Abstract
Diapause hormone (DH), which can terminate diapause in Helicoverpa armigera Hübner (Lepidoptera: Noctuidae), has shown promise as a pest control method. However, the main challenge in using DH as an insecticide lies in achieving effective oral delivery, since the peptide may be degraded by digestive enzymes in the gut. To improve the efficacy of oral DH application, the Clostera anastomosis (L.) (Lepidoptera: Notodontidae) diapause hormone (caDH) was fused to the Protein Transduction Domain (PTD) of the human immunodeficiency virus-1 transactivator of transcription (TAT). Cellular transduction of TAT-caDH was verified with the use of a green fluorescent protein fusion, and its ability to terminate diapause was verified by injection into diapausing H. armigera pupae. Orally administered TAT-caDH resulted in larval growth inhibition. In TAT-caDH–treated insects, larval duration was delayed and the pupation rates were decreased at both development promoting conditions [27°C, a photoperiod of 14:10(L:D) h] and diapause inducing conditions [20°C, a photoperiod of 10:14(L:D) h]. No significant difference in diapause rate was observed between the TAT-caDH–treated and caDH-treated or control pupae maintained at diapause inducing conditions. Our results show that treatment with a recombinant TAT-caDH protein can affect larval development in H. armigera, and it suggest that TAT-DH treatment may be useful for controlling pests. This study is the first record of oral DH application in insect.
Keywords: diapause hormone, Helicoverpa armigera, transactivator of transcription, fusion protein, growth inhibition
Diapause, genetically programed developmental arrest accompanied by a major decline in metabolic activity, has evolved as an adaption for surviving seasonally recurring adverse environments and permitting life cycle synchronization with favorable periods (Hahn and Denlinger 2011). Diapause can be occurred in any developmental stage, depending on the species (Denlinger et al. 2005). Diapause hormone (DH) is a member of the pyrokinin/PBAN(PBAN, pheromone biosynthesis activating neuropeptide) family of peptides, which share a conserved C-terminal motif, known as an FXPRLamide domain (X = G, T, S, or V) and range in size from 8 to 34 amino acids (aa) (Nachman et al. 2002). Although DH induces embryonic diapause in the commercial silkworm, Bombyx mori (L.) (Lepidoptera: Bombycidae) (Denlinger 1985, 2002; Imai et al. 1991, Yamashita 1996), DH is responsible for breaking pupal diapause after injection of DH in Heliothis virescens (F.) (Lepidoptera: Noctuidae) (Xu and Denlinger 2003), Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) (Zhang et al. 2004a) and Helicoverpa assulta Guenée (Lepidoptere: Noctuidae) (Zhao et al. 2004) and H. zea (Zhang et al. 2008). In species belonging to the Helicoverpa/Heliothis complex, DH activity is temperature dependent, with each species displaying a unique optimal temperature (Xu and Denlinger 2003, Zhang et al. 2004b, Zhao et al. 2004). Dose-response curves for H. assulta and H. zea in response to DH treatment show that the half-maximal effective dose (ED50) is ∼150 and 100 pmol, respectively (Zhao et al. 2004, Zhang et al. 2008).
Delivery routes for administering recombinant proteins to animals include injection, oral administration, and immersion treatment. Among these, oral administration appears to be the most practical route of delivery for pest management because it does not require handling insects individually. The main challenge for the practical application of orally administered DH is utilization efficiency, as the dose may be degraded by digestive enzymes. This type of inefficiency could be costly in large-scale applications.
Cell-penetrating peptides (CPPs) represent a potential mean of improving the utilization efficiency of orally administered recombinant proteins. Carrier peptides can deliver a variety of recombinant proteins to a diverse range of cells and tissues (Dietz and Bohr 2004, Simon et al. 2011, Yu et al. 2013). The transactivator of transcription (TAT) protein, an 86-aa protein that functions in the replication, neurotoxicity, and immune activation of human immunodeficiency virus-1 (HIV-1), is one of the most extensively studied CPPs (Fawell et al. 1994). The TAT protein transduction domain (TAT-PTD) is a short cluster of basic amino acids that are necessary for cell membrane transduction. Previous studies have shown that heterologous proteins containing the TAT-PTD are internalized by living cells (Fawell et al. 1994). Fusion proteins containing the TAT-PTD have been used to introduce a variety of physiologically and therapeutically active macromolecules (i.e., polypeptides, oligonucleotides, magnet beads, liposomes, phages, and antibodies) into living cells via cellular transduction (Harada and Hiraoka 2009). Systemic delivery of TAT-PTD–fused galactosidase has also been accomplished in mice via intraperitoneal injection (Schwarze et al. 1999), suggesting that TAT-PTD fusion proteins may allow the systemic administration of macromolecules in various organisms using similar techniques.
Cloan-DH consists of 29 amino acid residues in size from Clostera anastomosis (L.) (Lepidoptera: Notodontidae), the black-back prominent moth. Cloan-DH, PBAN, and a-SGNP (SGNP, suboesophageal ganglion neuropeptide), b-SGNP and g-SGNP are likely to be released from the Cloan-DH-PBAN precursor during post-endoproteolytic processing, as in other DH-PBAN cDNAs. Sequence of Cloan-DH-PBAN shares high structural similarity to other DH-PBAN members and is most homologous to DH-PBAN from species of Noctuoidea (Jing et al. 2007). Cloan-DH has the high homology to that of H. armigera with a similarity of 79.3%. A high level of DH-PBAN mRNA was detected in adult females of C. anastomsis, similar to those in adult females of H. zea (Ma et al. 1998) and H. armigera (Zhang et al. 2004a). We suspected that Cloan-DH may be able to induce similar physiological responses in H. armigera. We tried to develop a strategy for pest management based on the oral administration of the heterologous TAT-PTD fusion protein using the agriculturally important species H. armigera, the cotton bollworm, as the insect model for diapause induction and termination. The objectives of our study included: 1) production of a recombinant TAT-DH fusion protein of C. anastomosis DH in the active core of the TAT-PTD, 2) evaluation of cellular uptake of orally administered TAT-DH in H. armigera based on an analysis of gastric tissues, and 3) analysis of the effects of orally administered TAT-DH on H. armigera development.
Materials and Methods
Fusion Gene Sequences
The TAT-caDH sequence (GenBank accession no. KJ434109) was synthesized and inserted into the pUC57 vector by a commercial service provider (Genscript, Nanjing, China). The DH was based on the mRNA sequence (caDH) from C. anastomosis, the black-back prominent moth (Jing et al. 2007). The TAT sequence contained the codons encoding residues 47–57 (YGRKKRRQRRR) (Vivès et al. 1997), and the nucleotide sequence of caDH was 183 bp in size from ATG initiation codon to the end of caDH coding sequence of the Cloan-DH-PBAN cDNA.
Expression Plasmid Construction
The TAT-caDH sequence was amplified by PCR using the TcaDH forward primer (5′-CGGAATTCATGTACGGCCGCAAGAAGAGG-3′) and TcaDH reverse primer (5′-ACGCGTCGACGTCGACATTGTCTTCACTG-3′). PCR was performed using Taq DNA polymerase (Sangon, Shanghai, China), 2 µl of pUC57-TAT-caDH (1:100 dilution) as the template, 2 µl of 10× buffer, 0.2 mM dNTPs, and 0.25 mM of each primer in a final volume of 20 µl. Thermal cycling was performed at 94°C for 4 min, followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s, with a final extension at 72°C for 7 min.
A faint DNA band corresponding to the expected size of ∼225 bp was excised from an agarose gel after electrophoresis and purified using a DNA gel extraction kit (Sangon, Shanghai, China). The PCR product and the pGEX-4T(+) plasmid were digested using EcoRI and SalI (Sangon, Shanghai, China) at 37°C for 3 h. The digested DNA fragments were isolated using a PCR product purification kit (Sangon, Shanghai, China) and ligated using DNA ligase (Sangon, Shanghai, China). The ligation mixture was used to transform competent JM109 Escherichia coli. Recombinant pGEX-4T(+)-TAT-caDH plasmid sequences were confirmed by restriction endonuclease digestion and DNA sequencing (Sangon, Shanghai, China). The nucleotide sequence of the enhanced green fluorescent protein (eGFP) was PCR amplified using the peGFP-NI plasmid as a template and eGFPf (5′-ACGCGTCGACATGGTGAGCAAGGGCGAGGAGC-3′) and eGFPr (5′-AATGCGGCCGCTTACTTGTACAGCTCGTCCATG-3′) as primers. Thermal cycling was performed at 94°C for 4 min, followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 60 s, with a final extension at 72°C for 7 min. The sequence of the recombinant pGEX-4T(+)-TAT-caDH-eGFP plasmid was confirmed by restriction endonuclease digestion and DNA sequencing.
Expression, Purification, and Renaturation of TAT-caDH-eGFP
The expression of TAT-caDH-eGFP was carried out as described in Yu et al. (2013), but with the following modifications. The recombinant pGEX-4T(+)-TAT-caDH-eGFP plasmid was used to transform BL21 (DE3) E. coli, and protein expression was induced using 0.1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 20°C for 36 h. BL21 (DE3) E. coli in which TAT-caDH-eGFP expression had been induced using IPTG were collected by centrifugation, and suspended in 30 ml of sonication buffer containing 50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 10 mM imidazole, and 0.05% (v/v) Tween 20. The bacteria were lysed by sonication on ice for 30 min (3-s on/off pulses) using a JY92-2D Sonicator (Scientz, Ningbo, China). For the isolation of inclusion bodies containing the Tat-caDH-eGFP, the sonicated cells were centrifuged at 12,000 rpm for 10 min at 4°C. The cell pellet was washed twice with a solution containing 2 M urea, 50 mM Tris (pH 8.0), 300 mM NaCl, and 0.05% Tween 20, and suspended in denaturing buffer containing 8 M urea, 50 mM Tris (pH 8.0), and 300 mM NaCl, in which the concentrations of Tris and NaCl were equivalent to those used to purify proteins under native conditions.
In subsequent refolding experiments, proteins were renatured using a discontinuous urea gradient (4, 2, 1, and 0 M) in the dialysis buffer (10 mM Tris, pH 8.0) for 24 h at 4°C. For better renaturation, 10 mM oxidized glutathione and 1 mM reduced glutathione were added into dialysis buffer. After concentrated by PEG 20,000 and centrifugation, the supernatant containing TAT-caDH-eGFP was collected for further in vivo cellular transduction assay. The presence of target proteins was determined by subjecting the bacterial lysates to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in a 12% acrylamide gradient gel. Lysates were mixed with 2×sample loading buffer containing dithiothreitol. Protein Marker was BM201 (Sangon, Shanghai, China). The protein bands in the gel were stained using Coomassie Brilliant Blue R-250 (Sangon, Shanghai, China), and protein expression levels were estimated using Quantity One (Bio-Rad).
In Vivo Cellular Transduction Assay
The pGEX-4T(+)-TAT-caDH-eGFP plasmid was introduced into BL21 (DE3) E. coli, and recombinant protein expression was induced using 0.1 mM IPTG at 20°C for 36 h. The TAT-caDH-eGFP or eGFP (Sangon Biotech, RC323) were suspended in distilled water to a concentration of 8 × 10−7 M. The suspension was added to the commercial diet at a concentration of 1.6 × 10−6 mol/kg. The food was heated to 45°C to allow mixing because it was solid at room temperature. Food was withheld from the third-stage larvae of H. armigera for 12 h prior to providing the TAT-caDH-eGFP- or eGFP-enhanced diet. In total, five larvae were collected at each time-point of 4, 8, 12, 24, 48, 72, and 90 h post-feeding. The larvae were embedded in paraffin, as previously described (Wang et al. 2007), and cellular transduction was evaluated by observing thin sections of gastric tissue to detect intracellular eGFP using a Leica DM3000 fluorescence microscope (Wetzlar, Germany), as described by Kiernan (Kiernan 2006).
Insect Rearing
H. armigera larvae and a commercial diet were supplied by Baiyun Industry Co., Ltd. (Henan, China) at 27°C with a 14-h light and 10-h dark a photo period of 14:10 (L:D). Under these conditions, the pupae developed without entering diapause. Third-stage larvae were individually transferred to 2.5 × 10 cm glass test tubes containing the commercial diet and were maintained at 20°C with a photoperiod of 10:14(L:D) to induce diapause. Fresh food was supplied every 5 d until the larvae pupated. Under these conditions, >70% of the pupae entered diapause. Diapause status was ascertained based on an examination of the pupal stemmata and eclosion rate, as described previously (Zhang et al. 2004a). The pupae were collected and returned to 20°C with a photoperiod of 10:14(L:D) for 2 weeks prior to injection experiments.
Synthesis and Characterization of TAT, caDH, and TAT-caDH peptides
TAT, caDH, and TAT-caDH aldehyde (C-terminal) peptides were synthesized by Sangon Biotech (Shanghai) Co., Ltd. Sequence quality and quantity were characterized by HPLC and mass spectrometry analyses. The TAT sequence encoded residues 47–57 (YGRKKRRQRRR). The caDH sequence was synthesized based on the deduced amino acid sequence from the DH encoding sequence of C. anastomosis’s cDNA. TAT-caDH was fused by TAT and caDH aa.
Effects of Intra-Abdominally Injected TAT-caDH on Pupae Diapause
TAT, caDH, and TAT-caDH peptides were dissolved in distilled water to a final concentration of 20 pmol/l for injection. Diapausing pupae were maintained at 25°C for 5 d and checked for diapause status (Zhang et al. 2009). The TAT, caDH, or TAT-caDH solution was injected into diapausing pupae using a fine glass capillary tube, same volume distilled water were injected as control. The capillary tube was inserted into the first or second segment of the abdomen between 30° and 45° in relation to the abdomen and a microinjector (Hamilton, Bonaduz, Switzerland) was used to inject 5 µl (0.1 nmol peptide). After injection, the pupae were maintained in Petri dishes at 25°C with a photoperiod of 14:10(L:D) h. The diapause status of each pupa was evaluated on post-injection days 3 and 7. The diapause termination rate was calculated for each group by comparing the number of diapause-terminated individuals on day 7 post-injection to the number of total diapausing pupae on day 3 post-injection.
Effects of Orally Administered TAT-caDH on Development
TAT-caDH or caDH peptides were dissolved in distilled water and added to the commercial diet to a final concentration of 16 × 10−8 M. Early third-stage larvae of H. armigera were randomly divided into four groups, with 100 larvae in each group. The peptide solution was mixed with the commercial diet at a concentration of 6.4 × 10−8 mol/kg. One of the groups was maintained at 20°C with a photoperiod of 10:14(L:D) h, whereas the other group was maintained at 27°C with a photoperiod of 14:10(L:D) h. The other two groups of larvae received the equal concentration of caDH. The control diet was prepared by adding a diet with no added peptides. Two groups of larvae received the control diet. One group of control larvae was maintained at 20°C with a photoperiod of 10:14(L:D) h, and the other group was maintained at 27°C with a photoperiod of 14:10(L:D) h. These diets were offered every 3 d until the larvae pupated. The pupation rate, larval period, pupal weight and diapause rate were assayed. Three independent experiments were performed.
Statistical Analysis
The data are presented as the mean ± SE. All of the data were subjected to a one-way analysis of variance using the DPS software, version 7.05 (Zhejiang University, Hangzhou, China). The differences between the means were evaluated using the Least-significant difference test. The Student-Newman-Keuls test was used to evaluate the intergroup differences. The results where P < 0.05 were considered to represent statistically significant differences.
Results
Expression, Purification, and Characterization of TAT-caDH-eGFP
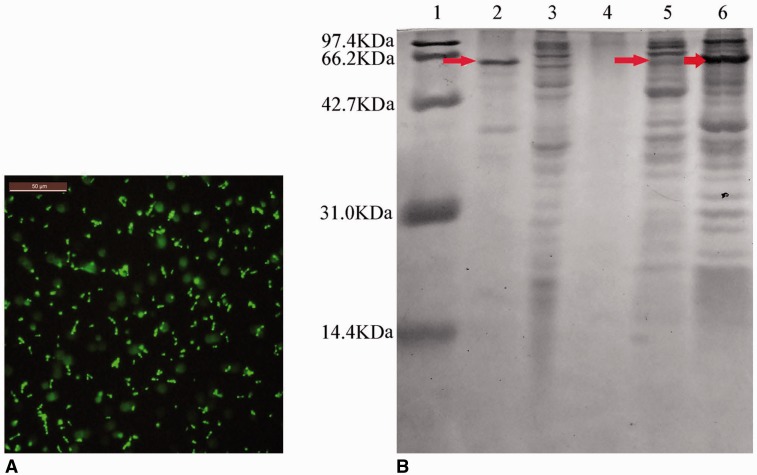
E. coli carrying the pGEX-4T(+)-TAT-caDH-eGFP plasmid in which protein expression had been induced using IPTG were observed using a fluorescence microscope. In these bacteria, intense intracellular fluorescence was observed (Fig. 1A). TAT-caDH-eGFP constituted 18.1% of the total cellular protein (Fig. 1B), and the level of soluble TAT-caDH-eGFP in the purified supernatant was 3.5% of the total soluble cellular protein.
Fig. 1.
Fluorescence microscopy and SDS-PAGE analyses of recombinant TAT-caDH-eGFP expression in E. coli. (A) Visualization of TAT-caDH-eGFP fluorescence. (B) SDS-PAGE and Coomassie Brilliant Blue staining of protein lysates from E. coli. Arrows indicate TAT-caDH-eGFP. Lane 1, protein size markers; lane 2, purified TAT-caDH-eGFP from E. coli BL21 (DE3) carrying pGEX-4T(+)-TAT-caDH-eGFP (+IPTG); lane 3, E. coli BL21 (DE3) carrying pGEX-4T(+)-TAT-caDH-eGFP (–IPTG); lane 4, supernatants of nutrient medium from E. coli BL21 (DE3) carrying pGEX-4T(+)-TAT-caDH-eGFP (–IPTG); lane 5, supernatants of TAT-caDH-eGFP from E. coli BL21 (DE3) carrying pGEX-4T(+)-TAT-caDH eGFP (+IPTG); lane 6, crude preparation of TAT-caDH-eGFP inclusion bodies from E. coli BL21 (DE3) carrying pGEX-4T(+)-TAT-caDH-eGFP (+IPTG). Bar = 50 µm.
Cellular Transduction of TAT-caDH-eGFP Through Gastric Tissues
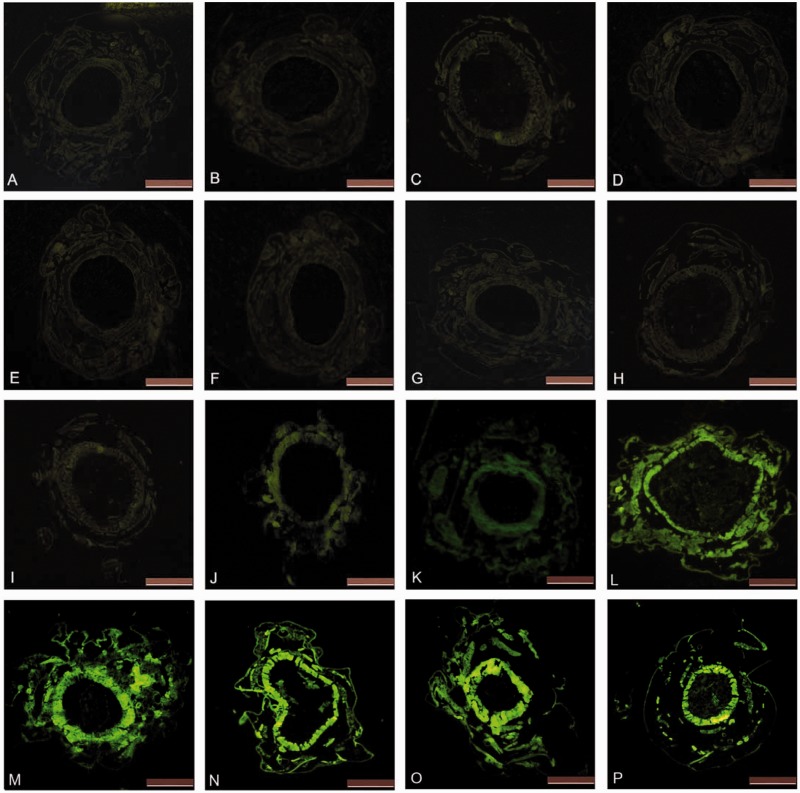
Paraffin-embedded sections of the midgut from larvae fed the diet enhanced with either TAT-caDH-eGFP– or eGFP-diet were examined using a fluorescence microscope (Fig. 2). No observable difference was observed in the fluorescence of the inner layer of the midgut before (Fig. 2I) versus after (Fig. 2J) feeding on the TAT-caDH-eGFP diet. However, a substantial increase in green fluorescence was observed in the inner layer of the midgut, the midgut muscle, and the larval cuticle after the consumption of the TAT-caDH-eGFP–enhanced diet (Fig. 2J). Fluorescence increased with a concomitant increase in the duration of TAT-caDH-eGFP feeding (Fig. 2J–P), with a maximum level of midgut fluorescence reached after 24 h eating. These results indicated that TAT-caDH-eGFP transduced across the cell membranes of the midgut cells and continued migrating into additional tissues.
Fig. 2.
Visualization of green fluorescence in midgut sections from larvae fed with TAT-caDH-eGFP– or eGFP-diet. A–H show the corresponding dark-field micrographs of the midgut at 0, 4, 8, 12, 24, 48, 72, and 90 h, respectively, after feeding eGFP-diet. I–P show the corresponding dark-field micrographs of the midgut at 0, 4, 8, 12, 24, 48, 72, and 90 h, respectively, after feeding TAT-caDH-eGFP diet. Bar = 500 µm.
Termination of Pupal Diapause With Injected TAT-caDH or caDH and Developmental Interfering Effects of Orally Administered TAT-caDH
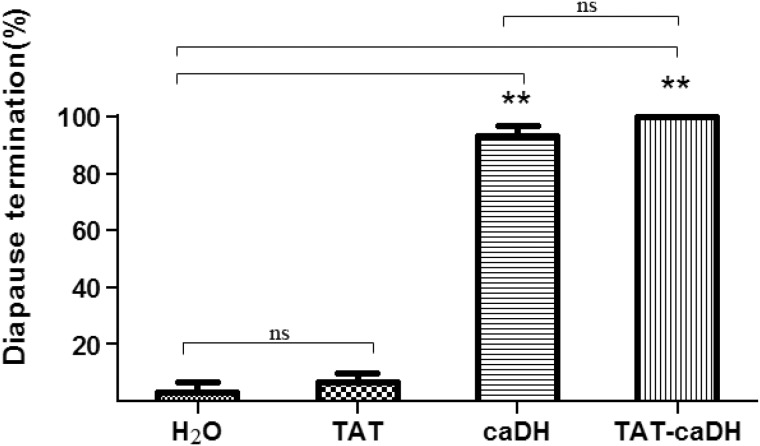
The diapause termination rate of pupae injected with 0.1 nmol TAT-caDH or caDH was significantly higher (100 and 97%, respectively; P < 0.05) than that of the pupae injected with 0.1 nmol TAT (7%; Fig. 3). These results indicated that either TAT-caDH or caDH could terminate diapause in H. armigera.
Fig. 3.
Pupal diapause termination in H. armigera in response to TAT-caDH or caDH Each column represents the mean ± SD. In all groups, for treatment compared with CK, **P < 0.01; ns denotes not significant.
TAT-caDH inhibited development at the two kinds of growth condicions. caDH had no role in development of H.armigera through oral administration. At development promoting conditions [27°C, a photoperiod of 14:10(L:D)h], the pupation rate for larvae fed a diet containing TAT-caDH was significantly lower (76.9 ± 2.2%; P < 0.01) than that of the larvae (84 ± 3.1%) fed a diet containing caDH (Fig. 4A). There was no difference between the control larvae (83 ± 3.9%) fed a normal diet and the larvae fed a diet containing caDH. At diapause inducing conditions [20°C, a photoperiod of 10:14(L:D) h], the pupation rate for larvae fed a diet containing TAT-caDH was significantly lower (64 ± 4.3%; P < 0.01) than that of the control larvae (79 ± 2.1%) too. There is no difference between the control larvae (78 ± 1.2%) fed a normal diet and the larvae fed a diet containing caDH.
Fig. 4.
Pupation rate (A), duration of larval stage (B), pupal weight (C) and diapause rate (D) of H. armigera at development promoting conditions [27°C, a photoperiod of 14:10 (L:D) h] and diapause inducing conditions [20°C, a photoperiod of 10:14 (L:D) h] in the presence of TAT-caDH or caDH. Each column represents the mean ± SD. In all groups, for treatment compared with CK, **P < 0.01; ns denotes not significant.
At development promoting conditions [27°C, a photoperiod of 14:10(L:D) h], the duration of larval development in larvae fed a diet containing TAT-caDH was significantly longer (22 ± 2.3 d; P < 0.01) than that of larvae fed a diet containing caDH larvae and the control larvae (19 ± 1.7 and 19 ± 1.3 d, respectively; Fig. 4B). At diapause inducing conditions [20°C, a photoperiod of 10:14(L:D) h], the duration of larval development in larvae fed a diet containing TAT-caDH was significantly longer (50 ± 11.4 d, P < 0.01) when compared with larvae fed a diet containing caDH larvae and the control larvae (38 ± 8.5 and 38 ± 8.9 d, respectively).
At development promoting conditions [27°C, a photoperiod of 14:10(L:D) h], the weight of pupae previously fed a diet containing TAT-caDH (0.243 ± 0.046, P < 0.01) was similar to that of the pupae previously fed a diet containing caDH and control pupae (0.240 ± 0.052 and 0.231 ± 0.048 g, respectively; Fig. 4C). Likewise, at diapause inducing conditions [20°C, a photoperiod of 10:14(L:D) h], the weight of pupae previously fed a diet containing TAT-caDH (0.230 ± 0.057 g) was similar to that of the pupae previously fed a diet containing caDH and control pupae (0.220 ± 0.058 and 0.219 ± 0.061 g, respectively).
The mean diapause incidence for the pupae previously fed a diet containing TAT-caDH and maintained at diapause inducing conditions [20°C, a photoperiod of 10:14(L:D) h] was 66 ± 1.9%. The diapause rates for the pupae previously fed a diet containing caDH and control diet were 68 ± 2.3% and 70 ± 3.7%, respectively (Fig. 4D). No significant difference in diapause rate was observed between the TAT-caDH–treated and caDH-treated or control pupae maintained at 20°C with a photoperiod of 10:14(L:D) h.
Discussion
DH functions in the regulation of the nervous system and is required for various physiological responses in insects (Denlinger 2002; Denlinger et al. 2005), thus affecting both the survival of individuals and of populations. DH of C. anastomosis (caDH) shares 93% sequence similarity to DH of H. armigera. caDH is 29 aa in size (Jing et al. 2007). DH of H.zea, a 24-aa neuropeptide, effectively terminates diapause in H. armigera and H. zea, at optimal temperatures. Studies using truncated and alanine-substituted analogs of the DH of H. zea have demonstrated that the active core consists of LWFGPRLa (Zhang et al. 2008). Because caDH has the same active core amino acid sequence, we hypothesized that caDH would terminate diapause effectively in H. armigera. When TAT-caDH and caDH proteins were injected into diapausing pupae of H. armigera, the diapause termination activity of caDH was confirmed in this system.
Other investigators have proposed that artificial or synthetic DH analogs might be capable of controlling insect pests. Zhang et al. (2008) examined the effects of structural DH analogs in H. zea and reported that certain structures were more potent with regard to ending diapause. An assay for diapause termination identified 13 active compounds suggest the feasibility of developing DH agonists that can be applied topically and suggest the identity of new lead molecules for development of additional topically active DH analogs. The ability to penetrate the insect epidermis and/or midgut lining is critical (Zhang et al. 2015). The exploitation of insect feeding behavior is an important aspect of pest control. To offset potential problems related to protein degradation in the digestive tract, we used the TAT-PTD (Schwarze et al. 1999, Harada and Hiraoka, 2009) to increase the absorption of the caDH fusion protein in the insect gut. TAT-caDH-eGFP, but not eGFP alone, passed across the intestinal wall and entered the hemolymph, in which it was transported to other tissues. As an answer to this potential issue, we used the TAT fusion partner to enhance the stability and bioavailability of the fusion protein.
When TAT-caDH and caDH proteins were fed to H. armigera larvae, only the bioactivity of TAT-caDH was visualized. Our results are consistent with those of Yu et al. (2013) who used TAT fused to the C terminus of catfish growth hormone and found that TAT greatly enhanced the biological activity of the hormone. To reduce the cost of the chemical synthesis and to increase DH stability, a TAT-caDH fusion protein produced in either E. coli or plants could be used in the future. Zhang et al. (2011) designed several agonists that are much more active than DH in breaking diapause. Their results suggest potential for using such agents or next-generation derivatives for derailing the success of overwintering in pest species by injection. Though the finding that DH upregulates the growth hormone ecdysteroid during the stage of pupal development (Chen and Xu 2014), we found that TAT-caDH inhibited larvae development either at development promoting conditions [27°C, a photoperiod of 14:10(L:D) h] or at diapause inducing conditions [20°C, a photoperiod of 10:14(L:D) h] by oral administration, which is the first discovery about the larvae growth inhibition of DH feeding. Our article is the first record of oral DH application in an insect.
In our experiments, feeding on a diet containing TAT-caDH affected the development in H. armigera. Termination of diapause was found after injection of TAT-caDH directly into the diapausing pupa. Though larval feeding had no effect on diapause incidence, our findings provide a method for enhancing the stability and bioavailability of agents used to manipulate development in agriculturally important insects.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (U1204324) and the National High Technology Research and Development Program of China (2012AA101503).
References Cited
- Chen W., Xu W. H. 2014. Wnt/β-catenin signaling regulates Helicoverpa armigera pupal development by up-regulating c-Myc and AP-4. Insect Biochem. Mol. Biol. 53: 44–53. [DOI] [PubMed] [Google Scholar]
- Denlinger D. L. 2002. Regulation of diapause. Annu. Rev. Entomol. 47: 93–122. [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., Yocum G. D., Rinehart J. P. 2005. Hormonal control of diapause, vol. 3, pp. 615–650. In Gilbert L. I., Kostas I., Gill S. S. (eds.), Comprehensive Molecular Insect Science, Elsevier Press, Amsterdam, NL. [Google Scholar]
- Denlinger D. L. 1985. Hormonal control of diapause, pp. 353–412. In Kerkut G. A., Gilbert L. I. (eds.), Comprehensive Insect Physiology, Biochemistry and Pharmacology, Pergamon Press, Oxford, UK. [Google Scholar]
- Dietz G. P. H., Bohr M. 2004. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol. Cell. Neurosci. 27: 85–131. [DOI] [PubMed] [Google Scholar]
- Fawell S., Seery J., Daikh Y., Moore C., Chen L. L., Pepinsky B., Barsoum J. 1994. TAT mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA. 91: 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn D. A., Denlinger D. L. 2011. Energetics of insect diapause. Annu. Rev. Entomol. 56: 103–121. [DOI] [PubMed] [Google Scholar]
- Harada H., Hiraoka M. 2009. Protein transduction domain-mediated delivery of anticancer proteins, pp. 297–319. In Lu Y., Mahato R. I. (eds.), Pharmaceutical Perspectives of Cancer Therapeutics. Springer US, New York, USA. [Google Scholar]
- Imai K., Konno T., Nakazawa Y., Komiya T., Isobe M., Koga K. 1991. Isolation and structure of diapause hormone of the silkworm, Bombyx mori. Proc. Jpn. Acad. 67: 98–101. [Google Scholar]
- Jing T. Z., Wang Z. Y., Qi F. H., Liu K. Y. 2007. Molecular characterization of diapause hormone and pheromone biosynthesis activating neuropeptide from the black-back prominent moth, Clostera anastomosis (L.) (Lepidoptera, Notodontidae). Insect Biochem. Mol. Biol. 37: 1262–1271. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. 2006. Fluorescence of GFP in sections of fixed tissue, Biotech. Histochem. 81: 159. [DOI] [PubMed] [Google Scholar]
- Ma P. W. K., Knipple D. C., Roelofs W. L. 1998. Expression of a gene that encodes pheromone biosynthesis activating neuropeptide in the central nervous system of corn earworm, Helicoverpa zea. Insect Biochem. Mol. Biol. 28: 373–385. [Google Scholar]
- Nachman R. J., Teal P. E., Strey A. 2002. Enhanced oral availability/pheromonotropic activity of peptidase-resistant topical amphiphilic analogs of pyrokinin/PBAN insect neuropeptides. Peptides. 23: 2035–2043. [DOI] [PubMed] [Google Scholar]
- Schwarze S. R., Ho A., Vocero-Akbani A., Dowdy S. F. 1999. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285: 1569–1572. [DOI] [PubMed] [Google Scholar]
- Simon M. J., Kang W. H., Gao S., Banta S., Morrison B. 2011. TAT is not capable of transcellular delivery across an intact endothelial monolayer in vitro. Ann. Biomed. Eng. 39: 394–401. [DOI] [PubMed] [Google Scholar]
- Vivès E., Brodin P., Lebleu B. 1997. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the nucleus. J. Biol. Chem. 272: 16010–16017. [DOI] [PubMed] [Google Scholar]
- Wang Y., Feng G. S., Tian H., Zhang Z. H., Xiong T. 2007. Fluorescent imaging of angiogenesis and spontaneous metastasis in transplanted hepatoma: experiment with mice. Natl. Med. J. China. 87: 3307–3309. [PubMed] [Google Scholar]
- Xu W. H., Denlinger D. L. 2003. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect. Mol. Biol. 12: 509–516. [DOI] [PubMed] [Google Scholar]
- Yamashita O. 1996. Diapause hormone of the silkworm, Bombyx mori: structure, gene expression and function. J. Insect. Physiol. 42: 669–979. [Google Scholar]
- Yu J. Y., Meng X. L., Xu J. P., Chen D. D., Meng M. X., Ni Y. W. 2013. Fusion of TAT-PTD to the C-terminus of catfish growth hormone enhances its cell uptakes and growth-promoting effects. Aquaculture 392: 84–93. [Google Scholar]
- Zhang Q. R., Nachman R. J., Zubrzak P., Denlinger D. L. 2009. Conformational aspects and hyperpotent agonists of diapause hormone for termination of pupal diapause in the corn earworm. Peptides. 30: 596–602. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zdarek J., Nachman R. J., Denlinger D. L. 2008. Diapause hormone in the corn earworm, Helicoverpa zea: optimum temperature for activity, structure-activity relationships, and efficacy in accelerating flesh fly pupariation. Peptides. 29: 196–205. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Nachman R. J., Kaczmarek K., Zabrocki J., Denlinger D. L. 2011. Disruption of insect diapause using agonists and an antagonist of diapause hormone. Proc. Natl. Acad. Sci. USA. 108: 16922–16926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T. Y., Sun J. S., Zhang L. B., Shen J. L., Xu W. H. 2004a. Cloning and expression of the cDNA encoding the FXPRL family of peptides and a functional analysis of their effect on breaking pupal diapause in Helicoverpa armigera. J. Insect Physiol. 50: 25–33. [DOI] [PubMed] [Google Scholar]
- Zhang T. Y., Sun J. S., Zhang Q. R., Xu J., Jiang R. J., Xu W. H. 2004b. The diapause hormone-pheromone biosynthesis activating neuropeptide gene of Helicoverpa armigera encodes multiple peptides that break, rather than induce, diapause. J. Insect Physiol. 50: 547–554. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Nachman R. J., Kaczmarek K., Kierus K., Zabrocki J., Denlinger D. L. 2015. Development of neuropeptide analogs capable of traversing the integument: a case study using diapause hormone analogs in Helicoverpa zea. Insect. Mol. Biol. pii: S0965-1748(15)00049-1 (doi:10.1016/j.ibmb.2015.02.015). [DOI] [PubMed] [Google Scholar]
- Zhao J. Y., Xu W. H., Kang L. 2004. Functional analysis of the SGNP I in the pupal diapause of the oriental tobacco budworm, Helicoverpa assulta (Lepidoptera: Noctuidae). Regul. Pept. 118: 25–31. [DOI] [PubMed] [Google Scholar]