Abstract
A copper-mediated radiofluorination of aryl- and vinylboronic acids with K18F is described. This method exhibits high functional group tolerance and is effective for the radiofluorination of a range of electron-deficient, -neutral, and -rich aryl-, heteroaryl-, and vinylboronic acids. This method has been applied to the synthesis of [18F]FPEB, a PET radiotracer for quantifying metabotropic glutamate 5 receptors.
There are over 1.5 million positron emission tomography (PET) scans performed annually in the US, in which patients are injected with a PET tracer (a bioactive molecule tagged with a positron-emitting radionuclide).1 The functional information obtained from these studies can be used to diagnose and stage disease as well as to predict and/or monitor response to therapy.2,3 The most common PET radionuclide is 18F, due to its convenient half-life (110 min), excellent imaging properties, and ready availability of large amounts of no-carrier-added [18F]fluoride from small medical cyclotrons.4 The growing prevalence of fluorine in pharmaceutical scaffolds5 also offers rich opportunities for the simultaneous development of PET radiotracers as companion diagnostics. However, despite these benefits, the development of new 18F radiotracers is complicated by the limited number of reactions available for the introduction of 18F into bioactive molecules, particularly on electron-rich aromatic rings.6
18F-labeled aromatics are most commonly prepared using SNAr reactions.6 These reactions typically require high temperatures (often >150 °C) and are restricted to electron-deficient substrates. Recent advances have expanded the scope of nucleophilic aromatic radiofluorination using triarylsulfonium,7 diarylselenone,8 and iodonium ylide9 precursors. Complementary studies from our group10 and others11 have focused on the transition-metal-mediated nucleophilic radiofluorination of aromatic substrates. However, despite these advances, there are still very few operationally simple nucleophilic radiofluorination reactions that are effective for electron-rich aromatic substrates and use stable, commercially available precursors.
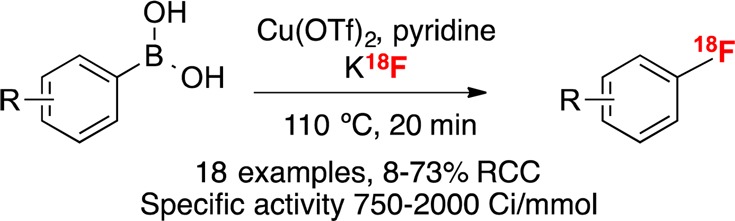
In 2013, the Sanford group disclosed the Cu-mediated fluorination of aryl trifluoroborates, arylboronate esters, and arylboronic acids with KF (Scheme 1a),12 and we immediately sought to translate this discovery to a radiofluorination of arylboron compounds with K18F.13 Our initial studies focused on conditions similar to those used for nonradioactive 19F fluorination, and we found that both potassium trifluoroborate salts and boronate esters undergo Cu-mediated radiofluorination. Preliminary optimization focused on arylpinacol ester 1-BPin (a substrate of particular interest in connection with a program developing radiotracers for glycogen synthase kinase-3).14 These studies uncovered conditions for the radiofluorination of 1-BPin with K18F to afford 2 in ∼5% unoptimized radiochemical conversion (RCC; % integrated area corresponding to product versus 18F– in a radio-HPLC or -TLC trace) (Scheme 1b and Scheme 2).
Scheme 1. Nucleophilic Fluorination of Aryl Boron Reagents.
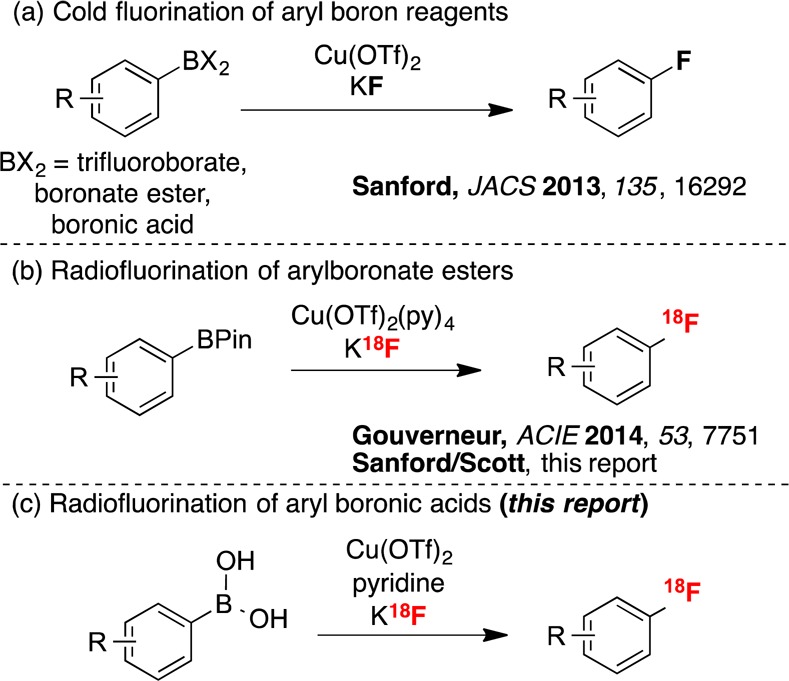
Scheme 2. Radiofluorination of 1-BPin To Form [18F]-4-Fluoroacetophenone 2.
Concomitant with our initial studies, Gouverneur reported a closely related radiofluorination of pinacol boronate esters and demonstrated its application to the synthesis of a range of [18F]arenes, including 2 in 66 ± 6% radiochemical yield.11e However, in our hands, exposing 1-BPin to Gouverneur’s conditions provided 2 in just 31 ± 13% RCC (n = 7, Scheme 2). In addition to the modest yield and reproducibility, we noted several other limitations of Gouverneur’s method. These include (1) the requirement for an expensive copper salt [Cu(OTf)2(py)4],15 (2) incompatibility with more abundant organoboron precursors such as boronic acids, and (3) incompatibility with automation using a commercial radiochemistry synthesis module.16 The latter point is particularly critical given that all modern radiopharmaceuticals in routine clinical use are prepared according to current good manufacturing practice (cGMP) via automated synthesis.
We report herein that these limitations have all been addressed through the development of a copper-mediated radiofluorination of boronic acids (Scheme 1c). These starting materials are particularly practical given their ready availability17 and stability (most boronic acids are white crystalline solids that can be handled in air without special precautions and typically possess long shelf lives).18 While the central theme of this paper is demonstrating the first efficient nucleophilic fluorination of a boronic acid (using 18F or 19F), the method also offers a general approach for the radiofluorination of boronate esters. Furthermore, we demonstrate the full automation of this organoboron radiofluorination reaction using a commercial radiochemistry synthesis platform.
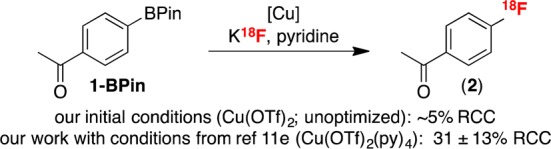
The radiofluorination of boronic acid 1-B(OH)2 was first examined under the conditions developed for the corresponding pinacol boronate ester 1-BPin. However, none of 2 was detected under either our or Gouverneur’s conditions (Table 1, entries 1 and 2, respectively). Similarly, the conditions that we recently reported for the Cu-catalyzed radiofluorination of (mesityl) (aryl)iodonium salts with K18F10a were also not applicable to 1-B(OH)2 (entry 3). A recent report by Neumaier has shown that the use of large quantities of strongly basic K2CO3 to elute 18F– from quaternary methylammonium (QMA) ion exchange cartridges can be problematic for downstream copper-mediated radiofluorination reactions.19 We therefore next evaluated 18F elution with alternative salts.20
Table 1. Optimization of Radiofluorination of 1-B(OH)2 To Form [18F]-4-Fluoroacetophenone 2.
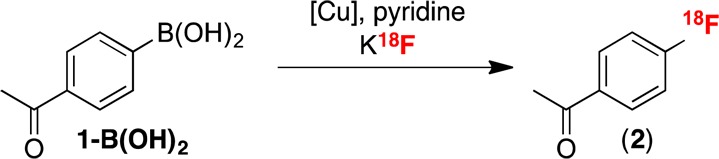
| entrya | QMA eluent | [Cu] | RCCb (%) |
|---|---|---|---|
| 1 | K2CO3 | Cu(OTf)2 | 0 |
| 2 | K2CO3 | Cu(OTf)2(py)4 | 0 |
| 3 | K2CO3 | (MeCN)4CuOTfc | 0 |
| 4 | PPTS | Cu(OTf)2 | 48 ± 2 |
| 5 | KOTf/K2CO3 | Cu(OTf)2 | 51 ± 5d |
| 6 | KOTf/K2CO3 | none | <1 |
| 7 | KOTf/K2CO3 | Cu(OTf)2 | <4e |
| 8 | KOTf/K2CO3 | Cu(OTf)2 | 61 ± 8 |
| 9 | KOTf/K2CO3 | Cu(OTf)2(py)4 | 51 ± 7 |
| 10 | KOTf/K2CO3 | Cu(OTf)2 | 10 ± 2f |
Conditions: 1:5:125 1-B(OH)2/Cu(OTf)2/py at 4 mM concentration of the boronic acid precursor in DMF, K18F, 110 °C, 20 min.
RCC was determined by radio-TLC (n ≥ 2).
Other Cu sources were tested as well. See the SI.
Best conditions 1:5:125:0.1 1-B(OH)2/Cu(OTf)2/py/PPTS.
Pyridine omitted.
Reaction automated using a GE TRACERLab FXFN.
We first examined elution with a solution of pyridinium p-toluenesulfonate (PPTS), given the importance of pyridine in these reactions. This led to recovery of 72% of the 18F– from the QMA cartridge. The eluted 18F– was then azeotropically dried and combined with Cu(OTf)2, 1-B(OH)2, and pyridine in DMF. The reaction was heated at 110 °C for 20 min, after which time radio-TLC and radio-HPLC confirmed the formation of 2 in 48 ± 2% (n = 3) RCC (entry 4).
While PPTS was effective for eluting 18F–, we noted significant losses (50–60%) of radioactivity during azeotropic drying, which is likely due to the formation of some volatile H18F under the acidic conditions.21 Inspired by the work of Katsifis,21 Oh,22 and Lemaire23 exploring alternate 18F– elution strategies, we next examined the elution of 18F– with a weakly basic combination of KOTf and K2CO3 (73/1 molar ratio). With this new eluent, 80% of the 18F– was recovered from the QMA. After azeotropic drying of K18F, PPTS and pyridine were added to the reaction mixture, along with Cu(OTf)2 and 1-B(OH)2. This modification eliminated loss of activity during the drying step and also resulted in a slightly improved RCC of 51 ± 5% (n = 3, entry 5).
We next optimized the reaction with respect to copper source. These studies revealed that copper is essential for the reaction (entry 6 and Table S1) and that CuI complexes do not promote radiofluorination (Table S2). A screen of different solvents showed that radiofluorination proceeds in DMF but not MeCN (Tables S3 and S4). Finally, an evaluation of different pyridine additives showed that pyridine is essential for reactivity (entry 7 and Table S1) and that many substituted pyridines afford yields comparable to that of pyridine (Table S5). As such, pyridine was utilized moving forward due to its low cost and ready availability.24 Reagent concentrations and ratios were also optimized, as well as reaction temperature (Tables S6–10), leading to optimal conditions as follows: a 1:5:125 ratio of 1-B(OH)2/Cu(OTf)2/pyridine at a 4 mM concentration of the boronic acid in DMF at 110 °C for 20 min, providing 61 ± 8% RCC to 2 (n = 7; entry 8). Notably, the analogous reaction with Cu(OTf)2(py)4 proceeded in 51 ± 7% RCC (n = 3, entry 9), confirming that the readily available and inexpensive combination of Cu(OTf)2 and pyridine promotes this reaction as effectively as the more costly Cu(OTf)2(py)4. The process was fully automated in one pot (entry 10), resulting in high specific activity 2 (∼2000 Ci/mmol), albeit in lower RCC of 10 ± 2% (n = 2).
Notably, we do observe competing formation of the protodeboronated byproduct in HPLC traces of the crude reaction mixtures. This byproduct is also formed in the radiofluorination of Bpin precursors. In general, 10–20% of the aryl boron precursor is converted to the protodeboronated byproduct. However, in all of the reported examples, the fluorinated products were readily separable from both the precursor and the protodeboronated byproduct by HPLC.
We next tested the compatibility of this radiofluorination method with water and strong bases. The reaction showed water tolerance, with water/boronic acid ratios of 16:1 resulting in only minor decreases in RCC (Table S11). Despite being tolerant of pyridine, the reaction was highly sensitive to stronger bases. For example, rapid declines in RCC were observed upon the addition of even small amounts of K2CO3 or Hunig’s base (Table S12).
The optimized radiofluorination conditions were applied to a series of different boronic acid substrates. As summarized in Figure 1, this method is compatible with a range of functional groups. The reaction proceeds in moderate to high RCC with arylboronic acids bearing electron-withdrawing (2–8), electron-neutral (9–12), and electron-donating (13–16) substituents. Electron-rich 1-[18F]fluoro-3,4,5-trimethoxybenzene (16) was formed in 36 ± 11% RCC (n = 6), significantly higher than that obtained in the corresponding copper-mediated (mesityl) (aryl)iodonium chemistry (14 ± 1%, n = 5).10aMeta (4, 14, and 16) and ortho substituents (5 and 11) were tolerated, although the latter resulted in lower RCC, likely due to slower transmetalation of the more sterically hindered aryl boronic acids.
Figure 1.
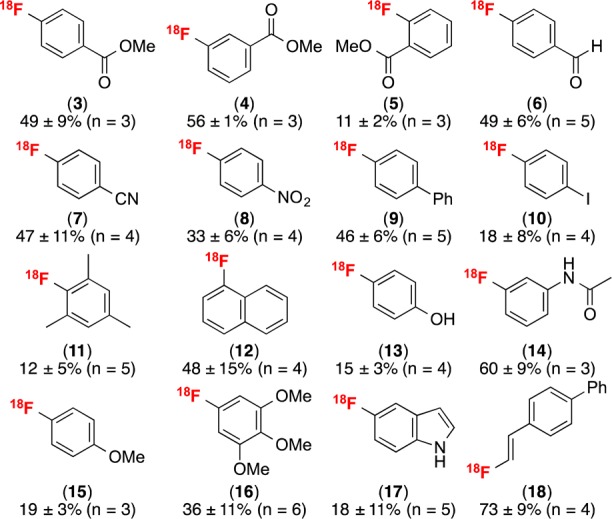
Substrate scope. Conditions: 1:5:125 boronic acid:CuOTf2:pyridine at 4 mM concentration of the boronic acid precursor in DMF, K18F, 110 °C, 20 min.
This method is also compatible with heteroaromatic (17) and vinyl (18) boronic acids. While the yield of [18F]5-fluoroindole (17) was lower than that for many of the other substrates (18 ± 11%; n = 5), this substrate is of significant interest given the importance of fluoroindoles as, for example, RNA analogs25 and cannabinoid CB2 receptor ligands.26 Moreover, the formation of [18F]5-fluoroindole (17), in addition to [18F]4-fluorophenol (13), demonstrate that this radiofluorination reaction proceeds without the need for protection of protic functional groups.
The radiofluorination of pinacol boronate esters and potassium trifluoroborates was also revisited using these optimized conditions. Using boronate esters, fluorinated products were formed in comparable yields to the boronic acid reactions (Table S13). For example, the radiofluorination of 1-BPin afforded product 2 in 69 ± 1% RCC (n = 3). Radiofluorination of aryltrifluoroborates also proceeded, albeit in low yields. For example, the radiofluorination of 7-BF3–K+ yielded 7 in up to 6% RCC (Table S14).27
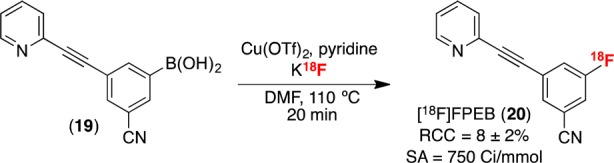
Finally, we applied this radiofluorination method to the synthesis of [18F]FPEB (Scheme 3). This is an important radiotracer for quantifying metabotropic glutamate 5 receptors,28 and it has been historically challenging to synthesize. Following optimization of the precursor amount (Table S15), the one-pot radiofluorination of FPEB boronic acid precursor 19 afforded [18F]FPEB (20) in 8 ± 2% (manual, n = 4) and 4 ± 1% (automated, n = 2) RCC, with specific activity of 750 Ci/mmol. These yields are within the range of that obtained in previously reported syntheses of [18F]FPEB (1–20%).28,29
Scheme 3. Synthesis of [18F]FPEB.
In conclusion, this paper reports a mild and general Cu-mediated method for the radiofluorination of organoboron compounds with K18F. This method represents the first high-yielding nucleophilic fluorination of boronic acids (using 18F or 19F), is compatible with aryl, heteroaryl, and vinyl boronic acids, and thus fills an important gap in the late-stage fluorination space. The method is also suitable for the radiofluorination of boronate esters and potassium trifluoroborates. Finally, this process can be automated on a commercial radiochemistry synthesis module and applied to clinically relevant radiotracers, such as [18F]FPEB. Validation of the method for cGMP clinical production of [18F]FPEB and other PET tracers is currently under investigation.
Acknowledgments
We acknowledge the NIH (GM073836 (M.S.S.) and T32-EB005172 (P.J.H.S.)), the US DOE (DE-SC0012484 (P.J.H.S.)), the Alzheimer’s Association (NIRP-14-305669 (P.J.H.S.)), and Merck (M.S.S. and P.J.H.S.) for financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.5b02875.
Experimental procedures, optimization details, radio-HPLC/TLC traces, and spectral data for all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For a review of PET, see:Ametamey S. M.; Honer M.; Schubiger P. A. Chem. Rev. 2008, 108, 1501. 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- Van der Veldt A. A. M.; Lubberink M.; Mathijssen R. H. J.; Loos W. J.; Herder G. J. M.; Greuter H. N.; Comans E. F. I.; Rutten H. B.; Eriksson J.; Windhorst A. D.; Hendrikse N. H.; Postmus P. E.; Smit E. F.; Lammertsma A. A. Clin. Cancer Res. 2013, 19, 4163. 10.1158/1078-0432.CCR-12-3779. [DOI] [PubMed] [Google Scholar]
- a Van den Abbeele A. D. Oncologist 2008, 13Suppl 28. 10.1634/theoncologist.13-S2-8. [DOI] [PubMed] [Google Scholar]; b Prior J. O.; Montemurro M.; Orcurto M. V.; Michielin O.; Luthi F.; Benhattar J.; Guillou L.; Elsig V.; Stupp R.; Delaloye A. B.; Leyvraz S. J. Clin. Oncol. 2008, 27, 439. 10.1200/JCO.2008.17.2742. [DOI] [PubMed] [Google Scholar]
- a Miller P. W.; Long N. J.; Vilar R.; Gee A. D. Angew. Chem., Int. Ed. 2008, 47, 8998. 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]; b Cai L.; Lu S.; Pike V. W. Eur. J. Org. Chem. 2008, 2008, 2853. 10.1002/ejoc.200800114. [DOI] [Google Scholar]; c Littich R.; Scott P. J. H. Angew. Chem., Int. Ed. 2012, 51, 1106. 10.1002/anie.201106785. [DOI] [PubMed] [Google Scholar]; d Brooks A. F.; Topczewski J. J.; Ichiishi N.; Sanford M. S.; Scott P. J. H. Chem. Sci. 2014, 5, 4545. 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Cole E. L.; Stewart M. N.; Littich R.; Hoareau R.; Scott P. J. H. Curr. Top. Med. Chem. 2014, 14, 875. 10.2174/1568026614666140202205035. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Liang S. H.; Vasdev N. Angew. Chem., Int. Ed. 2014, 53, 11416. 10.1002/anie.201407065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wang J.; Sánchez-Roselló M.; Aceña J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Chem. Rev. 2014, 114, 2432. 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]; b Gillis E. P.; Eastman K. J.; Donnelly D. J.; Meanwell N. A. J. Med. Chem. 2015, 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- a Adams D. J.; Clark J. H. Chem. Soc. Rev. 1999, 28, 225. 10.1039/a808707e. [DOI] [Google Scholar]; b Tredwell M.; Gouverneur V. Angew. Chem., Int. Ed. 2012, 51, 11426. 10.1002/anie.201204687. [DOI] [PubMed] [Google Scholar]
- a Mu L.; Fischer C. R.; Holland J. P.; Becaud J.; Schubiger P. A.; Schibli R.; Ametamey S. M.; Graham K.; Stellfeld T.; Dinkelborg L. M.; Lehman L. Eur. J. Org. Chem. 2012, 2012, 889. 10.1002/ejoc.201101730. [DOI] [Google Scholar]; b Sander K.; Gendron T.; Yiannaki E.; Cybulska K.; Kalber T. L.; Lythgoe M. F.; Årstad E. Sci. Rep. 2015, 5, 9941. 10.1038/srep09941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siméon F. G.; Lu S.; Pike V. W. J. Label. Compd. Radiopharm. 2015, 58Suppl 1S1. [Google Scholar]
- a Satyamurthy M.; Barrio J. R. Patent WO2010/117435 A2, 2010.; b Cardinale J.; Ermert J.; Humpert S.; Coenen H. H. RSC Adv. 2014, 4, 17293. 10.1039/c4ra00674g. [DOI] [Google Scholar]; c Rotstein B. H.; Stephenson N. A.; Vasdev N.; Liang S. H. Nat. Commun. 2014, 5, 4365. 10.1038/ncomms5365. [DOI] [PubMed] [Google Scholar]; d Stephenson N. A.; Holland J. P.; Kassenbrock A.; Yokell D. L.; Livni E.; Liang S. H.; Vasdev N. J. Nucl. Med. 2015, 56, 489. 10.2967/jnumed.114.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ichiishi N.; Brooks A. F.; Topczewski J. J.; Rodnick M. E.; Sanford M. S.; Scott P. J. H. Org. Lett. 2014, 16, 3224. 10.1021/ol501243g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ichiishi N.; Canty A. J.; Yates B. F.; Sanford M. S. Org. Lett. 2013, 15, 5134. 10.1021/ol4025716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lee E.; Kamlet A. S.; Powers D. C.; Neumann C. N.; Boursalian G. B.; Furuya T.; Choi D. C.; Hooker J. M.; Ritter T. Science 2011, 334, 639. 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee E.; Hooker J. M.; Ritter T. J. Am. Chem. Soc. 2012, 134, 17456. 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kamlet A. S.; Neumann C. N.; Lee E.; Carlin S. M.; Moseley C. K.; Stephenson N.; Hooker J. M.; Ritter T. PLoS One 2013, 8, e59187. 10.1371/journal.pone.0059187. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ren H.; Wey H.-Y.; Strebl M.; Neelamegam R.; Ritter T.; Hooker J. M. ACS Chem. Neurosci. 2014, 5, 611. 10.1021/cn500078e. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Tredwell M.; Preshlock S. M.; Taylor N. J.; Gruber S.; Huiban M.; Passchier J.; Mercier J.; Génicot C.; Gouverneur V. Angew. Chem., Int. Ed. 2014, 53, 7751. 10.1002/anie.201404436. [DOI] [PubMed] [Google Scholar]
- Ye Y.; Schimler S. D.; Hanley P. S.; Sanford M. S. J. Am. Chem. Soc. 2013, 135, 16292. 10.1021/ja408607r. [DOI] [PubMed] [Google Scholar]
- For examples of electrophilic fluorination of aryl boron compounds, see:; a Furuya T.; Ritter T. Org. Lett. 2009, 11, 2860. 10.1021/ol901113t. [DOI] [PubMed] [Google Scholar]; b Fier P. S.; Luo J.; Hartwig J. F. J. Am. Chem. Soc. 2013, 135, 2552. 10.1021/ja310909q. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Mazzotti A. R.; Campbell M. G.; Tang P.; Murphy J. M.; Ritter T. J. Am. Chem. Soc. 2013, 135, 14012. 10.1021/ja405919z. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Stenhagen I. S. R.; Kirjavainen A. K.; Forsback S. J.; Jørgensen C. G.; Robins E. G.; Luthra S. K.; Solin O.; Gouverneur V. Chem. Commun. 2013, 49, 1386. 10.1039/c2cc38646a. [DOI] [PubMed] [Google Scholar]; e Ye Y.; Sanford M. S. J. Am. Chem. Soc. 2013, 135, 4648. 10.1021/ja400300g. [DOI] [PubMed] [Google Scholar]
- Perez D. I.; Palomo V.; Pérez C.; Gil C.; Dans P. D.; Luque F. J.; Conde S.; Martínez A. J. Med. Chem. 2011, 54, 4042. 10.1021/jm1016279. [DOI] [PubMed] [Google Scholar]
- Aldrich price (accessed Sep 26, 2015) [Cu(OTf)2(py)4] (CAS: 113110-58-0) $143,075/mol; Cu(OTf)2 (CAS: 34946-82-2): $3154/mol.
- Gouverneur’s method is challenging to automate because it is sensitive to base (see ref (19)) and also because the requirement for O2 in the reaction is incompatible with the inert push gases used in modern automated radiochemistry synthesis modules.
- Aryl boronic acids are more widely available than the corresponding aryl boronate esters. For instance, 1089 aryl boronic acids are commercially available from Sigma-Aldrich versus 218 aryl boronate esters (www.sigmaaldrich.com; accessed August 2015).
- Hall D. G., Ed. Boronic Acids: Preparation and Applications in Organic Synthesis. Medicine and Materials, 2nd ed.; Wiley–VCH Verlag: Weinheim, 2011. [Google Scholar]
- Zlatopolskiy B. D.; Zischler J.; Krapf P.; Zarrad F.; Urusova E. A.; Kordys E.; Endepols H.; Neumaier B. Chem. - Eur. J. 2015, 21, 5972. 10.1002/chem.201405586. [DOI] [PubMed] [Google Scholar]
- Neumaier’s modified elution conditions (i.e., elution with 60 μg of K2CO3) were not practical in our system, since this small quantity of K2CO3 requires backflushing of the QMA cartridge. As such, this process is challenging to automate and can lead to the introduction of cyclotron byproducts to the reaction mixture.
- Katsifis A.; Hamacher K.; Schnitter J.; Stöcklin G. Appl. Radiat. Isot. 1993, 44, 1015. 10.1016/0969-8043(93)90005-U. [DOI] [Google Scholar]
- Lee S. J.; Oh S. J.; Chi D. Y.; Moon D. H.; Ryu J. S. Bull. Korean Chem. Soc. 2012, 33, 2177. 10.5012/bkcs.2012.33.7.2177. [DOI] [Google Scholar]
- Lemaire C. F.; Aerts J. J.; Voccia S.; Libert L. C.; Mercier F.; Goblet D.; Plenevaux A. R. P.; Luxen A. J. Angew. Chem., Int. Ed. 2010, 49, 3161. 10.1002/anie.200906341. [DOI] [PubMed] [Google Scholar]
- Pyridine is an accepted solvent for use in (radio) pharmaceutical synthesis with a residual concentration limit of 200 ppm.Impurities: Guideline for Residual Solvents Q3C(R5). International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2011, http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html.
- Eldrup A. B.; Prhavc M.; Brooks J.; Bhat B.; Prakash T. P.; Song Q.; Bera S.; Bhat N.; Dande P.; Cook P. D.; Bennett C. F.; Carroll S. S.; Ball R. G.; Bosserman M.; Burlein C.; Colwell L. F.; Fay J. F.; Flores O. A.; Getty K.; LaFemina R. L.; Leone J.; MacCoss M.; McMasters D. R.; Tomassini J. E.; Von Langen D.; Wolanski B.; Olsen D. B. J. Med. Chem. 2004, 47, 5284. 10.1021/jm040068f. [DOI] [PubMed] [Google Scholar]
- Frost J. M.; Dart J. M.; Tietje K. R.; Garrison T. R.; Grayson G. K.; Daza A. V.; El-Kouhen O. F.; Miller L. N.; Li L.; Yao B. B.; Hsieh G. C.; Pai M.; Zhu C. Z.; Chandran P.; Meyer M. D. J. Med. Chem. 2008, 51, 1904. 10.1021/jm7011613. [DOI] [PubMed] [Google Scholar]
- Aryl trifluoroborates are less desirable as radiofluorination precursors due to the potential for isotopic exchange.
- a Hamill T. G.; Krause S.; Ryan C.; Bonnefous C.; Govek S.; Seiders T. J.; Cosford N. D.; Roppe J.; Kamenecka T.; Patel S.; Gibson R. E.; Sanabria S.; Riffel K.; Eng W.; King C.; Yang X.; Green M. D.; O’Malley S. S.; Hargreaves R.; Burns H. D. Synapse 2005, 56, 205. 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]; b Wang J.-Q.; Tueckmantel W.; Zhu A.; Pellegrino D.; Brownell A.-L. Synapse 2007, 61, 951. 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]; c Lim K.; Labaree D.; Li S.; Huang Y. Appl. Radiat. Isot. 2014, 94, 349. 10.1016/j.apradiso.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liang S. H.; Yokell D. L.; Jackson R. N.; Rice P. A.; Callahan R.; Johnson K.; Alagille D.; Tamagnan G.; Collier T. L.; Vasdev N. MedChemComm 2014, 5, 432. 10.1039/C3MD00335C. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Stephenson N. A.; Holland J. P.; Kassenbrock A.; Yokell D. L.; Livni E.; Liang S. H.; Vasdev N. J. Nucl. Med. 2015, 56, 489. 10.2967/jnumed.114.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- While this manuscript was in preparation, a small-scale microfluidic method was published that reports 68% RCC to [18F]FPEB. See:Calderwood S.; Collier T. L.; Gouverneur V.; Liang S. H.; Vasdev N. J. Fluorine Chem. 2015, 178, 249. 10.1016/j.jfluchem.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


