Abstract
Inulin, a natural renewable polysaccharide resource produced by various plants in nature, has been reported to possess a significant number of diverse pharmaceutical and food applications. Recently, there has been rapid progress in high-throughput technologies and platforms to assay global mRNA, proteins, metabolites and gut microbiota. In this review, we will describe the current status of utilizing omics technologies of elucidating the impact of inulin and inulin-containing prebiotics at the transcriptome, proteome, metabolome and gut microbiome levels. Although many studies in this review have addressed the impact of inulin comprehensively, these omics technologies only enable us to understand physiological information at each different stage of mRNA, protein, metabolite and gut microbe. We believe that a synergistic approach is vital in order to fully illustrate the intricate beauty behind the relatively modest influence of food factors like inulin on host health.
Introduction: what is inulin?
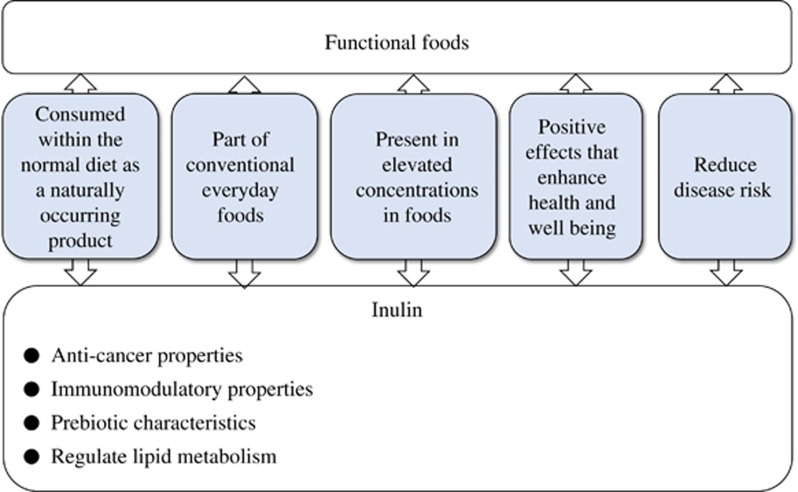
α-D-glucopyranosyl-[α-D-fructofuranosyl](n-1)-D-fructofuranoside or β-glucopyranosyl-[D-fructofuranosyl](n-1)-D-fructofuranoside, also more commonly known as inulin, is a natural renewable polysaccharide resource produced by various plants in nature with a significant number of diverse pharmaceutical and food applications. Plant bulbs of Asteraceae use it as a means for storing nutrients.1 Owing to its excellent nutritional properties, its use in food products has increased in recent years. Sometimes it is used in place of sugar, fat and flour. Inulin is included in Jerusalem artichoke, chicory (Cichorium intybus), dandelion and dahlia.2 Inulin is sometimes used as a fat or sugar replacement in the food industry;3, 4, 5, 6, 7 however, it also has important pharmaceutical applications, as an excipient or a stabilizer, and as an injectable for the clinical measurement of kidney function.6, 8 In addition, inulin possesses interesting biological effects, being a potent complement pathway activator when in a particulate form and having anticancer9, 10 and immunomodulatory properties11, 12, 13, 14 (Figure 1).
Figure 1.
Inulin or inulin-containing prebiotic fiber as a functional food. Inulin is a natural renewable polysaccharide resource produced by various plants in nature. Plant bulbs of Asteraceae: Jerusalem artichoke and chicory (Cichorium intybus) use it as a means for storing nutrients. Inulin is also included in dandelion and dahlia. Inulin fulfills the requirements of functional foods in the following manner: being a part of conventional everyday foods, to be consumed with the normal/usual diet as naturally occurring (as opposed to synthetic) components, sometimes in increased concentrations or present in foods that would not normally supply them, and having positive effects on target functions that may enhance health and well-being, as well as reduce disease risk.
Inulin as a prebiotic and functional food
Inulin is resistant to hydrolysis by small human gut digestive enzymes, as the anomeric C2 in fructose monomers is beta-configured and forms β 2–1 glyosidic linkages. As such, inulin is categorized as a ‘non-digestible' oligosaccharide.15 As reported by a large number of in vitro and in vivo studies, inulin is fermented by bacteria colonizing the large bowel with lactate and short-chain fatty acids (SCFAs), mainly acetate, as end products of the fermentation process.16 Furthermore, well-designed human studies have shown that inulin dietary intervention induced significant changes in the composition of human fecal microbiota, concluding that inulin possesses prebiotic properties.17, 18, 19 Apart from being a prebiotic, inulin also belongs to the category of functional foods, which, by reference to the European consensus,20 comprises several unique characteristics such as: being a part of conventional everyday foods, to be consumed with the normal/usual diet as naturally occurring (as opposed to synthetic) components, sometimes in increased concentrations or present in foods that would not normally supply them, and having positive effects on target functions that may enhance health and well-being, as well as reduce disease risk. As stipulated by the Japanese Ministry of Health, Labour and Welfare, oligofructose, an inulin-containing, prebiotic food product, is classified as a functional food or, as specified by ‘Food for Specified Health Uses', as a food that can modify gastrointestinal conditions (http://www.mhlw.go.jp/english/topics/foodsafety/fhc/02.html). Inulin is indeed present in commonly consumed plants and can be added to normal food products. In addition, inulin dietary intervention can regulate the key physiological functions such as lipid metabolism;21 they modulate the composition of gut microbiota, which has a major role in gastrointestinal physiology, and, finally, might have a role in reducing the risk of colon cancer.22
Nutrigenomics in food and nutrition research
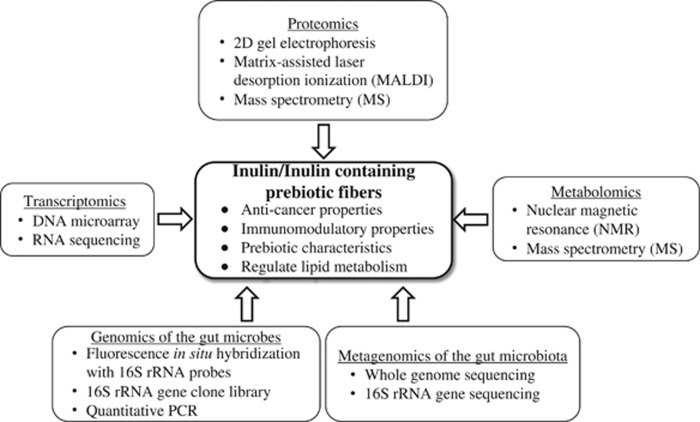
In recent years, there has been rapid progress in high-throughput technologies and platforms to assay global mRNA, proteins, metabolites and gut microbiota. As a consequence, today's researchers of food and nutrition science have been witnessing a rapid expansion of nutrigenomics, also known as nutriomics, comprising transcriptomics, proteomics, metabolomics and metagenomics.23 Nutrigenomics is a discipline in which all available lines of information about the genome and other biological molecules are effectively utilized to unveil every detail of the interactions between diets and the human body.24, 25 In this review, we describe the current status of omics approaches toward elucidating the molecular mechanisms of inulin supplementation in vivo (Figure 2 and Table 1).
Figure 2.
Application of omics technologies in functional evaluation of inulin and inulin-containing prebiotic fibers. Inulin dietary intervention can regulate the key physiological functions such as lipid metabolism and composition of gut microbiota, and can reduce cancer risk. Omics technologies such as transcriptomics, proteomics, metabolomics, metagenomics of the gut microbiota and genomics of the gut microbes have been employed to elucidate the functionality of inulin supplementation.
Table 1. Application of omics technologies in the functional evaluation of inulin and inulin-containing prebiotics dietary supplementation.
| No. | Type of omics evaluation | Summary of results | Reference |
|---|---|---|---|
| 1 | Transcriptomics | Immune-system-related genes, tumor necrosis factor receptor superfamily member D16, acyl-CoA synthetase long-chain family member 6 and peroxisome proliferator-activated receptor, alpha, were upregulated | (33) |
| Inulin supplementation is involved in chicken growth and performance while reinforcing the immune status of animals and fostering the production of long-chain fatty acids | |||
| Addition of prebiotics on chicken diets may be an useful alternative to antibiotics for improving performance and general immunity in poultry farming | |||
| 2 | Transcriptomics | Prebiotic fiber diets significantly lowered serum-cholesterol levels | (34) |
| Expression levels of hepatic genes primarily associated with cholesterol metabolism, cholesterol 7-alpha-monooxygenase, hydroxymethylglutaryl-CoA reductase, lecithin-cholesterol acyltransferase and sterol regulatory element-binding protein 2, were significantly decreased in rats fed on prebiotic fibers | |||
| Increase in cecal digesta as well as an upregulation of genes involved in cholesterol synthesis and bile production | |||
| Prebiotic fibers containing inulin may be considered as a potential dietary intervention for hypercholesterolemia | |||
| 3 | Transcriptomics | Cecal proglucagon and peptide YY mRNA levels throughout the entire gastrointestinal tract were upregulated | (35) |
| Ghrelin O-acyltransferase mRNA levels of the fundus in the stomach were higher in obese rats as compared with lean rats and had lowered gene expression in the OHF group | |||
| Food intake, satiety hormones and alterations in gut microbiota via which prebiotics acts were regulated in a dose-dependent manner | |||
| 4 | Transcriptomics | Dietary JA supplementation significantly improved insulin resistance and hepatic triglyceride accumulation | (36) |
| Transcriptomic profiling of the liver revealed that the expression of malic enzyme 1, associated with fatty-acid synthesis; decorin, related to fibrosis; and cytochrome P450, family 1, subfamily a, polypeptide 2 and nicotinamide phosphoribosyl transferase associated with inflammation was significantly improved by JA supplementation | |||
| 10 % JA supplementation may be beneficial for the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease | |||
| 5 | Transcriptomics | Incorporation of KGM and inulin into the high-fat fiber-free diet beneficially reduced the malondialdehyde levels of the colon and liver and DNA damage in blood lymphocytes | (37) |
| Antioxidative defense systems were enhanced by upregulating the gene expressions of colonic mucosa glutathione peroxidase and catalase and of hepatic superoxide dismutase and catalase | |||
| In vivo utilization of KGM and inulin activates both the local and systemic antioxidative defense systems in rats | |||
| 6 | Transcriptomics | High-fat diet feeding significantly decreased the expression of regenerating islet-derived 3-gamma (Reg3g) and phospholipase A2 group-II (PLA2g2) in the jejunum | (38) |
| Prebiotic treatment increased Reg3g expression and improved gut homeostasis as suggested by the increase in the expression of intectin, a key protein involved in gut epithelial cell turnover | |||
| 8 | Proteomics | Low temperatures influence harvesting of chicory root, as 7 of the 21 most intense protein spots observed were associated with cold acclimation | (43) |
| 9 | Metabolomics | Inulin intake attenuated some of the HFD-induced metabolic changes in both myocardium (3-HB, lactate and guanosine) and testicle tissues (3-HB, inosine and betaine) | (48) |
| 10 | Metabolomics | Concentrations of esters, acids and some aldehydes in fecal samples from healthy volunteers were significantly increased with increasing doses of inulin | (49) |
| Inulin dose-dependently inhibited the formation of S-compounds and the generation of other protein fermentation metabolites such as phenolic compounds | |||
| 11 | Metagenomics of gut microbiota | Deep metagenomic sequencing analysis revealed that HFD and prebiotic treatment significantly affected the gut microbiota at different taxonomic levels | (38) |
| Gut microbiota modulations induced by prebiotics counteracted HFD-induced inflammation and related metabolic disorders | |||
| 12 | Genomics of gut microbes | Significant increase in Faecalibacterium prausnitzii after inulin intake | (62) |
| The presence of Bifidobacterium adolescentis and B. longum in all volunteers, and B. pseudocatenulatum, B. animalis, B. bifidum and B. dentium was observed | |||
| B. adolescentis showed the strongest response to inulin consumption, significantly increasing from 0.89 to 3.9% of the total gut microbiota | |||
| Numbers of B. bifidum were remarkably increased from 0.22 to 0.63% in the five volunteers for whom this species was present | |||
| 13 | Genomics of gut microbes | In pigs that fed on diets containing inulin, higher colonic bifidobacteria, lower total colonic SCFA concentrations due to reduced acetate but higher proportions of colonic butyrate were observed | (63) |
| Inulin did not stimulate increase in lactobacilli and bifidobacteria numbers irrespective of the basal diet, although 20–50% of inulin was degraded in the jejunum | |||
| 14 | Genomics of gut microbes | Consumption of prebiotic inulin/partially hydrolyzed guar gum mixture produced clinical results comparable to placebo in constipated females, but had additional protective effects on gut microbiota by decreasing the amount of pathological bacteria of the Clostridium genera | (64) |
| 15 | Genomics of gut microbes | In anaerobic fermenters containing different carbohydrate souces, including inulin, the growth of a greater proportion of Bacteroides and a lower proportion of Gram-positive anaerobes related to Faecalibacterium prausnitzii, Ruminococcus flavefaciens–R. bromii, Eubacterium rectale–Clostridium coccoides and E. cylindroides than the proportions in the starting fecal inoculum were supported | (65) |
| Inulin from dahlia resulted in a significant increase in the number of bacteria related to R. flavefaciens–R. bromii and E. cylindroides | |||
| 16 | Genomics of gut microbes | Inulin treatment had moderate effects on lactate, propionate and butyrate levels | (66) |
| Denaturing gradient gel electrophoresis analysis revealed that inulin changed microbial metabolism by modulating the microbial community composition | |||
| Inulin has a lower potency than AXOS to shift part of the sugar fermentation toward the distal colon parts | |||
| 17 | Genomics of gut microbes | A mixture of OF and OF-lcIN- and lcIN-containing diets resulted in larger numbers of cecal, colonic and fecal bacteria of the C. coccoide–E. rectale cluster. | (67) |
| Higher amounts of lactobacilli were found in cecal and colonic contents of Mix OF-lcIN-fed rats and in feces of OF-fed rats | |||
| Mix OF-lcIN and OF resulted in significantly smaller numbers of cecal, colonic and fecal bacteria belonging to the C. histolyticum and C. lituseburense as compared with Con. Counts of Bacteroides–Prevotella and Enterobacteriaceae were similar between the groups | |||
| OF and/or lcIN-containing diets significantly increased the butyrate concentration and its relative molar proportion in the cecal and colonic contents | |||
| Only lcIN-containing diets resulted in a higher fecal concentration of butyrate than Con. Higher molar proportions of fecal butyrate were observed with all diets that had been supplemented with OF and/or lcIN |
Abbreviations: AXOS, arabinoxylan oligosaccharides; HFD, high-fat diet; JA, Jerusalem artichoke; KGM, konjac glucomannan; OHF, obese 20% fiber; OF, oligofructose; lcIN, long-chain inulin; SCFA, short-chain fatty acids.
Transcriptomic evaluation of inulin supplementation
Transcriptomics is the most widely employed omics technology, as compared with others in food research, because of the many merits of the DNA microarray technology, which include the comprehensiveness of the gene expression data, established protocols and high reliability and reproducibility of the data.26, 27, 28, 29 Another upcoming popular approach in transcriptomics is RNA-sequencing, also known as whole-transcriptome sequencing.30 As compared with microarrays, RNA-sequencing at sufficient coverage captures a wider range of expression values. As a digital measure (count data), it scales linearly even at extreme values, whereas microarrays only show saturation of analog-type fluorescent signals.31 RNA-sequencing further provides information on RNA splice events, which are not readily detected with standard microarrays.32
Recently, Sevane et al.33 reported about the differences in the hepatic transcriptome profiles between chickens supplemented with inulin (5 g of inulin per kg diet) and controls for 34 days. Overall, 148 differentially expressed genes were identified because of inulin dietary supplementation, and Kyoto Encyclopedia of Genes and Genomes pathway visual analysis identified that three immune-system-related genes, tumor necrosis factor receptor superfamily member 1B, acyl-CoA synthetase long-chain family member 6 and peroxisome proliferator-activated receptor, alpha, were upregulated with inulin supplementation. Comprehensive gene expression analyses have revealed that inulin supplementation, while reinforcing the immune status of animals, and fostering the production of long-chain fatty acids, is also involved in chicken growth and performance. This study is useful in the application of promoting the use of prebiotics on chicken diets as a useful alternative to antibiotics for producing chickens with a healthier meat lipid profile and also for improving performance and general immunity in poultry farming. In addition, Parnell and Reimer34 identified possible mechanisms through which prebiotic fibers improve serum lipids. Lean and obese male JCR:La-cp rats were fed with the following three diets: 0, 10 or 20% prebiotic fiber comprising 1:1 mix of inulin and oligofructose for 10 weeks. Both diets of prebiotic fiber significantly lowered serum-cholesterol levels by 24% in the obese hyperlipidemic rats. Expression levels of hepatic genes primarily associated with cholesterol metabolism, cholesterol 7-alpha-monooxygenase, hydroxymethylglutaryl-CoA reductase, lecithin-cholesterol acyltransferase and sterol regulatory element-binding protein 2, were significantly decreased in rats fed on prebiotic fibers. There was also an increase in cecal digesta as well as an upregulation of genes involved in cholesterol synthesis and bile production. As such, prebiotic fibers containing inulin may be considered as a potential dietary intervention for hypercholesterolemia.34 In another study conducted by the same group, dose-dependent effects of prebiotics (inulin and oligofructose) on gut satiety hormones, energy expenditure, gastric-emptying and gut microbiota were investigated. Male lean and obese JCR:LA-cp rats were divided into groups and administered the following diets: lean 0% fiber (LC), lean 10% fiber (LF), lean 20% fiber (LHF), obese 0% fiber (OC), obese 10% fiber (OF) or obese 20% fiber (OHF) for 10 weeks. Biochemical parameters measured include body composition, gastric-emptying, energy expenditure, plasma satiety hormone concentrations and presence of gut microbiota detected using quantitative PCR. It was observed that cecal proglucagon and peptide YY mRNA levels throughout the entire gastrointestinal tract were upregulated twofold in the LF, OF and OHF groups and threefold in the LHF group. Ghrelin O-acyltransferase mRNA levels of the fundus in the stomach were higher in obese rats as compared with lean rats, and had lowered gene expression in the OHF group. It was revealed that prebiotics regulated several of the mechanisms including food intake, satiety hormones and alterations in gut microbiota in a dose-dependent manner. As such, the results of this study conclude that the combined effects of prebiotics may have a therapeutic potential for obesity.35 Jerusalem artichoke (JA), which mainly comprises inulin, has been reported to potentially attenuate lipid disturbances and insulin resistance; however, the underlying mechanisms are not well understood. In a study by Chang et al.,36 the physiological responses and mechanisms of JA intervention were elucidated via a comprehensive transcriptome analysis. Wistar rats were fed a control diet (CT), a 60% fructose-enriched diet (FRU) or a FRU with 10% JA (n=6–7) for 4 weeks. Dietary JA supplementation significantly improved insulin resistance and hepatic triglyceride accumulation. Transcriptomic profiling of the liver revealed that FRU significantly altered the expression of malic enzyme 1, associated with fatty-acid synthesis; decorin, related to fibrosis; cytochrome P450, family 1, subfamily a, polypeptide 2; and nicotinamide phosphoribosyl transferase associated with inflammation, whereas dietary JA supplementation significantly modulated the expression of these genes. As such, it was proposed that 10% JA supplementation may be beneficial for the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease. Wu and Chen37 attempted to compare and investigate the effects of konjac glucomannan (KGM). Male Sprague–Dawley rats were fed a high-fat (25% corn oil, w/w), fiber-free diet or that supplemented with either KGM or inulin fiber (5%, w/w) for 4 weeks, after which the index of pro-oxidative status, malondialdehyde levels, blood lymphocyte DNA damage, colonic mucosa, hepatic concentrations of antioxidant enzymes (glutathione peroxidase, superoxide dismutase and catalase) and the plasma antioxidant levels were measured. It was observed that the incorporation of KGM and inulin into the high-fat, fiber-free diet beneficially reduced the malondialdehyde levels of the colon and liver and DNA damage in blood lymphocytes. In addition, the gene expressions of colonic mucosa glutathione peroxidase and catalase and of hepatic superoxide dismutase and catalase were upregulated by dietary intervention of both fibers. These results suggest that in vivo utilization of KGM and inulin activates both the local and systemic antioxidative defense systems in rats. In a novel study by Everard et al.,38 the targets involved in host response during obesity in oligofructose-treated mice were investigated by feeding 10-week-old C57BL/6J mice CT, a CT supplemented with oligofructose (0.3 g per mouse per day) added in tap water (CT-Pre), a high-fat diet (HFD) (60% fat and 20% carbohydrates (kcal per 100 g) or a HFD supplemented with oligofructose (0.3 g per mouse per day) added in tap water (HFD-Pre) for 8 weeks. It was observed that, whereas HFD feeding significantly decreased the expression of regenerating islet-derived 3-gamma (Reg3g) and phospholipase A2 group-II (PLA2g2) in the jejunum, prebiotic dietary intervention upregulated Reg3g expression and improved gut homeostasis as suggested by the increase in the protein expression levels of intectin that has a main role in gut epithelial cell turnover. In addition, prebiotic supplementation also counteracted gut microbiota modulations induced by HFD-induced inflammation and related metabolic disorders.
Proteomic evaluation of inulin supplementation
The separation, quantification and identification of proteins are the steps commonly involved in proteome analyses.39 In conventional protein detection methods, proteins in biological samples are separated using two-dimensional gel electrophoresis, in which separated proteins are visualized and quantified after silver or fluorescent staining. However, the differential imaging gel electrophoresis method is a more sophisticated strategy based on the same principle of separation in which proteins of different samples are pre-labeled with different fluorescent dyes. Gel-free separation usually relies on chromatography that includes two-dimensional chromatography.40 Recently, a novel, tandem mass spectrometry (MS)-based approach for the relative quantification of proteins, relying on isobaric tag for relative and absolute quantitation, has been reported. Isobaric tag for relative and absolute quantitation reagents are designed in a manner in which their mass is isobaric. As such, differentially labeled proteins do not differ in mass. In MS scans, the corresponding proteolytic peptides appear as single peaks.41 Despite the advances in quantitative proteomics, MS remains the most widely used method for the identification of proteins with evaporation of peptides and proteins using matrix-assisted laser desorption/ionization and electrospray ionization.42 The evaluation of inulin dietary interventions in disease prevention using proteomic analyses is yet to be reported. As such, in this section, we would focus on using proteomic technologies to analyze components and stability of the inulin compound.
Two-dimensional electrophoretic analysis of proteins from chicory root was performed before the first freezing period. After protein digestion with trypsin, the peptides were analyzed using MS (matrix-assisted laser desorption/ionization-time-of-flight/time-of-flight). From the 881 protein spots analyzed, 714 proteins corresponded to a database accession, 619 of which were classified into the following functional categories: metabolism, energy, protein synthesis, cell structure, folding and stability, proteolysis and stress response. The importance of abiotic stress response was attested, as 7 of the 21 most intense protein spots observed are known to be involved in cold acclimation, suggesting the major effect of the low-temperature period that preceded root harvesting.43
Metabolomic evaluation of inulin supplementation
Metabolomics is widely used in the identification of disease biomarkers. By using nuclear magnetic resonance and/or MS, we can capture the metabolome using a wide range of analytical methods by providing robust and sensitive identification of metabolites produced by microbiota and host cells in fecal, blood, urine and tissue samples.44 Ranging from targeted to untargeted methods, methodologies have been reported for screening biochemical pathways, for example, central carbon metabolism, glycolysis, tricarboxylic acid cycle, amino-acid pathways, lipid pathways and selected secondary metabolism pathways.45, 46 These tools allow researchers to determine the effects that treatments have on the host's metabolic profile by analyzing the presence and quantity of thousands of metabolites simultaneously. There are various challenges in the metabolite analysis because of the diversity of the chemical properties of metabolites, which is larger in number than those of transcripts and proteins. In addition, another difficulty derives from the width of the abundance of metabolites. As such, metabolite analysis usually requires the use of techniques requiring a high skill level. Despite these difficulties, metabolome analysis is a powerful tool in the field of food and nutrition science.47
Duan et al.48 comprehensively analyzed the effects of HFD and inulin intake on the metabolite compositions of myocardium and testicle using nuclear magnetic resonance spectroscopy. Multiple univariate data analysis is a high-throughput method utilized to visualize and efficiently extract information on metabolites significantly affected by an intervention. Using multiple univariate data analysis, it was reported that HFD induced metabolic changes in rat testicles and myocardium that are involved in fatty-acid β-oxidation, together with the metabolisms of choline, amino acids, purines and pyrimidines, even before HFD resulted in significant body-weight increases. Inulin intake attenuated some of the HFD-induced metabolic changes in both myocardium (3-HB, lactate and guanosine) and testicle tissues (3-HB, inosine and betaine). These observations suggest that inulin intervened HFD-induced metabolic changes and illustrated that multiple univariate data analysis is a power-alternative method in metabolomics analysis.
De Preter et al.49 demonstrated that inulin supplementation modulates the fecal metabolite profile in vitro. Fecal samples obtained from healthy subjects were anaerobically incubated at 37 °C with or without increasing doses of inulin, and changes in the metabolite pattern and pH were assessed. According to their chemical classes, a total of 107 different volatile organic compounds were identified and classified. The concentrations of esters, acids and some aldehydes were significantly increased with increasing doses of inulin. On the contrary, inulin dose-dependently inhibited the formation of S-compounds. In addition, the generation of other protein fermentation metabolites such as phenolic compounds was inhibited in the presence of inulin.
Gut microbiome evaluation of inulin supplementation via metagenomic approach
The gut microbiota consists of all the microorganisms inhabiting the gastrointestinal tract. In most mammals, the gut microbiota is dominated by four bacterial phyla, Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria, which perform roles that define the health of the host.50 The gut is mainly populated by bacteria amounting to ~100 trillion cells, which is approximately threefold larger than the number of human body cells51 and has extensive metabolic capabilities.50 Thus, the gut microbiota that creates the unique gut ecosystem together with the host eukaryotic cells consists of prokaryotic cells and is frequently referred to as a measurable and functional organ.52 In recent years, the gut microbiome can be comprehensively analyzed using next-generation whole-DNA sequencing and 16S rRNA gene sequencing.53, 54, 55 One of the advantages is that microbial community profiling using 16S rRNA genes allows us to gain deep views into hundreds of microbial communities simultaneously.56 According to the lifestyle and nutritional status of the host, the bacterial communities vary in composition along the digestive tract and adapt through life.57
In a novel study by Everard et al., to investigate the targets involved in host response during obesity in oligofructose-treated mice, 10-week-old C57BL/6J mice were fed a CT, a CT supplemented with oligofructose (0.3 g per mouse per day) added in tap water (CT-Pre), a HFD (60% fat and 20% carbohydrates (kcal per 100 g) or a HFD supplemented with oligofructose (0.3 g per mouse per day) added in tap water (HFD-Pre) for 8 weeks. Results from deep metagenomic sequencing revealed that HFD and prebiotic treatment significantly altered the gut microbiome at different taxonomic levels. Distinct profiles for the HFD, Pre, HFD-Pre and CT groups were observed from the functional analyses based on the occurrence of clusters of orthologous groups of proteins. Finally, the modulations in gut microbiota induced by prebiotic intervention counteracted HFD-induced inflammation and related metabolic disorders.38
Genomic evaluation of the impact of inulin supplementation on the gut microbes
In this review, we have highlighted several studies that have analyzed the impact of inulin supplementation using various omics technologies such as transcriptomics, proteomics, metabolomics and metagenomics. Genomic technologies, for example, quantitative polymerase chain reaction,58 fluorescent in situ hybridization59 and in situ hybridization with 16S rRNA-targeting probes,60 are also some of the popular omics technologies that have been used in the interpretation of inulin intervention. Another genomic approach includes the application of 16S rRNA gene clone library sequencing using capillary sequencer.61
In a study by Ramirez-Farias et al.,62 variations in the fecal microbiota composition were analyzed using real-time PCR in human volunteers after inulin ingestion (10 g per day) for 16 days in comparison with a control period without any supplement intake. The prevalence of most bacterial groups examined was not affected. However, there was a significant increase in Faecalibacterium prausnitzii after inulin intake. Using clone library analysis, the composition of the genus Bifidobacterium was studied in four of the volunteers. There were between three and five Bifidobacterium spp. observed in each volunteer. B. adolescentis and B. longum were present in all the volunteers and B. pseudocatenulatum, B. animalis, B. bifidum and B. dentium were also observed. Using real-time PCR, the four most prevalent Bifidobacterium spp., B. adolescentis, B. longum, B. pseudocatenulatum and B. bifidum, in volunteers carrying detectable levels of bifidobacteria and B. adolescentis showed the strongest response to inulin consumption, significantly increasing from 0.89 to 3.9% of the total microbiota. In addition, the amount of B. bifidum was remarkably increased from 0.22 to 0.63% in the five volunteers for whom this species was present. The effects of inulin on the gut microbiota were investigated using fluorescent in situ hybridization in growing pigs. For this study, pigs (n=8 per group) were assigned to two types of basal diets (wheat and barley or corn and wheat gluten) with or without 3% inulin for 3 and 6 weeks to evaluate whether naturally occurring dietary fibers influence the inulin effect. Bifidobacteria were observed in less than half of the pigs and in pigs that were administered inulin-containing diets, higher colonic bifidobacteria and lower total colonic SCFA concentrations due to reduced acetate, but higher proportions of colonic butyrate were observed. Inulin did not stimulate increase in lactobacilli and bifidobacteria numbers irrespective of the basal diet, although 20–50% of inulin was degraded in the jejunum. As such, inulin supplementation affected gut luminal SCFA and the number of pigs harboring bifidobacteria.63 In a clinical study to assess the tolerance and effectiveness of a prebiotic inulin/partially hydrolyzed guar gum mixture (I-PHGG) for the treatment of constipation in females, as well as its influence on the composition of gut microbiota and production of short-chain fatty acids, female health volunteers were randomized to treatment with I-PHGG or placebo. Treatment consisted of 3-week supplementation with 15 g per day I-PHGG (fiber group) or maltodextrin (placebo group). Changes in fecal bacterial population and SCFAs were assessed using real-time PCR and gas chromatography, respectively. Consumption of I-PHGG produced clinical results comparable to placebo in constipated females, but had additional protective effects on gut microbiota by decreasing the amount of pathological bacteria of the Clostridium genera.64 As reported by Duncan et al., duplicate anaerobic fermentor systems were used to examine changes in a community of human fecal bacteria supplied with different carbohydrate energy sources and a panel of group-specific fluorescent in situ hybridizationprobes targeting 16S rRNA sequences were used. It was reported that the fermentors supported growth of a lower proportion of Gram-positive anaerobes related to Faecalibacterium prausnitzii, Ruminococcus flavefaciens–R. bromii, Eubacterium rectale–Clostridium coccoides and E. cylindroides and a greater proportion of Bacteroides as compared with the starting fecal inoculum. Inulin from dahlia resulted in a significant increase in the number of bacteria related to R. flavefaciens–R. bromii and E. cylindroides. Roseburia-related strain A2-183 F was able to grow on all substrates, despite the fact that it was unable to utilize complex carbohydrates in pure culture, and it was assumed that this organism survived by cross-feeding. In contrast, Roseburia gutis L1-82 R and Eubacterium sp. strain A2-194 R survived less well, despite the fact that they were able to utilize polysaccharides in pure culture, except that A2-194 R was stimulated 100-fold by inulin. This suggests that many low-G+C-content Gram-positive anaerobes may be selected against during in vitro incubation, although several groups were stimulated by inulin.65 In another study, the prebiotic potential of inulin was compared against arabinoxylan oligosaccharides in two simulators of the human gut microbial ecosystem. Microbial breakdown of both oligosaccharides and SCFA production was colon compartment-specific, with ascending and transverse colon being the predominant site of inulin and arabinoxylan oligosaccharide degradation, respectively. Inulin dietary intervention slightly affected concentrations of lactate, propionate and butyrate. Using denaturing gradient gel electrophoresis, it was also revealed that inulin supplementation modulated microbial metabolism by modifying the microbial community composition. The results indicate that inulin has a lower potency than arabinoxylan oligosaccharide to shift part of the sugar fermentation toward the distal colon parts. Furthermore, as AXOS has a stronger propionate-stimulating effect, it may be suitable for lowering cholesterol and beneficially affecting host lipid metabolism as a prebiotic candidate.66 As reported by Kleessen et al., one of four following treatments: (1) commercial standard diet as a control (Con); (2) Con+50-g short-chain oligofructose per kg (OF); (3) C+50-g long-chain inulin per kg (lcIN); or (4) Con+50 g OF-lcIN/kg (Mix OF-lcIN) were assigned to germ-free rats inoculated with human fecal microbiota. At the end of the treatment period, 16S rRNA-targeted probes applied in in situ hybridization were used to observe the changes in bacterial population groups in response to feeding these diets. It was observed that there were larger numbers of cecal, colonic and fecal bacteria of the C. coccoides–E. rectale cluster in rats that fed on Mix OF-lcIN- and lcIN-containing diets, whereas OF alone did not affect this bacterial group in the cecum, colon or feces. Higher amounts of lactobacilli were found in cecal and colonic contents of Mix OF-lcIN-fed rats and in feces of OF-fed rats compared with Con. Mix OF-lcIN and OF resulted in significantly smaller numbers of cecal, colonic and fecal bacteria belonging to the C. histolyticum and C. lituseburense as compared with Con. Counts of Bacteroides–Prevotella and Enterobacteriaceae were similar between the groups. OF- and/or lcIN-containing diets significantly increased the butyrate concentration and its relative molar proportion in cecal and colonic contents. The results of this study revealed that there was higher fecal concentration of butyrate in only lcIN-containing diets than in Con. In addition, higher molar proportions of fecal butyrate were observed with all diets that had been supplemented with OF and/or lcIN. As such, the incorporation of fermentable, indigestible fructans may be beneficial to gastrointestinal health by providing SCFAs, stimulating the proliferation of bifidobacteria or lactobacilli and suppressing potential pathogenic organisms in the gut.67
Adverse effects of high doses of inulin
Although we have mainly reviewed studies highlighting the beneficial properties of inulin supplementation, it is reported that high dosage of inulin intake has several side effects. The effects of daily intake of 14 g inulin added to a low-fat spread on fasting blood lipids and gastrointestinal symptoms were investigated in 64 young healthy women in a randomized double-blind crossover study. Inulin showed no effect on blood lipid concentrations. Interestingly, gastrointestinal symptoms assessed with questionnaires showed that during the inulin intake period there was a significantly higher degree of gastrointestinal symptoms such as discomfort from flatulence, bloatedness, stomach and gut cramps and rumbling in the stomach and gut than in the control period.68 Bonnema et al.69 conducted a doubled-blind, randomized, placebo-controlled and crossover study on the gastrointestinal tolerance of two inulin fibers: shorter-chain-length oligofructose and native inulin at 5- and 10-g doses on 26 healthy men and women aged 18–60 years. It was observed that doses up to 10 g per day of native inulin and up to 5 g per day of oligofructose were well tolerated.
Conclusions
Although many studies in this review have addressed the impact of inulin solely at the transcriptome, proteome, metabolome and gut microbiome level, they should be interpreted with reservation that these omics technologies individually enable us to understand physiological information at each different stages of mRNA, protein, metabolite and gut microbe. However, a synergistic omics approach is of significant importance as no single omics methodology can fully illustrate the intricate beauty behind the relatively modest influence of food factors such as inulin, as addressed in this review. In addition, as the gastrointestinal tract is a complex ecosystem that harbors various microorganisms that influence many aspects of health, including inflammatory bowel disease, cancer and obesity in adults.52 The intricate interaction among gut microbiota-derived metabolites, the gut microbiota itself and the host immune system is transmitted via a large array of signaling pathways that extend beyond the immune system. Conjointly, the direct chemical interactions between gut microbiota and the host and the immune-mediated signaling mechanisms influence various organs such as the gut, skeletal muscle, liver and the brain. These complex inter-relationships come together to form a series of host–microbe metabolic axes.70 As such, integrating metagenome, metabolome, proteome and transcriptome information on a systems biology-wide approach would also allow us to better understand this interplay between inulin, inulin-containing nutraceuticals and host metabolism.
The authors declare no conflict of interest.
References
- 1Leroy G, Grongnet JF, Mabeau S, Corre DL, Baty-Julien C. Changes in inulin and soluble sugar concentration in artichokes (Cynara scolymus L.) during storage. J Sci Food Agri 2010; 90: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 2Roberfroid MB. Functional foods: concepts and application to inulin and oligofructose. Br JNutr 2007; 87: S139. [DOI] [PubMed] [Google Scholar]
- 3Roberfroid MB, Delzenne NM. Dietary fructans. Annu Rev Nutr 1998; 18: 117–143. [DOI] [PubMed] [Google Scholar]
- 4Ronkart SN, Blecker CS, Fourmanoir H, Fougnies C, Deroanne C, Van Herck JC et al. Isolation and identification of inulooligosaccharides resulting from inulin hydrolysis. Anal Chim Acta 2007; 604: 81–87. [DOI] [PubMed] [Google Scholar]
- 5Anikó Matusek, Péter Merész, Thi Khanh Diem Le, Ferenc Örsi. Effect of temperature and pH on the degradation of fructo-oligosaccharides. Eur Food Res Technol 2008; 228: 355–365. [Google Scholar]
- 6Dan A, Ghosh S, Moulik SP. Physicochemical studies on the biopolymer inulin: a critical evaluation of its self-aggregation, aggregate-morphology, interaction with water, and thermal stability. Biopolymers 2009; 91: 687–699. [DOI] [PubMed] [Google Scholar]
- 7Ronkart Sébastien N, Claude D, Michel P, Christian F, Christoph SB. Impact of the crystallisation pathway of inulin on its mono-hydrate to hemi-hydrate thermal transition. Food Chem 2010; 119: 317–322. [Google Scholar]
- 8Phelps CF. The physical properties of inulin solutions. Biochem J 1965; 95: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Cooper PD, Carter M. The anti-melanoma activity of inulin in mice. Mol Immunol 1986; 23: 903–908. [DOI] [PubMed] [Google Scholar]
- 10Korbelik M, Cooper PD. Potentiation of photodynamic therapy of cancer by complement: the effect of gamma-inulin. Br J Cancer 2007; 96: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Cooper PD, Steele EJ. Algammulin, a new vaccine adjuvant comprising gamma inulin particles containing alum: preparation and in vitro properties. Vaccine 9: 351–357. [DOI] [PubMed] [Google Scholar]
- 12Cooper PD, Steele EJ. The adjuvanticity of gamma inulin. Immunol Cell Biol 1988; 66: 345–352. [DOI] [PubMed] [Google Scholar]
- 13Cooper PD, McComb C, Steele EJ. The adjuvanticity of Algammulin, a new vaccine adjuvant. Vaccine 1991; 9: 408–415. [DOI] [PubMed] [Google Scholar]
- 14Silva DG, Cooper PD, Petrovsky N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol Cell Biol 2004; 82: 611–616. [DOI] [PubMed] [Google Scholar]
- 15Ranawana V. Inulin: a review of its functional properties in relation to calcium absorption in humans. J Food Agri 2008; 1: 26–35. [Google Scholar]
- 16Alles MS, Hautvast JG, Nagengast FM, Hartemink R, Van Laere KM, Jansen JB. Fate of fructo-oligosaccharides in the human intestine. Br J Nutr 1996; 76: 211–221. [DOI] [PubMed] [Google Scholar]
- 17Buddington RK, Williams CH, Chen SC, Witherly SA. Dietary supplement of neosugar alters the fecal flora and decreases activities of some reductive enzymes in human subjects. Am J Clin Nutr 1996; 63: 709–716. [DOI] [PubMed] [Google Scholar]
- 18Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J Nutr 1999; 129(7 Suppl): 1438–1441. [DOI] [PubMed] [Google Scholar]
- 19Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995; 108: 975–982. [DOI] [PubMed] [Google Scholar]
- 20Diplock AT, Aggett PJ, Ashwell M, Bornet F, Fern EB, Roberfroid MB. Scientific concepts of functional foods in europe: consensus document. Br J Nutr 1999; 81: 1–28. [PubMed] [Google Scholar]
- 21Beylot M. Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br J Nutr 2005; 93(Suppl 1): S163–S168. [DOI] [PubMed] [Google Scholar]
- 22Pool-Zobel B, van Loo J, Rowland I, Roberfroid MB. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br J Nutr 2007; 87: S273. [DOI] [PubMed] [Google Scholar]
- 23Kato H. Nutrigenomics: the cutting edge and Asian perspectives. Asia Pacific J Clin Nutr 2008; 17(Suppl 1): 12–15. [PubMed] [Google Scholar]
- 24Kato H, Takahashi S, Saito K. Omics and integrated omics for the promotion of food and nutrition science. J Tradit Complement Med 2011; 1: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Müller M, Kersten S. Nutrigenomics: goals and strategies. Nat Rev Genet 2003; 4: 315–322. [DOI] [PubMed] [Google Scholar]
- 26Jia H, Saito K, Aw W, Takahashi S, Hanate M, Hasebe Y et al. Transcriptional profiling in rats and an ex vivo analysis implicate novel beneficial function of egg shell membrane in liver fibrosis. J Funct Foods 2013; 5: 1611–1619. [Google Scholar]
- 27Jia H, Takahashi S, Saito K, Kato H. DNA microarray analysis identified molecular pathways mediating the effects of supplementation of branched-chain amino acids on CCl4-induced cirrhosis in rats. Mol Nutr Food Res 2013; 57: 291–306. [DOI] [PubMed] [Google Scholar]
- 28Jia H, Aw W, Egashira K, Takahashi S, Aoyama S, Saito K et al. Coffee intake mitigated inflammation and obesity-induced insulin resistance in skeletal muscle of high-fat diet-induced obese mice. Genes Nutr 2014; 9: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Takahashi S, Egashira K, Saito K, Jia H, Abe K, Kato H. Coffee intake down-regulates the hepatic gene expression of peroxisome proliferator-activated receptor gamma in C57BL/6J mice fed a high-fat diet. J Funct Foods 2014; 6: 157–167. [Google Scholar]
- 30Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 2008; 5: 621–628. [DOI] [PubMed] [Google Scholar]
- 31Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res 2008; 18: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Wolf JB. Principles of transcriptome analysis and gene expression quantification: an RNA-seq tutorial. Mol Ecol Resour 2013; 13: 559–572. [DOI] [PubMed] [Google Scholar]
- 33Sevane N, Bialade F, Velasco S, Rebole A, Rodriguez ML, Ortiz LT et al. Dietary inulin supplementation modifies significantly the liver transcriptomic profile of broiler chickens. PLoS One 2014; 9: e98942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Parnell JA, Reimer RA. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose-response study in JCR:LA-cp rats. Br J Nutr 2010; 103: 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr 2012; 107: 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Chang WC, Jia H, Aw W, Saito K, Hasegawa S, Kato H. Beneficial effects of soluble dietary Jerusalem artichoke (Helianthus tuberosus in the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease in high-fructose diet-fed rats. Br J Nutr 2014; 112: 709–717. [DOI] [PubMed] [Google Scholar]
- 37Wu WT, Chen HL. Konjac glucomannan and inulin systematically modulate antioxidant defense in rats fed a high-fat fiber-free diet. J Agri Food Chem 2011; 59: 9194–9200. [DOI] [PubMed] [Google Scholar]
- 38Everard A, Lazarevic V, Gaia N, Johansson M, Stahlman M, Backhed F et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J 2014; 8: 2116–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Kussmann M, Panchaud A, Affolter M. Proteomics in nutrition: status quo and outlook for biomarkers and bioactives. J Proteome Res 2010; 9: 4876–4887. [DOI] [PubMed] [Google Scholar]
- 40Swatton JE, Prabakaran S, Karp NA, Lilley KS, Bahn S. Protein profiling of human postmortem brain using 2-dimensional fluorescence difference gel electrophoresis (2-D DIGE). Mol Psychiatry 2004; 9: 128–143. [DOI] [PubMed] [Google Scholar]
- 41Wiese S, Reidegeld KA, Meyer HE, Warscheid B. Protein labeling by iTRAQ: a new tool for quantitative mass spectrometry in proteome research. Proteomics 2007; 7: 340–350. [DOI] [PubMed] [Google Scholar]
- 42Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem 1997; 69: 4751–4760. [DOI] [PubMed] [Google Scholar]
- 43Degand H, Faber AM, Dauchot N, Mingeot D, Watillon B, Cutsem PV et al. Proteomic analysis of chicory root identifies proteins typically involved in cold acclimation. Proteomics 2009; 9: 2903–2907. [DOI] [PubMed] [Google Scholar]
- 44Dettmer K, Aronov PA, Hammock BD. Mass spectrometry based metabolomics. Mass Spectrom Rev 2007; 26: 51–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Buescher JM, Moco S, Sauer U, Zamboni N. Ultrahigh performance liquid chromatography-tandem mass spectrometry method for fast and robust quantification of anionic and aromatic metabolites. Anal Chem 2010; 82: 4403–4412. [DOI] [PubMed] [Google Scholar]
- 46Moco S, Bino RJ, Vorst O, Verhoeven HA, de Groot J, van Beek TA et al. A liquid chromatography-mass spectrometry-based metabolome database for tomato. Plant Physiol 2006; 141: 1205–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Sugimoto M, Kaneko M, Onuma H, Sakaguchi Y, Mori M, Abe S et al. Changes in the charged metabolite and sugar profiles of pasteurized and unpasteurized Japanese sake with storage. J Agri Food Chem 2012; 60: 2586–2593. [DOI] [PubMed] [Google Scholar]
- 48Duan Y, An Y, Li N, Liu B, Wang Y, Tang H. Multiple univariate data analysis reveals the inulin effects on the high-fat-diet induced metabolic alterations in rat myocardium and testicles in the preobesity state. J Proteome Res 2013; 12: 3480–3495. [DOI] [PubMed] [Google Scholar]
- 49De Preter V, Falony G, Windey K, Hamer HM, De Vuyst L, Verbeke K. The prebiotic, oligofructose-enriched inulin modulates the faecal metabolite profile: an in vitro analysis. Mol Nutr Food Res 2010; 54: 1791–1801. [DOI] [PubMed] [Google Scholar]
- 50Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F et al. An estimation of the number of cells in the human body. Ann Hum Biol 2013; 40: 463–471. [DOI] [PubMed] [Google Scholar]
- 52Fukuda S, Ohno H. Gut microbiome and metabolic diseases. Semin Immunopathol 2014; 36: 103–114. [DOI] [PubMed] [Google Scholar]
- 53Zhu XY, Zhong T, Pandya Y, Joerger RD. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl Environ Microbiol 2002; 68: 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS et al. Metagenomic analysis of the human distal gut microbiome. Science 2006; 312: 1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6: e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 2008; 5: 235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol 2007; 5: e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Smith CJ, Osborn AM. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol Ecol 2009; 67: 6–20. [DOI] [PubMed] [Google Scholar]
- 59Aminov RI, Walker AW, Duncan SH, Harmsen HJM, Welling GW, Flint HJ. Molecular diversity, cultivation, and improved detection by fluorescent in situ hybridization of a dominant group of human gut bacteria related to Roseburia Spp. or eubacterium rectale. Appl Environ Microbiol 2006; 72: 6371–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60Harmsen HJM, Raangs GC, He T, Degener JE, Welling GW. Extensive Set of 16S rRNA-based probes for detection of bacteria in human feces. Appl Environ Microbiol 2002; 68: 2982–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61DeSantis TZ, Brodie EL, Moberg JP, Zubieta IX, Piceno YM, Andersen GL. High-density universal 16S rRNA microarray analysis reveals broader diversity than typical clone library when sampling the environment. Microb Ecol 2007; 53: 371–383. [DOI] [PubMed] [Google Scholar]
- 62Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 2009; 101: 541–550. [DOI] [PubMed] [Google Scholar]
- 63Loh G, Eberhard M, Brunner RM, Hennig U, Kuhla S, Kleessen B et al. Inulin alters the intestinal microbiota and short-chain fatty acid concentrations in growing pigs regardless of their basal diet. J Nutr 2006; 136: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 64Linetzky Waitzberg D, Alves Pereira CC, Logullo L, Manzoni Jacintho T, Almeida D, Teixeira da Silva ML et al. Microbiota benefits after inulin and partially hydrolized guar gum supplementation: a randomized clinical trial in constipated women. Nutr Hosp 2012; 27: 123–129. [DOI] [PubMed] [Google Scholar]
- 65Duncan SH, Scott KP, Ramsay AG, Harmsen HJM, Welling GW, Stewart CS et al. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl Environ Microbiol 2003; 69: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Grootaert C, Van den Abbeele P, Marzorati M, Broekaert WF, Courtin CM, Delcour JA et al. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol 2009; 69: 231–242. [DOI] [PubMed] [Google Scholar]
- 67Kleessen B, Hartmann L, Blaut M. Oligofructose and long-chain inulin: influence on the gut microbial ecology of rats associated with a human faecal flora. Br J Nutr 2007; 86: 291. [DOI] [PubMed] [Google Scholar]
- 68Pedersen A, Sandström B, Van Amelsvoort J. The effect of ingestion of inulin on blood lipids and gastrointestinal symptoms in healthy females. Br J Nutr 1997; 78: 215–222. [DOI] [PubMed] [Google Scholar]
- 69Bonnema AL, Kolberg LW, Thomas W, Slavin JL. Gastrointestinal tolerance of chicory inulin products. J Am Diet Assoc 2010; 110: 865–868. [DOI] [PubMed] [Google Scholar]
- 70Aw W, Fukuda S. Toward the comprehensive understanding of the gut ecosystem via metabolomics-based integrated omics approach. Semin Immunopathol 2015; 37: 5–16. [DOI] [PubMed] [Google Scholar]