Abstract
During neural development, regulation of microtubule stability is essential for proper morphogenesis of neurons. Recently, the striatin-interacting phosphatase and kinase (STRIPAK) complex was revealed to be involved in diverse cellular processes. However, there is little evidence that STRIPAK components regulate microtubule dynamics, especially in vivo. Here, we show that one of the core STRIPAK components, Strip, is required for microtubule organization during neuronal morphogenesis. Knockdown of Strip causes a decrease in the level of acetylated α-tubulin in Drosophila S2 cells, suggesting that Strip influences the stability of microtubules. We also found that Strip physically and genetically interacts with tubulin folding cofactor D (TBCD), an essential regulator of α- and β-tubulin heterodimers. Furthermore, we demonstrate the genetic interaction between strip and Down syndrome cell adhesion molecule (Dscam), a cell surface molecule that is known to work with TBCD. Thus, we propose that Strip regulates neuronal morphogenesis by affecting microtubule stability.
Microtubules are crucial building blocks of axons and dendrites. They provide physical support, establish polarization, and serve as ‘tracks’ for intracellular transport that enable trafficking of various molecules between cell body and neurite tips. However, microtubules are intrinsically dynamic, which means that the microtubule cytoskeleton can rapidly rearrange in response to internal or external cues. Various molecules support microtubule dynamics, which involves the proper regulation of polymerization and depolymerization of microtubules. Numerous microtubule-associated proteins (MAPs) including structural MAPs, plus-end tracking proteins (+TIPs), and motor proteins directly regulate microtubule assembly and depolymerization1,2,3. Furthermore, post-translational modifications of microtubules are also important for their dynamics by forming a biochemical ‘tubulin code’ that can be ‘read’ by microtubule-interacting factors4. For example, acetylation of α-tubulin influences the degree of kinsesin-1 interaction with the microtubules5. Acetylation of α-tubulin also influences microtubule sensitivity to be severed by katanin6, another mode of regulation of microtubule dynamics that is particularly important for branch formation7. Thus, the stringently regulated microtubule dynamics are crucial for both development and maintenance of neurites, and many neuronal diseases are linked to microtubules8.
The striatin-interacting phosphatase and kinase (STRIPAK) complex is an evolutionarily conserved complex that is recently revealed to have roles in various cellular processes including, signaling, cell cycle control, apoptosis, vesicular trafficking, Golgi assembly, cell polarity and cell migration9. Moreover, STRIPAK complexes have been linked to clinical conditions, including cardiac disease, diabetes, autism, and cerebral cavernous malformation. The core of the STRIPAK complex is the striatin family of proteins that serve as B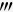 subunits (one of the subfamily of regulatory B subunits) of protein phosphatase 2A (PP2A) complex. In addition to Striatins, A and C subunits of PP2A, Mob3, Mst3, Mst4, Ysk1, Ccm3, and Strip1 and 2 are known to form the core mammalian STRIPAK complex10,11. This core complex binds additional proteins in a mutually exclusive manner to form distinct STRIPAK complexes involved in diverse functions9. Although our knowledge of the composition of the striatin family complexes has increased greatly, much remains to be determined regarding the function of these complexes and the roles of the various striatin family-associated proteins9. There have been some indications that STRIPAK complexes might affect microtubule organization. For example, dMob4, the Drosophila Mob3 homolog is required for microtubule morphology at neuromuscular junctions, peripheral nerves and muscles12. Furthermore, STRIP2 knockdown in PC3 prostate cancer cells altered microtubule organization and induced cell elongation13. It was also reported that Dynein, a minus-end directed motor, associates with the STRIPAK complex using an affinity-purification mass spectrometry analysis10. However, the precise molecular mechanism of microtubule regulation by STRIPAK complexes in vivo is unclear.
subunits (one of the subfamily of regulatory B subunits) of protein phosphatase 2A (PP2A) complex. In addition to Striatins, A and C subunits of PP2A, Mob3, Mst3, Mst4, Ysk1, Ccm3, and Strip1 and 2 are known to form the core mammalian STRIPAK complex10,11. This core complex binds additional proteins in a mutually exclusive manner to form distinct STRIPAK complexes involved in diverse functions9. Although our knowledge of the composition of the striatin family complexes has increased greatly, much remains to be determined regarding the function of these complexes and the roles of the various striatin family-associated proteins9. There have been some indications that STRIPAK complexes might affect microtubule organization. For example, dMob4, the Drosophila Mob3 homolog is required for microtubule morphology at neuromuscular junctions, peripheral nerves and muscles12. Furthermore, STRIP2 knockdown in PC3 prostate cancer cells altered microtubule organization and induced cell elongation13. It was also reported that Dynein, a minus-end directed motor, associates with the STRIPAK complex using an affinity-purification mass spectrometry analysis10. However, the precise molecular mechanism of microtubule regulation by STRIPAK complexes in vivo is unclear.
We previously demonstrated that Drosophila Strip, the homolog of mammalian Strip1 and 2, regulates dendrite branching and axon elongation in Drosophila olfactory projection neurons14. We revealed that Strip serves as a platform for early endosome organization during axon elongation. The shorter axon phenotype caused by strip knockdown was suppressed by the expression of constitutive active form of Rab5, one of the key regulators of early endosome fusion. However, the suppression was partial, and the dendrite overbranching phenotype was not suppressed. We hypothesized that Strip might also form complexes with other molecules that affect axon elongation and dendrite branching. Thus, we speculated that Strip might affect microtubule organization, since microtubules are crucial components of axons and dendrites, and microtubule dynamics should be strictly regulated during neural development.
Here we show that Strip forms a complex with microtubules and affects their stabilization. Furthermore, we reveal that strip genetically interacts with tubulin folding cofactor D (TBCD), one of the tubulin-folding cofactors that assist the tubulin heterodimer formation and control the availability of tubulin subunits and microtubule stability15, to regulate neuronal morphogenesis. Moreover, we show that Strip cooperates with Down syndrome cell adhesion molecule (Dscam), a cell surface molecule whose intracellular domain binds to TBCD and it is likely that TBCD mediates Dscam functions by affecting microtubule dynamics16.
Results
Strip is localized along microtubules
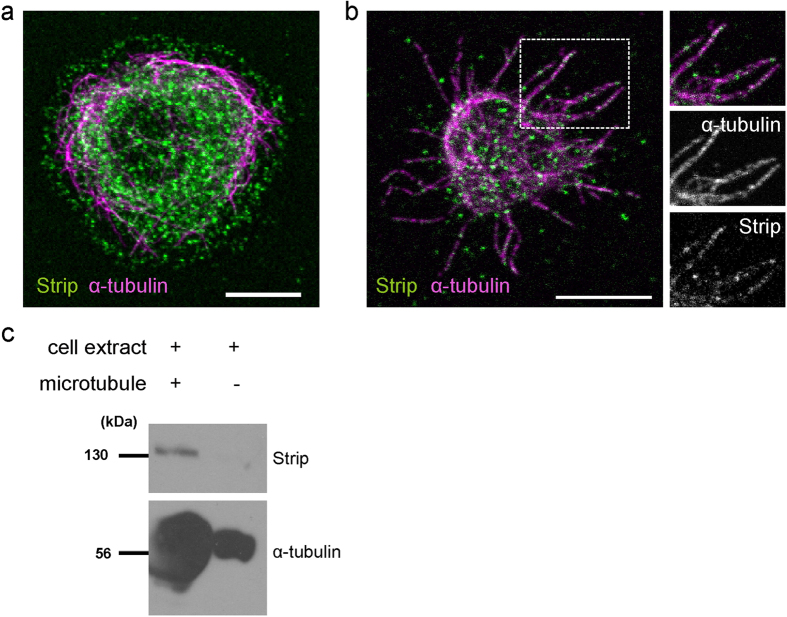
To understand the relationship between Strip and microtubule, we first examined the subcellular localization of Strip in Drosophila S2 cells. It is difficult to evaluate whether Strip is localized on microtubules because endogenous Strip seems to be distributed throughout the cytoplasm (Fig. 1a). Therefore, we utilized the extraction method17 to remove the cytosolic components and visualize cytoskeleton-associated proteins. As we expected, Strip was localized along microtubules (Fig. 1b). To further investigate this relationship, we performed the microtubule co-sedimentation assay. When in vitro-polymerized microtubules with no Strip were incubated with the extracts of S2 cells and pelleted by centrifugation through a glycerol cushion, S2 cell-derived Strip was associated with the microtubules (Fig. 1c). These data demonstrate that Strip forms a complex with microtubules.
Figure 1. Strip interacts with microtubule.
(a) Drosophila S2 cells plated on a concanavalin-A coated cover slip were immunostained with anti-Strip (green) and anti-α-tubulin (magenta) antibodies. Scale bar is 7.5 μm. (b) S2 cells were treated with the extraction method for clear observation of microtubules. S2 cells are immunostained with anti-Strip (green) and anti-α-tubulin (magenta) antibodies. Dotted rectangle area is enlarged in the right panels. Scale bar is 7.5 μm. (c) S2 cell extract was incubated with in vitro-polymerized microtubules or buffer only and pelleted by centrifugation. The pellet was denatured and subjected to western blotting using anti-Strip and anti-α-tubulin antibodies.
Strip affects organization and stability of microtubule
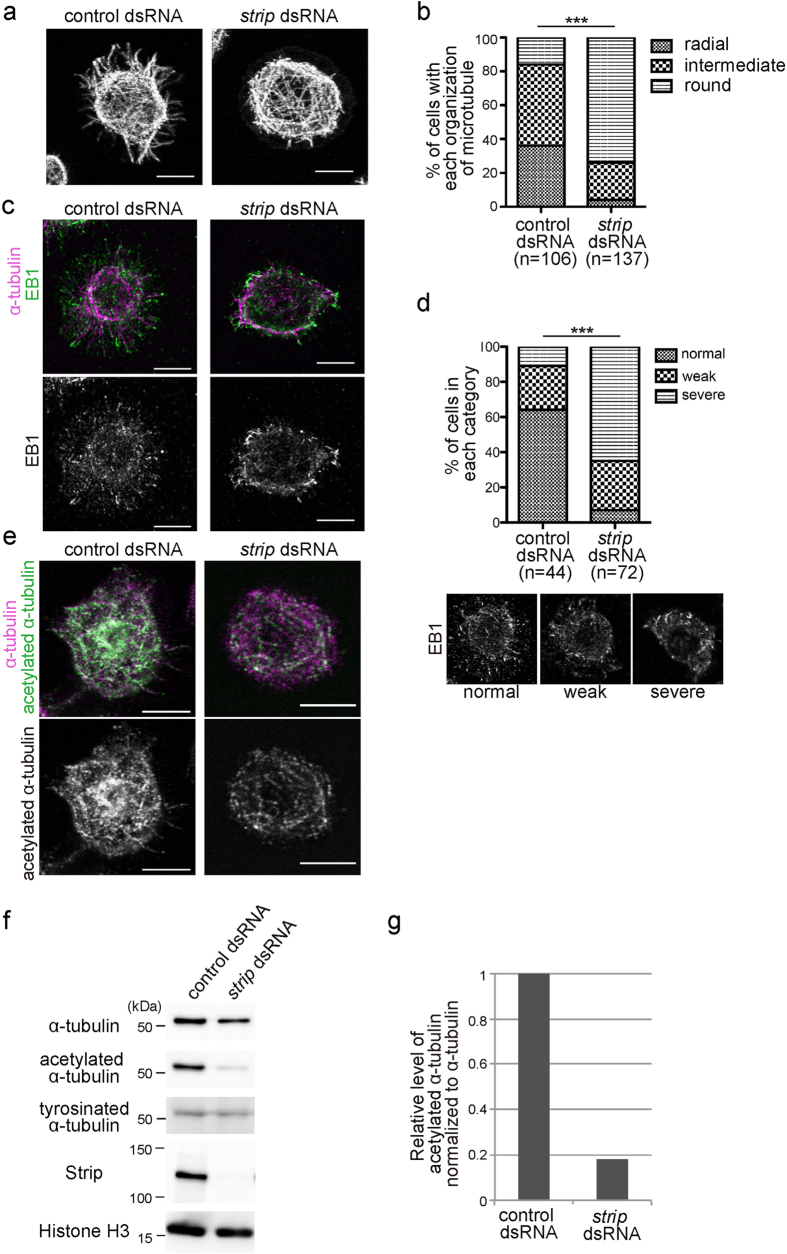
We next examined the role of Strip on microtubule by treating S2 cells with strip dsRNA. Most of the microtubules showed radial projections from the cell center to the periphery in control dsRNA-treated S2 cells (Fig. 2a,b). In contrast, radial projections towards cell periphery were mostly absent and overall microtubule morphology was rounded in strip dsRNA-treated S2 cells (Fig. 2a,b). We further investigated the localization of microtubule plus end tracking protein, EB1 to monitor the microtubule organization and found that EB1 distribution was obviously altered in strip dsRNA-treated S2 cells (Fig. 2c,d). EB1 was localized at the periphery of wild-type S2 cells in a small comet-like pattern while the number of EB1 comets was significantly reduced and sometime irregular EB1 accumulations were observed in strip dsRNA-treated S2 cells.
Figure 2. strip knockdown affected microtubule stability.
(a) S2 cells were treated with control or strip dsRNA for 8 days and immunostained with anti-α-tubulin antibody. Scale bar is 7.5 μm. (b) All S2 cells are classified into having radial projections to cell periphery, not having radial projection (overall microtubule morphology was rounded) or intermediate by a blind test. ***P < 0.0001, chi-square test. (c) S2 cells were treated with control or strip dsRNA for 8 days and immunostained with anti-EB1 (green) and anti-α-tubulin (magenta) antibodies. (d) All cells were blindly classified into three categories, scattered and small EB1 comets (normal), intermediate (weak), fewer comets/irregular EB1 accumulation (severe). ***P < 0.0001, chi-square test. Scale bar is 7.5 μm. (e) S2 cells were treated with control or strip dsRNA for 8 days and immunostained with anti-acetylated-α-tubulin (green) and anti-α-tubulin (magenta) antibodies. Scale bar is 7.5 μm. (f) Immunoblots of lysate of S2 cells treated with control or strip dsRNA for 8 days. Since Strip seems to slightly affect the total α-tubulin level, Histone H3 is used as a loading control. (g) Densitometric analysis of acetylated-α-tubulin. The level of acetylated-α-tubulin is normalized to α-tubulin. This reduction of the level of acetylated-α-tubulin in strip dsRNA-treated cells was confirmed for 10 times.
Multiple post-translational modifications in tubulins are crucial for dynamics and organization of microtubules18 and can be used to monitor different populations of microtubules. Therefore, we tried to investigate the level of acetylated and tyrosinated α-tubulin in strip dsRNA-treated S2 cells. Acetylated α-tubulins exist in stable, long-lived microtubules that are resistant to nocodazole19,20. Tyrosinated α-tubulins are found in newly formed microtubules since tubulin detyrosination occurs after the incorporation of tubulin subunits into the microtubule lattice21. Interestingly, the level of acetylated α-tubulin was greatly reduced when cells were treated with strip dsRNA, indicating that microtubule stability was decreased when strip was knocked down (Fig. 2e–g). The level of tyrosinated tubulin was not drastically changed in strip dsRNA-treated cells (Fig. 2f).
strip and tubulin folding cofactor D cooperatively regulate neuronal morphogenesis
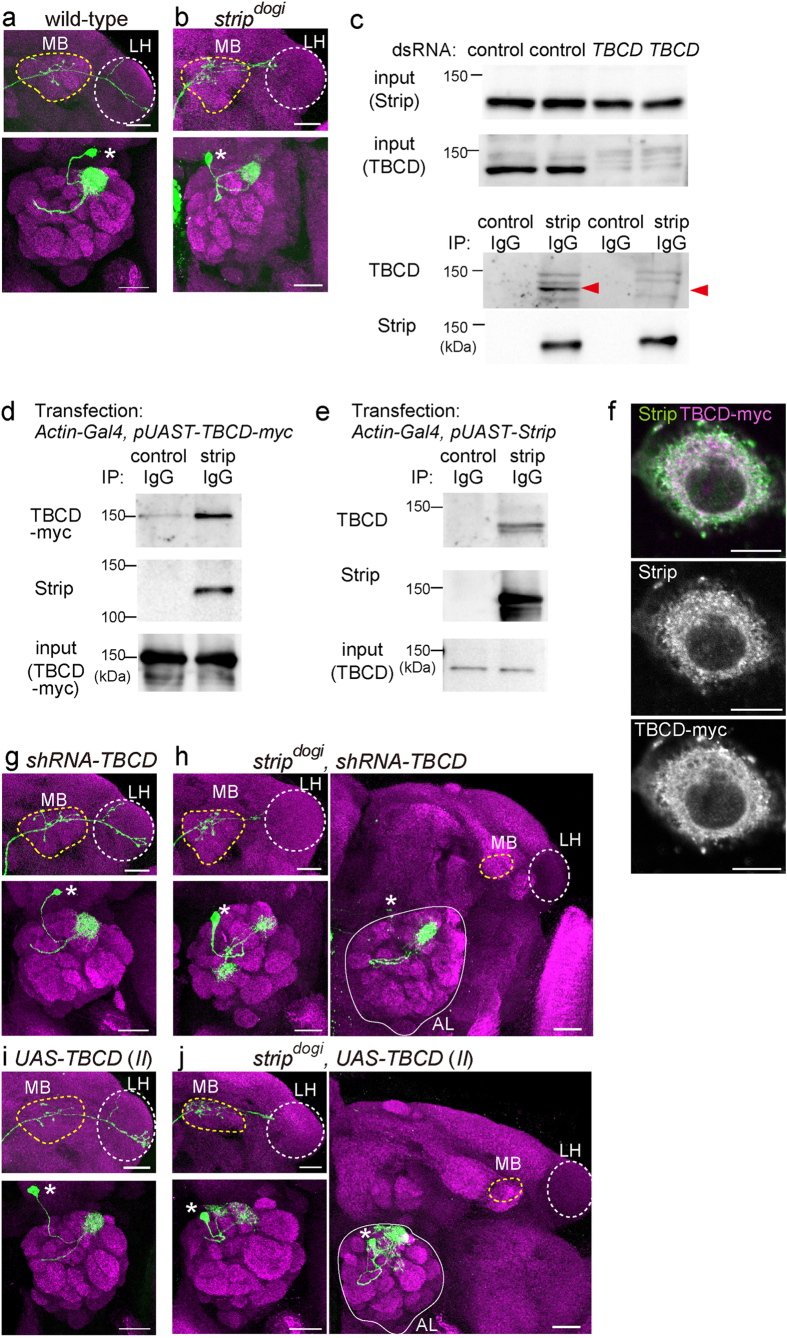
To investigate whether Strip also influences the stability of microtubules in vivo, we performed a mosaic analysis with a repressible cell marker (MARCM)-based analysis22 in Drosophila olfactory projection neurons (PNs), an excellent model system for studying the molecular mechanisms of neuronal morphogenesis23,24,25. We analyzed MARCM single-cell clones of PNs whose dendrites target the DL1 glomerulus in the antennal lobe (DL1 PNs, Fig. 3a). Axons of the DL1 PNs target the mushroom body and the lateral horn where the axon exhibits stereotypical L-shape branching (Fig. 3a). As we reported previously, single-cell clones of PNs homozygous for stripdogi (hereafter, stripdogi PN) show defects in axon elongation and dendrite branching14. In stripdogi PNs, an additional dendrite branch appeared from the proximal side of the dendrite shaft (overbranching phenotype: 10.26%, n = 4/39, Fig. 3b) and two-glomerular-targeting dendrites were also observed at a low frequency14. The axon of stripdogi PNs did not elongate or form L-shaped branches in the lateral horn (97.2%, n = 35/36, Fig. 3b).
Figure 3. Strip physically and genetically interacts with TBCD.
(a,b) Representative images of wild-type (a) or stripdogi (b) DL1 PN clones (green). Bruchpilot (Brp) staining in magenta. Scale bar is 25 μm. Asterisk: cell body. Yellow and white dotted circles indicate the mushroom body (MB) and the lateral horn (LH), respectively. (c) Immunoprecipitation of S2 cell lysates treated with control or TBCD dsRNA for 6 days. Endogenous TBCD was co-immunoprecipitated when endogenous Strip was precipitated by anti-Strip antibody in control dsRNA-treated S2 cells but not in TBCD dsRNA-treated cells. Red triangles indicate endogenous TBCD. (d) Immunoprecipitation of S2 cell lysates expressing c-myc tagged TBCD (TBCD-myc). TBCD-myc was co-immunoprecipitated when endogenous Strip was precipitated by anti-Strip antibody. (e) Immunoprecipitation of S2 lysates expressing Strip. Endogenous TBCD was co-immunoprecipitated when Strip was precipitated by anti-Strip antibody. (f) Representative image of S2 cells expressing TBCD-myc. Magenta: TBCD-myc, green: endogenous Strip. (g,h) Representative images of single-cell clones of DL1 PNs expressing shRNA against TBCD (shRNA-TBCD) in wild type (g) or stripdogi (h) PNs. (i,j) Representative images of single-cell clones of DL1 PNs overexpressing TBCD in wild type (i) or stripdogi (j) PNs. The right panels in (h) and (j) show PNs exhibiting the ‘extremely short axon’ phenotype, i.e., the axon did not exit the antennal lobe (white circle) and did not enter the mushroom body (MB, yellow dotted circle) or the lateral horn (LH, white dotted circle). Scale bar is 25 μm. Genotypes: (a) y w, hs-FLP122, UAS-mCD8GFP/y w (or Y); GH146-Gal4, UAS-mCD8-GFP/+; tubP-Gal80, FRT2A/FRT2A, (b) y w, hs-FLP122, UAS-mCD8GFP/y w (or Y); GH146-Gal4, UAS-mCD8-GFP/+; tubP-Gal80, FRT2A/stripdogi, FRT2A, y+, (g) y w, hs-FLP122, UAS-mCD8GFP/y w (or Y); GH146-Gal4, UAS-mCD8-GFP/UAS-shRNA-TBCD#1; tubP-Gal80, FRT2A/FRT2A, (h) y w, hs-FLP122, UAS-mCD8GFP/y w (or Y); GH146-Gal4, UAS-mCD8-GFP/UAS-shRNA-TBCD#1; tubP-Gal80, FRT2A/stripdogi, FRT2A, y+, (i) y w, hs-FLP122, UAS-mCD8GFP/y w (or Y); GH146-Gal4, UAS-mCD8-GFP/UAS-TBCD; tubP-Gal80, FRT2A/FRT2A, (j) y w, hs-FLP122, UAS-mCD8GFP/y w (or Y); GH146-Gal4, UAS-mCD8-GFP/UAS-TBCD; tubP-Gal80, FRT2A/stripdogi, FRT2A, y+.
To determine whether Strip is involved in microtubule dynamics during neuronal morphogenesis, we examined the relationship between strip and TBCD. TBCD is one of the five tubulin-folding cofactors and assists in the formation of tubulin heterodimers26. Recently, we reported that an optimum level of TBCD is crucial for neuronal morphogenesis. Interestingly, PNs homozygous for TBCD1 mutation or overexpressing TBCD show similar phenotypes with stripdogi PNs; both PNs exhibit dendrite overbranching phenotype16. Furthermore, TBCD1 PNs exhibits shorter axon phenotype as stripdogi PNs. Based on these phenotypic similarities between strip and TBCD mutant, we hypothesized that Strip could function with TBCD to regulate neuronal morphogenesis. First, we examined the physical interaction between Strip and TBCD by performing a co-immunoprecipitation assay using S2 cell lysate, and found that Strip and TBCD can form a protein complex (Fig. 3c–e). Next, we examined the subcellular localization of Strip and TBCD-myc in S2 cells and found that endogenous Strip and TBCD-myc are mostly co-localized (Fig. 3f). We then examined the genetic interaction between strip and TBCD by expressing short-hairpin RNA for TBCD (shRNA-TBCD) or overexpressing TBCD in stripdogi PNs. Although PNs homozygous for TBCD1 mutation exhibited defects in dendrite branching and axon elongation, shRNA-TBCD expression in DL1 PNs did not cause obvious phenotypes either in dendrites or axons, probably owing to its weak knockdown efficiency (Fig. 3g). In addition, TBCD overexpression also did not cause obvious phenotype (Fig. 3i), which was different from our previous report16 that used different UAS-TBCD transgenic line (See Methods for more details). When shRNA-TBCD or TBCD was expressed in stripdogi PNs, the phenotypes of stripdogi PNs were significantly enhanced. The penetrance of two-glomeruli-targeting dendrites was increased (2.56%, n = 1/39 for stripdogi PNs, Fig. 3b; 37.5% n = 9/24 for shRNA-TBCD expressing stripdogi PNs, Fig. 3h; 30% n = 3/10 for TBCD expressing stripdogi PNs, Fig. 3j), and some axons showed ‘extremely short axon’ phenotype, that is, the axons did not exit the antennal lobe; this was never observed in stripdogiPNs (9.52%, n = 2/21, for shRNA-TBCD expressing stripdogi PNs, Fig. 3h; 20%, n = 2/10, for TBCD expressing stripdogi PNs, Fig. 3j). These results suggest that Strip interacts with TBCD during neuronal morphogenesis of PNs.
Strip cooperates with Dscam in mushroom body neurons
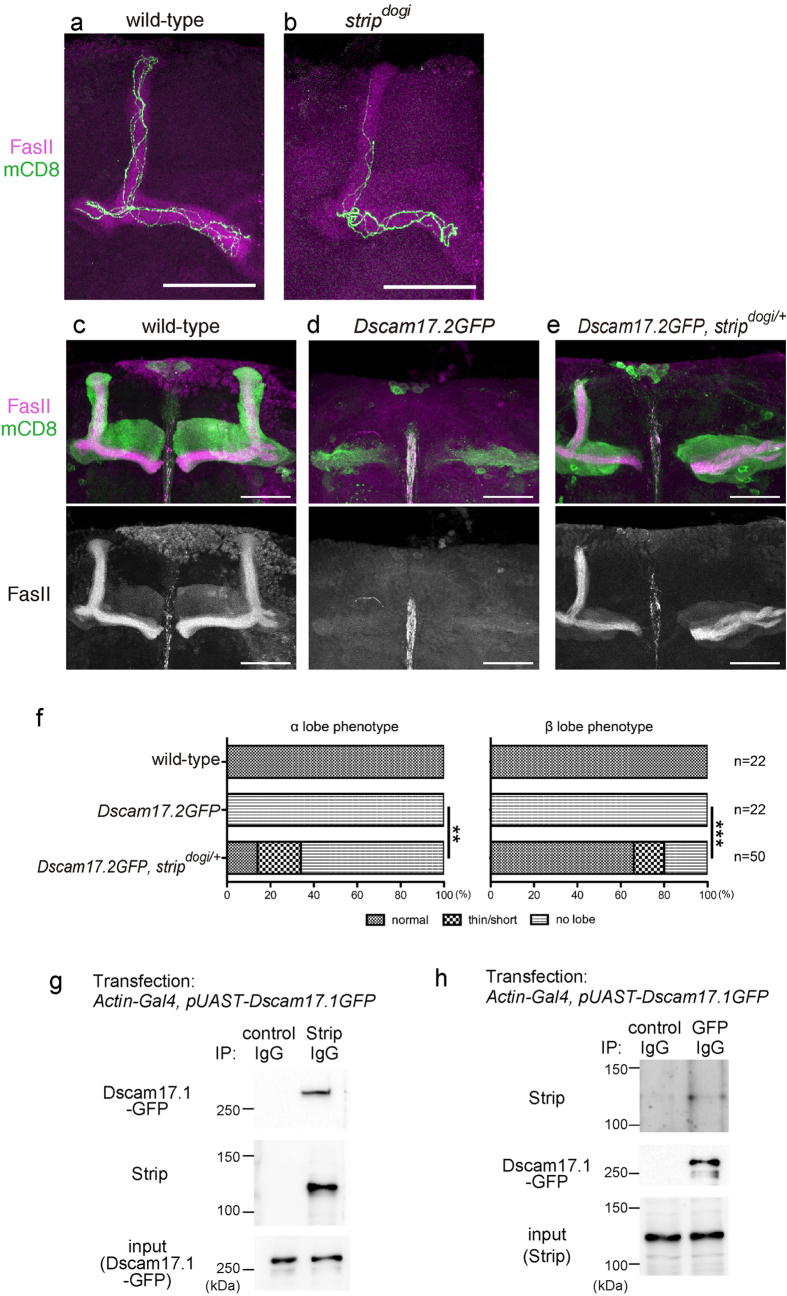
As we reported previously, TBCD forms complex with Dscam and cooperates to regulate morphology of mushroom body neurons16. The mushroom body is an olfactory learning and memory centre27 and consists of three types of neurons, α/β, α′/β′, and γ neurons28. α/β neurons extend one axon into the α lobe and the other axon to the β lobe, that are labelled by anti-FasII antibody (Fig. 4a). Here we hypothesized that Strip, like TBCD, also acts in the downstream of Dscam. To test this hypothesis, we first generated MARCM clones of mushroom body α/β neurons homozygous for stripdogi(stripdogimushroom body neurons). We found that stripdogimushroom body neurons exhibited axon segregation phenotypes (Fig. 4b; 11.8% (n = 8/68) of stripdogi clones exhibited less axons into the α lobes) that were similar to those in Dscam mutant mushroom body neurons29,30. We further provide the genetic evidence that Dscam and strip interact in mushroom body neurons. The ‘α and β lobes missing’ phenotypes were observed when Dscam was overexpressed in the mushroom body neurons (Fig. 4c,d,f), which was suppressed in the TBCD1 heterozygous background16. Similarly, stripdogiheterozygous background could also suppress ‘α and β lobes missing’ phenotype (Fig. 4e,f). Next we performed co-immunoprecipitation experiment. Anti-Strip antibody could co-immunoprecipitated endogenous Strip with Dscam-GFP (Fig. 4g). We could further confirm this interaction by immunoprecipitating Dscam-GFP by anti-GFP antibody; endogenous Strip was co-immunoprecipitated with Dscam-GFP (Fig. 4h). From these results, we concluded that Strip cooperates with Dscam in mushroom body neurons, possibly through interacting with TBCD.
Figure 4. Strip cooperates with Dscam in the morphogenesis of mushroom body neurons.
(a,b) Representative images of control (a) or stripdogi (b, 11.8%, n = 8/68) homozygous clones of mushroom body α/β neurons. UAS-mCD8-GFP (green) is expressed by OK107-Gal4. Anti-FasII antibody was used to visualize MB α/β lobes (magenta). Scale bar is 50 μm. (c–e) Representative images of mushroom body neurons for each genotype. UAS-mCD8-GFP (green) is expressed by OK107-Gal4. Anti-FasII antibody was used to visualize MB α/β lobes (magenta). Scale bar is 50 μm. (f) Quantification of phenotypes of α and β lobes. **P = 0.0075, ***P < 0.0001, chi-square test. (g,h) Immunoprecipitation of S2 lysates expressing Dscam17.1-GFP. Dscam17.1-GFP was co-immunoprecipitated when Strip was precipitated by anti-Strip antibody (g). Endogenous Strip was co-immunoprecipitated when Dscam17.1-GFP was precipitated by anti-GFP antibody (h). Genotypes: (a) y w, hs-flp, UAS-mCD8GFP/y w (or Y); UAS-βtub56D-myc/+; tub-Gal80, FRT2A/FRT2A, y(+); OK107-Gal4/+ (b) y w, hs-flp, UAS-mCD8GFP/y w (or Y); UAS-βtub56D-myc/+; tub-Gal80, FRT2A/stripdogi, FRT2A, y(+); OK107-Gal4/+ (c) y w, hs-FLP, UAS-mCD8GFP/w (or Y); +/Cy, y+; OK107-Gal4/+, (d) y w, hs-FLP, UAS-mCD8GFP/y w (or Y); Dscam17.2GFP/Cy, y+;; OK107-Gal4/+, (e) y w, hs-FLP, UAS-mCD8GFP/y w (or Y);; stripdogi, FRT2A, y+/TM3, Sb; OK107-Gal4/+.
Discussion
To form a functional neural circuit, the morphology of each neuron should be strictly regulated to wire with the correct synaptic partners. Microtubules are fundamental structural components of axons and dendrites and their dynamics directly affect neuronal morphology. In this report, we show that Strip, one component of the STRIPAK complex, affects microtubule stability in Drosophila S2 cells. Furthermore, we report that Strip regulates dendrite branching and axon elongation by interacting with TBCD in the olfactory projection neurons. Moreover, we observed genetic interactions between strip and Dscam in the mushroom body neurons, suggesting that Strip and TBCD regulate microtubule stability in the downstream of Dscam.
We recently found that TBCD can bind to the intracellular domain of Dscam and it is likely that TBCD mediates Dscam functions by affecting microtubule dynamics16. Although Dscam has been extensively studied, the downstream pathways are not well known. The Dock/Pak signaling pathway, one of the known pathways acting downstream of Dscam, does not seem to be required for dendrite targeting and axon guidance of olfactory projection neurons31. Strip seems to act with TBCD in the downstream of Dscam during neuronal morphogenesis, and link outer cellular environment with microtubules.
strip and TBCD mutants show similar phenotypes in axon elongation and dendrite branching, and Strip and TBCD genetically and physically interact with each other. TBCD assists in the formation of tubulin heterodimers, and affects microtubule stability by controlling the availability of tubulin subunits because concentration of free tubulin dimers can affect microtubule dynamics15. Although mammalian TBCD does not bind to microtubules32, Alp1, a homolog of TBCD in Schizosaccharomyces pombe, is colocalized with microtubules33. Furthermore, overproduction of Cin1p, the homolog of TBCD in Saccharomyces cerevisiae, resulted in an increased sensitivity to benomyl34, an antifungal compound that weakly inhibits microtubule assembly35. Thus, TBCD and its homologs have been shown to regulate microtubule dynamics.
Strip seems to affect microtubule stability by interacting with several molecules in addition to TBCD. We previously reported that Strip also forms a complex with Glued, the homolog of mammalian p150Glued,14. Glued is one of the components of the dynactin complex required for dynein motor-mediated retrograde transport along microtubules. Glued has the CAP-Gly domain that is common in several +TIPs and regulates initiation of retrograde transport in neuronal cells36,37. Interestingly, mammalian TBCB, another member of tubulin-folding cofactor family, also has the CAP-Gly domain and interacts with p150Glued, 38. +TIPs are a structurally and functionally diverse group of proteins that are distinguished by their specific accumulation at microtubule plus ends, or growing ends39,40. +TIPs control different aspects of microtubule dynamics and form links between microtubule ends and other cellular structures2. Some of +TIPs also participate in microtubule-actin crosstalk, such as CLIP-170–formin interaction and EB1-RhoGEF2 interaction2. There is accumulating evidence that Strip and also other STRIPAK components (Mst3, Mst4, and Ccm3) regulate the actin network9,13. For example, Strip1 and 2, homologs of Drosophila Strip, were recently identified as regulators of the actomyosin contraction that regulates cell migration in cancer cells41. Taken together, Strip and STRIPAK seem to form a giant complex with TBCD and Glued at growing ends of microtubule to stabilize them and serve as a linker between microtubules and actin networks to regulate proper neurite branching and elongation.
strip knockdown resulted in a decrease in the level of acetylated α-tubulin. Therefore, we examined the possibility that Strip directly affects the acetylated tubulin level by regulating acetylation or deacetylation enzymes. We investigated the genetic interaction between strip and HDAC6, one of the enzymes responsible for the deacetylation of α-tubulin42, however, the shorter axon phenotype of strip knockdown was not suppressed when combined with a HDAC6 mutation (data not shown). Thus, Strip does not seem to directly affect the acetylation of α-tubulin, but affect the stabilization of microtubule by interacting with TBCD, Glued, and other molecules. Although controversial, some reports indicate that acetylation is the result, and not the cause, of stabilization19,43,44.
Many diseases are linked to microtubules. For example, defects in retrograde transports along microtubules cause neurodegenerative diseases, such as motor neuropathy 7B45 and Perry syndrome46. Furthermore, Dscam is implicated in the cognitive disabilities in Down syndrome. Moreover, STRIPAK complexes have been linked to a number of clinical conditions and diseases9. Cancer genome sequencing also identified frequent mutations in human STRIP2, and based on the mutation frequency and types, STRIP2 was classified as an oncogene47. Thus, further investigation of Strip, STRIPAK, and the microtubule complex would yield new insights into the mechanisms of various diseases.
Methods
Fly strains
Flies were maintained under standard laboratory condition (25 °C). UAS-Dscam17.2-GFP29 was a kind gift from Tzumin Lee. We previously generated stripdogi 14. UAS-TBCD used in previous study16 is different from the one we used in this report. They are independent transgenic lines generated from the same construct16. The former one is inserted in third chromosome, and the one we use in this study is on second chromosome (UAS-TBCDweak). To generate UAS-shRNA-TBCD#1, we used the protocol previously described48. The target sequence of the shRNA-TBCD#1 is 5′- GGAGCTGAATGAACTAATAATA -3′, which is different from the shRNA we used in the previous study16. The fragment was subcloned into the pUAST-attB vector. Transgenic flies were raised by BestGene.
Clonal analysis
We used the MARCM method49. Briefly, we crossed MARCM-ready flies that contain FLP recombinase, an FRT site, GAL4, tubulin 1α promoter-GAL80, and UAS-mCD8GFP to a line containing the corresponding FRT and mutation of interest for MARCM analysis. To analyze DL1 PNs, we used the GH146-Gal4 driver and heat-shocked flies (37 °C, 1 h) at 0–24 h after larval hatching (ALH). The anterodorsal PN single-cell clones generated during this time period innervated the DL1 glomerulus. The penetrance of overbranching phenotype of stripdogiPNs was considerably reduced in this study compared to the previous report14. This was because of the difference in the heat-shock timing. In the previous study14, we heat-shocked flies 0–30 h ALH for the phenotype analysis of stripdogiPNs. To analyze mushroom body α/β neurons, we used the OK107-Gal4 driver and heat-shocked flies (37 °C, 1h) at 0–24 h after puparium formation. Dissections were performed on both sexes of adults aged 1–10 days.
Immunohistochemistry
The fixation, immunostaining, and imaging of the fly brains were carried out as previously described50. Briefly, the brains were dissected in 0.3% Triton X-100 (vol/vol) in phosphate-buffer (PBT), followed by fixing with 4% paraformaldehyde (wt/vol) in PBT at room temperature (RT) for 20 min, and subsequently blocked in PBT with 5% normal goat serum (vol/vol) for 1 h at RT. Primary and secondary antibody incubations were carried out in PBT for overnight at 4 °C.
For Drosophila S2 cells, cells were cultured on concanavalin A-coated coverslips, fixed, and immunostained as previously described51. Briefly, S2 cells were grown for 1 h at 26 °C on concanavalin A-coated coverslips, and were fixed with 4% paraformaldehyde (wt/vol) in 0.3% Triton X-100 (vol/vol) in phosphate-buffer saline (PBST) at RT for 10 min, and subsequently blocked in PBST with 5% normal goat serum (vol/vol) for 30 min. For investigating the colocalization of Strip and TBCD, we transfected S2 cells with Gal4-actin promoter and pUAST-TBCD-myc16 by utilizing Effectene Transfection Reagent (Qiagen) and cultured for 24 h at 26 °C before plating on concanavalin A-coated coverslips. For clear observation of the microtubules, we performed the extraction method17. We rinsed S2 cells cultured on concanavalin A-coated coverslips with PEM buffer (100 mM PIPES[pH 6.9], 1 mM EGTA, 1 mM MgSO4) once, and treated them with extraction buffer (100 mM PIPES [pH 6.9], 1 mM EGTA, 1 mM MgSO4, 1% Triton X-100, 2% paraformaldehyde, 10 μM taxol). After incubation for 4 min at RT, the cells were fixed with 4% paraformaldehyde (wt/vol) in PEM buffer for 20 min at RT and subsequently blocked in PBST with 5% normal goat serum (vol/vol) for 30 min. Primary and secondary antibody incubations were carried out in blocking solution for 1 h at RT. We used the following antibodies: anti-Strip14 (rat, 1:50), anti-α-tubulin (mouse, 1:2000, Sigma T6199), anti-acetylated-α-tubulin (mouse, 1:1000, Sigma T7451), anti-EB152 (rabbit, 1:1000, a kind gift from S. L. Rogers), anti-myc (mouse, 1:1000, Invitrogen), anti-Brp (mouse, 1:40, DSHB nc82), anti-mCD8 (rat, 1:200, Invitrogen), anti-FasII (mouse, 1:40, DSHB 1D4) antibodies. For the anti-acetylated-α-tubulin staining in S2 cells, we co-stained cells with anti-α-tubulin (mouse) and anti-acetylated-α-tubulin (mouse) antibodies by utilizing the Zenon labelling technology. S2 cells were incubated with anti-acetylated-α-tubulin for 1 h and subsequently incubated with anti-mouse IgG conjugated with Alexa Flour 488 for 1 h. Lastly, S2 cells were cultured with anti-α-tubulin that was conjugated with Alexa Flour 647 beforehand by the Zenon Alexa Flour 647 Mouse IgG1 labeling kit (life, Z-25008).
dsRNA generation and treatment
The dsRNA design, production, and treatment were performed as follows51. Briefly, 700–900 bp gene-specific sequences with T7 RNA polymerase sequence at both ends were amplified by PCR, and in vitro transcription was performed using T7 RiboMAX Express RNAi System (Promega) according to the manufacturer’s protocol. For strip dsRNA, templates for in vitro transcription were generated using the primers 5′-TAATACGACTCACTATAGGGCCTGCATAAACCTGCTGCGC-3′ and 5′-TAATACGACTCACTATAGGGCTAGAGGGCGTCCCAGTCGG-3′. For TBCD dsRNA, templates for in vitro transcription were generated using the primers 5′-TAATACGACTCACTATAGGGGTGGTTTACCTCTCCAACCAACGG-3′ and 5′-TAATACGACTCACTATAGGGCTGTATGCCTGGATGTTCTCGCGG-3′. For control dsRNA, primer sequences were used to amplify the sequence from the bacterial cloning plasmid pBluescript SK51. For Fig. 2, Drosophila S2 cells (1.0 × 106) were cultured in a 35-mm dish; 30 μg control or strip dsRNA was applied every 2 days for 8 days. For Fig. 3c, Drosophila S2 cells (1.0 × 107) were cultured in a 100-mm dish; 120 μg control or TBCD dsRNA was applied every 2 days for 6 days.
Immunoprecipitation
Gal4-actin promoter and desired UAS construct (UAS-TBCD-myc, UAS-strip or UAS-Dscam17.1GFP) were co-transfected with Effectene Transfection Reagent (Qiagen); 6.0 × 106 Drosophila S2 cells per 60-mm dish were plated, and transfection was performed according to the manufacturer’s protocol. After 48 h in culture, S2 cells were collected, sonicated in the lysis buffer (25 mM Tris-HCl, pH 7.9, 10 mM NaCl, 2 mM EDTA, 0.5% Triton X-100, 10 mM DTT, and 1 x cOmplete (cocktail of protease inhibitors, Roche)), and incubated with anti-Strip antibody (rat, 1:100), control IgG, or anti-GFP (rabbit, 1:500, MBL) overnight. Protein G agarose (Roche) was added, and immunoprecipitation was performed according to the manufacturer’s protocol.
Immunoblotting
Western blotting was performed according to standard techniques. Briefly, we subjected 2–10 μg of S2 cell lysates to SDS-PAGE analysis (12.5% for Histone H3; for others, 7.5% or 10%) and immunoblotting. The following antibodies were used for immunoblotting: the anti-Strip antibody14 (rabbit, 1:200), the anti-Strip antibody14 (rat, 1:50), the anti-α-tubulin (mouse, 1:2000, Sigma T6199), the anti-acetylated-α-tubulin (mouse, 1:1000, Sigma T7451), the anti-tyrosinated-α-tubulin (mouse, 1:1000, Sigma T9028), the anti-Histone H3 antibody (rabbit, 1:2000, Active motif 39163) and the anti-TBCD antibody16 (guinea pig, 1:1000). All data are representative of more than three biological replicates.
Microtubule co-sedimentation assay
This assay was carried out as previously described53. Porcine tubulin was polymerized in a tubulin buffer (40 mM PIPES pH6.9, 1 mM MgCl2, 1 mM EGTA, 1 mM GTP, 1 mM DTT) by adding Taxol (20 μM) for 20 min at RT. Drosophila S2 cells from two 100-mm confluent dishes were collected, rinsed with PBS, re-suspended in a buffer (40 mM PIPES pH6.9, 1 mM MgCl2, 1 mM EGTA, 1 mM DTT, 1% NP40, 20μM Latrunculin B with protease inhibitors), and centrifuged at 156,500 g for 20 min. The supernatant of this S2 extract was split in half, incubated either with in vivo-polymerized microtubules or buffer only for 30 min at RT and pelleted through 30% glycerol cushion (30% glycerol, 40 mM PIPES pH6.9, 1 mM MgCl2, 1 mM EGTA, protease inhibitors, 20 μM Taxol only for the vial that contains microtubule) by centrifugation at 194,000 g for 90 min at 4 °C. The pellet was collected and separated by SDS-PAGE and analyzed by western blotting.
Image acquisition
For all experiments, images were obtained using TCS-SP5 confocal laser scanning confocal microscopy (Leica) with a PL APO CS 40×/1.25 Oil lens (Leica) for PN and mushroom body neuron imaging or a PL APO CS 63x/1.40 Oil lens (Leica) for imaging S2 cells. Fields of view were randomly selected and each biologically independent experiment was repeated more than two times.
Image analysis
For Fig. 2a–d, all images were blinded prior to classification to avoid experimental bias. The investigator who conducted the blind test was different from the investigator who performed the experiments.
Statistical analysis
Statistical analysis was performed using Prism 5 software (GraphPad). The chi-square test was used to compare data variations between control and strip dsRNA-treated S2 cells and data variations of phenotypes of mushroom α and β lobes.
Additional Information
How to cite this article: Sakuma, C. et al. A STRIPAK component Strip regulates neuronal morphogenesis by affecting microtubule stability. Sci. Rep. 5, 17769; doi: 10.1038/srep17769 (2015).
Acknowledgments
We thank T. Lee for the fly stock, S.L. Rogers for anti-EB1 antibody, V.I. Gelfand for advising us on the microtubule sedimentation assay, C.-H. Chen for advising us on shRNA construction, Y. Hiromi for actin-Gal4 plasmid, and all members of the Miura laboratory for their comments on this study. This work was supported by grants from the Japanese Ministry of Education, Science, Sports, Culture, and Technology (MEXT), the Japan Society for the Promotion of Science, and the Japan Science and Technology Agency (to C.S., M.O., M.M. and T.C.).
Footnotes
Author Contributions C.S., M.O. and T.C. designed the study with the help of M.M., C.S., M.O. and T.U. conducted the experiments. C.S. and T.C. analysed the data, wrote the manuscript with contributions from all other authors.
References
- Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell 6, 74–81 (1994). [DOI] [PubMed] [Google Scholar]
- Akhmanova A. & Steinmetz M. O. Microtubule +TIPs at a glance. J Cell Sci 123, 3415–3419 (2010). [DOI] [PubMed] [Google Scholar]
- Wu X., Xiang X. & Hammer J. A. 3rd. Motor proteins at the microtubule plus-end. Trends Cell Biol 16, 135–143 (2006). [DOI] [PubMed] [Google Scholar]
- Verhey K. J. & Gaertig J. The tubulin code. Cell Cycle 6, 2152–2160 (2007). [DOI] [PubMed] [Google Scholar]
- Reed N. A. et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16, 2166–2172 (2006). [DOI] [PubMed] [Google Scholar]
- Sudo H. & Baas P. W. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci 30, 7215–7226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol 71, 201–220 (2011). [DOI] [PubMed] [Google Scholar]
- Millecamps S. & Julien J. P. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci 14, 161–176 (2013). [DOI] [PubMed] [Google Scholar]
- Hwang J. & Pallas D. C. STRIPAK complexes: structure, biological function, and involvement in human diseases. Int J Biochem Cell Biol 47, 118–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudreault M. et al. A PP2A Phosphatase High Density Interaction Network Identifies a Novel Striatin-interacting Phosphatase and Kinase Complex Linked to the Cerebral Cavernous Malformation 3 (CCM3) Protein. Mol Cell 8, 157–171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean M. J. et al. Structure-function analysis of core STRIPAK Proteins: a signaling complex implicated in Golgi polarization. J Biol Chem 286, 25065–25075 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte J. et al. DMob4/Phocein regulates synapse formation, axonal transport, and microtubule organization. J Neurosci 30, 5189–5203 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S. W. et al. Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol 9, 54 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma C. et al. Drosophila Strip serves as a platform for early endosome organization during axon elongation. Nat commun 5, 5180 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski D. Tubulin Folding Cofactors: Half a Dozen for a Dimer. Curr Biol 12, F767–769 (2002). [DOI] [PubMed] [Google Scholar]
- Okumura M., Sakuma C., Miura M. & Chihara T. Linking Cell Surface Receptors to Microtubules: Tubulin Folding Cofactor D Mediates Dscam Functions during Neuronal Morphogenesis. J Neurosci 35, 1979–1990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura N. et al. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev Cell 16, 675–686 (2009). [DOI] [PubMed] [Google Scholar]
- Wloga D. & Gaertig J. Post-translational modifications of microtubules. J Cell Sci 123, 3447–3455 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A., Ackerman B. & Gundersen G. G. Cell biology: Tubulin acetylation and cell motility. Nature 421, 230 (2003). [DOI] [PubMed] [Google Scholar]
- Fukushima N., Furuta D., Hidaka Y., Moriyama R. & Tsujiuchi T. Post-translational modifications of tubulin in the nervous system. J Neurochem 109, 683–693 (2009). [DOI] [PubMed] [Google Scholar]
- Kumar N. & Flavin, M. Preferential action of a brain detyrosinolating carboxypeptidase on polymerized tubulin. J Biol Chem 256, 7678–7686 (1981). [PubMed] [Google Scholar]
- Lee T. & Luo L. Mosaic Analysis with a Repressible Neurotechnique Cell Marker for Studies of Gene Function in Neuronal Morphogenesis. Neuron 22, 451–481 (1999). [DOI] [PubMed] [Google Scholar]
- Jefferis G. S. & Hummel T. Wiring specificity in the olfactory system. Semin Cell Dev Biol 17, 50–65 (2006). [DOI] [PubMed] [Google Scholar]
- Marin E. C., Watts R. J., Tanaka N. K., Ito K. & Luo L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development 132, 725–737 (2005). [DOI] [PubMed] [Google Scholar]
- Sakuma C., Anzo M., Miura M. & Chihara T. Development of olfactory projection neuron dendrites that contribute to wiring specificity of the Drosophila olfactory circuit. Genes Genet System 89, 17–26 (2014). [DOI] [PubMed] [Google Scholar]
- Tian G. et al. Pathway Leading to Correctly Folded beta-Tubulin. Cell 86, 287–296 (1996). [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci 4, 266–275 (2003). [DOI] [PubMed] [Google Scholar]
- Lee T., Lee A. & Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 126, 4065–4076 (1999). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron 43, 663–672 (2004). [DOI] [PubMed] [Google Scholar]
- Zhan X.-L. et al. Analysis of Dscam Diversity in Regulating Axon Guidance in Drosophila Mushroom Bodies. Neuron 43, 673–686 (2004). [DOI] [PubMed] [Google Scholar]
- Sekine S. U. et al. Meigo governs dendrite targeting specificity by modulating ephrin level and N-glycosylation. Nat Neurosci 16, 683–691 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L., Fanarraga M. L., Aloria K. & Zabala J. C. Tubulin folding cofactor D is a microtubule destabilizing protein. FEBS letters 470, 93–95 (2000). [DOI] [PubMed] [Google Scholar]
- Hirata D., Masuda H., Eddison M. & Toda T. Essential role of tubulin-folding cofactor D in microtubule assembly and its association with microtubules in fission yeast. EMBO J 17, 658–666 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Macke J. P., Roberts B. T. & Geiser J. R. Saccharomyces cerevisiae PAC2 functions with CIN1, 2 and 4 in a pathway leading to normal microtubule stability. Genetics 146, 849–857 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Rathinasamy K., Mohan R. & Panda D. Microtubule assembly dynamics: an attractive target for anticancer drugs. IUBMB life 60, 368–375 (2008). [DOI] [PubMed] [Google Scholar]
- Lloyd T. E. et al. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron 74, 344–360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughamian A. J. & Holzbaur E. L. Dynactin is required for transport initiation from the distal axon. Neuron 74, 331–343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh G. F. et al. Tubulin-binding cofactor B is a direct interaction partner of the dynactin subunit p150(Glued). Cell Tissue Res 350, 13–26 (2012). [DOI] [PubMed] [Google Scholar]
- Schuyler S. C. & Pellman D. Microtubule “plus-end-tracking proteins”: The end is just the beginning. Cell 105, 421–424 (2001). [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Shiina N. & Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol 10, 865–868 (2000). [DOI] [PubMed] [Google Scholar]
- Madsen C. D. et al. STRIPAK components determine mode of cancer cell migration and metastasis. Nat Cell Biol 17, 68–80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A. L. et al. dSIR2 and dHDAC6: two novel, inhibitor-resistant deacetylases in Drosophila melanogaster. Exp Cell Res 265, 90–103 (2001). [DOI] [PubMed] [Google Scholar]
- Gaertig J. et al. Acetylation of lysine 40 in alpha-tubulin is not essential in Tetrahymena thermophila. J Cell Biol 129, 1301–1310 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol 28, 1688–1701 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls I. et al. Mutant dynactin in motor neuron disease. Nat Genet 33, 455–456 (2003). [DOI] [PubMed] [Google Scholar]
- Farrer M. J. et al. DCTN1 mutations in Perry syndrome. Nat Genet 41, 163–165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T. et al. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell 155, 948–962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H. et al. A Synthetic Maternal-Effect Selfish Genetic Element Drives Population Replacement in Drosophila. Science 316, 597–600 (2007). [DOI] [PubMed] [Google Scholar]
- Wu J. S. & Luo L. A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nat Protoc 1, 2583–2589 (2006). [DOI] [PubMed] [Google Scholar]
- Wu J. S. & Luo L. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc 1, 2110–2115 (2006). [DOI] [PubMed] [Google Scholar]
- Rogers S. L. & Rogers G. C. Culture of Drosophila S2 cells and their use for RNAi-mediated loss-of-function studies and immunofluorescence microscopy. Nat Protoc 3, 606–611 (2008). [DOI] [PubMed] [Google Scholar]
- Rogers S. L., Rogers G. C., Sharp D. J. & Vale R. D. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J Cell Biol 158, 873–884 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. et al. Microtubule binding by dynactin is required for microtubule organization but not cargo transport. J Cell Biol 176, 641–651 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]