Abstract
Cystoisospora suis is a coccidian species that typically affects suckling piglets. Infections occur by oral uptake of oocysts and are characterized by non-hemorrhagic transient diarrhea, resulting in poor weight gain. Apparently, primary immune responses to C. suis cannot readily be mounted by neonates, which contributes to the establishment and rapid development of the parasite, while in older pigs age-resistance prevents disease development. However, the presence of extraintestinal stages, although not unequivocally demonstrated, is suspected to enable parasite persistence together with the induction and maintenance of immune response in older pigs, which in turn may facilitate the transfer of C. suis-specific factors from sow to offspring. It is assumed that neonates are particularly prone to clinical disease because infections with C. suis interfere with the establishment of the gut microbiome. Clostridia have been especially inferred to profit from the altered intestinal environment during parasite infection. New tools, particularly in the area of genomics, might illustrate the interactions between C. suis and its host and pave the way for the development of new control methods not only for porcine cystoisosporosis but also for other mammalian Cystoisospora infections. The first reference genome for C. suis is under way and will be a fertile ground to discover new drugs and vaccines. At the same time, the establishment and refinement of an in vivo model and an in vitro culture system, supporting the complete life cycle of C. suis, will underpin the functional characterization of the parasite and shed light on its biology and control.
Keywords: swine, piglets, Cystoisospora suis, immunology, microbiome, coccidian
Introduction
Cystoisospora suis (syn. Isospora suis), an apicomplexan parasite of swine, is the causative agent of neonatal porcine cystoisosporosis (coccidiosis). The parasite was first described in 1934 (1), but it received recognition only after the introduction of intensive, high-throughput pig breeding facilities in the mid-1970s (2–4). Suckling piglets are the most affected age group and frequently show pasty-to-watery non-hemorrhagic diarrhea and marked weight loss, while older pigs are less susceptible and excrete few or no oocysts without clinical signs upon infection. Despite high rates of morbidity, piglets exhibit high individual variability in the development of disease (5, 6), which leads to uneven weaning weights (7, 8). Infected piglets usually recover within 2 weeks post-infection (9–11). Although cystoisosporosis has a ubiquitous distribution (12–15), the diagnosis is still cumbersome because of variations in the excretion intensity (16) and short individual oocyst excretion periods (10).
Several species of the genera Eimeria and Cystoisospora can infect swine. Unlike in other livestock, where mixed infections with various Eimeria species are common (17–20), C. suis is the predominant pathogen in pigs (15, 21). Economic losses associated with coccidiosis in livestock are mainly due to impaired performance, retarded growth, mortality, and cost of treatment. Moreover, cystoisosporosis is thought to predispose the piglet to infection with secondary bacterial and viral pathogens, which subsequently increase morbidity, mortality, and managerial costs (22). There are no vaccines available so far, and toltrazuril is the only licensed drug for metaphylaxis that can effectively suppress oocyst excretion and improve piglet health both under experimental conditions (8, 23) and in the field (24). However, rapid emergence of resistance against all introduced anticoccidials in chicken Eimeria (25) is also of concern regarding porcine cystoisosporosis, and there is an urgent need to develop new and sustainable intervention strategies against C. suis for combating neonatal porcine cystoisosporosis in the future.
An experimental model mimicking the field situation (10) in conventional piglets gave deeper insight into neonatal porcine cystoisosporosis. This was further strengthened by the establishment of an in vitro culture system supporting the entire lifecycle of C. suis in intestinal porcine epithelial cells (26). Moreover, gnotobiotic piglets are available as infection models for specific applications (3, 21). Taken together, C. suis may serve as a representative infection model for comparative research on mammalian cystoisosporosis.
Cystoisospora – What Do We Really Know about the Life Cycle?
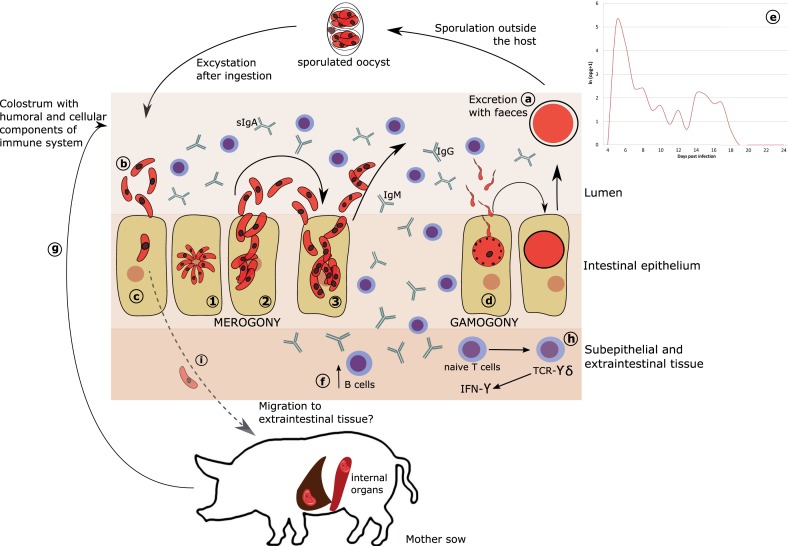
Like other Cystoisospora species, C. suis entirely develops in one host (26, 27) (Figure 1). Directly after ingestion, sporulated oocysts undergo excystation and sporozoites invade the small intestine epithelium (12, 28) to reproduce within a parasitophorus vacuole (29, 30). Asexual reproduction (merogony) peaks at day 4 and 5 post-infection. Unlike Eimeria, merogonic stages are not assigned to generations but to types defined by the number of nuclei, shape, size, and time of appearance (26, 27, 31). From day 5, mature sexual stages can be identified (3, 31). After fusion to form a zygote, the unsporulated oocyst is excreted with the feces and undergoes sporogony outside the host (27, 28, 32).
Figure 1.
Proposed model of C. suis development and immunity. (A) Oocysts are excreted with feces and undergo sporulation in the environment. (B) Sporulated oocysts excyst upon ingested by host to release sporozoites. (C) Sporozoites invade intestinal epithelium and develop to become merozoites (1). In contrast to Eimeria, merogony in C. suis is not synchronized but rather stages are defined as types (2–3). It is currently not known which type could act as extraintestinal resting stage. (D) Merogony is followed by gamogony resulting in fusion of macro- and micro-gametes to form a zygote and subsequently an oocyst. (E) The desynchronization of the merogonic development may also be responsible for the characteristic oocyst excretion occurring in two (or more) peaks, when some of the merozoites may undergo rapid development to gamogonic stages, while others enter into a development lag phase to undergo the sexual maturation for the next peak. (F) In response to infection, naïve B cells proliferate and produce IgA, IgG, and IgM. (G) Intake of colostrum and milk, rich in antibodies and cellular components could partially confer passive humoral immunity against C. suis from sow to piglets. (H) Following infection, TCR-γδ T cells show an almost 30-fold increase in the epithelium and are assumed to be the major producers of IFN-γ, which could support the termination of primary infection in pigs harboring sufficient numbers of these cells in the gut, which is age dependent. (I) The existence of extraintestinal stages of C. suis in liver and spleen of adult pig has been proposed, but viable stages in these tissues have not been demonstrated yet.
Various environmental conditions influence the sporulation time. Lindsay et al. (33) found that the most rapid sporulation takes place between 30 and 37°C, which is well supported by the conditions prevailing in a modern farrowing unit. Rapid multiplication of sporozoites and merozoites inside the intestinal epithelium leads to massive histological alterations including atrophy, necrosis, and fusion of villi, hyperplasia of crypts, and desquamation of epithelial cells (12, 29, 34, 35). These changes persist for a considerable time after parasite development (8), which may contribute to the reduction in body weight gain due to lasting impairment of nutrient absorption.
Cystoisospora suis completes its life cycle within 5–6 days (36). Clinical signs can be seen as early as 3 days post-infection (dpi), shedding of oocysts typically starts on fifth dpi (6, 10, 21, 28, 31, 35). However, these periods may differ, probably due to the age and health condition of the piglets and the virulence of the parasite strain (3, 10, 35). Oocyst excretion and symptoms show typical peaks at 5th–9th and 11th–14th dpi (21, 28, 30), which might be due to extraintestinal stages re-entering the intestines (3).
It has been shown for several Cystoisospora species (C. felis, C. rivolta, C. canis, and C. ohioensis) that sporozoites enter extraintestinal tissues, most often mesenteric lymph nodes but also liver, spleen, other lymph nodes or skeletal muscle, and form monozoic cysts. These extraintestinal stages have been found in definitive as well as in paratenic hosts (3, 27, 37). Also, C. belli extraintestinal cysts were described in humans (38). Paratenic hosts do not show clinical signs but act as carriers, since parasites can survive for at least 2 years within their tissues (32).
However, no study could so far unequivocally demonstrate the existence of C. suis extraintestinal stages in infected piglets or in potential paratenic hosts. Previous studies (31, 37, 39) could not provide evidence of extraintestinal stages in tissues of experimentally infected piglets or mice. Still, gnotobiotic piglets shed oocysts after intraperitoneal inoculation of liver, spleen, and lymph node homogenates from experimentally infected piglets (3).
In a preliminary study, C. suis-specific PCR of tissues from experimentally infected piglets revealed the presence of parasite DNA in several organs. In spleen and mesenteric lymph nodes, it could first be detected on the second dpi. In kidney tissues, it was detected on the second and in kidney and liver tissue from the fifth to the ninth dpi. In jejunal mucosa, it was found from the 1st dpi until the end of the study on 13th dpi (40). Although detection of DNA does not prove the presence of viable, infectious parasitic cells, these results indicate trafficking of C. suis to extraintestinal tissue, either by active migration or after phagocytosis, e.g., by macrophages, and still warrant further studies.
Immunity and Age Resistance Against Cystoisospora – What is What?
Many aspects, such as age, maturation of the gut immune system, as well as the immune status of the infected piglet, influence resistance to C. suis. Stuart et al. (41) showed that piglets infected during the first 3 days of their life develop severe clinical signs compared to 2-week-old infected piglets. Also, when piglets were infected at 3rd vs. 9th day of their life (or on both days) with high doses of C. suis, the clinical signs and oocyst output were most notable in early infection, while piglets infected on the 3rd and on 9th day of life and those infected for the first time on the day 9th of life did not significantly differ (42). Therefore, the authors concluded that age resistance (based on the maturation of the innate immune system) plays a more important role than acquired immunity. Age resistance seems to be a general feature in coccidiosis and is probably due to an increase of T cells and IFN-γ production in the spleen of mice with increasing age that became resistant to primo infections with Cryptosporidium parvum (43). However, in some mammalian species, susceptibility increases with the age of the animal before it decreases again (44, 45), which may also be related to changing immune responses in older animals (46).
Maturation of the porcine immune system can also influence the clinical outcome. Piglets are born with a premature immune system, which only starts to develop during the first few weeks of age. Neonatal piglets do not have well-developed Peyer’s patches; CD2+CD4− and CD8− T cells make most of the intraepithelial lymphocytes and CD8+ cells are not present until the seventh week of life. Also, T cells within the lamina propria of the small intestine and interfollicular areas of Peyer’s patches were found to be fewer compared to older pigs. Likewise, the small intestinal mucosa of new-born piglets is characterized by the absence of lymphoid cells with the exception of a few antigen presenting cells and T cells (47), which may explain the severity of the disease in young piglets due to the inability to adequately respond to the parasite. In older piglets, by contrast, Worliczek et al. (16) detected changes in the T-cell populations of infected piglets, which displayed decreased cell numbers in blood, spleen, and mesenteric lymph nodes and increased T-cell numbers in the epithelium and the lamina propria of the jejunum of C. suis infected piglets, indicating a specific immune response to infection. The most prominent subpopulation in the gut epithelium was T-cell receptor-γδ (TcR-γδ) cells, which are engaged in the primary immune response to pathogens (16, 34). TcR-γδ T-cells were also found to be involved in the immune response against other coccidian parasites, e.g., Eimeria vermiformis of mice (48).
For other coccidian parasites, humoral immune response seems to have a minor role in the protection mechanism. Schito et al. (49) suggested that primary infection with different Eimeria spp. is controlled by innate immune response. Stimulation of humoral immunity by Eimeria is known but its effectiveness in controlling the infection is still unclear (50). Immune sera from E. tenella-infected chicken and E. falciformis in mice enhanced the phagocytic activity of macrophages (51, 52). In spite of the fact that piglets are born with an immature immune system (47), cellular immune responses might be involved in the development of immunity against coccidian parasites including C. suis (16, 53–55). The role of passive immune response and the transmission of immune components from infected sows to piglets had been neglected by many authors (41, 56, 57). However, earlier works have shown that colostral antibodies may participate in resistance against natural infections with C. suis (58, 59). Recently, Schwarz et al. (60) demonstrated that naturally acquired C. suis-specific antibodies (IgA, IgM, and IgG) were transferred from sows to their piglets via colostrum, which in turn provided partial protection against the outcome of experimental infection (clinical disease and oocyst shedding) in the presence of high IgA titers in colostrum as well as milk and serum of superinfected sows. It is currently unclear whether the detected immunoglobulins have a protective function by themselves or are merely markers for protection conveyed by other, not yet explored, mechanisms.
Can Comparative Genomics Help to Unravel Biology and Support New Intervention Strategies?
While the genomes of many coccidian species are available in the ToxoDB database (61), C. suis is still lacking a reference genome. Moreover, the number of chromosomes is also unknown. To date, only few ribosomal and mitochondrial sequences of Cystoisospora species were generated for phylogenetic studies, which established that the genus Cystoisospora constitutes a monophyletic clade with the Sarcocystidae, and it is closely related to Toxoplasma and Neospora (62–64). These studies also confirmed the hypothesis that heteroxeny is an evolutionary derived character in Cystoisospora (62).
Current Next Generation Sequencing (NGS) technologies allow assembling of new genomes in a rapid and inexpensive way. First estimates based on NGS data showed that the genome of C. suis is about 84 Mb and contains more than 8000 genes (65). These numbers are comparable to other coccidian species; however, comparative genomics analyses revealed that only about 60% of the C. suis genes have orthologs in T. gondii (65), implying a greater divergence than expected between these two species. Thus, to generate a comprehensive gene catalog of C. suis it will be crucial to integrate gene predictions with RNA-Seq data from different developmental stages. This will also allow for identification of genes involved in life stage transitions, as similarly performed in E. tenella (66). Finally, RNA-Seq can elucidate the molecular changes of C. suis and pig during infection, as exemplified in experiments in N. caninum (67) and T. gondii (68).
Intervention strategies can also be aided by genomics. Currently, the drug toltrazuril is the only treatment available against C. suis; however, resistance has already emerged in Eimeria (69), implying the necessity to find new effective drugs. The availability of the gene catalog of C. suis will be a starting point to detect drug targets, based on the functional annotation of protein-coding genes. Typically, annotation of gene function can be inferred on the basis of orthologous proteins, using tools such as Blast2GO (70). Afterwards, screening for drug targets can be performed on the basis of the functions of candidates identified as drug targets in other coccidia. These include protein kinases (71–73), apicoplast proteins (74), enzymes involved in fatty acid biosynthesis (75) and shikimate metabolism (76), mitochondrial proteins (77), and others, reviewed in Ref. (78). Another approach to identify drug targets involves comparing the metabolic pathways of parasite and host (79), for example, selecting pathways that are present in C. suis but absent in pig.
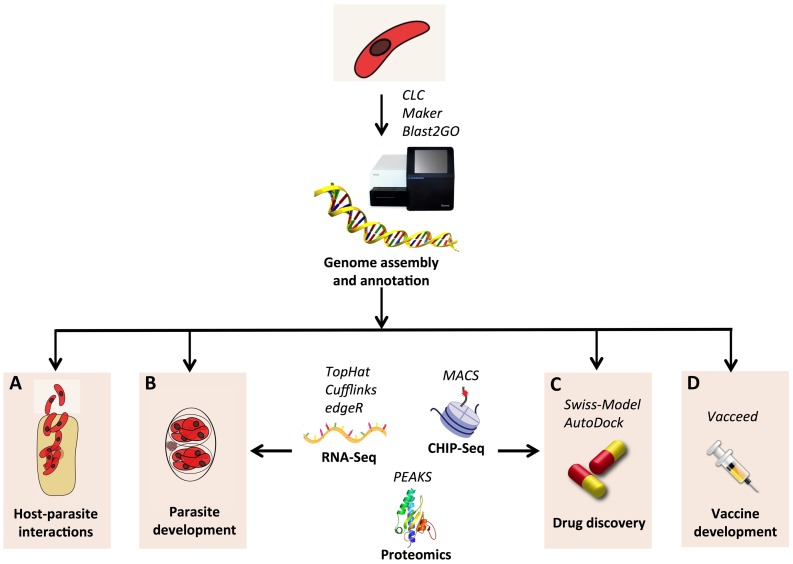
An alternative control route might be vaccination; however, there is at present no vaccine available against C. suis. Although early attempts using the merozoite attachment protein SAP induced a 96–99% reduction in merozoites (80), the resulting vaccine patent was withdrawn. In this regard, genomics can also contribute to vaccine discovery: using the reverse vaccinology paradigm (81), it is possible to screen the genome for vaccine candidates by identifying proteins with immunogenic features. This approach has been successfully applied in various bacterial species (82). However, the inherent complexity of eukaryotic pathogens has hindered the application of this strategy in such organisms. Recently, the feasibility of reverse vaccinology has been reviewed in the coccidian parasite N. caninum (83). In parallel, bioinformatics tools and pipelines have finally emerged to address the specific issue of detecting vaccine candidates in eukaryotic pathogens (84–87). An overview of the in silico analysis of the C. suis genomics data is depicted in Figure 2.
Figure 2.
Schematic view of the in silico analysis of genomic data for C. suis. The genome sequence can be assembled with next generation sequencing using a combination of short and long reads libraries. Tools such as CLC (CLC Bio-Qiagen, Aarhus, Denmark), Maker (88), and Blast2GO (70) can be applied for de novo assembly, annotation, and functional annotation, respectively. Other NGS technologies, such as RNA-Seq (89) and CHIP-Seq (90), together with proteomics (91), can be used to unravel the biology of the parasite and to discover new drugs and vaccines. Changes involved in host–parasite interaction (A) and developmental switches (B) can be identified both at the genetic and epigenetic level by RNA-Seq and CHIP-Seq, respectively: transcripts are reconstructed using the programs TopHat (92) and Cufflinks (93); differentially expressed genes are detected by edgeR (94); CHIP-Seq data are processed with the MACS software (95). (C) 3D structure of drug candidates can be reconstructed by homology using Swiss-Model (96); screening of virtual libraries of compounds can be performed with AutoDock (97). (D) Vaccine candidates can be identified using Vacceed (86) and validated by proteomics approaches, such as mass spectrometry, with the aid of the software PEAKS (Bioinformatics Solutions Inc., Waterloo, ON, Canada).
Interactions of C. suis with the Gut Microbiota
The gut ecosystem is maintained by close cross-talk between host, intestinal microbiota, and parasites (98), and ultimately this has implications on host health and diseases (99). Excretory and secretory products of intestinal parasites may continuously disrupt the balance between the gut microbiota and the body (100), whereas on the other hand, metabolic products of the microbiota may also interfere with the establishment and survival of parasites, subsequently changing the outcome of parasitic infection (100).
The digestive tract of piglets is sterile at birth and becomes rapidly colonized with microorganisms from the surrounding environment (101, 102). Strict anaerobes predominate in the normal flora and this microbial composition and diversity underpins the health status of the pigs (103, 104), especially during the suckling and post-weaning period. Symbiotic interactions between host and gut microbiota mainly occur along the intestinal mucosa (105). Since C. suis is mainly localized in the intestinal mucosa, more precisely in the epithelial cells of the villi and, in heavier infections, also the crypts (12), it is prudent to assume that it may strongly interact with the gut microbiota of the host. It is well documented that coccidiosis in chickens highly influences the diversity of gut microbiota (106–108). Damage of intestinal epithelium during intracellular multiplication of Eimeria enhances mucus secretion from goblet cells together with leakage of glycoproteins and mannose residues, which favors growth and adherence of pathogenic bacteria-like Clostridium perfringens (109, 110) and Salmonella typhimurium in germ-free chickens (111). More recently, Kirino et al. (112) reported significantly higher Eimeria OPG count in fecal samples of Japanese beef cattle suffering from hemorrhagic enteritis compared to the control animals. Based on microbiological examination, the authors also found that the mean fecal coliform count was also significantly higher in the cattle harboring both Eimeria zuernii and Cl. perfringens.
Cystoisosporosis is characterized by high morbidity and low mortality within a litter. Increased mortality, however, may be related to coinfection and/or secondary bacterial infection (103, 113). Entry of pathogenic microorganism following disruption of mucosal barrier as a result of multiplication of C. suis has also been demonstrated in pigs (114). Results obtained by Mengel et al. (103) highlighted a correlation between clostridial infection and clinical cystoisosporosis, which further confirms the hypothesis that C. suis creates a suitable environment for extensive development of Cl. perfringens, as severe clinical signs and mortality occurred only in pigs that harbored both pathogens.
Klaus (115) examined the fecal flora of piglets from three groups, one infected with C. suis on the first day of life, one infected with C. suis and treated with toltrazuril 2 days later, and one uninfected group. It was evident that the fecal flora of young piglets undergoes significant changes during the first weeks of life, with an initial high excretion of E. coli and other enterobacteriaceae, followed by an increase of lactobacilli, which appeared to stabilize the intestinal environment. Irrespective of treatment groups, high numbers of enterococci were excreted during the period of parasitic invasion. The average excretion of Cl. perfringens was highest in the infected untreated group and lowest in the uninfected animals, indicating that infection with C. suis seems to alter the succession of bacterial colonization and that this effect can be partially reversed by toltrazuril treatment (115). These results are in accordance with a study conducted by Alnassan et al. (116), where prophylactic medication of chickens with toltrazuril before infection caused less severe coccidial and subsequent necrotic enteric lesions in treated individuals. Further research on the development of the gut microbiota during the first weeks of life is needed to understand the role of bacterial colonization in the pathogenesis of coccidiosis in young animals including piglets.
Moreover, as pigs may serve as an animal model for many human pathologies (117), interactions between C. suis, the gut microbiota, and the intestinal immune system in piglets may also help to understand the pathogenesis of other neonatal diarrheal diseases in mammals.
Conclusion
Although current knowledge on the immunity and host–pathogen interactions of neonatal porcine cystoisosporosis is still fragmentary, recent findings indicate that sustainable control must focus on immunity-based methods and new drug targets, taking into consideration the interaction of the parasite with the gut microbiome. As the immature immune system in new-born piglets seems to be incapable of controlling the parasite, the role of maternal immunity should be reconsidered. The presence of a single available compound against cystoisosporosis calls for urgent development of new drugs and vaccines as sustainable control methods against C. suis. We prospect that genomics and transcriptomics analyses will certainly play a major role in finding new drug targets and vaccines. Moreover, since C. suis significantly disturbs the composition of the microbial gut community, intervention strategies must focus on a more holistic approach to piglet health. We anticipate that a deeper understanding of the biology C. suis will favor the flourishing of studies in other mammalian hosts, where coccidiosis is often enigmatic and frequently neglected. Since new tools are available to carry out research on porcine cystoisosporosis, we propose that C. suis can serve as a model for cystoisosporosis in other mammals.
Author Contributions
AS drafted the introduction and compiled the manuscript; AA-E compiled the chapter on immunology and age resistance, BF drafted the chapter about the life cycle, NP drafted the chapter about the genomics analyses, AJ, BR, and BH devised the outline, added unpublished data, and revised and edited the MS together with all other authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Biester BW, Murray C. Studies in infections enteritis of swine. J Am Vet Med Assoc (1934) 85:207–19. [Google Scholar]
- 2.Sangster LT, Seibold HR, Mitchell FE. Coccidial infection in suckling pigs. Proc Am Assoc Vet Lab Diagn (1976) 19:51–5. [Google Scholar]
- 3.Harleman JH, Meyer RC. Life cycle of Isospora suis in gnotobiotic and conventionalized piglets. Vet Parasitol (1984) 17:27–9. 10.1016/0304-4017(84)90062-1 [DOI] [PubMed] [Google Scholar]
- 4.Stuart B, Lindsay D, Ernest J, Gosser H. Isospora suis enteritis in piglets. Vet Pathol (1980) 17:84–93. [DOI] [PubMed] [Google Scholar]
- 5.Worliczek HL, Ruttkowski B, Joachim A, Saalmüller A, Gerner W. Faeces, FACS, and functional assays – preparation of Isospora suis oocyst antigen and representative controls for immunoassays. Parasitology (2010) 137(11):1637–43. 10.1017/S0031182010000557 [DOI] [PubMed] [Google Scholar]
- 6.Joachim A, Schwarz L, Hinney B, Ruttkowski B, Vogl C, Mundt HC. Which factors influence the outcome of experimental infection with Cystoisospora suis? Parasitol Res (2014) 113(5):1863–73. 10.1007/s00436-014-3834-8 [DOI] [PubMed] [Google Scholar]
- 7.Lindsay D, Current W, Taylor J. Effects of experimentally induced Isospora suis infection on morbidity, mortality and weight gains in nursing pigs. Am J Vet Res (1985) 46(7):71511–2. [PubMed] [Google Scholar]
- 8.Mundt HC, Mundt-Wustenberg S, Daugschies A, Joachim A. Efficacy of various anticoccidials against experimental porcine neonatal isosporosis. Parasitol Res (2007) 100(2):401–11. 10.1007/s00436-006-0314-9 [DOI] [PubMed] [Google Scholar]
- 9.Stuart BP, Sisk DB, Bedell DM, Gosser HS. Demonstration of immunity against Isospora suis in swine. Vet Parasitol (1982) 9(3–4):185–91. 10.1016/0304-4017(82)90063-2 [DOI] [PubMed] [Google Scholar]
- 10.Mundt HC, Joachim A, Becka M, Daugschies A. Isospora suis: an experimental model for mammalian intestinal coccidiosis. Parasitol Res (2006) 98(2):167–75. 10.1007/s00436-005-0030-x [DOI] [PubMed] [Google Scholar]
- 11.Sotiraki S, Roepstorff A, Nielsen JP, Maddox-Hyttel C, Enoe C, Boes J, et al. Population dynamics and intra-litter transmission patterns of Isospora suis in suckling piglets under on-farm conditions. Parasitology (2008) 135(3):395–405. 10.1017/S0031182007003952 [DOI] [PubMed] [Google Scholar]
- 12.Niestrath M, Takla M, Joachim A, Daugschies A. The role of Isospora suis as a pathogen in conventional piglet production in Germany. J Vet Med B Infect Dis Vet Public Health (2002) 49(4):176–80. 10.1046/j.1439-0450.2002.00459.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gualdi V, Vezzoli F, Luini M, Nisoli L. The role of Isospora suis in the ethiology of diarrhoea in suckling piglets. Parasitol Res (2003) 90(Suppl 3):S163–5. 10.1007/s00436-003-0928-0 [DOI] [PubMed] [Google Scholar]
- 14.Leten J, Smets K, Claerebout E, Mundt H, Heesen H, Vercruysse J. Isosporose bij zuigende biggen in Vlaanderen. Vlaams Diergeneesk Tijdschr (2002) 71:63–7. [Google Scholar]
- 15.Mundt HC, Cohnen A, Daugschies A, Joachim A, Prosl H, Schmäschke R, et al. Occurrence of Isospora suis in Germany, Switzerland and Austria. J Vet Med B Infect Dis Vet Public Health (2005) 52(2):93–7. 10.1111/j.1439-0450.2005.00824.x [DOI] [PubMed] [Google Scholar]
- 16.Worliczek HL, Buggelsheim M, Alexandrowicz R, Witter K, Schmidt P, Gerner W, et al. Changes in lymphocyte populations in suckling piglets during primary infections with Isospora suis. Parasite Immunol (2010) 32(4):232–44. 10.1111/j.1365-3024.2009.01184.x [DOI] [PubMed] [Google Scholar]
- 17.Haug A, Gjevre A-G, Thebo P, Mattsson JG, Kaldhusdal M. Coccidial infections in commercial broilers: epidemiological aspects and comparison of Eimeria species identification by morphometric and polymerase chain techniques. Avian Pathol (2008) 37(2):161–70. 10.1080/03079450801915130 [DOI] [PubMed] [Google Scholar]
- 18.Györke A, Pop L, Cozma V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite (2013) 20:50. 10.1051/parasite/2013052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicek H, Sevimli F, Kozan E, Köse M, Eser M, Doğan N. Prevalence of coccidia in beef cattle in Western Turkey. Parasitol Res (2007) 101(5):1239–43. 10.1007/s00436-007-0627-3 [DOI] [PubMed] [Google Scholar]
- 20.Reddy S, Sivajothi S, Rayulu V. Clinical coccidiosis in adult cattle. J Parasit Dis (2015) 39(3):557–9. 10.1007/s12639-013-0395-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vítovec J, Koudela B. Double alteration of the small intestine in conventional and gnotobiotic piglets experimentally infected with the coccidium Isospora suis (Apicomplexa, Eimeriidae). Folia Parasitol (Praha) (1990) 37(1):21–3. [PubMed] [Google Scholar]
- 22.Chae C, Kwon D, Kim O, Min K, Cheon DS, Choi C, et al. Diarrhoea in nursing piglets associated with coccidiosis: prevalence, microscopic lesions and coexisting microorganisms. Vet Rec (1998) 143(15):417–20. 10.1136/vr.143.15.417 [DOI] [PubMed] [Google Scholar]
- 23.Joachim A, Mundt HC. Efficacy of sulfonamides and Baycox® against Isospora suis in experimental infections of suckling piglets. Parasitol Res (2011) 109(6):1653–9. 10.1007/s00436-011-2438-9 [DOI] [PubMed] [Google Scholar]
- 24.Kreiner T, Worliczek HL, Tichy A, Joachim A. Influence of toltrazuril treatment on parasitological parameters and health performance of piglets in the field – an Austrian experience. Vet Parasitol (2011) 183(1–2):14–20. 10.1016/j.vetpar.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 25.Chapman HD. Biochemical, genetics and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol (1997) 26(2):221–44. 10.1080/03079459708419208 [DOI] [PubMed] [Google Scholar]
- 26.Worliczek HL, Ruttkowski B, Schwarz L, Witter K, Tschulenk W, Joachim A. Isospora suis in an epithelial cell culture system – an in vitro model for sexual development in coccidia. PLoS One (2013) 8(7):e69797. 10.1371/journal.pone.0069797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsay DS, Dubey JP, Blagburn BL. Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin Microbiol Rev (1997) 10:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harleman JH, Meyer RC. Isospora suis infection in piglets. A review. Vet Q (1983) 5(4):178–85. 10.1080/01652176.1983.9693894 [DOI] [PubMed] [Google Scholar]
- 29.Vítovec J, Koudela B. Pathology of natural isosporosis in nursing piglets. Folia Parasitol (Praha) (1987) 34(3):199–204. [PubMed] [Google Scholar]
- 30.Worliczek HL, Mundt HC, Ruttkowski B, Joachim A. Age, not infection dose, determines the outcome of Isospora suis infections in suckling piglets. Parasitol Res (2009) 105(Suppl 1):S157–62. 10.1007/s00436-009-1507-9 [DOI] [PubMed] [Google Scholar]
- 31.Lindsay DS, Stuart BP, Wheat BE, Ernst JV. Endogenous development of the swine coccidium, Isospora suis Biester 1934. J Parasitol (1980) 66(5):771–9. 10.2307/3280667 [DOI] [PubMed] [Google Scholar]
- 32.Dubey JP, Lindsay DS, Lappin MR. Toxoplasmosis and other intestinal coccidial infections in cats and dogs. Vet Clin North Am Small Anim Pract (2009) 39(6):1009–34. 10.1016/j.cvsm.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 33.Lindsay DS, Current WL, Ernst JV. Sporogony of Isospora suis Biester, 1934 of swine. J Parasitol (1982) 68(5):861–5. 10.2307/3280994 [DOI] [PubMed] [Google Scholar]
- 34.Gabner S, Worliczek HL, Witter K, Meyer FR, Gerner W, Joachim A. Immune response to Cystoisospora suis in piglets: local and systemic changes in T-cell subsets and selected mRNA transcripts in the small intestine. Parasite Immunol (2014) 36(7):277–91. 10.1111/pim.12116 [DOI] [PubMed] [Google Scholar]
- 35.Worliczek HL, Buggelsheim M, Saalmüller A, Joachim A. Porcine isosporosis: infection dynamics, pathophysiology and immunology of experimental infections. Wien Klin Wochenschr (2007) 119(19–20 Suppl 3):33–9. 10.1007/s00508-007-0859-3 [DOI] [PubMed] [Google Scholar]
- 36.Worliczek HL, Joachim A. Neonatal porcine coccidiosis. In: Mehlhorn H, editor. Progress in Parasitology. Parasitology Research Monographs. (Vol. 2), Berlin: Springer; (2011). p. 79–91. [Google Scholar]
- 37.Pinckney RD, Lindsay DS, Toivio-Kinnucan MA, Blagburn BL. Ultrastructure of Isospora suis during excystation and attempts to demonstrate extraintestinal stages in mice. Vet Parasitol (1993) 47(3–4):225–33. 10.1016/0304-4017(93)90024-H [DOI] [PubMed] [Google Scholar]
- 38.Lindsay D, Houk A, Mitchell S, Dubey J. Developmental biology of Cystoisospora (Apicomplexa: Sarcocystidae) monozoic tissue cysts. J Parasitol (2014) 100(4):392–8. 10.1645/13-494.1 [DOI] [PubMed] [Google Scholar]
- 39.Stuart BP, Bedell DM, Lindsay DS. Coccidiosis in swine: a search for extraintestinal stages of Isospora suis. Vet Rec (1982) 110:82–3. 10.1136/vr.110.4.82 [DOI] [PubMed] [Google Scholar]
- 40.Joachim A, Schwarz L, Worliczek H, editors. Superinfection of sows with Cystoisospora suis ante partum leads to a milder course of cystoisosporosis in suckling piglets. In: 11th International Coccidiosis Conference; 2014 September 26-30. Dresden (2014). [DOI] [PubMed] [Google Scholar]
- 41.Stuart B, Gosser H, Allen C, Bedell D. Coccidiosis in swine: dose and age response to Isospora suis. Can J Comp Med (1982) 46(3):317–20. [PMC free article] [PubMed] [Google Scholar]
- 42.Koudela K, Kučerová S. Role of acquired immunity and natural age resistance on course of Isospora suis coccidiosis in nursing piglets. Vet Parasitol (1999) 82(2):93–9. 10.1016/S0304-4017(99)00009-6 [DOI] [PubMed] [Google Scholar]
- 43.Harp J, Sacco R. Development of cellular immune functions in neonatal to weanling mice: relationship to Cryptosporidium parvum infection. J Parasitol (1996) 82(2):245–9. 10.2307/3284155 [DOI] [PubMed] [Google Scholar]
- 44.Daugschies A, Najdrowski M. Eimeriosis in cattle: current understanding. J Vet Med B Infect Dis Vet Public Health (2005) 52(10):417–27. 10.1111/j.1439-0450.2005.00894.x [DOI] [PubMed] [Google Scholar]
- 45.Pakandl M, Hlásková L, Poplstein M, Neveceralová M, Vodicka T, Salát J, et al. Immune response to rabbit coccidiosis:a comparison between infections with Eimeria flavescens and E. intestinalis. Folia Parasitol (Praha) (2008) 55(1):1–6. 10.14411/fp.2008.001 [DOI] [PubMed] [Google Scholar]
- 46.Pakandl M, Hlásková L, Poplštein M, Chromá V, Vodička T, Salát J, et al. Dependence of the immune response to coccidiosis on the age of rabbit suckling. Parasitol Res (2008) 103(6):1265–71. 10.1007/s00436-008-1123-0 [DOI] [PubMed] [Google Scholar]
- 47.Becker B, Misfeldt M. Evaluation of the mitogen-induced proliferation and cell surface differentiation antigens of lymphocytes from pigs 1 to 30 days of age. J Anim Sci (1993) 71(8):2073–8. [DOI] [PubMed] [Google Scholar]
- 48.Ramsburg E, Tigelaar R, Craft J, Hayday A. Age-dependent requirement for γδ T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med (2003) 198(9):1403–14. 10.1084/jem.20030050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schito M, Barta J, Chobotar B. Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J Parasitol (1996) 82(2):255–62. 10.2307/3284157 [DOI] [PubMed] [Google Scholar]
- 50.Lillehoj H, Trout J. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev (1996) 9(3):349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onaga H, Ishii T. Effects of chicken anti-Eimeria tenella serum on the phagocytosis of sporozoites and merozoites by chicken peritoneal macrophages. Jpn J Vet Sci (1980) 42(2):211–9. 10.1292/jvms1939.42.211 [DOI] [PubMed] [Google Scholar]
- 52.Bekhti K, Pery P. In vitro interactions between murine macrophages and Eimeria falciformis sporozoites. Res Immunol (1989) 140(7):697–709. 10.1016/0923-2494(89)90023-0 [DOI] [PubMed] [Google Scholar]
- 53.Rommel M, Heydorn A. Transfer of immunity to Eimeria infections by lymphocytes. Z Parasitenkd (1971) 36(3):242–50. [PubMed] [Google Scholar]
- 54.Yun CH, Lillehoj H, Lillehoj E. Intestinal immune responses to coccidiosis. Dev Comp Immunol (2000) 24(2–3):303–24. 10.1016/S0145-305X(99)00080-4 [DOI] [PubMed] [Google Scholar]
- 55.Buggelsheim M. Interaktionen von Isospora suis mit dem Immun-system des Schweins: Auswirkungen der Infektion auf die lokale Immunantwort im Dünndarm. Doctoral thesis, Vetmeduni, Vienna: (2008). [Google Scholar]
- 56.Taylor J. Immune Response of Pigs to Isospora suis (Apicomplexa, Eimeriidae) Doctoral thesis, Auburn University, Auburn, AL: (1984). [Google Scholar]
- 57.Baekbo P, Christensen J, Henriksen S, Nielsen K, editors. Attempts to induce colostral immunity against Isospora suis infections in piglets. In: Proc Int Pig Vet Soc Cong Bangkok (1994). [Google Scholar]
- 58.O’Neill P, Parfitt J. Observations on Isospora suis infection in a minimal disease pig herd. Vet Rec (1976) 16:321–3. 10.1136/vr.98.16.321 [DOI] [PubMed] [Google Scholar]
- 59.Greve E. Isospora suis species in a Danish SPF-herd. Nord Vet Med (1985) 37(3):140–4. [PubMed] [Google Scholar]
- 60.Schwarz L, Worliczek HL, Winkler M, Joachim A. Superinfection of sows with Cystoisospora suis ante partum leads to a milder course of cystoisosporosis in suckling piglets. Vet Parasitol (2014) 204(3–4):158–68. 10.1016/j.vetpar.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 61.Gajria B, Bahl A, Brestelli J, Dommer J, Fischer S, Gao X, et al. ToxoDB: an integrated Toxoplasma gondii database resource. Nucleic Acids Res (2008) 36:553–6. 10.1093/nar/gkm981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carreno R, Schnitzler B, Jeffries A, Tenter A, Johnson A, Barta J. Phylogenetic analysis of coccidia based on 18S rDNA sequence comparison indicates that Isospora is most closely related to Toxoplasma and Neospora. J Eukaryot Microbiol (1998) 45(2):184–8. 10.1111/j.1550-7408.1998.tb04523.x [DOI] [PubMed] [Google Scholar]
- 63.Franzen C, Müller A, Bialek R, Diehl V, Salzberger B, Fatkenheuer G. Taxonomic position of the human intestinal protozoan parasite Isospora belli as based on ribosomal RNA sequences. Parasitol Res (2000) 86(8):669–76. 10.1007/PL00008550 [DOI] [PubMed] [Google Scholar]
- 64.Samarasinghe B, Johnson J, Ryan U. Phylogenetic analysis of Cystoisospora species at the rRNA ITS1 locus and development of a PCR-RFLP assay. Exp Parasitol (2008) 118(4):592–5. 10.1016/j.exppara.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 65.Palmieri N, Worliczek H, Blake D, Joachim A, editors. The genome of the protozoan parasite Cystoisospora suis and its implications for vaccine discovery. In: Society for Molecular Biology and Evolution; 2015 July 12-16. Vienna: (2015). [Google Scholar]
- 66.Walker R, Sharman P, Miller C, Lippuner C, Okoniewski M, Eichenberger R, et al. RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genomics (2015) 16:94. 10.1186/s12864-015-1298-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishimura M, Tanaka S, Ihara F, Muroi Y, Yamagishi J, Furuoka H, et al. Transcriptome and histopathological changes in mouse brain infected with Neospora caninum. Sci Rep (2015) 5(1):7936. 10.1038/srep07936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pittman KJ, Aliota M, Knoll L. Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genomics (2014) 15:806. 10.1186/1471-2164-15-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephan B, Rommel M, Daugschies A, Haberkorn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet Parasitol (1997) 69(1–2):19–29. 10.1016/S0304-4017(96)01096-5 [DOI] [PubMed] [Google Scholar]
- 70.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics (2005) 21:3674–6. 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- 71.Donald RGK, Zhong T, Wiersma H, Nare B, Yao D, Lee A, et al. Anticoccidial kinase inhibitors: identification of protein kinase targets secondary to cGMP-dependent protein kinase. Mol Biochem Parasitol (2006) 149(1):86–98. 10.1016/j.molbiopara.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 72.Miranda-Saavedra D, Gabaldón T, Barton GJ, Langsley G, Doerig C. The kinomes of apicomplexan parasites. Microbes Infect (2012) 14(10):796–810. 10.1016/j.micinf.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 73.Wei F, Wang W, Liu Q. Protein kinases of Toxoplasma gondii: functions and drug targets. Parasitol Res (2013) 112(6):2121–9. 10.1007/s00436-013-3451-y [DOI] [PubMed] [Google Scholar]
- 74.Wiesner J, Reichenberg A, Heinrich S, Schlitzer M, Jomaa H. The plastid-like organelle of apicomplexan parasites as drug target. Curr Pharm Des (2008) 14(9):855–71. 10.2174/138161208784041105 [DOI] [PubMed] [Google Scholar]
- 75.Goodman C, McFadden G. Fatty acid biosynthesis as a drug target in apicomplexan parasites. Curr Drug Targets (2007) 8(1):15–30. 10.2174/138945007779315579 [DOI] [PubMed] [Google Scholar]
- 76.Derrer B, Macheroux P, Kappes B. The shikimate pathway in apicomplexan parasites: implications for drug development. Front Biosci (Landmark Ed) (2013) 18:944–69. 10.2741/4155 [DOI] [PubMed] [Google Scholar]
- 77.Mather MW, Henry K, Vaidya A. Mitochondrial drug targets in apicomplexan parasites. Curr Drug Targets (2007) 8(1):49–60. 10.2174/138945007779315632 [DOI] [PubMed] [Google Scholar]
- 78.Coombs GH, Müller S. Recent advances in the search for new anti-coccidial drugs. Int J Parasitol (2002) 32(5):497–508. 10.1016/S0020-7519(01)00354-X [DOI] [PubMed] [Google Scholar]
- 79.Chaudhary K, Roos DS. Protozoan genomics for drug discovery. Nat Biotechnol (2005) 23(9):1089–91. 10.1038/nbt0905-1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quick DP, Steger AM, Welter CJ, Welter LM, MW W. Isospora suis Vaccine. Google Patents (1998).
- 81.Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity (2010) 33(4):530–41. 10.1016/j.immuni.2010.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science (2000) 287:1816–20. 10.1126/science.287.5459.1816 [DOI] [PubMed] [Google Scholar]
- 83.Goodswen SJ, Kennedy P, Ellis J. Discovering a vaccine against neosporosis using computers: is it feasible? Trends Parasitol (2014) 30(8):401–11. 10.1016/j.pt.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 84.Goodswen SJ, Kennedy P, Ellis J. A novel strategy for classifying the output from an in silico vaccine discovery pipeline for eukaryotic pathogens using machine learning algorithms. BMC Bioinformatics (2013) 14:315. 10.1186/1471-2105-14-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodswen S, Kennedy P, Ellis J. Enhancing in silico protein-based vaccine discovery for eukaryotic pathogens using predicted peptide-MHC binding and peptide conservation scores. PLoS One (2014) 9(12):e115745. 10.1371/journal.pone.0115745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goodswen SJ, Kennedy P, Ellis J. Vacceed: a high-throughput in silico vaccine candidate discovery pipeline for eukaryotic pathogens based on reverse vaccinology. Bioinformatics (2014) 15(30):2381–3. 10.1093/bioinformatics/btu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phan IH, Stacy R, Myler PJ. Selecting targets from eukaryotic parasites for structural genomics and drug discovery. In: Anderson WF, editor. Structural Genomics and Drug Discovery. Methods in Molecular Biology. (Vol. 1140), New York, NY: Springer; (2014). p. 53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cantarel B, Korf I, Robb S, Parra G, Ross E, Moore B, et al. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res (2008) 18(1):188–96. 10.1101/gr.6743907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet (2009) 10(1):57–63. 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furey T. ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet (2012) 13(12):840–52. 10.1038/nrg3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wasinger V, Zeng M, Yau Y. Current status and advances in quantitative proteomic mass spectrometry. Int J Proteomics (2013) 2013:180605. 10.1155/2013/180605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg S. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol (2013) 14(4):R36. 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trapnell C, Williams B, Pertea G, Mortazav IA, Kwan G, van Baren M, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol (2010) 28(5):511–5. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robinson MD, McCarthy DJ, Smyth G. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (2010) 26(1):139–40. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol (2008) 9(9):R137. 10.1186/gb-2008-9-9-r137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwede T, Kopp J, Guex N, Peitsch M. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res (2003) 31(13):3381–5. 10.1093/nar/gkg520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morris G, Huey R, Lindstrom W, Sanner M, Belew R, Goodsell D, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem (2009) 30(16):2785–91. 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cantacessi C, Giacomin P, Croese J, Zakrzewski M, Sotillo J, McCann L, et al. Impact of experimental hookworm infection on the human gut microbiota. J Infect Dis (2014) 210(9):1431–4. 10.1093/infdis/jiu256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dheilly NM. Holobiont-holobiont interactions: redefining host-parasite interactions. PLoS Pathog (2014) 10(7):e1004093. 10.1371/journal.ppat.1004093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berrilli F, Di Cave D, Cvallero S, D’Amelio S. Interactions between parasites and microbial communities in the human gut. Front Cell Infect Microbiol (2012) 2:141. 10.3389/fcimb.2012.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zoetendal E, Akkermans A, Akkermans-van Vliet W, Arjan J, de Visser G, de Vos W. The host genotype affects the bacterial community in the human gastrointestinal tract. Micro Ecol Health Dis (2001) 13:129–34. 10.1080/089106001750462669 [DOI] [Google Scholar]
- 102.Katouli M, Lund A, Wallgren P, Kühn I, Söderlind O, Möllby R. Phenotypic characterization of intestinal Escherichia coli of pigs during suckling, postweaning, and fattening periods. Appl Environ Microbiol (1995) 61(2):778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mengel H, Krüger M, Krüger MU, Westphal B, Swidsinski A, Schwarz S, et al. Necrotic enteritis due to simultaneous infection with Isospora suis and clostridia in newborn piglets and its prevention by early treatment with toltrazuril. Parasitol Res (2012) 110(4):1347–55. 10.1007/s00436-011-2633-8 [DOI] [PubMed] [Google Scholar]
- 104.Katouli M, Wallgren P. Chapter 2 metabolism and population dynamics of the intestinal microflora in the growing pig. In: Holzapfel WH, Naughton PJ, Salek E, editors. Biology of Growing Animals. (Vol. 2), Amsterdam: Elsevier; (2005). p. 21–53. [Google Scholar]
- 105.Cabreiro F, Gems D. Worms need microbes too: microbiota, health and aging in Caenorhabditis elegans. EMBO Mol Med (2013) 5(9):1300–10. 10.1002/emmm.201100972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perez V, Jacobs C, Barnes J, Jenkins M, Kuhlenschmidt M, Fahey GJ, et al. Effect of corn distillers dried grains with solubles and Eimeria acervulina infection on growth performance and intestinal microbiota of young chicks. Poult Sci (2011) 90:958–64. 10.3382/ps.2010-01066 [DOI] [PubMed] [Google Scholar]
- 107.Martynova-Van Kley M, Oviedo-Rondon E, Dowd S, Hume M, Nalian A. Effect of Eimeria infection on cecal microbiome of broilers fed essential oils. Int J Poult Sci (2012) 11(12):747–55. 10.3923/ijps.2012.747.755 [DOI] [Google Scholar]
- 108.Stanley D, Wu S, Rodgers N, Swick R, Moore R. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a nectrotic enteritis challenge model in chickens. PLoS One (2014) 9(8):e104739. 10.1371/journal.pone.0104739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, et al. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol (2008) 122(1–2):104–15. 10.1016/j.vetimm.2007.10.014 [DOI] [PubMed] [Google Scholar]
- 110.Williams RB. Intercurrent coccidiosis and nectrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol (2005) 34(3):159–80. 10.1080/03079450500112195 [DOI] [PubMed] [Google Scholar]
- 111.Baba E, Tsukamoto Y, Fukata T, Sasai K, Arakawa A. Increase of mannose residues, as Salmonella typhimurium-adhering factor, on the cecal mucosa of germ-free chickens infected with Eimeria tenella. Am J Vet Res (1993) 54:1471–5. [PubMed] [Google Scholar]
- 112.Kirino Y, Tanida M, Hasunuma H, Kato T, Irie T, Horii Y, et al. Increase of Clostridium perfringens in association with Eimeria in haemorrhagic enteritis in Japanese beef cattle. Vet Rec (2015) 177(8):202. 10.1136/vr.103237 [DOI] [PubMed] [Google Scholar]
- 113.Mundt H, Krüger M, Westphal B, Mengel H, Dittmar K, Kuhnert Y, editors. Investigations into the synergistic effect of postnatal experimental infection with Isospora suis and natural infection with Clostridium perfringens ß 2 for the development of necrotic enteritis in piglets. In: International Pig Veterinary Congress. Durban: (2008). [Google Scholar]
- 114.Bach U, Kalthoff V, Mundt H-C, Popp A, Rinke M, Daugschies A, et al. Parasitological and morphological findings in porcine isosporosis after treatment with symmetrical triazintriones. Parasitol Res (2003) 91(1):27–33. 10.1007/s00436-003-0828-3 [DOI] [PubMed] [Google Scholar]
- 115.Klaus R. Influence of an Isospora suis-Infection on the Development of the Faecal Flora of Suckling Piglets in the First Weeks of Life Diploma thesis, Vetmeduni, Vienna (2012). [Google Scholar]
- 116.Alnassan AA, Shehata AA, Kotsch M, Schrödl W, Krüger M, Daugsschies A, et al. Efficacy of early treatment with toltrazuril in prevention of coccidiosis and necrotic enteritis in chickens. Avian Pathol (2013) 42(5):482–90. 10.1080/03079457.2013.823476 [DOI] [PubMed] [Google Scholar]
- 117.Bassols A, Costa C, Eckersall PD, Osada J, Sabrià J, Tibau J. The pig as an animal model for human pathologies: a proteomics perspective. Proteomics Clin Appl (2014) 8(9–10):715–31. 10.1002/prca.201300099 [DOI] [PubMed] [Google Scholar]