Abstract
Purpose of review
In allergic disease, dendritic cells (DCs) play a critical role in orchestrating immune responses to innate stimuli and promoting the formation of TH2 effector versus T regulatory cells. Here, we review recent advances in our understanding of how current forms of immunotherapy modulate DC responses.
Recent Findings
Sublingual (SLIT) and oral (OIT) immunotherapy for peanut allergy alter the expression of co-stimulatory molecules on DCs that leads to reduced expression of TH2 effector cytokines in an antigen non-specific manner. SLIT and OIT also modulate DC innate immune responses to Toll like receptor (TLR) agonists, including enhanced production of IFN-α and reduced expression of pro-inflammatory cytokines that may serve to promote the development of tolerance. DCs isolated from subjects post-OIT promoted hypomethylation of the FOXP3 locus in effector T cells. Reduced methylation of the FOXP3 locus has been associated with more persistent clinical desensitization following OIT. Recent studies have additionally highlighted a role for B cells in inducing tolerogenic DC populations and T regulatory cells during immunotherapy. Epicutaneous immunotherapy may also elicit immunosuppressive populations of cutaneous DCs, although in some cases, antigen exposure through the skin can lead to sensitization. Finally, efforts have focused on identifying pharmacologic and/or antigen-independent strategies of altering DC function to enhance the immunosuppressive effects of immunotherapy.
Summary
Dendritic cells are a critical target of immunotherapy. Alterations in both adaptive and innate immunity likely underlie the immunosuppressive effects of this treatment.
Keywords: Dendritic cells, innate immunity, adaptive immunity, allergy, immunotherapy, desensitization, tolerance
INTRODUCTION
Dendritic cells (DCs) are professional antigen presenting cells that sit at the interface between innate and adaptive immunity where they help control the balance between inflammation and tolerance at mucosal sites. Following ligation of Toll like receptors (TLRs), DCs are an important source of pro-inflammatory cytokines, including IL-6 and tumor necrosis factor (TNF-α), as well as Type I interferons (IFNs) that are critical in eradicating pathogens. However, DCs also contribute to adaptive immunity by regulating the differentiation of naïve T cells, as well as memory responses by antigen-specific T cells.
Several studies have suggested that innate immune responses may be impaired in patients with allergic disease[1–3]. DCs from allergic subjects show reduced IFN-α secretion following treatment with TLR7 or 9 ligands, including viral stimulation[1,4]. The development of innate immune function in allergic children also appears to be altered relative to their nonallergic peers[2]. Exactly how these alterations in innate immunity might contribute to exaggerated T helper 2 (TH2) adaptive immune responses and allergic disease remain to be fully elucidated. Pro-inflammatory cytokines secreted by DCs favor commitment to Th2 phenotypes, while IFN-α inhibits IL-4 driven Th2 differentiation of CD4+ T cells and destabilizes the Th2 phenotype by suppressing expression of GATA3 [5–7]. In the setting of helminth infection and allergy, DCs are believed to be activated by epithelial cell-derived thymic lymphopoetin (TSLP) via OX40 ligand to induce a TH2 directed response[8]. However, normally DCs capture proteins at mucosal surfaces and play a tolerizing role via their expression of ICOSL, which induces the generation of T regulatory (Treg) cells [9]. Recently, DCs from patients with allergic rhinitis and asthma were found to display impaired expression of ICOSL and thereby promote T cells to acquire TH2 effector function, underscoring a potential role for this pathway in the pathogenesis of allergic disease [10].
Two major types of immature DCs are found in the peripheral circulation in humans: Blood Dendritic Cell Antigen BDCA2+, BDCA4+, CD123hi, CD11c− plasmacytoid DCs (pDCs), which express TLR-7 and 9 and produce IFN-α, and BDCA1+ BDCA3+ CD123lo CD11c+ myeloid DCs (mDCs), which express TLR-2 and 4 and produce IL-12 [11]. DC subsets, especially of the mDC lineage and Langerhans cells (LCs), are also found in the oral mucosa and skin and can be further differentiated by the expression of CD207 (langerin), CD103 and epithelial cell adhesion molecule (Ep-CAM) [12]. LCs are believed to be of hematopoetic origin and differentiate from progenitor cells in response to transforming growth factor (TGF-β) and TNF-α [13]. Recently it was shown that the stimulation of BDCA-1+ BDCA-3− circulating DCs with thymic stromal lymphopoietin (TSLP) and transforming growth factor (TGF-β) may be a novel pathway for LC differentiation [14].
Given the complex interplay between innate and adaptive immunity and the central role for DCs in orchestrating this interaction, the ability of allergen immunotherapy to modulate allergic immune responses almost certainly involves alterations in DC function. This review provides an update on the most recent publications related to the role of DCs in immunotherapy for allergic disease.
EFFECT OF ORAL AND SUBLINGUAL IMMUNOTHERAPY ON DCs
Sublingual (SLIT) and oral (OIT) immunotherapy are two modalities currently being explored for treatment of food allergy, and SLIT is also increasingly being utilized as a therapy for rhinoconjunctivitis and asthma. With SLIT, an allergen preparation is held under the tongue for a period of time before swallowing, while OIT involves immediate ingestion of the allergen in a food vehicle.
A recent open-label, randomized trial of SLIT and OIT for the treatment of IgE-mediated cow’s milk allergy evaluated DC innate and adaptive functions before, during and after therapy [15]. In this study, OIT and SLIT were found to produce differing effects on DC subsets, with OIT having the greatest effect on pDC function and SLIT on mDC responses. OIT appeared to partially correct the impaired pDC secretion of IFN-α that has been reported in allergic populations. On the other hand, SLIT uniquely decreased TLR-induced IL-6 production by mDCs, and this latter finding appeared to be associated with the development of sustained unresponsiveness to milk. Notably, a similar enhancement in pDC responsiveness to TLR9 stimulation was observed in allergic patients receiving subcutaneous immunotherapy for house dust mite and other aeroallergens [16].
A second study [17], which evaluated DC and other immunologic responses to peanut immunotherapy in school-aged children, found that OIT and SLIT suppressed Th2 cytokine responses to peanut in DC-T cell co-cultures. These changes were associated with reduced expression of CD40, HLA-DR, and CD86 expression on DCs, and were most robust in mDC DC-T cell cultures from subjects undergoing OIT. Interestingly, expression of CD80 demonstrated a reverse pattern of change, consistent with the opposing role of CD80 and CD86 in T cell suppression and activation. The alterations in DC behavior induced by SLIT and OIT were not antigen-specific, as synonymous changes in DC responses were seen after stimulation with an irrelevant antigen (house dust mite). The changes were also not persistent, as most markers of immunologic suppression reversed after withdrawal from therapy and in some cases during ongoing maintenance therapy.
In a phase 1 single-site study, immunologic responses from participants undergoing 24 months of peanut OIT were studied [18]. The investigators found that Tregs from clinically “tolerant” subjects had greater suppressive function and higher levels of FOXP3 hypomethylation, and that DCs isolated from subjects post-therapy were capable of decreasing the percentage of FOXP3 methylation in effector T cells. After a period off therapy, patients who lost clinical “tolerance” demonstrated a reversion of methylation patterns at the FOXP3 locus in peanut-specific Tregs.
Collectively, these studies suggest that SLIT and OIT for food allergy are able to modulate the phenotype and function of circulating DCs. Whether these effects are driven by the small quantities of allergen that reach the systemic circulation, or are an indirect consequence of the other immune changes exerted by IT (e.g. changes in allergen-specific IgE or IgG), requires further investigation.
ROLE of DCs IN EPICUTANEOUS IMMUNOTHERAPY
Within the last few years, the skin has emerged as a novel route of allergen delivery in the form of epicutaneous immunotherapy (EPIT). Recent studies by Dioszeghy and colleagues [19,20] have implicated a key role for DCs in intact skin in mediating antigen uptake and the induction of tolerogenic responses during EPIT in mice. They found that OVA antigen was transported by DCs in the superficial layers of the stratum corneum to draining lymph nodes, and that after EPIT, local and systemic responses were downregulated, as evidenced by a decrease in local allergic inflammation, systemic T cell responses to OVA, and an increase in the number of splenic Treg cells. These Tregs appeared to persist even after EPIT has been discontinued, and they may protect against sensitization to new allergens. In additional studies of mice sensitized to milk [21], milk-specific EPIT prevented subsequent sensitization to peanut and house dust mite via a Treg-dependent mechanism. EPIT was also associated with reduced expression of CD86 on allergen-activated DCs.
In contrast to these findings, Tordesillas et al [22] recently found that application of peanut extract alone (no additional adjuvant) to intact skin of mice promoted allergic sensitization and the development of anaphylaxis upon subsequent oral exposure to peanut. The authors went on to show that DCs purified from draining lymph nodes following epicutaneous exposure to peanut were sufficient for driving TH2 responses to this allergen. Allergic responses to peanut were also dependent upon the innate cytokine, IL-33, since blockade of the IL33 receptor following topical exposure mitigated Th2 priming responses to peanut. Importantly, these observations were not true for all allergens. Topical application of less potent allergens, including milk and soy, failed to elicit an allergic response.
ROLE OF B CELLS IN PROMOTING TOLEROGENIC DCs
While the T cell - DC relationship has received the most focus in efforts to understand how immunotherapy suppresses allergic inflammation or induces tolerance, another major cell population that has garnered interest is the B cell. Recent findings suggest that certain subpopulations of B cells, known as regulatory B cells, may play a critical role in supporting immunologic tolerance [23].
In the field of immunotherapy, it was recently observed that human subjects with egg allergy treated with allergen specific immunotherapy appear to generate suppressor/regulatory B cells. Wu [24] found that, following treatment with immunotherapy, subjects exhibited an increased frequency of CD27+CD35+ memory B cells that had strong immunosuppressive properties. This B cell population was found to produce TGFβ, and when co-cultured in the presence of OVA with immature DCs, induced the development of a tolerogenic TGFβ+ DC population. Subsequent co-culturing of these DCs with T cells was able to induce a Treg population.
A role for CD35+ B cells and DCs in promoting tolerogenic changes in the context of immunotherapy has also been seen in murine studies. Zhang et al. sensitized mice with OVA to induce intestinal food allergy, and then treated the mice with OIT [25]. They found that OIT induced the expansion of CD35+ B cells, which promoted the differentiation of Tregs in the intestines, downregulated expression of CD80 and CD86 on DCs, and suppressed ongoing allergic inflammation via expression of thrombospondin 1 (TSP1). In a second study by the same group [26], the investigators further examined the relationship between B cells, DCs and T cells in directing tolerogenic responses. The authors found that by using a CD20 monoclonal antibody to deplete B cells, mice sensitized to OVA generated a lower frequency of TGFβ+ tolerogenic DCs, defined as CD11c+TGFβ+. Bone marrow-derived DCs from this B cell-depleted model were then activated with LPS, and found to be positive for precursor/latent TGFβ (LTGFβ), but not active TGFβ. Co-culture of activated B cells with these BMDCs was able to restore expression of active TGFβ. The investigators went on to show that expression of TSP1 by B cells was critical to induce conversion of latent to active TGFβ via its proteolytic activity and thereby generate tolerogenic DCs. Interestingly, IL13 (but not IL-4 or IL-5) promoted TSP1 gene methylation in B cells, reducing their production of TSP1. Antagonism of IL-13 during immunotherapy improved the efficacy of this treatment and enhanced production of tolerogenic DCs in their mouse model of food allergy. Collectively, these studies point to a critical role for CD35+ B cells in promoting tolerogenic responses to food allergens.
Another recent study examined the effect of peanut OIT on the antigen-specific repertoire of B cells in humans. Patil et al. [27] found that peanut OIT led to the expansion of peanut-specific memory B cells whose receptor repertoire was oligoclonal and somatically hypermutated. These changes occurred early during the course of treatment. Unrelated individuals were found to share similar peanut-specific B cell clones, consistent with convergent selection. Whether these shared clonal groups play a role in the clinical efficacy of immunotherapy requires further investigation.
OTHER EXPERIMENTAL APPROACHES THAT MAY MODULATE ADAPTIVE AND INNATE DC FUNCTION DURING IMMUNOTHERAPY
A number of studies have recently explored novel ways of modulating DC responses for potential application in allergen immunotherapy. Many of these approaches utilize antigen-independent strategies or pharmacologic methods of downregulating DC responses.
The generation of tolerogenic DC cells via incubation with glucocorticoids was investigated in latex allergy [28]. In this study, peripheral blood mononuclear cells were obtained from human subjects with latex allergy, and DCs were generated from isolated monocytes. DCs from allergic subjects treated with dexamethasone (dx-DC) acquired an immature phenotype with low expression of MHCII and costimulatory molecules including CD40, CD80 and CD86. The dx-DCs exhibited reduced ability to present antigen, enhanced expression of IL-10 upon CD40L stimulation, and an increased propensity to promote development of latex-specific regulatory T cells. The authors concluded that dexamethasone-primed DCs may be a useful tool in immunotherapy for latex allergic subjects via their ability to downregulate allergen-specific T cell responses.
Bakdash et al [29] evaluated the potential for using calcidiol (25 dihydroxy vitamin D3) as a modulating agent to induce tolerogenic DCs. The authors found that priming of maturing DCs with calcidiol promoted generation of a population of IL-10- and IL-12-producing DCs, which in turn induced the development of T cells that produced IL- 10 and IFN-γ. The authors hypothesize that these Treg-like cells may promote a TH1 and Treg immunologic skewing, and may be useful in counteracting a TH2 directed immune response as is seen in allergic disease, and thus “potentiate” allergen-specific immunotherapy.
Strategies for an antigen-independent immunotherapy were investigated by the use of mycobacterial antigens to modulate house dust mite (HDM)-induced allergic airway inflammation [30]. HDM sensitized mice (via subcutaneous injection) were treated with mycobacterial antigens via intramuscular injection at 2 week intervals. Mice were challenged with HDM both before and after treatment, and cellular and humoral markers of inflammation and airway hyperresponsiveness were assessed. Investigators found that mycobacterial antigens downregulated TH2 responses and promoted IL-10 and IFN-γ production. Adoptive transfer of spleen cells from mice immunized with mycobacterial antigens recapitulated these changes in allergic recipients, but transfer from MyD88-deficient mice did not. In sum, the authors demonstrated that allergen-free immunotherapy can modulate innate immune responses in a way that reduces TH2 adaptive immunity and allergic clinical responses in mice.
The use of bystander immunotherapy to generate tolerogenic DCs was investigated by Navarro et al [31]. In this model, mice were tolerized to OVA and subsequently sensitized to another antigen, Leishmania homolog of receptors for activated c kinase (LACK). Mice were then challenged to aerosols of LACK alone or OVA and LACK in concert. The authors found that airway inflammation and hyperresponsiveness decreased following co-treatment with both antigens. Protection required interaction between CTLA-4 on T cells and ICOS on DCs. Thus, in sum, exposure of the animal to a tolerogenic stimulus in concert with an antigen can provoke a bystander effect that results in decreased allergic inflammation to the antigen. The authors propose that this may explain the protective effects of allergen-specific immunotherapy against antigens that are different than those used during the desensitization.
CONCLUSION
While DCs have long been recognized to play a critical role in regulating innate and adaptive immune responses, several studies in recent years highlight a central role for these cells in driving the immunologic suppression that accompanies immunotherapy (fig 1). In the setting of sublingual and oral immunotherapy for food allergy in school aged children and adolescents, DCs isolated from treated subjects exhibited enhanced secretion of IFN-α and reduced expression of pro-inflammatory cytokines following TLR stimulation. DCs also showed evidence of reduced activation as indicated by lower expression of co-stimulatory molecules, which was correlated with reduced expression of Th2 effector cytokines in DC-T cell co-cultures. At least some of these changes in DC phenotype and function are evident only transiently. The fleeting nature of the immunologic suppression in OIT and SLIT may not be surprising as only a minority of participants in these studies achieve long-lasting clinical tolerance. Whether more persistent changes would result if immunotherapy were begun earlier in life or were continued for longer periods remains to be seen, but recent studies suggest this may be the case [32]. Fascinating studies over the last few years examining the role of the skin in driving tolerogenic versus allergic responses to external antigens leave many questions unanswered, and further research into exactly how the skin can drive responses in either direction are clearly warranted. The role of the B cell in augmenting tolerogenic responses during immunotherapy represents another exciting area of investigation that deserves further attention. A greater understanding of how innate and adaptive immunity can be manipulated to promote tolerance in allergic disease will lead to novel and more effective immunotherapy strategies for these common conditions.
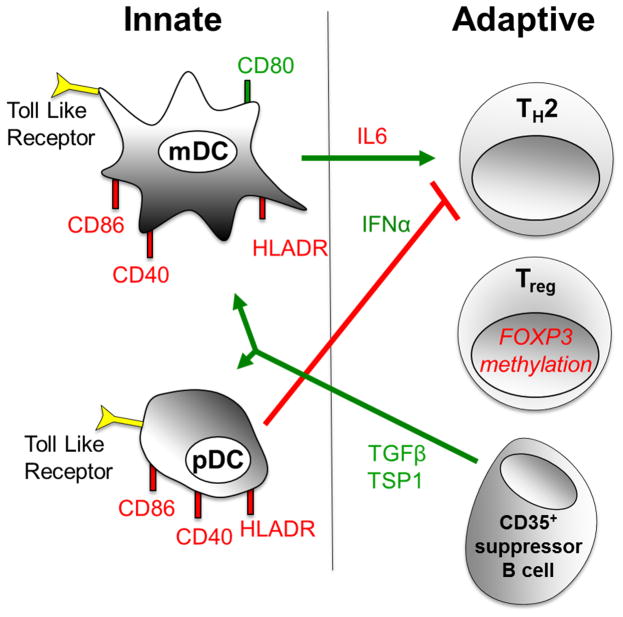
Fig 1.
Recent mechanistic insights into the role of DCs in regulating innate and adaptive responses to immunotherapy
Several recent studies have provided new insight into the mechanisms by which immunotherapy alters plasmacytoid (pDC) and myeloid (mDC) dendritic cell phenotype and function to produce innate and adaptive immune changes that suppress allergic inflammation. SLIT and OIT have been found to enhance IFN-α secretion by pDCs and suppress IL-6 expression by mDCs following stimulation with Toll-like receptor (TLR) agonists. This may contribute to the decrease in T helper 2 (Th2) effector cytokine responses during IT. Following OIT, DCs also enhance the development of T regulatory (Treg) cells by promoting hypomethylation at the Foxp3 locus. The beneficial effects of immunotherapy have been associated with reduced expression of HLA-DR, CD86, and CD40 on DCs, and up-regulation of CD80. Recent findings suggest that immunotherapy might also induce a population of CD35+ suppressor B cells that produce TGFβ and thrombospondin 1 (TSP), which promotes the development of tolerogenic DCs. Immune changes that have been found to be increased during immunotherapy are depicted in green, and those that are decreased are depicted in red.
KEY POINTS.
Sublingual and oral immunotherapy for food allergy elicit changes in DC innate and adaptive immune functions that may serve to reduce allergic inflammation and promote tolerance; in some cases, these changes are transient
The role of the skin in induction of tolerance versus sensitization to allergens is complex and requires further study.
Recent evidence suggests that interactions among DCs, regulatory T cells, and CD35+ B cells are central to the immunosuppressive effects of immunotherapy
Antigen non-specific modulation of innate immune responses may provide a novel means of augmenting the tolerogenic effects of immunotherapy
Acknowledgments
Financial support and sponsorship. The Research Training in Pediatric Allergy and Immunology, Grant # 5T32AI007007 (M.G). Winkelstein fellowship (M.G.). P. A. F.-G. is supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Disclosures: No conflicts of interest to report.
Other substantial contributions to the article. None.
Conflicts of interest. none.
References
- 1.Durrani SR, Montville DJ, Pratt AS, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012 Aug;130(2):489–95. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tulic MK, Hodder M, Forsberg A, et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J Allergy Clin Immunol. 2011 Feb;127(2):470–478. e1. doi: 10.1016/j.jaci.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010 Jun 1;184(11):5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tversky JR, Le TV, Bieneman AP, et al. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-alpha via Toll-like receptor 9. Clin Exp Allergy. 2008 May;38(5):781–8. doi: 10.1111/j.1365-2222.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber JP, Ramos HJ, Gill MA, Farrar JD. Cutting edge: Type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J Immunol. 2010;185:813–817. doi: 10.4049/jimmunol.1000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon M, Anguita J, Nakamura T, et al. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exper Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodge IL, Carr MW, Cernadas M, Brenner MB. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J Immunol. 2003;170:4457–4464. doi: 10.4049/jimmunol.170.9.4457. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005 Nov 7;202(9):1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007 Sep 27;449(7161):419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 10*.Shen C, Hupin C, Froidure A, et al. Impaired ICOSL in human myeloid dendritic cells promotes Th2 responses in patients with allergic rhinitis and asthma. Clin Exp Allergy. 2014 Jun;44(6):831–41. doi: 10.1111/cea.12308. This study demonstrates a role for ICOSL in suppressing Th2 responses. [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001 Sep 17;194(6):863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovav AH. Dendritic cells of the oral mucosa. Mucosal Immunol. 2014 Jan;7(1):27–37. doi: 10.1038/mi.2013.42. [DOI] [PubMed] [Google Scholar]
- 13.Ito T, Inaba M, Inaba K, et al. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 1999 Aug 1;163(3):1409–19. [PubMed] [Google Scholar]
- 14*.Martínez-Cingolani C, Grandclaudon M, Jeanmougin M, et al. Human blood BDCA-1 dendritic cells differentiate into Langerhans-like cells with thymic stromal lymphopoietin and TGF-β. Blood. 2014 Oct 9;124(15):2411–20. doi: 10.1182/blood-2014-04-568311. This manuscript demonstrates a novel pathway for langerhans cell differentiation that is driven by thymic stromal lymphopoietin and TGF-β. [DOI] [PubMed] [Google Scholar]
- 15**.Frischmeyer-Guerrerio PA, Keet CA, Guerrerio AL, et al. Modulation of dendritic cell innate and adaptive immune functions by oral and sublingual immunotherapy. Clin Immunol. 2014 Nov;155(1):47–59. doi: 10.1016/j.clim.2014.08.006. This study demonstrates that sublingual and oral immunotherapy for milk allergy invoke striking changes in innate immune functions mediated by DCs that may suppress allergic responses and, at least in part, depend on the route of allergen delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Tversky JR, Bieneman AP, Chichester KL, et al. Subcutaneous allergen immunotherapy restores human dendritic cell innate immune function. Clin Exp Allergy. 2010 Jan;40(1):94–102. doi: 10.1111/j.1365-2222.2009.03388.x. These authors demonstrate that subcutaneous immunotherapy enhances Toll-like receptor (TLR9)-mediated innate immune functions mediated by plasmacytoid DCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Gorelik M, Narisety SD, Guerrerio AL, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol. 2014 Dec 24; doi: 10.1016/j.jaci.2014.11.010. This study demonstrates that while SLIT and OIT can induce rapid suppression of DC activation and Th2 cytokine responses to allergen, these immunosuppressive effects are often transient. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Syed A, Garcia MA, Lyu SC, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014 Feb;133(2):500–10. doi: 10.1016/j.jaci.2013.12.1037. This study demonstrates a mechanistic pathway by which dendritic cells may promote the induction of regulatory T cells via hypomethylation of the FOXP3 locus during oral immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dioszeghy V, Mondoulet L, Dhelft V, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011 May 15;186(10):5629–37. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 20.Dioszeghy V, Mondoulet L, Dhelft V, et al. The regulatory T cells induction by epicutaneous immunotherapy is sustained and mediates long-term protection from eosinophilic disorders in peanut-sensitized mice. Clin Exp Allergy. 2014 Jun;44(6):867–81. doi: 10.1111/cea.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Mondoulet L, Dioszeghy V, Puteaux E, et al. Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model. J Allergy Clin Immunol. 2015 Jun;135(6):1546–1557. e4. doi: 10.1016/j.jaci.2014.11.028. Using murine models, the authors demonstrate that epicutaneous immunotherapy for a specific allergen may reduce the risk of becoming sensitized to additional allergens via a T regulatory cell-dependent mechanism. [DOI] [PubMed] [Google Scholar]
- 22*.Tordesillas L, Goswami R, Benedé S, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014 Nov;124(11):4965–75. doi: 10.1172/JCI75660. This study demonstrates that peanut allergens can activate innate immune pathways in the skin that promote allergic sensitization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosser EC, Mauri C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity. 2015 Apr 21;42(4):607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Wu Z. Antigen specific immunotherapy generates CD27(+) CD35(+) tolerogenic dendritic cells. Cell Immunol. 2013 May-Jun;283(1–2):75–80. doi: 10.1016/j.cellimm.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 25**.Yang G, Geng XR, Liu ZQ, et al. TSP1-producing B cells restore Ag specific immune tolerance in an allergic environment. J Biol Chem. 2015 Apr 3; doi: 10.1074/jbc.M114.623421. This study elucidates novel mechanistic pathways whereby B cells may promote immune tolerance by secreting thrombospondin-1 (TSP1) to induce the generation of tolerogenic dendritic cells in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang HP, Wu Y, Liu J, et al. TSP1-producing B cells show immune regulatory property and suppress allergy-related mucosal inflammation. Sci Rep. 2013 Nov 26;3:3345. doi: 10.1038/srep03345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Patil SU, Ogunniyi AO, Calatroni A, et al. Peanut oral immunotherapy transiently expands circulating Ara h 2-specific B cells with a homologous repertoire in unrelated subjects. J Allergy Clin Immunol. 2015 May 15; doi: 10.1016/j.jaci.2015.03.026. New findings that demonstrate immunotherapy may induce an oligoclonal population of B cells and that clonal groups are shared among unrelated subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escobar A, Aguirre A, Guzmán MA, et al. Tolerogenic dendritic cells derived from donors with natural rubber latex allergy modulate allergen-specific T-cell responses and IgE production. PLoS One. 2014 Jan 22;9(1):e85930. doi: 10.1371/journal.pone.0085930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakdash G, van Capel TM, Mason LM, et al. Vitamin D3 metabolite calcidiol primes human dendritic cells to promote the development of immunomodulatory IL-10-producing T cells. Vaccine. 2014 Oct 29;32(47):6294–302. doi: 10.1016/j.vaccine.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca DM, Wowk PF, Paula MO, et al. Requirement of MyD88 and Fas pathways for the efficacy of allergen-free immunotherapy. Allergy. 2015 Mar;70(3):275–84. doi: 10.1111/all.12555. [DOI] [PubMed] [Google Scholar]
- 31.Navarro S, Lazzari A, Kanda A, et al. Bystander immunotherapy as a strategy to control allergen-driven airway inflammation. Mucosal Immunol. 2014 Nov 26; doi: 10.1038/mi.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015 Feb 26;372(9):803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]