Abstract
Respiratory syncytial virus (RSV) is estimated to claim more lives among infants <1 year old than any other single pathogen, except malaria, and poses a substantial global health burden. Viral entry is mediated by a type I fusion glycoprotein (F) that transitions from a metastable prefusion (pre-F) to a stable postfusion (post-F) trimer. A highly neutralization-sensitive epitope, antigenic site Ø, is found only on pre-F. We determined what fraction of neutralizing (NT) activity in human sera is dependent on antibodies specific for antigenic site Ø or other antigenic sites on F in healthy subjects from ages 7 to 93 years. Adsorption of individual sera with stabilized pre-F protein removed >90% of NT activity and depleted binding antibodies to both F conformations. In contrast, adsorption with post-F removed ~30% of NT activity, and binding antibodies to pre-F were retained. These findings were consistent across all age groups. Protein competition neutralization assays with pre-F mutants in which sites Ø or II were altered to knock out binding of antibodies to the corresponding sites showed that these sites accounted for ~35 and <10% of NT activity, respectively. Binding competition assays with monoclonal antibodies (mAbs) indicated that the amount of site Ø–specific antibodies correlated with NT activity, whereas the magnitude of binding competed by site II mAbs did not correlate with neutralization. Our results indicate that RSV NT activity in human sera is primarily derived from pre-F–specific antibodies, and therefore, inducing or boosting NT activity by vaccination will be facilitated by using pre-F antigens that preserve site Ø.
INTRODUCTION
Human respiratory syncytial virus (RSV) infects virtually every child by 2 years of age (1) and annually accounts for an estimated 33 million lower respiratory tract infections in children less than 5 years of age (2). Of 11 proteins expressed by this paramyxovirus, the F and G glycoproteins are known to generate protective neutralizing (NT) antibody responses (3). However, F displays more NT epitopes, is highly conserved, is required for fusion and entry of RSV into host cells, and therefore is a primary target for vaccine-induced protection (4). Currently, at least four described antigenic sites on F are associated with virus neutralization. Site I is a target for monoclonal antibodies (mAbs) such as 2F, 44F, or 45F (5) with weak or negligible NT activity and is defined by a P389 escape mutation. Site II comprises the epitope for palivizumab, a licensed mAb administered prophylactically to infants at high risk of severe disease (6). Site IV is recognized by mAbs such as mAb19 (7) or 101F (8) with moderate NT activity. All the mAbs that recognize these three sites can bind the stable postfusion (post-F) conformation (9). The recent structural definition of the prefusion (pre-F) trimer revealed a new antigenic site (site Ø), which is targeted by mAbs such as D25, AM22, and 5C4 that have NT potency 10- to 100-fold greater than palivizumab (10). Another epitope on F is recognized by the mAb MPE8 (11), which has been mapped to a region adjacent to antigenic site II but binds almost exclusively to the pre-F conformation of the molecule. Other pre-F–specific antibodies such as AM14 (12), which binds to a quaternary epitope only present in stable trimers (13), have been recently identified.
Immunization with a stabilized version of the pre-F trimer induces significantly higher NT responses than immunization with a post-F immunogen (14), suggesting that pre-F–specific antibodies are more readily elicited and potent than antibodies targeting sites shared by post-F. Therefore, despite the success achieved by passive immunoprophylaxis with palivizumab, which targets the shared antigenic site II, other pre-F–specific surfaces are likely to induce antibody responses with more potent RSV neutralization. Also, there are some limitations in the use of palivizumab. For example, treatment is only recommended for premature infants, those with congenital heart disease, and other select populations at high risk of severe disease (6). Because most hospitalizations occur in infants without identified risk factors (15) and there is a continuing high burden of disease in older children and the frail elderly (16), there remains a need to understand the basis for RSV immunity to develop approaches for preventing RSV disease in the entire birth cohort.
A previous study by Melero and colleagues demonstrated that depletion of antibodies to the post-F conformation does not remove NT activity from the sera of rabbits immunized with RSV. In the same study, pooled polyclonal human sera screened for high levels of NT activity (RSVIG) was shown to retain most of NT activity after adsorption with post-F (17), suggesting the importance of unique NT epitopes present on alternative conformations of F (17). The ability to stabilize pre-F using a structure-guided atomic-level design (14) has enabled the generation of reagents that can detect differential NT activity against the two major conformations of F. We characterized human serum responses to pre-F and post-F and further defined serum NT antibody responses that target antigenic sites Ø and II in a population spanning ages 7 to 93 years.
RESULTS
RSV F–specific binding antibodies in human sera are primarily specific for pre-F
To evaluate the level of antibody response to pre-F and post-F (expressed from RSV A2 constructs) in human sera, we used both proteins in a kinetic enzyme-linked immunosorbent assay (ELISA) analysis. Untreated human serum samples bound pre-F and post-F with similar magnitude [geometric mean titers (GMTs) of 75.8 and 75.4 milli–optical density (mOD)/min, respectively; n = 140] (Fig. 1A). Serum adsorption with pre-F removed nearly all binding activity to both F conformations (7.6% residual binding to pre-F and 10% residual binding to post-F after pre-F adsorption; P < 0.0001 for both cases compared to unadsorbed sera, n = 140, Wilcoxon signed-rank test) (Fig. 1A). In contrast, adsorption with post-F did not substantially affect serum binding to pre-F (77% residual binding) but, as expected, removed binding to post-F (8.7% residual binding, P < 0.0001, n = 140, Wilcox-on signed-rank test), which suggests that about 90% of antibodies in human sera that bindtoRSVFare abletorecognize pre-F, whereas <10% can exclusively bind post-F. ELISA confirmed negligible residual pre-F or post-F following adsorption (table S1 and fig. S1), and a hemagglutination inhibition assay (HAI) showed that F proteins did not non-specifically remove influenza-specific antibodies in sera (table S3). The differential antibody binding activityofseratopre-F and post-F is not proportional to the exposed surfaces of each F conformation: ~50% is shared between pre-F and post-F and ~50% is unique (Fig. 1B) (14). Therefore, either the unique surfaces on pre-F are more immunogenic than those on post-F, or post-F is less abundant or less exposed during natural infection. Whereas there was variation in the binding ratio of pre-F to post-F among individuals, age stratification by decade revealed no major differences in relative binding specificity across age groups (fig. S2).
Fig. 1. Serum binding activity to the pre-F and post-F conformations of the RSV F glycoprotein.
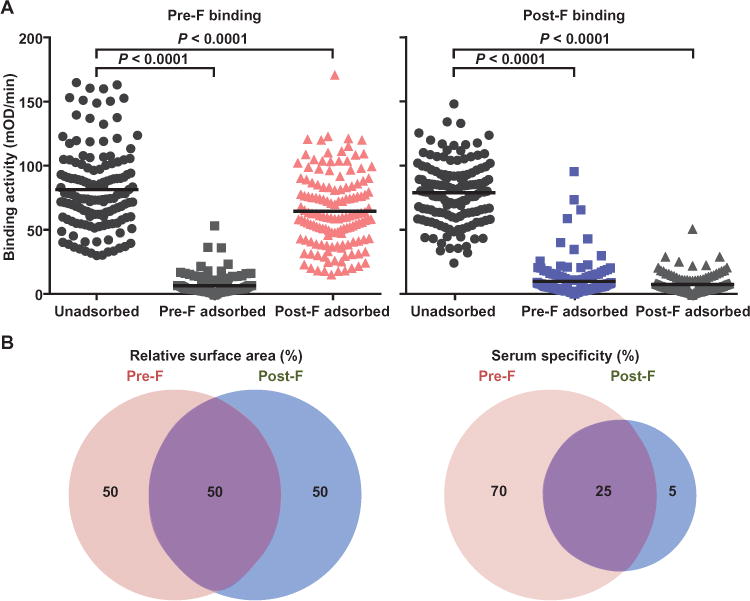
(A) Human sera were analyzed for binding to pre-F and post-F conformations by kinetic ELISA (black circles, left and right panels, respectively). Binding of unadsorbed sera to pre-F and post-F binding was similar (75.8 and 75.4 mOD/min, respectively). Sera were also analyzed for binding to either F conformation after adsorption with pre-F or post-F subunit proteins. As expected, pre-F adsorption removed antibodies that could bind to pre-F, and post-F adsorption removed antibodies that could bind post-F. Post-F adsorption did not remove a substantial amount of pre-F binding antibodies (pink triangles, left panel), but pre-F adsorption removed nearly all post-F binding antibodies (light blue squares, right panel). (B) Pre-F and post-F conformations share about 50% of their surface, and about 50% of the surface is unique to each. On the basis of the binding data, about 70% of antibodies in human sera bind pre-F–specific surfaces, about 25% bind the shared surfaces, and about 5% bind post-F–specific surfaces, which is disproportionate to the available surface area.
Adsorption of antibodies directed against pre-F removes NT activity from human sera
We assessed sera for their ability to block HEp-2 infection by RSV A2. Serum adsorption with pre-F removed nearly all serum NT activity, reducing the reciprocal median effective concentration (EC50) GMT from 511 to 9 (Fig. 2A, P < 0.0001, n = 121, Wilcoxon signed-rank test). Adsorption with post-F reduced serum NT activity by 29% (EC50 GMT from 511 to 362; P< 0.0001, Wilcoxon signed-rank test) (Fig. 2A). Therefore, these results suggest that pre-F–specific antigenic sites account for most NT antibody targets, and shared sites (I, II, and IV) on both F conformations must account for most of what is removed by the post-F adsorption. The pattern of pre-F and post-F adsorption on NT activity was consistent across all age groups (Fig. 2, B and C, and fig. S4). Despite the dependence of neutralization on pre-F–specific antibodies, we could not establish a correlation between overall pre-F binding and NT activity, and we therefore assessed binding to individual antigenic sites (Ø and II).
Fig. 2. Serum NT activity against RSV after adsorption with pre-F and post-F.
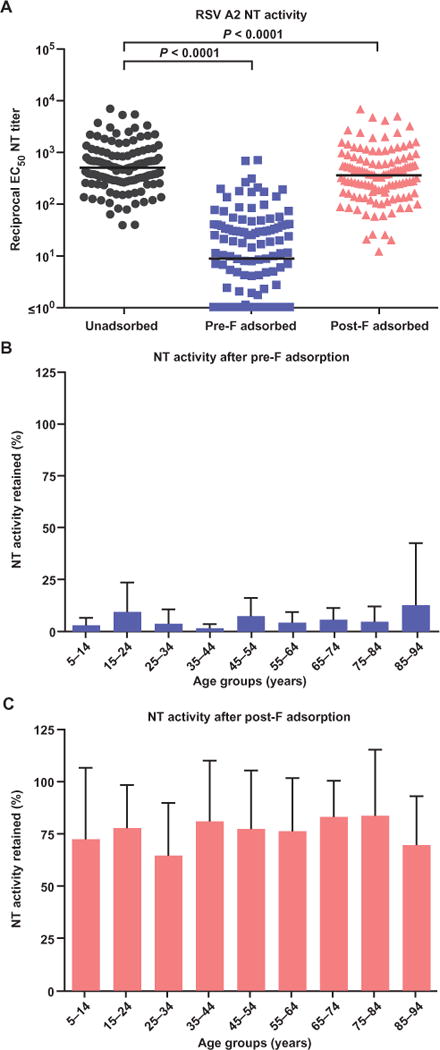
(A) Geometric mean reciprocal EC50 NT titers for unadsorbed sera and sera adsorbed with pre-F or post-F were 511, 9, and 362, respectively. (B) Evaluation of samples by age groups showed that less than 5% of NT activity was retained after adsorption with pre-F in all age groups. (C) Adsorption with post-F removed 20 to 45% of the original NT activity, and there were no trends according to age group.
RSV F knockout proteins serve as a platform to define antibody specificity
To evaluate the contribution of serum antibodies to specific antigenic sites on pre-F to the overall NT activity in sera, we designed knockout mutations that abolished mAb binding to antigenic sites Ø and II (Fig. 3 and table S2). These sites were chosen for analysis primarily because site Ø is pre-F–specific and known to induce potent NT antibodies, whereas site II is present on both F conformations and the target of the currently licensed mAb for prophylactic treatment, palivizumab. RSV F molecules that did not bind to antigenic site Ø–specific antibodies (D25, AM22, and 5C4) or site II–specific antibodies (motavizumab and palivizumab) were designed using two approaches: (i) addition of N-glycan sequons and (ii) mutation of critical contact residues. For N-glycan sequon design, surfaceexposed residues were identified on the RSV F molecule that were in close proximity to the D25 binding site (10) or the motavizumab binding site (18). N-Glycan sequon (Asn-X-Ser/Thr) mutations were modeled at these sites, and the resulting models were assessed. Three residue positions in RSV F antigenic site Ø and in antigenic site II were chosen on the basis of the prediction that they will allow N-glycan introduction with minimal clashes, minimal disruption of hydrogen bonding patterns, and a high likelihood of N-glycan addition as assessed by NGlycPred (19). In addition, four residues in antigenic site Ø and three residues in antigenic site II that displayed extensive interactions with either D25 or motavizumab and various mutations to these residues were modeled. Mutants that were chosen exhibited maximal disruption of antibody binding as judged by clash scores and charge incompatibility, and designated site Ø or site II knockout (KO). Octet biolayer interferometry (BLI) was used to assess binding of mAbs to the pre-F variants, and two mutants were chosen that fully disrupted site Ø or site II antibody binding while preserving the binding of mAbs to other pre-F–specific antigenic sites associated with NT activity (Fig. 3C). These mutations were also introduced to RSV F glycoproteins from a B-subtype strain (18537) to knock out binding of the site Ø–specific antibodies (Fig. 3C and table S2). Successful knockout of these sites enabled the analysis of site-specific serum neutralizing activity.
Fig. 3. Design and characterization of F constructs with mutated antigenic sites Ø and II.
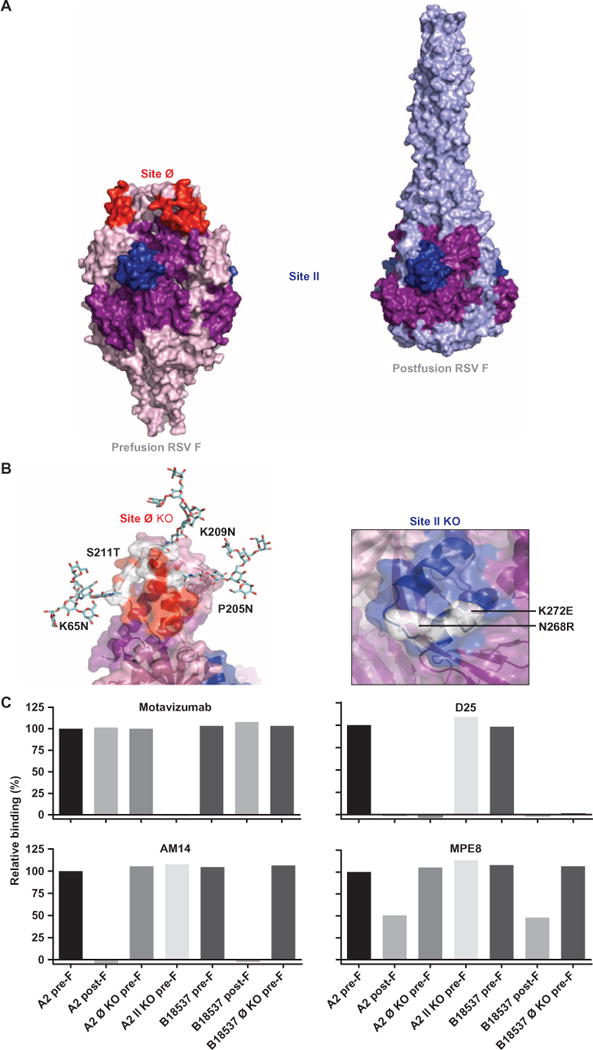
(A) The RSV fusion glycoprotein is shown in the stabilized pre-F (left) and post-F (right) forms in surface representation with antigenic sites Ø and II highlighted in red and dark blue, respectively. The purple surface shown on the post-F and the dark blue motavizumab epitope are common to the surface on pre-F as mentioned in Fig. 1B. (B) Close-up views of the antigenic sites Ø and II with the mutated residues are shown. The three nonnative glycans at residues 65, 205, and 209 introduced to block site Ø binding are shown in stick representation. The surface representation is set to 60% transparency with mutated residues shown in stick representation, with a single protomer shown for clarity. (C) BLI was used to measure binding by mAbs that bind pre-F: D25, motavizumab, AM14, and MPE8. Antigenic site Ø KO variant Ø-C (A2 strain) and Ø KO of the B18537 strain showed typical binding to all of the pre-F–targeting antibodies except D25, whereas antigenic site II KO variant II-E bound to all antibodies except motavizumab.
Antigenic site Ø is a major but not the sole target for NT antibodies exclusive to pre-F
The selected pre-F variants with mutations to sites Ø and II, along with wild-type pre-F and post-F, were used in neutralization competition assays to determine what fraction of the pre-F–specific NT activity adsorbed by pre-F could be attributed to sites Ø and II. Assays were performed on samples that exhibited NT titers at the 25th, 50th, 75th, and 95th percentiles within each 10-year age group. Stabilized RSV A2 pre-F, post-F, or pre-F with either site Ø or site II KO were incubated with heat-inactivated serum samples (n = 32) before treatment with virus. As observed with the adsorption assay, competition with stabilized wild-type pre-F inhibited >90% of serum NT activity, whereas competition with wild-type post-F removed <30% of NT activity (Fig. 4A). This difference between pre-F– and post-F–specific NT activity was consistent across all subjects. Competition with a site Ø KO pre-F restored 35% (GMT) of NT activity (Fig. 4A; median, 47%; n = 32), indicating that antibodies targeting the membrane-distal antigenic site Ø account for a substantial fraction of the overall NT activity in human sera (Fig. 4A). Consistent with these results, incubation of sera (n = 24) with pre-F and post-F of the B18537 strain before treatment with virus removed >85 and ~30% of NT activity, respectively. Competition with the site Ø KO pre-F of the B18537 strain restored ~47% of NT activity (Fig. 4B), suggesting the importance of unique pre-F antigenic sites across RSV subtypes in the generation of functional antibody response. In some individuals, nearly all the NT activity was restored when sera were competed with site Ø KO pre-F (Fig. 4C, red bars at the right end). However, in others, the NT activity was largely determined by pre-F–specific antigenic sites other than site Ø (Fig. 4C, extreme left end). These would be sites recognized by MPE8- or AM14-like antibodies or other as yet unidentified pre-F–specific antibodies. Sera competed with the RSV A2 site II KO pre-F had ~8% restoration of NT activity compared to wild-type pre-F–competed sera, suggesting that site II–specific antibodies account for <10% of RSV NT activity in human sera. Similar to site Ø, the relative contribution of site II–specific antibodies varied between individuals, but they contributed a minor fraction of NT activity in most individuals (Fig. 4C, dark blue bars).
Fig. 4. Serum NT activity against RSV after competition with stabilized pre-F, post-F, and pre-F with site Ø and site II KO.
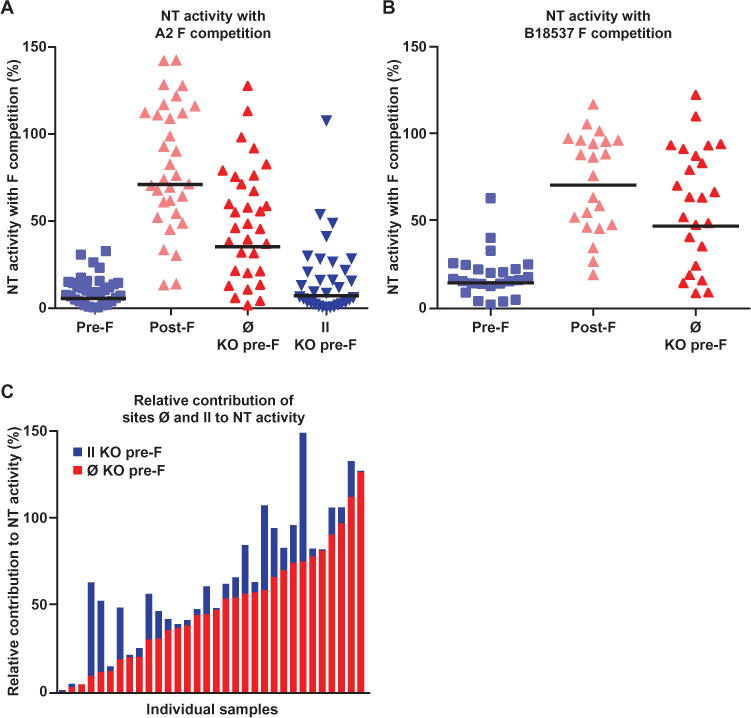
(A) Protein competition neutralization assays show that pre-F removes nearly all (>90%) NT activity from human sera, in contrast to post-F, which only minimally removed NT activity (<30%). This assay also showed that antibodies specific for site Ø present on pre-F account for ~35% of NT activity. (B) Consistent with the results obtained with the RSV A2 strain, protein competition neutralization assays with pre-F of the B18537 strain removes most (>85%) NT activity from human sera, while post-F removed only ~30% of NT activity. Antibodies specific for site Ø on the B18537 pre-F are shown to account for ~45% of NT activity. (C) Results from individual samples are arranged according to increasing site Ø activity in a stacked bar graph to show the relative amount of NT activity attributed to site Ø or site II. Site Ø–specific antibodies accounted for majority of the NT activity in most subjects (red bars). However, there was significant variation between individuals, and in some subjects, most of NT activity could not be attributed to either site Ø or II (bars on the extreme left) but was clearly pre-F–specific. Although in most individuals, site II antibodies account for some NT activity (dark blue bars), it was a small fraction of the overall activity.
Competitive binding assays confirm the presence of unique antigenic sites other than site Ø on pre-F
We next evaluated the fraction of pre-F–specific antibodies binding to individual epitopes by using selected mAbs (Fig. 5A and fig. S6) (D25 and motavizumab) to compete with serum binding to wild-type pre-F by the BLI technique. The assay was validated by six mAbs to RSV, and a cross-competition table was generated (Fig. 5A). We selected the mAbs D25 (site Ø) and motavizumab (site II) for serum antibody competition. The median level of serum antibody blocked by D25 alone was 40% (Fig. 5B). D25 blocked serum binding to a greater extent in higher-NT groups (that is, 75th and 95th percentile samples) than in lower-NT groups (25th and 50th percentiles). The fraction of inhibition by D25 was directly correlated to reciprocal log10 EC50 NT titers (r = 0.45, P = 0.0234, n = 25, Pearson’s correlation) (Fig. 5C). Motavizumab inhibited about 60% of serum antibody binding, even though antigenic site II antibodies account for <10% of NT activity. In this assay, pre-F–bound motavizumab prevented serum antibodies from binding not only to its epitope but also to proximal sites such as the MPE8 binding site. This explains the higher overall inhibition of serum antibody binding by motavizumab despite its lack of correlation with NT activity. There was an inverse correlation that did not reach statistical significance between the percent of serum antibody binding inhibited by motavizumab and reciprocal log10 EC50 NT titers (r= −0.26, P = 0.20, n = 25, Pearson’s correlation) (Fig. 5C), suggesting that there are non-NT antibodies blocked by motavizumab that would also be potentially inhibitory to other more potent MPE8-like NT antibodies in sera. Non-NT antibodies that compete with motavizumab binding to site II were also found in animals immunized with scaffolded motavizumab epitope immunogens (20).
Fig. 5. Octet BLI competition assay using D25 and motavizumab.
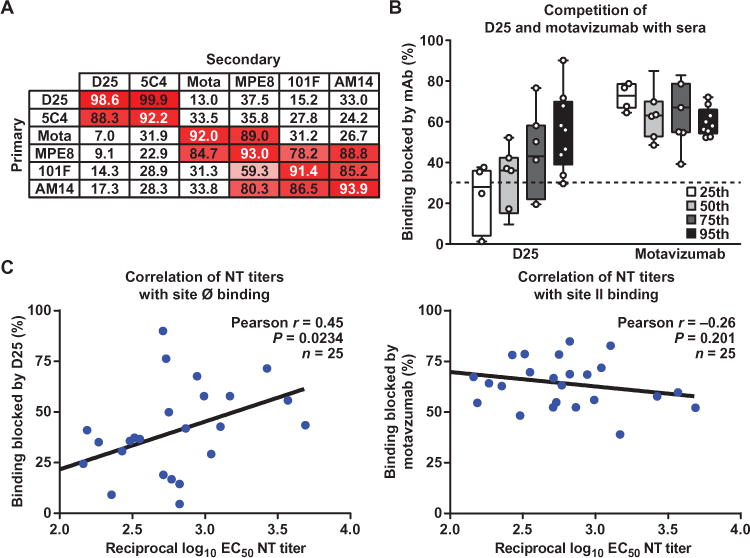
(A) In a BLI assay, biosensor tips were dipped in pre-F, then into analyte, and then into competing antibody. D25 does not inhibit motavizumab binding to pre-F and vice versa, making these mAbs eligible for use in serum analysis. (B) Biosensor tips were dipped into pre-F, then into D25 or motavizumab, and then into sera. The mean percent inhibition of serum antibody binding by D25 is ~40%, and that by motavizumab is ~60%. Unlike motavizumab, there is increasing inhibition by D25 with increasing NT titers. The dashed line represents background binding inhibition. (C) A moderate correlation is found between percent binding inhibited by D25 and reciprocal EC50 NT titers, and a weak inverse correlation was found between percent binding blocked by motavizumab and NT titers.
Adsorption of sera with pre-F permits RSV infection of human airway epithelium
Testing neutralization in an immortalized cell line such as HEp-2 is required for large-scale screening but may not completely reflect neutralization in the in vivo target cell. Primary well-differentiated human airway epithelial (HAE) cultures may be better for this purpose (21). RSV uses heparan sulfate as its receptor in immortalized cells (22), but HAE cultures, which display tight junctions, do not display detectable amounts of heparan sulfate on their apical surface where infection is initiated (23). To examine RSV neutralization in HAE cultures, we chose two sera with high levels of antibody to the RSV A2 G glycoprotein, matching the virus stock used for neutralization assays. Unadsorbed and pre-F adsorbed versions of both samples were evaluated for the presence of antibodies targeting the G glycoprotein associated with preventing RSV infection of HAE cells. The NT curves for unadsorbed and adsorbed sera were similar to the NT curves in HEp-2 cells, suggesting that antibodies to F neutralize at a similar level in both cell types (fig. S7). However, the shapes of the NT curves differed, with a steeper Hill slope for pre-F adsorbed than for unadsorbed sera, a difference particularly obvious in the HAE cultures. Overall, the NT activity that was not removed by pre-F adsorption and therefore potentially attributable to G was 2 to 3% in serum 1 and 14 to 20% in serum 11, further suggesting that antibodies to F are the primary determinants of NT activity, even in primary HAE cells.
DISCUSSION
Understanding the human immune response to natural infection can inform vaccine development because this enables an insight into what protein(s) must be included in a vaccine to generate protective immunity. An earlier study showed that in high-titer pooled human immunoglobulin, NT activity could not be removed with post-F, suggesting that pre-F–specific antibodies may be important in RSV neutralization (17). Here, we sought to determine the specificity of antibodies in individual human sera for the two major conformations of the RSV F glycoprotein. Therefore, a cross-sectional serological analysis was performed to evaluate the neutralization potency and specificity of sera from healthy volunteers ranging in age from 7 to 93 years. We found that unadsorbed sera had similar binding activity to both conformations of RSV F, which was not surprising because ~50% of the surface area of pre-F and post-F conformations is shared, including well-characterized antigenic sites II and IV to which the prototypic NT mAbs palivizumab/motavizumab and 101F bind, respectively (10). In contrast, the NT activity in human sera is nearly all removed by adsorption with pre-F, whereas only a small fraction is removed by adsorption with post-F. As noted, the moderately neutralization-sensitive antigenic sites II and IV are present on both conformations of F, whereas pre-F also displays the more neutralization-sensitive sites such as antigenic site Ø and the MPE8 and AM14 epitopes. Given the overlap of epitopes, post-F presentation—in the context of vaccination or natural infection—will induce a significant amount of antibodies that bind pre-F. This is consistent with our finding that there is no correlation between pre-F or post-F binding (or their ratio) and overall NT activity or NT activity within a particular age group. It is likely that the level of NT activity in human serum is a more complex function of epitope access, binding affinity and specificity for epitopes associated with different levels of neutralization, non-NT antibodies that may interfere with binding to more functionally relevant sites, and synergy or interference of different antibodies when multiple sites are targeted simultaneously. Therefore, a simple ELISA assay using pre-F or post-F will not be sufficient to predict the NT activity in serum.
The analysis of sera by competition with site Ø and site II KO probes demonstrated that there are diverse patterns of binding to the various sites associated with NT activity. Whereas a substantial amount of NT activity in most human sera can be accounted for by antigenic site Ø–specific antibodies, there are some individuals whose predominant response is to other pre-F–specific sites (Fig. 4C), because some samples that lost NT activity after competition with wild-type pre-F did not regain this activity when site Ø KO pre-F was used in competition. As the complete antigenic topology of pre-F and the antibody content of human serum become more fully defined, it may be possible to predict NT activity on the basis of the magnitude and composition of epitope-specific antibodies. About 10% of samples had an increase in NT activity after adsorption with post-F. One potential explanation for this phenomenon is that non-NT antibodies that recognize post-F surfaces may also bind pre-F and block the access of antibodies with more potent NT activity. For example, the abundance of antibodies binding to site II (Fig. 5B) or site IV with relatively little NT activity could potentially interfere with the access of potent pre-F–specific antibodies in sera with similar characteristics as MPE8 or AM14, which are both potent pre-F–specific mAbs. This may explain our finding that site II–specific antibodies in human sera had a 60% inhibition of pre-F binding when competed with site II–specific mAbs and yet accounted for <10% of NT activity.
A limitation of our analysis was the use of immortalized human epithelial cells (HEp-2) for the neutralization assay cellular substrate. Immortalized cells have a large amount of heparan sulfate on surface proteoglycans that act as a receptor for both the F and G proteins and diminish the need for attachment via G proteins. However, heparan sulfate is not present on the apical surface of primary well-differentiated HAE cultures, strongly suggesting that a different receptor is used by RSV to infect these cultures, and therefore, antibodies against the G protein may neutralize differently in HEp-2 and HAE cultures. We partially addressed this by selecting two subjects with high levels of G protein–specific binding (fig. S7). One subject had a low (2 to 3%) and the other a moderate (16 to 20%) NT activity remaining after adsorption with pre-F, which was likely attributable to G-specific antibodies. However, the overall pattern of neutralization was similar in both cell systems, indicating that antibodies against pre-F are the dominant NT antibodies in human sera. More individual sera will need to be tested on primary and immortalized cell lines to fully define the contribution of G protein–specific antibodies to RSV neutralization.
Addition of guinea pig complement to sera has been shown to increase RSV NT titers in sera of naturally infected adults (24). The adsorption and neutralization assays in this study were performed without and with heat inactivation (Fig. 2A and fig. S3, respectively). The level of NT activity in heat-inactivated samples (n = 117) was lower than that of serum not heat-inactivated (n = 121) (Fig. 2A and fig. S3). With or without heat inactivation of sera, the effect of removing antibodies specific to pre-F through adsorption and competition assays remained the same (Figs. 2A, 4, A and B, and fig. S3). In all age groups, including the 65-to 84-year-old cohort, NT activity was retained after sera were adsorbed with post-F, and a previous study showed that children, young adults, and the elderly had comparable levels of NT activity to RSV (25). Therefore, the cumulative impact of repeated RSV infections with multiple subtypes over the course of a lifetime could promote the maintenance of pre-F–specific NT antibodies. It is not known whether the susceptibility of humans to recurrent infection with RSV throughout life is related to the overall magnitude of NT activity, the specificity of antibodies mediating neutralization, or other factors involving innate or adaptive cellular responses.
Although natural infection with RSV occurs throughout life, young infants and children <5 years old are much more likely than healthy individuals >5 years old to require hospitalizations due to lower respiratory tract infections. We speculate that antibodies specific for pre-F account for most of the NT activity that limits infection to the upper airway in older children and adults. We therefore suggest that the pre-F protein may be better suited than post-F as a vaccine antigen on the basis of the observation that most NT activity induced by natural infection is pre-F–specific. This is of particular importance for target populations that have had prior natural infection where the intent is to boost serum NT activity, such as pregnant women or the elderly. In addition, there are neutralization-sensitive antigenic sites unique to pre-F, whereas post-F–binding antibodies may be distracted from the functional pre-F trimers in lieu of binding post-F that has no functional value to the virus except as a decoy, supported by the observation that adsorption with post-F resulted in an increase in serum NT activity in about 10% of individuals. These observations suggest that induction of pre-F–specific responses may be more favorable for antigen-naïve populations to prime for more functionally relevant antibody responses. As antibody epitopes on RSV F are further defined, it will be important to characterize the pattern of antibody specificities induced in children under 5 years of age experiencing primary or secondary RSV infections.
MATERIALS AND METHODS
Study design
With the stabilization of the pre-F conformation of RSV F and the availability of human serum samples, we sought to understand the antibody responses to RSV. All samples for this study were collected with informed consent of volunteers, and approval for this study was obtained under protocol number VRC 700 (Clinicaltrials.gov NCT01262079). All volunteers were at least 6 years of age, healthy, and ranged in age from 7 to 93 years. Of all samples collected, 140 were systematically selected to fill up nine 10-year age groups with at least 15 per age group, first through blinded selection from stock and later by randomization. Actual ages were concealed until results were obtained and analyzed for each individual subject.
Viruses and cells
Virus stocks were generated in HEp-2 cells cultured with 10% minimum essential medium (MEM) according to previously described methods (26). Briefly, T175 flasks of cells at 80% confluence were infected with 1 ml of RSV A2 or B18537 master stock and allowed to incubate on a shaker for 1 hour at room temperature (RT). Fifty milliliters of 10% MEM was then added to the adherent cultures, which were incubated at 37°C for an additional 4 days. When substantial syncytia had formed, cells were scraped loose from the flask and transferred to 50-ml tubes for sonication (six 1-s bursts) and centrifugation at 4°C and 1000 rpm for 15 min. The supernatant containing virus was aliquoted into dram vials, quick-frozen in an alcohol–dry ice slurry, and stored at −80°C.
Serum adsorption assay
Each serum sample (300 μl) was diluted 1:9.7 in 1× phosphate-buffered saline (PBS) and split into three parts. Twenty microliters of pre-F or post-F (0.5 mg/ml) expressed from an RSV A2 construct using previously described methods (9, 14) was added to 970 μl of diluted samples, whereas the positive control, 970 μl of diluted unadsorbed serum, was further diluted 1:10 with 1× PBS. All samples were then incubated at RT for 2 hours, and positive control samples were stored at 4°C until use. To F-treated samples, 10 μl of reconstituted Strep-tag II mAb at 0.5 mg/ml (GenScript) was added, and the mixture was placed on a 360° rotator at 4°C for 2 hours. Eighty microliters of sheep anti-mouse immunoglobulin G Dynabeads (2 mg/ml) (Life Technologies) was washed twice with wash buffer [1% bovine serum albumin (BSA), 2 mM EDTA in 1× PBS (pH 7.4)]. The wash buffer was separated from the beads using a DynaMag-2 magnet (Life Technologies). The Sera-RSV F–strep tag mAb mixture was added to corresponding labeled tubes containing Dynabeads and incubated for 1 hour at 4°C on a rotator. The beads were separated from supernatant using DynaMag-2; the supernatant was stored at 4°C.
Kinetic ELISA
Plates (96-well) were coated with 100 μl of stabilized pre-F (1 μg/ml) or post-F (1 μg/ml) diluted in 1× PBS (pH 7.4) and incubated overnight at 4°C. The plates were washed with wash buffer (0.2% Tween 20 in 1× PBS) and had 200 μl of 2% BSA (in 1×PBS) added per well as a blocking buffer. Plates were then incubated for 1 hour at RT. Three tubes containing 100 μl (1:10 dilution) of unadsorbed serum, serum adsorbed with pre-F, and serum adsorbed with post-F were added to pre-F– and post-F–coated wells. The plates were incubated for 1 hour at RT, washed, and coated with 100 μl of goat anti-human IgG (1:5000, Santa Cruz Biotechnology). After 1 hour of incubation, the plates were washed, and 100 μl of Super AquaBlue substrate (eBioscience Inc.) was added to each well. An ELISA plate reader (Molecular Devices LLC) was used to read signals at 538-nm wavelength.
Neutralization assay
Fourfold dilutions were done on unadsorbed and pre-F– and post-F–adsorbed serum samples (with or without heat inactivation) starting at 1:10 through 1:40,960 with 45 μl in each well. Each dilution was mixed with 45 μl of diluted RSV strain A2 carrying the Katushka fluorescent protein mKate (27). The mixture was incubated for 1 hour at 37°C. Fifty microliters of the serum dilution/virus mixture was then added to HEp-2 cells that had been seeded at a concentration of 1.8 × 104 cells in 30 μl of 10% MEM in each well of 384-well black clear-bottom plates. The plates were incubated for 22 to 26 hours at 37°C before spectrophotometric reading at 588-nm excitation and 635-nm emission (Molecular Devices LLC).
Production of KO RSV F proteins
RSV F mutations were carried out by site-directed mutagenesis using Quik-Change (Stratagene), as was the addition of the AviTag sequence at the C terminus of the RSV F protein. Expi293F cells were transiently transfected with plasmids expressing RSV F site Ø and site II KO pre-F constructs and grown in suspension. The culture supernatants were harvested 5 days after transfection and centrifuged at 10,000 rpm to remove cell debris. The culture supernatants were sterile-filtered, and RSV F glycoproteins were purified by a Ni2+–nitrilotriacetic acid resin (Qiagen) and a Strep-Tactin resin (Novagen). Relevant fractions containing the RSV F variants were pooled, concentrated, and subjected to size-exclusion chromatography. Fractions corresponding to the trimer peak (fig. S5) were concentrated, and frozen at −80°C.
Assessment of KO RSV F proteins
RSV F KO proteins with an introduced N-glycan sequon were initially examined by SDS–polyacrylamide gel electrophoresis to assess whether the novel glycan had been added to the RSV F protein and whether the glycan addition was uniform. RSV F KO proteins that expressed the glycan (9 of 10) were assessed for binding to antigen-binding fragments (Fabs) specific for site Ø or site II by BLI with an Octet Red384 (FortéBio Corp.). Assays were performed with agitation at 1000 rpm in PBS (100 μl per well) supplemented with 1% BSA to minimize nonspecific interactions at 30°C in solid black 96-well plates (Greiner Bio-One). Anti-mouse Fc probes were sequentially incubated for 300 s with Strep-tag II mAb (35 μg/ml) in PBS buffer, and in RSV F KO proteins that bound via their C terminus (location of the Strep-tag II purification tag). Typical capture levels were between 0.7 and 1 nm, and variability within a row of eight tips did not exceed 0.1 nm for each of these steps. Biosensor tips were equilibrated for 300 s in 1% BSA in 1× PBS before measuring association with Fabs in solution (1 μM) for 300 to 600 s followed by dissociation for 100 to 600 s.
Competition neutralization assay
A total of 32 samples with NT titers closest to the 25th, 50th, 75th, and 95th percentiles for each age group were randomly selected. Fourfold dilutions were done on uncompeted sera, and sera mixed with 6 mg of different versions of RSV A2 F (starting concentration of sera, 1:10): stabilized pre-F, post-F, site Ø KO pre-F, and site II KO pre-F (starting concentration of F constructs, 0.1 μg/ml). Dilutions were done through 1:40,960 with 45 μl in each well. Plates were incubated for 1 hour at 37°C, after which 45 μl of diluted RSV A2 virus was added to each well. This was followed by another hour of incubation at 37°C. Fifty microliters of serum-virus or of serum-F protein–virus mixture was added to 384-well black clear-bottom plates that had been seeded with HEp-2 cells at 1.8 × 104 in 30 μl of 10% MEM, and plates were incubated for 22 to 26 hours at 37°C, followed by NT assessment as previously described. Competition neutralization assays were also performed with different versions of RSV B18537 F: stabilized pre-F, post-F, and site Ø KO pre-F for n = 24 samples as described above. Both virus strains used in these assays expressed the Katushka fluorescent protein, mKate.
Biolayer interferometry
Inhibition of serum antibody binding to pre-F–specific antigenic sites on the pre-F trimer was carried out using the Octet Red384 instrument (FortéBio Corp). The RSV pre-F trimer was loaded onto HIS biosensors through a polyhistidine tag for 300 s in 1% BSA in 1× PBS. Typical capture levels were between 0.9 and 1.2 nm, and variability within a row of eight tips did not exceed 0.2 nm. Biosensor tips were then equilibrated for 60 s in 1% BSA in 1× PBS before capturing competing mAbs. Pre-F was diluted to 30 μg/ml and mAbs D25, motavizumab, and isotype control VRC01 were diluted to 35 μg/ml in the same buffer. Binding of competing mAbs was assessed for 300 s followed by a baseline equilibration for 120 s in buffer before dipping into serum samples. Sera were diluted 1:50 in 1% BSA in 1× PBS and binding was assessed for 300 s. After the pre-F load was normalized, serum antibody binding was calculated by subtracting the binding of competing mAbs. Percent inhibition of serum antibody binding to pre-F by competing mAbs was determined by the following equation: inhibition (%) = 100 − [(serum antibody binding in the presence of competitor mAb)/(serum antibody binding in the presence of isotope mAb)] × 100. All the assays were done twice at 30°C, and the values reported were the average of two independent experiments.
Statistical analysis
Comparisons between unadsorbed and adsorbed serum samples (Figs. 1A and 2A) were done using the Wilcoxon signed-rank test. Thirty-five samples were selected from all 140 for further analysis by protein and antibody competition assays. Four samples were chosen within each age group that represented the 25th, 50th, 75th, and 95th percentiles of the geometric mean for the corresponding group, which allowed a spread-out of EC50. Thirty-two samples were analyzed in the RSV A2 competition neutralization assays, 24 were analyzed in the RSV B18537 competition neutralization assays, and 25 were analyzed in the competition binding assays. Correlations between continuous variables were assessed using Pearson’s correlation. All P values were compared to a two-sided α level of 0.05. All statistical analyses were done with GraphPad Prism v6.
Supplementary Material
Fig. S1. Adsorption assay validation.
Fig. S2. RSV F–specific binding activity in human sera by decade.
Fig. S3. Neutralization activity with heat inactivation of sera.
Fig. S4. Neutralization activity in human sera by decade.
Fig. S5. Characterization of RSV A2 site Ø and site II KO pre-F proteins.
Fig. S6. Analysis of RSV F–specific mAbs by binding competition.
Fig. S7. NT activity in HEp-2 versus primary HAE cells.
Table S1. ELISA assay readings to validate adsorption assay and show negligible residual pre-F/post-F and Strep-tag mAb in sera after adsorption.
Table S2. RSV F antigenic site Ø and site II KO variant mutations, production, and characterization.
Table S3. HAI assay shows that RSV F proteins did not non-specifically remove influenza-specific antibodies in human sera.
Acknowledgments
We thank all members of the Viral Pathogenesis Laboratory, the Biodefense and Structural Biology Sections, and the Clinical Trials Program of the National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center, NIH, as well as the study sites and participants that provided serum samples for the analysis. The RSV A2 construct used in this study was derived from a recombineering system for producing molecular clones of RSV provided by A. Hotard Lopez-Ona and M. Moore from Emory University. We also thank M. Teng (University of South Florida) for the RSV expressing Renilla luciferase (RSV-Luc) used in the neutralization studies in HAE cultures, and B. Hartman of the NIH Vaccine Research Center for preparing the manuscript for submission.
Funding: Funding for the VRC 700 trial was provided by the American Recovery and Reinvestment Act of 2009 (Recovery Act) through contract #HHSN2722010000491 awarded to the EMMES Corporation by the NIH.
Footnotes
Author contributions: J.O.N., M.C., K.M., and B.S.G. planned the study. J.O.N. performed adsorption, binding, and neutralization assays and prepared working RSV stocks for this study. A.K. purified and expressed native RSV A2 pre-F and post-F proteins. J.O.N., M.K., and A.M.K. performed BLI experiments. H.M.Y. designed and performed the hemagglutinin assay. S.M.M. engineered and made the reporter RSV A2 master stock. M.C.N. was our consulting statistician, and M. Enama and J.E.L. made human samples available for this study. A.K., K.M., J.S.M., and M.G.J. expressed and purified the mAbs used for the competition binding assays. RSV A2 and B18537 pre-F knockout proteins were designed and characterized by J.S.M., M.G.J., G.B.E.S.-J., G.-Y.C, I.S.G., and P.D.K.; expressed (RSVA2) by Y.Y., B.Z., A.D., and J.O.N.; and purified (RSV A2) by M.G.J., G.B.E.S.-J., M.S., E.J.R., A.K., and J.O.N. J.O.N. and A.K. expressed and purified RSV B18537 pre-F, post-F, and site Ø KO pre-F proteins. C.C. and M.E.P. performed and analyzed neutralization in the HAE cultures and were supported by grants AI095684 and AI093848 from the NIH. J.O.N., M.C., M.K., and B.S.G. analyzed the data; J.O.N., K.M., and B.S.G. wrote the manuscript with contributions from M.G.J., M.K., and M.E.P. All authors reviewed and edited the manuscript.
Competing interests: M.C., J.S.M., M.G.J., M.K., G.B.E.S.-J., G.-Y.C., I.S.G., M.S., Y.Y., B.Z., P.D.K., and B.S.G. are named as inventors on a patent pending entitled “Prefusion RSV F proteins and their use,” WO 2014160463.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/7/309/309ra162/DC1
Methods
REFERENCES AND NOTES
- 1.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson LJ. Respiratory syncytial virus vaccine development. Semin Immunol. 2013;25:160–171. doi: 10.1016/j.smim.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Melero JA, Moore ML. Influence of respiratory syncytial virus strain differences on pathogenesis and immunity. Curr Top Microbiol Immunol. 2013;372:59–82. doi: 10.1007/978-3-642-38919-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldera LJ, González-Reyes L, García-Barreno B, Wharton SA, Skehel JJ, Wiley DC, Melerob JA. Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology. 2000;271:122–131. doi: 10.1006/viro.2000.0279. [DOI] [PubMed] [Google Scholar]
- 6.Rogovik AL, Carleton B, Solimano A, Goldman RD. Palivizumab for the prevention of respiratory syncytial virus infection. Can Fam Physician. 2010;56:769–772. [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor G, Stott EJ, Furze J, Ford J, Sopp P. Protective epitopes on the fusion protein of respiratory syncytial virus recognized by murine and bovine monoclonal antibodies. J Gen Virol. 1992;73:2217–2223. doi: 10.1099/0022-1317-73-9-2217. [DOI] [PubMed] [Google Scholar]
- 8.Magro M, Andreu D, Gómez-Puertas P, Melero JA, Palomo C. Neutralization of human respiratory syncytial virus infectivity by antibodies and low-molecular-weight compounds targeted against the fusion glycoprotein. J Virol. 2010;84:7970–7982. doi: 10.1128/JVI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. 2011;85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, Guarino B, Silacci C, Marcandalli J, Marsland BJ, Piralla A, Percivalle E, Sallusto F, Baldanti F, Lanzavecchia A. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501:439–443. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 12.Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, van Geelen CMM, Lukens MV, van Bleek GM, Widjojoatmodjo MN, Bogers WMJM, Mei H, Radbruch A, Scheeren FA, Spits H, Beaumont T. Generation of stable monoclonal antibody–producing B cell receptor– positive human memory B cells by genetic programming. Nat Med. 2010;16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilman MS, Moin SM, Mas V, Chen M, Patel NK, Kramer K, Zhu Q, Kabeche SC, Kumar A, Palomo C, Beaumont T, Baxa U, Ulbrandt ND, Melero JA, Graham BS, McLellan JS. Characterization of a prefusion-specific antibody that recognizes a quaternary, cleavage-dependent epitope on the RSV fusion glycoprotein. PLOS Pathog. 2015;11:e1005035. doi: 10.1371/journal.ppat.1005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang GY, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko SY, Todd JP, Rao S, Graham BS, Kwong PD. Structure-based design of a fusion glyco-protein vaccine for respiratory syncytial virus. Science. 2013;342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce TG, BG Mellen, EF Mitchel, Jr, PF Wright, MR Griffin. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 16.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 17.Magro M, Mas V, Chappell K, Vázquez M, Cano O, Luque D, Terrón MC, Melero JA, Palomo C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci USA. 2012;109:3089–3094. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol. 2010;17:248–250. doi: 10.1038/nsmb.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang GY, Boyington JC, Joyce MG, Zhu J, Nabel GJ, Kwong PD, Georgiev I. Computational prediction of N-linked glycosylation incorporating structural properties and patterns. Bioinformatics. 2012;28:2249–2255. doi: 10.1093/bioinformatics/bts426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLellan JS, Correia BE, Chen M, Yang Y, Graham BS, Schief WR, Kwong PD. Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus. J Mol Biol. 2011;409:853–866. doi: 10.1016/j.jmb.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol. 2000;74:10508–10513. doi: 10.1128/jvi.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol. 2005;79:1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoder SM, Zhu Y, Ikizler MR, Wright PF. Role of complement in neutralization of respiratory syncytial virus. J Med Virol. 2004;72:688–694. doi: 10.1002/jmv.20046. [DOI] [PubMed] [Google Scholar]
- 25.Cherukuria A, Pattona K, Gasser RA, Jr, Zuoa F, Wooa J, Esserc MT, Tanga RS. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol. 2013;20:239–247. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 27.Hotard AL, Shaikh FY, Lee S, Yana D, Teng MN, Plemper RK, Crowe JE, Jr, Moore ML. A stabilized respiratory syncytial virus reverse genetics system amenable to recombination-mediated mutagenesis. Virology. 2012;434:129–136. doi: 10.1016/j.virol.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Adsorption assay validation.
Fig. S2. RSV F–specific binding activity in human sera by decade.
Fig. S3. Neutralization activity with heat inactivation of sera.
Fig. S4. Neutralization activity in human sera by decade.
Fig. S5. Characterization of RSV A2 site Ø and site II KO pre-F proteins.
Fig. S6. Analysis of RSV F–specific mAbs by binding competition.
Fig. S7. NT activity in HEp-2 versus primary HAE cells.
Table S1. ELISA assay readings to validate adsorption assay and show negligible residual pre-F/post-F and Strep-tag mAb in sera after adsorption.
Table S2. RSV F antigenic site Ø and site II KO variant mutations, production, and characterization.
Table S3. HAI assay shows that RSV F proteins did not non-specifically remove influenza-specific antibodies in human sera.


