. L., Cuperusi G. W. 1990. Pesticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) in wheat. J. Econ. Entomol. 83: 1677–1681. [Google Scholar]