Abstract
Our laboratory has previously demonstrated that melanoma draining lymph node (MDLN) samples from stage III patients contained both CD4+ and CD8+ T cells that can be readily expanded to mediate tumor cell apoptosis in vitro and improve survival in mice bearing human melanoma xenografts. In this study, we investigated whether MDLN T cells contain melanoma-reactive CD4+ T cell compartment and what they are. In order to test this, we performed multi-parametric (11-color and 6-color) FACS analyses to monitor phenotypic and functional property of CD4+ T cells in response to melanoma cell antigen re-exposure. Our results have demonstrated that the antigen re-exposure could result in a generation of CD4+CCR7+CD62L+CD27− T cell subsets with various effector cell-like properties. Within the CD4+CCR7+CD62L+CD27− T cell compartment, in response to antigen re-exposure, some of the cells expressed significantly up-regulated CD40L and/or CXCR5, and some of them expressed significantly up-regulated IL-2 and/or TNF-α. This may suggest the existence of melanoma reactive CD4+ “effector-precursor” cells within the expanded MDLN cells and their differentiation into various effector lineages in response to antigen re-stimulation. Recent clinical trials have demonstrated that effective adoptive cellular immunotherapy (ACI) maybe enhanced by antigen specific CD4+ T cells. Therefore, results of this study may significantly benefit innovative design of ACI that can potentially mediate enhanced and durable clinical responses.
Keywords: Tumor draining lymph node (TDLN), Adoptive immunotherapy, antigen-experienced T cells, multi-color FACS, CD4 T cells
Introduction
Recent clinical trials have demonstrated that adoptive cellular immunotherapy (ACI) using antigen specific T cells can result in regression of bulky metastatic disease in patients with malignant melanoma (1). Despite the antitumor efficacy, clinical trials have demonstrated difficulty with long-term persistence of the transferred T cells, which may impact the ability to generate memory immune responses, which correlate to the most durable clinical responses (2). Recently, studies have shown that the effective ACI maybe enhanced by the use of antigen specific CD4+ T cells. For examples, patients who received infusion of primarily CD4+ tumor-infiltrating lymphocytes (TILs) demonstrated significant regression of diffuse metastatic disease (3,4); patients who were treated with melanoma-specific CD4+ T cells clones were observed with regression of metastatic melanoma (5). CD4+ T cells are known to play an important role in shaping adaptive immune response, the fundamental of which is to establish long-lived T cells that are ready-to-react upon second encounter with the specific antigens (6). Therefore, study of antigen specific CD4+ T cells is very important. It will not only advance our understanding of adaptive immunity but also lead to innovative design of ACI that can potentially mediating enhanced and the most durable clinical responses.
As an IRB-approved protocol (CASE3610), we have recently investigated melanoma draining lymph nodes (MDLNs) from 25 patients with stage III malignant melanoma (7, 8). Our results have demonstrated that the MDLN samples contain antigen specific CD4+ and CD8+ T cells that could mediate melanoma cell apoptosis in vitro and improve mice survival in vivo (7, 8). MDLN is secondary lymphoid organ where both CD4+ and CD8+ T cells experience melanoma antigens in vivo in the presence of APCs, i.e., dendritic cells (DCs) and relevant cytokines (9). Such the environment has been shown to be able to promote T cell priming, differentiation into effector phase, and survival of contraction phase and development of a memory state (9, 10). Therefore, MDLN may represent an excellent T cell resource to investigate antigen specific CD4+ T cells.
In this study, we applied multi-parametric FACS analysis to monitor phenotypic and functional properties of various CD4+ T cell subsets within MDLNs before and after melanoma antigen re-exposure. Our results identified a subset of CD4+ T cells that exhibited significantly increased surface markers and intracellular cytokine production in response to melanoma cell antigen re-exposure. Identification of tumor-reactive CD4+ T cells will not only advance current understanding of immune memory but also lead to innovative design of ACI that can potentially mediate enhanced and/or durable immune responses for cancers.
Methods
Culture and dissociation media
Unless otherwise stated, all cell cultures were maintained in complete medium (CM) comprising AIM-V medium (AIM-V Media, Gibco, Grand Island, NY) supplemented with 5.0% pooled human AB serum (Innovative Research, Novi, MI).
Patients and MDLN processing
All patients had informed consent on a protocol that was approved by the Case Western Reserve University IRB (Case 3610). Adult patients underwent completion lymphadenectomy of the lymph node basin draining the primary cutaneous melanomas as part of their standard care for stage III melanoma. Portions of MDLNs were removed from the lymphadenectomy specimen and used for this study. MDLN samples were washed twice with sterile PBS. After fat tissue was trimmed, MDLNs samples were cut into pieces with a surgical scalpel and then mechanically separated creating a single cell suspension. Cells were washed twice with CM and counted using a cytometer and Trypan-blue exclusion to determine yield and viability. T cell activation and expansion were performed according to our previous publication (7, 8). Briefly, freshly harvested MDLN cells were incubated overnight with CM containing 30 I.U. of human recombinant IL-2 (Invitrogen™) prior to cryopreservation. After thawing, cultures of MDLN cells started with 2 × 106 cells/well in a 24-well plate (2mL CM per well) at 1×106 cells/mL. Anti-CD3/anti-CD28 beads (Dynal AS, Oslo, Norway) were added according to manufacturer’s instructions as well as IL-2 100 I.U./ml. Cells were split to a concentration of 0.25×106 cells/mL by adding fresh CM and IL-2 on days 4, 7 and 11. Cells were cultured in a humidified 5% CO2 incubator at 37°C.
Cancer Cell Lines
Human cancer lines A375 (melanoma), U87 (glioma) and MDA-MB-231 (breast cancer) were obtained from Dr. Bingcheng Wang, Case Western Reserve University, Cleveland, OH. Cells were cultured in DMEM medium with 5% FBS (Gibco).
Selection of patients’ MDLN samples and tumor lines for tumor cell antigen re-exposure
In order to dissect tumor-reactive CD4+ T subsets within ex vivo expanded MDLN cells, we needed to monitor CD4+ cells that react to tumor antigen re-exposure. The re-stimulation of day-14 MDLN cells with melanoma antigen overlapping peptides has been conducted in our previous study and the results indicated that the MDLN CD4+ T cells could mediate diversified response to a broad range of antigens (7). Because of that, we determined to use whole tumor cells to re-stimulate MDLN cells as that whole tumor cells carry multiple and diversified antigens. Ideally we would re-stimulate MDLN T cells with autologous tumor cells. However, the MDLN samples investigated in our study were from patients with stage III melanoma, most of whom had the primary cutaneous melanoma previously resected and had no evidence clinically-evident melanoma in the regional lymph nodes. Because of this limitation, we had chosen to perform HLA typing (HLA-A, -DR and –DQ) for ten patients’ MDLN T cells and nine tumor cell lines in previous study (7). Our results of HLA-typing demonstrated that the patient 1–4 exhibited high degree of MHC-matching (50–87.5%) to A375 melanoma cells and low or no degree of MHC-matching to both U87 brain cancer cells (0–25%) and MDA-MB-231 breast cancer cells (12.5–25%) (Table 1). Since it is unlikely that all MHC molecules of patients’ MDLN T cells match those of the allogeneic tumor cell lines used in the study, we have aimed to identify tumor-reactive CD4+ T subsets that responded to high degree MHC-matching A375 significantly different from that responded to low/no degree MHC-matching U87 and MDA-MB-231. In other words, the low/no degree MHC-matching tumor cells served as control for allogeneic responses (Table 1).
Table 1.
Patients 1–4 show high degree MHC-matching to A375 and low/no degree MHC-matching to U87 and MDA-MB-231.
Co-culture of day-14 MDLN cells with medium, A375, U87 and MDA-MB-231
The day-14 MDLN cells were co-cultured with medium for 72 hours, which served as non-stimulated control. The day-14 MDLN cells were co-cultured with A375, U87 and MDA-MB-231 cells at effector : target = 2 : 1 for 72 hours. The T cell co-cultures with U87 and MDA-MB-231 served as controls for allogeneic responses. At the end of co-cultures, total numbers of cells was determined by cell counting using trypan blue. The cells were also stained and analyzed by FACS to quantify percentage of T cells based on the significant size difference from tumor cells. Therefore, total number of T cells in the co-cultures could be calculated by the following formula:
Phenotypic 11-color FACS analysis of MDLN cultures
At the end of co-culture, cells were stained and analyzed by 11-color FACS to monitor significant changes of various CD4+ subsets. 11-color FACS analysis was developed to characterize surface expression of CD4, CD8, CD25, CD69, CD185 (CXCR5), CD154 (CD40L), CD62L, CCR7, CD27, CD279 (PD-1) and Tim-3. Design of 11-color FACS and significant function of the markers of interest are shown in Table 2. The mouse anti-human fluorescence-conjugated antibodies for 11-color analysis were purchased from BD Biosciences. The staining panel consisted of 11 different antibodies with different fluorescent markers of various staining index was shown in Table 2. Cells were incubated with mouse IgG (BD Biosciences) and mouse anti-human Fc antibodies for 30 minutes, respectively, to block non-specific binding. After that, cells were washed and stained using cocktail containing antibodies listed in 11-color staining panel. All data were acquired on an 18-color LSR II flow cytometer equipped with four diode lasers (405nm, 488nm, 532nm and 640nm), and modified with optimal bandpass and dichronic filters (BD Biosciences). All data were acquired in FCS file format (FACia) and analyzed using Winlist 3D 7.1 Software (Verity House Software). Computer-assisted digital compensation was done using single color staining controls via the Hyperlog transform algorithim (Verity House Software) (11, 12). Fluorescence minus one controls were used to set hinged-gating and define histogram regions that distinguished positive from negative events for each fluorescent parameter (13, 14). Fidelity controls ensured that there was no loss of staining frequency and intensity between lower order panels and the corresponding fluorescence for each monoclonal antibody (mAb) in the 11-color panel.
Table 2.
Design of 11-color FACS to quantify surface markers with significant T cell functions.
| Laser | Dye | Stain Index | Surface marker | Significant function |
|---|---|---|---|---|
| Violet laser _405nm | BD Horizon V450 | 65 | CD25 | T cell activation and proliferation |
| BD Horizon V500 | 27 | CD8 | T cell marker | |
|
| ||||
| Blue laser_488nm | AF488 | 43 | CD185(CXCL5) | Activation of B cells |
| PerCP | 99 | Tim-3 | Tim3+PD-1+ defines exhausted T cells | |
|
| ||||
| Green laser_561nm | PE | 305 | CD62L | L-selectin marker |
| PE-Cy5 | CD154(CD40L) | Activation of dendritic cells | ||
| PE-Cy7 | 122 | CD197(CCR7) | Lymphatic tissue homing marker | |
| PE-Cy5.5 | CD4 | T helper cell marker | ||
|
| ||||
| Red Laser_640nm | APC | 184 | CD279 (PD-1) | Tim3+PD-1+ defines exhausted T cells |
| AF700 | 64 | CD27 | Antigen experiencing | |
| APC-H7 | 25 | CD69 | T cell Proliferation | |
Intracellular cytokine functional 6-color FACS analysis of MDLN cultures
Mouse anti-human fluorescence-conjugated antibodies for 6-color FACS analysis were purchased from BD Biosciences. The staining panel consisted of 6 different antibodies with different fluorescent markers of various staining index was listed in Table 3. Cells were incubated with mouse IgG (BD Biosciences) and mouse anti-human Fc antibodies, respectively, for 30 minutes to block non-specific binding. After that, cells were washed and staining with fluorescence-conjugated CD4, CD62L, CCR7 and CD27 antibodies followed by permeabilization using fix/perm buffer (Invitrogen). The permeabilized cells were further stained with fluorescence-conjugated IL-2 and IL-4, or TNF-α and IFN-γ for 30 minutes and then analyzed by FACS. Data acquisition, analyses and compensation were performed as described in 11-color FACS analysis. Briefly, the day-14 MDLN cells co-cultured with medium, A375, U87 and MDA-MB-231 were respectively stained using 6-color staining panel (Table 3), and then analyzed by FACS to quantify intracellular cytokine production in response to different tumor cell antigen re-exposure.
Table 3.
Design of 6-color FACS to quantify intracellular cytokines with Th1 and Th2 functions.
| Laser | Dye | Stain Index | Marker Group 1 | Marker Group 2 |
|---|---|---|---|---|
| Violet laser _405nm | BD Horizon V450 | 65 | IL-2 (Th1) | |
| BD Horizon V500 | 27 | CD62L | CD62L | |
|
| ||||
| Blue laser_488nm | AF488 | 43 | CD4 | CD4 |
| PerCP | 99 | IFN-γ (Th2) | ||
|
| ||||
| Green laser_561nm | PE | 305 | CCR7 | CCR7 |
| PE-Cy7 | 122 | TNF-α (Th1) | ||
|
| ||||
| Red Laser_640nm | AF700 | 64 | CD27 | CD27 |
| APC | 25 | IL-4 (Th2) | ||
Results
The 14-day culture process resulted in an expansion of non-regulatory CD4+ T cells
Increasing evidence has shown that regulatory T cells can mediate immune suppression (15), and PD-1+ T cells can result in apoptosis of T cells following engagement with tumor expressing PD L-1 (16). In order to determine whether our previously developed culture process resulted in generation of any regulatory and exhausted CD4+ T cells, we tracked the dynamic growth of CD4+, CD4+Tim3+PD-1+ and CD4+FoxP3+ subsets during the 14-day culture process. The CD4+FoxP3+ subsets have been used to define regulatory T cells (15) and the Tim3+PD-1+ to define exhausted T cells (16).
FACS analyses were performed to quantify percentage of CD4+ and CD8+ T cells in 14 patients day-0, -7, -11 and -14 MDLN cultures (FACS histograms not shown). Average percentages of CD4+ and CD8+ T cells within MDLN cultures of 14 patients during the ex vivo expansion were shown in Table 4. Total numbers of CD4+ and CD8+ were calculated by multiplying the total number of MDLN cells with the average percentages of CD4 and CD8, respectively, and shown in Table 4. The kinetic percentage and total number of CD4+ and CD8+ T cells during the culture expansion demonstrated that both CD4 and CD8 T cells proliferated during the 14-day culture, and the CD4+ T cells proliferate at higher expansion rate than the CD8+ T cells as that the CD4/CD8 ratio increases during the entire culture.
Table 4.
Kinetic growth of MDLN cultures and several T cell subsets. n = 14.
| MDLN | CD4 | CD8 | CD4/CD8 ratio | % CD4+Tim3+PD-1+ | % CD4+FoxP3+ * | % CD4+CD25+FoxP3+ * | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Cell number/million | Expansion rate | Cell number/million | Expansion rate | Cell number/million | Expansion rate | |||||
| Day-0 | 12 ± 2.1 | n.a. | 4.8 ± 1.9 | n.a. | 3.6 ± 1.6 | n.a. | 1.3 ± 0.7 | 0.9 % ± 0.6% | 3.2 % ± 3.3% | 0.5 % ± 0.3% |
| Day-7 | 108 ± 16 | 9.0 | 43.2 ± 3.5 | 9.0 | 21.6 ± 2.9 | 6 | 2.0 ± 0.5 | 2.3 % ± 1.2% | 5.9 % ± 1.8% | 0.6 % ± 0.1% |
| Day-11 | 710 ± 24 | 59.2 | 426 ± 31 | 88.45 | 178 ± 21 | 55.0 | 2.4 ± 0.5 | 3.5 % ± 2.2% | 4.6 % ± 3.1% | 0.4 % ± 0.3% |
| Day-14 | 1420 ±110 | 118.3 | 890 ± 45 | 185.4 | 286 ± 29 | 79.4 | 3.2 ± 1.6 | 4.1% ± 1.0% | 4.4% ± 3.3% | 0.7% ± 0.2% |
n = 6.
FACS analyses were also performed to quantify percentages of CD4+Tim3+PD-1+ and CD4+FoxP3+ subsets (FACS histograms not shown). Total numbers of these subsets were calculated by multiplying the total number of MDLN cells with percentages of the subsets, and shown in Table 4. The CD4+Tim3+PD-1+ and CD4+FoxP3+ subsets were < 5% and < 6%, respectively, during the entire culture expansion. These results demonstrated that the day-0 MDLN samples contain few regulatory and exhausted T cells, and the 14-day culture process does not result in a generation of these cells.
In summary, results of Table 4 confirmed our previous finding (7, 8) that the 14-day culture process result in a generation of large lymphocyte pool of proliferating CD4+ T cells; these results also demonstrated that the day-14 CD4+ T cells are non-regulatory T cells.
The CD4+CCR7+CD62L+ T cell subset significantly increased in number in response to melanoma cell antigen re-exposure
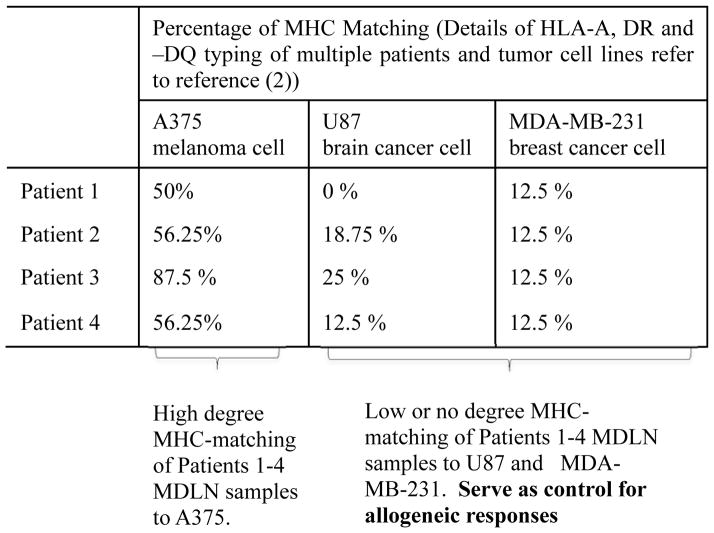
In order to dissect melanoma-reactive CD4+ T cells in the expanded lymphocyte pool, we characterize various CD4+ subsets before and after MDLN cell re-exposure to tumor cell antigens by 11-color FACS. The 11-color FACS analysis allowed us to quantify co-expression of CD4 marker with any other listed markers in Table 2. In our previous work, we have co-cultured MDLN cells with tumor cells and monitored the nature of the co-culture conditions by FACS using markers including annexin V-FITC, 7AAD and CD3-pacific blue7. In this previous setting, it was observed that the T cells underwent a low percentage of apoptosis (<5%) after co-culture; and the gating of T cells from tumor cells using CD3 stain was consistent with the gating of non-CD3-stained T cells from tumor cells using FSC vs SSC histograms with optimized parameters based on the different size and granularity7. Therefore, in this current experiment of co-cultures, the MDLN T cells were gated from tumor cells using FSC vs SSC histograms. For example, the FACS histograms of MDLN cells from a representative patient in response to fresh medium, A375, U87 and MDA-MB-231 were shown in Figure 1. Due to the different distribution of T cells and tumor cells in FSC vs SSC, all cell events can be gated to separate T cells from tumor cells. The P1-gated T cells were interrogated to quantify percentage of CD4 and CD8 T cells. The P2-gated CD4 T cells were interrogated to quantify surface expression of CD62L and CCR7. Among all the CD4+ subsets with 1–3 surface markers listed in Table 2, the CD4+CCR7+CD62L+ T cell subsets showed significant increase in number in response to melanoma cell antigen re-exposure.
Figure 1.
FACS analysis of T cell subsets in day-14 MDLN co-cultures with medium, A375, U87 and MDA-MB-231. Various MDLN co-cultures were stained and analyzed by 11-color FACS. All events were gated by P1 to exclude tumor cells of greater sizes. The P1-gated T cells were analyzed using CD4-PCy5.5 vs CD8-V500. The P2-gated CD4+ T cells were analyzed using CD62L-PE vs CD8-Pe-Cy7. Unstained MDLN co-culture with A375 served as negative control to gate positive cells. MDLN co-culture with medium served as non-stimulated control to quantify baseline expression of various surface markers. MDLN co-cultures with low degree MHC-matching U87 and MDA-MB-231 served as controls to monitor allogeneic responses.
Percentage and number of CD4+, CD4+CCR7+CD62L+ T cells and CD4+CCR7−CD62L− T cell subsets were summarized in Table 5. The MDLN cell co-culture with medium control (antigen non-stimulated control) contained a majority (>50%) of CD4+CCR7+CD62L+ T cells. As compare to the MHC-mismatched controls (U87 and MDA-MB-231 controls for alloresponses), the MDLN cell co-culture with A375 resulted in a significant number increase of the CD4+CCR7+CD62L+ T cell subsets. Based on current models of central memory and effector memory T cells, the CD4+CCR7+CD62L+ T cells are defined to be central memory T cells due to the co-expression of lymph node homing marker CCR7 and L-selectin marker CD62L. The CD4+CCR7+CD62L+ T cells are considered to be capable of homing to lymphoid organ and responding to secondary encounter of antigens (17, 18). Since the patients’ MDLN cells had been ex vivo expanded in IL-2-containing medium for 14 days, the polyclonally expanded CD4+CCR7+CD62L+ T cells may not truly represent the central memory phenotype. However, our observation that the melanoma cell antigen re-exposure resulted in a significant increase of the CD4+CCR7+CD62L+ T cells suggested that this is a very important CD4+ T cell compartment that can actively respond to melanoma antigens.
Table 5.
Percentage and total number of various CD4+ T cell subsets within the day-14 MDLN co-cultures with medium, A375, U87 and MDA-MB-231. n = 4.
| Day-14 MDLN cells + medium | Day-14 MDLN cells + A375 | Day-14 MDLN cells + U87 | Day-14 MDLN cells + MDA-MB-231 | ||
|---|---|---|---|---|---|
| T cell number/million | 1580 ± 120 | 1590 ± 210 | 1455 ± 230 | 1585 ± 180 | |
| CD4+ | Cell percentage | 63.4% ± 3.3% | 82.2% ± 4.8% * | 59.2% ± 5.1% | 63.4% ± 2.3% |
| Cell number/million | 1002 ± 76 | 1306 ± 60 * | 861 ± 110 | 1004 ± 130 | |
| CD4+CD62L+CCR7+ | Cell percentage | 50.7% ± 1.8% | 78.1% ± 2.1% * | 50.3% ± 1.6% | 52.0% ± 1.7% |
| Cell number/million | 801 ± 28 | 1241 ± 34 * | 732 ± 23 | 522 ± 25 | |
| CD4+CD40L+ | Cell percentage | 2.3% ± 3.4% | 26.7% ± 7.8% * | 9.9% ± 7.1% | 10.3% ± 5.5% |
| Cell number/million | 36 ± 54 | 425 ± 124 * | 144 ± 103 | 163 ± 87 | |
| CD4+CXCR5+ | Cell percentage | 6.7% ± 5.1% | 51.3% ± 9.7% * | 7.6% ± 6.3% | 9.4% + 5.5% |
| Cell number/million | 106 ± 81 | 816 ± 154 * | 111 ± 92 | 149 ± 87 | |
| CD4+CD27− | Cell percentage | 15.3% ± 2.8% | 46.4% ± 3.6% * | 30.6% ± 2.2% | 23.2% ± 7.1% |
| Cell number/million | 241 ± 44 | 483 ± 57 * | 271 ± 32 | 368 ± 113 | |
| CD4+CD62L+CCR7+CD27− | Cell percentage | 12.5% ± 2.1% | 43.1% ± 3.9% * | 28.2% ± 15.6% | 15.5% ± 4.3% |
| Cell number/million | 197 ± 33 | 685 ± 62 * | 453 ± 240 | 245 ± 68 | |
| CD4+CD62L+CCR7+CD27+ | Cell percentage | 39.9% ± 5.6% | 26.7% ± 7.5% | 25.4% ± 13.7% | 37.2% ± 5.1% |
| Cell number/million | 630 ± 88 | 425 ± 120 | 370 ± 188 | 590 ± 81 | |
| CD4+CD62L+CCR7+CD27−CXCR5+ | Cell percentage | Not detected | 36.6% ± 3.3% * | Not detected | 0.9% ± 0.4% |
| Cell number/million | Not detected | 582 ± 52.1 * | Not detected | 14 ± 6.2 | |
| CD4+CD62L+CCR7+CD27−CD40L+ | Cell percentage | Not detected | 23.4% ± 3.5% * | 10.2% ± 5.2% | Not detected |
| Cell number/million | Not detected | 373 ± 55.3 * | 162 ± 82.4 | Not detected | |
| CD4+CD62L+CCR7+CD27−IL-2+ | Cell percentage | 1.1% ± 5.7% | 18.2% ± 10.2% * | 14.3% ± 3.3% | 2.7% ± 0.9% |
| Cell number/million | 17 ± 90 | 289 ± 162 * | 208 ± 48 | 43 ± 14 | |
| CD4+CD62L+CCR7+CD27−TNF-α+ | Cell percentage | 2.1% ± 1.1% | 12.3% ± 2.6% * | 9.1% ± 1.5% | 2.6% ± 0.9% |
| Cell number/million | 33 ± 17 | 196 ± 41 * | 132 ± 22 | 41 ± 14 | |
| CD4+CD62L+CCR7+CD27−IL-4+ | Cell percentage | Not detected | |||
| Cell number/million | |||||
| CD4+CD62L+CCR7+CD27−IFN-γ+ | Cell percentage | Not detected | |||
| Cell number/million | |||||
| CD4+CD62L+CCR7+CD27+IL-2+ | Cell percentage | 41.3% ± 5.3% | 37.2% ± 4.1% | 35.2% ± 3.7% | 34.3% ± 6.5% |
| Cell number/million | 653 ± 84 | 591 ± 65 | 512 ± 54 | 541 ± 103 | |
| CD4+CD62L+CCR7+CD27+TNF-α+ | Cell percentage | 32.9% ± 6.1% | 41.2% ± 1.1% | 37.8% ± 3.3% | 39.4% ± 2.5% |
| Cell number/million | 520 ± 96 | 655 ± 16 | 549 ± 48 | 624 ± 40 | |
| CD4+CD62L+CCR7+CD27+IL-4+ | Cell percentage | 6.6% ± 2.1% | 8.6% ± 4.7% | 9.5% ± 4.3% | 9.9% ± 2.1% |
| Cell number/million | 104 ± 33 | 136 ± 75 | 138 ± 63 | 157 ± 35 | |
| CD4+CD62L+CCR7+CD27+IFN-γ+ | Cell percentage | Not detected | |||
| Cell number/million | |||||
significantly different from the non-stimulated control (medium) and the controls for allo-responses (U87 and MDA-MB-231).
Melanoma cell antigen re-exposure of ex vivo expanded MDLN cells resulted in significant number increase of CD4+ cells positively expressing CD40L and CXCR5 and negatively expressing CD27
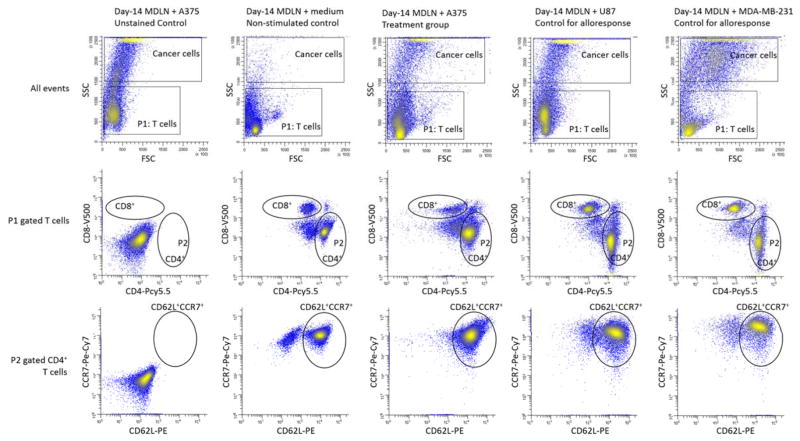
In order to monitor the CD4+ T cell function in response to melanoma cell antigen re-exposure, we characterized CD40L, CXCR5 and CD27 surface markers that associated with T cell functions. Surface expressions of CD40L and CXCR5 have been shown to mediate T helper cell functions through activating dendritic cells and B cells, respectively (19, 20). And it has been recently shown that antigen specific CD4+ T cells can be isolated from lymphocyte pool by capturing CD154+ (CD40L+) cells upon antigen re-exposure (21). The negative expression of CD27 by CD4+ cells has been discovered to associate with repeated exposure of T cells to antigens (22, 23). Based upon these, we chose to characterize surface expression of these markers monitor CD4+ T cell function. For example, FACS histograms of a representative patient on the surface expression of CD40L, CXCR5 and CD27 by CD4+ cells in MDLN co-cultures with fresh medium, A375, U87 and MDA-MB-231 were shown in Figure 2. The gating of T cells from tumor cells was conducted in the same way as described in Figure 1. The T cells were interrogated to quantify the CD4 expression of CD40L, CXCR5 and CD27.
Figure 2.
FACS analysis of surface expression of CD40L, CXCR5 and CD27 by CD4+ cells in day-14 MDLN co-cultures with medium, A375, U87 and MDA-MB-231. Various MDLN co-cultures were stained and analyzed by 11-color FACS. The gating of T cells from various tumor cells was the same as described in Figure 2. The gated T cells were analyzed using CD4-PCy5.5 vs CD40L-PE-Cy5, CD4-PCy5.5 vs 0CXCR5-AF488, and CD4-PCy5.5 vs CD27-AF700. Controls were designed in the same way as described in Figure 2.
Total number of CD4+CD40L+, CD4+CXCR5+ and CD4+CD27− in MDLN co-cultures with medium, A375, U87 and MDA-MB-231 were summarized in Table 5. Although the non-stimulated baseline expression of CD40L and CXCR5 in MDLN co-cultures with medium was low, a baseline expression of CD27− marker was found to be ~15.3±2.8% in the non-stimulated MDLN co-cultures. As compare to the non-stimulated control (medium) and controls for alloresponses (U87 and MDA-MB-231), the MDLN cell co-culture with A375 resulted in a percentage increase of CD4+CD40L+ from baseline ~2.3±3.4% to ~26.7±7.8%, percentage increase of CD4+CXCR5+ from baseline 6.7±5.1% to 51.3±9.7%, and percentage increase of CD4+CD27− from baseline 15.3±2.8% to 46.4±3.6%. Although the U87- and MDA-MB-231-stimulated MDLN co-cultures resulted in percentage increase of the CD4+CD40L+ subsets as compare to the non-stimulated MDLN cultures, yet the increases were not statistically significant. Based on the T cell numbers in MDLN co-cultures, the percentage increases in response to melanoma cell antigen re-exposure resulted in total number increase of the CD4+CD40L+, CD4+CXCR5+ and CD4+CD27− subsets from baseline level ~36±54, ~106±81 and ~241±44 million to ~425±124, 816±154 and 483±57 million (Table 5).
The CD4+CD62L+CCR7+ T cells with negative CD27 expression associated with markedly increased expression of CXCR5 and CD40L in response to melanoma cell re-exposure
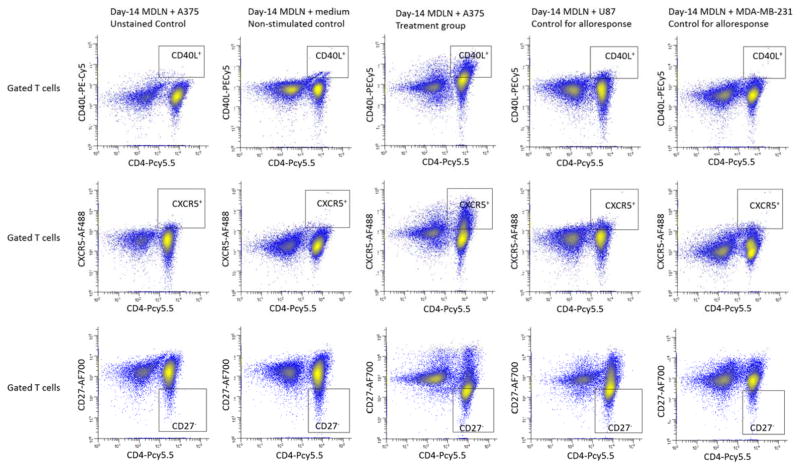
Having shown the number alteration of the CD4+CD62L+CCR7+, CD4+CD40L+, CD4+CXCR5+ and CD4+CD27− T cell subsets in response to melanoma cell antigen re-exposure (Figure 2 and 3, Table 5), we further investigated whether the CD4+CD62L+CCR7+ T cell subsets correlate with the up-regulation of CXCR5 and/or CD40L and/or down-regulation of CD27. In order to test this, the MDLN cells were re-exposed to melanoma cell antigens and controls, followed by a quantification of the co-expression of the CD4+CD62L+CCR7+ T cells with CD27, CXCR5 and CD40L surface markers. 11-color FACS analysis allowed us to investigate MDLN T cell expression of these markers of all different combinations. For examples, we have investigated and quantified various subsets including the CD4+CD62L+CCR7+CD27+, CD4+CD62L+CCR7+CD27−, CD4+CD62L+CCR7+CD27+CXCR5+CD40L+, CD4+CD62L+CCR7+CD27+CXCR5+CD40L−, CD4+CD62L+CCR7+CD27+CXCR5−CD40L+, CD4+CD62L+CCR7+CD27+CXCR5−CD40L−, CD4+CD62L+CCR7+CD27−CXCR5+CD40L+, CD4+CD62L+CCR7+CD27−CXCR5+CD40L−, CD4+CD62L+CCR7+CD27−CXCR5−CD40L+, et al. Among these subsets, the CD4+CD62L+CCR7+CD27− subsets in MDLN co-culture with A375 exhibited significant changes as compare to the subsets in MDLN co-cultures with medium, U87 and MDA-MB-231. For example, FACS histograms of CD40L and CXCR5 expression by CD4+CD62L+CCR7+CD27− cells in MDLN co-cultures with fresh medium, A375, U87 and MDA-MB-231 from a representative patient were shown in Figure 3. Gating of the CD4+CD62L+CCR7+ T cells was conducted in the same way as described in Figure 1. The CD4+CD62L+CCR7+ cells were gated and analyzed for CD27 expression. Then, the gated CD4+CD62L+CCR7+CD27− cells were further interrogated to quantify surface expression of CXCR5 and CD40L. Unstained MDLN co-culture with A375 served as control.
Figure 3.
FACS analysis of surface expression of CXCR5 and CD40L by central memory T cells (CD4+CD62L+CCR7+) with negative CD27 expression in day-14 MDLN co-cultures with medium, A375, U87 and MDA-MB-231. MDLN cultures were from a representative patient. Various MDLN co-cultures were stained and analyzed by 11-color FACS. The gating of central memory T cells (CD4+CD62L+CCR7+) was the same as described in Figure 2. The gated central memory T cells were analyzed using FSC vs CD27-AF700 to quantify the CD4+CD62L+CCR7+CD27+ and the CD4+CD62L+CCR7+CD27− cell subsets. The gated CD4+CD62L+CCR7+CD27− subsets were analyzed using FSC vs CD40L-PE-Cy5 and FSC vs CXCR5-AF488. Controls were designed in the same way as described in Figure 2.
The number of the CD4+CD62L+CCR7+CD27− subsets was shown in Table 5. As compare to controls, the MDLN co-culture with A375 exhibited a significant percentage and number increase of the CD4+CD62L+CCR7+ cells with negative CD27 expression (CD4+CD62L+CCR7+CD27−). Studies have suggested that repeated antigen exposure of T cells could result in loss of CD27 expression from CD27+ T cells (22, 23). Therefore, there is a possibility that the generation of CD27− T cell subsets may be caused by an antigen re-exposure-induced differentiation of CD27+ cells into CD27− cells. In order to test this, we quantified percentage and number of the CD4+CD62L+CCR7+CD27+ cell subsets in various MDLN co-cultures and the results were shown in Table 5. However, significant percentage and number change of the CD4+CD62L+CCR7+CD27+ subsets were not observed. This suggested that the generation of the CD4+CD62L+CCR7+CD27− cell subsets might be independent of the CD4+CD62L+CCR7+CD27+ T cell subsets.
As compare to controls, the MDLN co-culture with A375 also resulted in surface expression of CXCR5 by ~85% CD4+CD62L+CCR7+CD27− cells and surface expression of CD40L by ~53% CD4+CD62L+CCR7+CD27− cells (Figure 3). Since the percentage of CD4+CD62L+CCR7+CD27− cells within MDLN co-culture with A375 is ~43.1%, it resulted in a generation of ~36.6% (43.1 × 85% = 36.6 %) CD4+CD62L+CCR7+CD27−CXCR5+ cells and ~23.4% (43.1 × 53% = 23.4%) CD4+CD62L+CCR7+CD27−CD40L+ cells (Table 5). Since the number of T cells within MDLN co-culture with A375 is ~1590 million, the generation of ~36.6% CD4+CD62L+CCR7+CD27−CXCR5+ and ~23.4% CD4+CD62L+CCR7+CD27−CD40L+ cells represented a generation of ~582 million CD4+CD62L+CCR7+CD27−CXCR5+ cells and ~272 million CD4+CD62L+CCR7+CD27−CD40L+ cells in the MDLN cultures after re-exposure to melanoma cell antigens (Table 5). These changes were not observed in MDLN co-cultures with medium, U87 and MDA-MB-231. Considering the role of CXCR5 and CD40L for the activation of B cells and dendritic cells, these results suggested that the CD4+CD62L+CCR7+CD27− cell subset could express activation markers in response to melanoma cell antigen re-exposure.
The CD4+CD62L+CCR7+CD27− subsets exhibited increased intracellular cytokine production in response to melanoma cell antigen re-exposure
In order to determine whether the CD4+CD62L+CCR7+CD27− subsets exhibited functional responses in response to melanoma cell antigen re-exposure, we developed 6-color FACS analysis (Table 3) to quantify intracellular production of cytokines. Since our preliminary results demonstrated no intracellular expression of IL-17, we focused on the determination of Th1 and Th2 cytokines including IL-2, IL-4, TNF-α and IFN-γ. Results of intracellular cytokine production by the CD4+CD62L+CCR7+CD27− and CD4+CD62L+CCR7+CD27+ subsets were shown in Table 5. The CD4+CD62L+CCR7+CD27+ subsets served as control and showed high constitutive production of IL-2, IL-4, TNF-α and low production of IFN-γ. By contrast, the CD4+CD62L+CCR7+CD27− subsets showed little constitutive intracellular production of these four cytokines. As compare to the controls, the MDLN co-culture with A375 resulted in significant increase of intracellular production of IL-2 and TNF-α by the CD4+CD62L+CCR7+CD27− subsets. Since the total number of T cells within MDLN co-culture with A375 is ~1590 million, the generation of ~18.2±10.2% CD4+CD62L+CCR7+CD27−IL-2+ and ~12.3±2.6% CD4+CD62L+CCR7+CD27−TNF-α+ cells represented a generation of ~289±162 million CD4+CD62L+CCR7+CD27−cells producing intracellular IL-2 and 196±41 million CD4+CD62L+CCR7+CD27−cells producing intracellular TNF-α.
The ex vivo culture expansion of MDLN cells resulted in a slightly percentage and number increase of the CD4+CCR7+CD62L+CD27− T cells
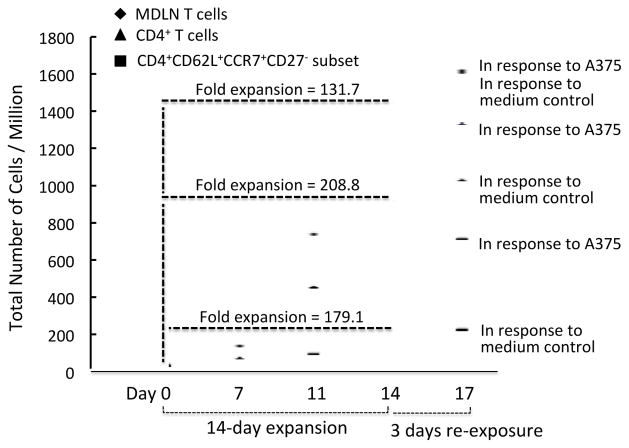
In order to monitor the CD4+CCR7+CD62L+CD27− subsets in human MDLN samples during the ex vivo expansion, we have conducted 11-color FACS analyses to determine the kinetic changes of the cell subsets and the results were shown in Table 6. The subsets exhibited percentage increase from day-0 ~8.5±1.7% to day-11 ~9.2±2.3% to day-14 ~12.7±3.4%. Additional 72 hours culture of MDLN cells resulted in ~ 12.5 ± 2.1% of the subsets, suggesting that further 3-day culture did not result in further increase of the subsets. Since the MDLN cultures on day-0, -11, -14, day-17 contained 12±2.1, 710±24, 1420±110 and 1580±120 million cells, the ~8.5±1.7%, ~9.2±2.3%, ~12.7±3.4% and 12.5±2.1% on these days represented 1.1±0.2, 65±16.3, 180±48 and 197±33 million cells of the CD4+CCR7+CD62L+CD27− subsets, respectively. The fold expansions of the CD4+CCR7+CD62L+CD27− subsets as well as the MDLN T cells and the CD4+ T cells during the 14-day ex vivo expansion and in response to A375 re-exposure were also shown in Figure 5. These results demonstrated that the CD4+CCR7+CD62L+CD27− subsets increased in number during the ex vivo culture expansion following antigen re-exposure.
Table 6.
Kinetic percentage and number of the CD4+CD62L+CCR7+CD27− subsets in MDLN cultures during the 14-day culture-expansion process and in response to A375 melanoma cell antigen re-exposure.
| Day-0 | Day-11 | Day-14 | Day-17 (Day-14 co-culture with medium for 72 hours) | Day-17 (Day-14 o-culture with A375 for 72 hours) | ||
|---|---|---|---|---|---|---|
| CD4+CD62L+CCR7+CD27− | Cell percentage | 8.5% ± 1.7% | 9.2% ± 2.3% | 12.7% ± 3.4% | 12.5% ± 2.1% | 43.1% ± 3.9% |
| Cell number/million | 1.1 ± 0.2 | 65 ± 16.3 | 180 ± 48 | 197 ± 33 | 685 ± 62 |
Figure 5.
The fold expansions of MDLN T cells, CD4+ T cells and the CD4+CD62L+CCR7+CD27− cell subsets during the 14-day culture expansion and in response to A375 re-exposure.
Discussion
Increasing evidence from animal studies and clinical trials have suggested that effective adoptive cellular immunotherapy (ACI) is not limited to the use of CD8+ T cells and may be enhanced by the use of antigen-specific CD4+ T cells (24–25). For examples, immunotherapy using tumor-specific CD8+ T cells in CD4-deficient MHC II−/− mice resulted in regression of pulmonary metastases, but did not result in long-term antitumor immunity and tumor eventually recurred (26, 27). Two studies using MART-1 and/or gp-100-specific HLA class I restricted TCR gene transfer for treatment of metastatic melanoma patients resulted in objective clinical response rates of 13% (2/15) and 30% (6/30), which were lower than the response rates achieved using bulk CD4+ and CD8+ tumor infiltrating T cells (TILs) (51–71%) (28–31). These suggest a therapeutic benefit of using tumor-reactive CD4+ T cells for ACI. Since CD4+ T cells are capable of activating and regulating many aspects of innate and adaptive immunities, the use of tumor-reactive CD4+ T cells for ACI may impact the ability to generate memory immune responses that are correlated with the most durable clinical responses.
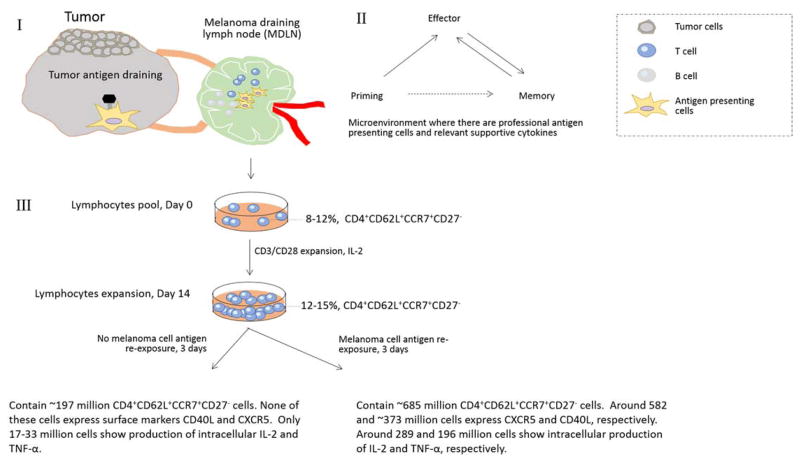
This study focuses on the investigation of relevant tumor-reactive CD4+ T cell subsets from MDLNs. We have previously shown that the in vitro expansion of patients’ MDLN samples result in the generation of antigen specific CD4+ T cells, which mediate MHC II-restricted protective immune responses both in vitro and in vivo in human melanoma xenograft model (7, 8). In addition, MDLN cultures contained majority of CD4+ T cells (~40–60%) both before and after activation and expansion (7), which made MDLN samples an excellent resource to study CD4+ T cells. More importantly, it has been shown that MDLNs are secondary lymph node organs where T cell sees an antigen in vivo during the primary immune responses (Figure 4, I). The microenvironment within MDLN around the primary tumor cells contains multiple antigen presenting cells (APCs), such as dendritic cells (DCs) and various cytokines, where T cells may undergo priming, effector, contraction and memory phases (Figure 4, II). These provide strong rationale to use MDLN samples as T cell resource to investigate tumor-reactive CD4+ subsets. However, if suppressive factors are accumulated in microenvironment of MDLN, T cells may differentiate into regulatory T cells (9, 10).
Figure 4.
Schematic Figure of the 14-day expansion of MDLN samples from Stage III melanoma patients. I. Cells within MDLN sample have experienced tumor antigens. I and II. In MDLN microenvironment where there are functional antigen presenting cells and supportive cytokines, T cells can undergo priming, effector and memory phase. III. The ex vivo activation by CD3/CD28 beads and expansion by IL-2 resulted in a kinetic growth of CD4+ TCM subset with negative CD27 expression (CD4+CD62L+CCR7+CD27−). Although the subsets do not proliferate or exhibit functional property in cultures with no antigen re-stimulation, the subsets exhibited a dramatic increase of total cell number in response to melanoma cell antigen re-exposure. The subsets also exhibited dramatically increased surface expression of CD40L and CXCR5 and intracellular production of IL-2 and TNF-α.
In order to test this, in this study we determined the proportion of CD4+Tim3+PD-1+, CD4+FoxP3+ and CD4+CD25+FoxP3+ subsets in the activated and expanded MDLN lymphocytes pool (Table 4). The FoxP3 expression by CD4 cells is suggested to be regulatory T cell marker and the Tim3+PD-1+ cells have been used as markers for exhausted T cells (15, 16). The expanded MDLN cells from multiple patients demonstrated < 5% CD4+Tim3+PD-1+ cells, suggesting that the MDLN samples from patients with stage III MM contained statistically low exhausted T cells (Table 4). In literature (15, 16), it showed that the Foxp3 could be transiently expressed in human activated non-regulatory CD4+ T cells that were derived from human peripheral blood. In contrast, our T cells were derived from tumor draining lymph nodes and the Foxp3 expression by CD4+ T cells did undergo expansion during the culture process based on results in Table 4. The %CD4+Foxp3+ were 3.2% on day 0, 5.9% on day 7, 4.6% on day 11, and 4.4% on day 14. The percentage slightly dropped from 5.9% (day 7) to 4.6% and 4.4% (day 11 and 14), however, since the CD4+ T cells underwent a significant number expansion (from 4.8 million of day 0 to 43.2 million of day 7 to 426 million on day 11 to 890 million on day 14) during the culture process, this resulted in the expansion of CD4+Foxp3+ from 1.54 million (day 0) to 2.56 million (day 7) to 19.6 million (day 11) and 39.2 million (day 14). Based on the observation o.f peripheral blood-derived T cells, the expansion of CD4+Foxp3+ T subset was transient and mediated by the non-specific activation), therefore, might not correlate to TReg (15,16). In this study, our observation of MDLN-derived T cells indicated the number expansion of CD4+Foxp3+ T cells during the entire culture process. Since it has been shown that the Foxp3 expression restricted by CD4+CD25+ subsets can represent TReg of helper cells, we monitored kinetic growth of the CD4+CD25+Foxp3+ subsets (Table 4). The results indicated that the MDLN samples from patients with stage III MM contained statistically low or no CD4+CD25+Foxp3+ subsets that are traditionally defined as TReg.
Noted that the MDLN samples we investigated in this study are from patients with stage III melanoma. At this stage, patients do not have melanoma available for biopsy. The primary cutaneous melanoma had been excised at the time of the original sentinel lymph node biopsy so that autologous tumors are not available as resources of tumor cells for the research. Because of this limitation, we chose to test MHC of several patients’ T cells and various melanoma and non-melanoma cell lines to look for evidence of matching at major loci. Since it is unlikely that all MHC molecules of MDLN T cells match those of the allogeneic tumor cell lines used in our study, we aimed to investigate the antitumor responses of MDLN T cells by comparing T cells of high degree of MHC matching to T cells of low or no degree of MHC matching, which could serve as control for alloresponses. Results of our previous study indicated that, when there was a high degree of MHC matching, MDLN T cells mediated higher antitumor responses than the low/no degree of MHC matching ones, suggesting that the MHC-matched locus may contribute to antitumor responses above any alloresponses seen with mis-matched T cells (7). In addition, we have incubated multiple patients’ T cells with overlapping melanoma peptides to determine the functional production of IL-2, TNF-a and IFN-g in response to peptide antigens (7). Moreover, we have previously shown that there are CD11c+ autologous antigen presenting cells within the MDLN cultures even after 14 days of expansion (7). These antigen presentation cells would allow alloantigens from MHC mismatched tumor cells to be presented to T cells in the proper MHC context. Based upon these results, our belief is, if the increased percentage of specific subsets was expanded by MHC-matched A375 but not by MHC-mismatched U87 and MDA231, it suggested that the responses were MHC-restricted; although the MHC-restricted responses could be responses against A375 tumor cell antigens and/or non-self antigens, yet our previous observation of the specific responses against melanoma overlapping peptide (7) suggested that the altered subsets could represent the subsets expanded in response to A375 cell re-exposure.
In this study, after ex vivo expansion of MDLN cells in IL-2-containing medium, we observed that the antigen non-re-stimulated MDLN cells were consisted of over 50% CD4+CD62L+CCR7+ T cells. Since the T cells have been expanded polyclonally in IL-2-containing medium that may up- or down-regulate surface markers, the CD4+CD62L+CCR7+ T cell subset may not truly represent the central memory phenotype. However, after being re-exposed to melanoma cell antigens, the MDLN cells contained over 75% CD4+CD62L+CCR7+ T cells, which were not observed in controls. This suggested that the CD4+CD62L+CCR7+ T cell compartment contained T cell subsets that could actively respond to melanoma. Within the CD4+CD62L+CCR7+ T cell compartment, we made further observation that the CD4+CD62L+CCR7+ subsets expressed up-regulated CD40L and CXCR5 and down-regulated CD27 in response to melanoma cell antigen re-exposure (Figure 2, 3 and Table 5). FACS analyses of ex vivo expanded and antigen re-stimulated MDLN cells using multiple surface markers (Table 2) demonstrated that some of the CD4+CD62L+CCR7+CD27− cell subsets associated with significantly up-regulated CD40L and some of them associated with significantly up-regulated CXCR5 in response to melanoma cell antigen re-exposure, as compared to controls. More interestingly, as compared to controls, FACS analysis using various surface and intracellular markers (Table 3) demonstrated that some of the CD4+CD62L+CCR7+CD27− cell subsets exhibited up-regulated production of IL-2 and some of them exhibited up-regulated production of TNF-α in response to melanoma cell antigen re-exposure (Table 5). Although further studies are needed, yet these results strongly suggested that melanoma cell antigen re-exposure could result in the generation of various functional markers and cytokines by the CD4+CD62L+CCR7+CD27− subsets.
Limited knowledge is available regarding the CD4+CD62L+CCR7+CD27− T cell subset. CD27 negative expression has been found to associate with repeated exposure to antigens and considered to be a marker for effector cells. This is evidenced by observations such as that the CD4+CD27− T cell subsets derived from persistent virus-infected human blood samples were found to contain functionally differentiated T cells (32, 33). Interestingly, in influenza virus-infected human blood samples, the frequency of CD4+CCR7+CD27+ and CD4+CCR7−CD27− T cell subsets are ~70% and ~5%, respectively; the frequency of CD4+CCR7+CD27− is ~2–3% and the function is currently unknown (33). In contrast, results of this study demonstrated that the frequency of CD4+CD62L+CCR7+CD27− T cell subsets is ~8–9% in non-activated and non-expanded MDLN cells, which was higher than that of the CD4+CCR7+CD27− cell subsets (2–3%) in infected blood samples. The CD4+CCR7−CD27− T cell subsets were interpreted to be the CD4+ effector T cell subsets because their negative expression of CD27 suggests that they may have experienced antigens and their negative expression of lymphoid homing marker CCR7 suggests that they may home to infected tissue but not lymphoid organ (32, 33). Based upon literature and results of this current study, the CD4+CCR7+CD27− T cells appear to represent the subsets that have experienced antigens but still carry lymphoid homing marker CCR7 to possibly migrate to secondary lymphoid organ.
In the ex vivo expanded and antigen re-stimulated MDLN cells, the CD4+CD62L+CCR7+CD27− T cell subsets increased in number and contained subsets that associated with significantly up-regulated CD40L, CXCR5, IL-2 and TNF-α. Since the CD4+CD62L+CCR7+CD27− cell subsets were expanded from MDLNs and associated with an up-regulation of CD40L in response to melanoma cell antigen re-exposure, this subset possibly could be follicular T helper (Tfh) cells, because Tfh cells have been demonstrated to co-express CD40L marker that can activate B cells. However, analysis of cytokine profile of this subset indicated the secretion of IL-2 and TNF-α, but not IL-4. Since IL-4 is a major cytokine secreted by Tfh, our results may suggest that the CD4+CD62L+CCR7+CD27− cells are different from Tfh. Based on these characteristics, it is still hard to category the CD4+CD62L+CCR7+CD27− subsets because they undergo up-regulation of both central memory-related markers (CD40L+, CXCR5+ and CCR7+) and effector-related markers (CD27−, IL-2+ and TNF-α+) in response to antigen re-exposure. Whether these T cells were self-proliferated from the CD4+CD62L+CCR7+CD27− cells or differentiated from the CD4+CD62L+CCR7+CD27+ and/or other type of T cell subsets are unknown. However, results of this study demonstrated the existence of melanoma specific CD4+ “effector-precursor” cells in the ex vivo expanded MDLN cultures. They were the CD4+ T cells that could actively respond to antigen re-exposure and differentiate into the CD4+CD62L+CCR7+CD27− cells expressing up-regulated CD40L, CXCR5, IL-2 and TNF-α. Although this study did not identify what the melanoma-reactive CD4+ “effector-precursor” cells are, yet the results suggested significance of the subsets and encouraged further investigation.
Thus, ongoing studies in our laboratory include the use of CFSE to track the precursors of the CD4+CD62L+CCR7+CD27− T cells expressing up-regulated CD40L, CXCR5, IL-2 and TNF-α in the antigen non-re-stimulated MDN cells; ongoing studies also include the use of ImmunoSEQ® to investigate whether the CD4+CD62L+CCR7+CD27− cells expressing up-regulated CD40L, CXCR5, IL-2 and TNF-α express unique T cell receptor sequences that overlap with certain CD4+ T cell subsets in the expanded and antigen non-stimulated MDLN cultures; we will also also determine functional property of the identified CD4+ T cell subsets in response to multiple defined melanoma- and non-melanoma antigens, and assess in vivo therapeutic effect using melanoma model and re-constituted humanized NSG mice. Successful identification of tumor reactive CD4+ T cell will greatly benefit innovative design of ACI that can potentially mediate enhanced and more durable clinical responses.
Acknowledgments
This study was supported by NIH 1 K23 CA109115-01A5 (J.A.K), CTSC (Clinical Translational Scientific Collaboration) grant of Coulter CON501510-69361 (M.Z.) and by Immunogene Therapy Fund from Seidman Cancer Center (J.A.K.). We thank Case Comprehensive Cancer Center-Flow Core Lab for the kind assistance and support on FACS analysis.
References
- 1.Rosenberg SA. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman KM, Prieto PA, Devillier LE. Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J Immunother. 2012;35:400–408. doi: 10.1097/CJI.0b013e31825898c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Yang JC. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunder NN, Wallen H, Cao J. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vesely M, Kershaw M, Schreiber R, Smyth M. Natural Innate and Adaptive Immunity to Cancer. Annual Rev Immun. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Graor H, Strohl M, Caja K, Yan L, Kim J. T cells Expansions from Human Melanoma Draining Lymph Nodes mediate melanoma specific antitumor responses in vitro and in vivo. Journal of Immunotherapy. 2015;38:229–238. doi: 10.1097/CJI.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 8.Visioni A, Zhang M, Graor H, Kim J. Expansion of melanoma-specific T cells from lymph nodes of patients in stage III: Implications for adoptive immunotherapy in treating cancer. Surgery. 2012;152:557–565. doi: 10.1016/j.surg.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode U, Lorchner M, Ahrendt M, Blessenohl M, Kalies K, Claus A, Overbeck S, Rink L, Pabst R. Dendtitic cell subsets in lymph nodes are charcaterized by the specific draining area and influence the phenotype and fate of primed T cells. Imunology. 2008;123:480–490. doi: 10.1111/j.1365-2567.2007.02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mempel T, Henrickson S, Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 11.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-color flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 12.Bagwell CB. Hyperlog, a flexible log-like transform for negative, zero, and positive valued data. Cytometry A. 2005;64:34–42. doi: 10.1002/cyto.a.20114. [DOI] [PubMed] [Google Scholar]
- 13.Petrausch U, Haley D, Miller W, Floyd K, Urba WJ, Walker E. Polychromatic flow cytometry: a rapid method for the reduction and analysis of complex multiparameter data. Cytometry A. 2006;69:1162–73. doi: 10.1002/cyto.a.20342. [DOI] [PubMed] [Google Scholar]
- 14.Tung JW, Parks DR, Moore WA, Herzenberg LA, Herzenberg LA. New approaches to fluorescence compensation and visualization of FACS data. Clin Immunol. 2004;110:277–283. doi: 10.1016/j.clim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Viguier M, Lemaitre F, Verola O, Cho MS. Foxp3 Expressing CD4+CD25high Regulatory T Cells Are Overrepresented in Human Metastatic Melanoma Lymph Nodes and Inhibit the Function of Infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 16.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepper M, Jenkin M. Origins of CD4+ effector and central memory T cells. Nat Immun. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 19.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nat. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 20.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay P, Roederer J. Live–cell assay to detect antigen-specific CD4+ T cell responses by CD154 expression. Nature Protocol. 2006;1:1–6. doi: 10.1038/nprot.2006.1. [DOI] [PubMed] [Google Scholar]
- 22.Fritsch R, Shen X, Sims G, Hathcock K, Hodes R, Lipsky P. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175:6489–6497. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 23.Schiott A, Lindstedt M, Johansson-Lindhom B, Roggen E, Borrebaeck CA. CD27-CD4+ memory T cells define a differentiated memory population at both the functional and transcriptional levels. Immunology. 2004;113:363–70. doi: 10.1111/j.1365-2567.2004.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon E, Muranski P. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapetti L, Meunier S, Pontoux C, Tanchot C. CD4 help regulates expression of crucial genes involved in CD8 T cell memory and sensitivity to regulatory elements. J Immunol. 2008;181:299–308. doi: 10.4049/jimmunol.181.1.299. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu HM, Winter H, Urba WJ, Fox B. Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy. J Immunol. 2000;165:4246–4253. doi: 10.4049/jimmunol.165.8.4246. [DOI] [PubMed] [Google Scholar]
- 28.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F. Gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herve D, Cariou M, Simonetta A, Taoufik Y. Hetero-specific CD4 help to rescue CD8 T cell killers. J Immunol. 2008;181:5974–5980. doi: 10.4049/jimmunol.181.9.5974. [DOI] [PubMed] [Google Scholar]
- 30.Wang RF, Johnson SL, Zeng G, Topalian SL, Schwartzentruber DJ, Rosenberg SA. A breast and melanoma-sahred tumor-antigen: T cell responses to antigenic peptides translated from different open reading frames. J Immunol. 1998;161:3598–3606. [PubMed] [Google Scholar]
- 31.Wen W, Zhang L, Peng J, Chen J, Hao J, Li X, Qian X, Zeng P, Zhang Y, Yin Y. Identification of promiscuous HLA-DR-restricted CD4+ T-cell epitopes on the cancer testis antigen HCA587. Cancer Sci. 2011;102:1455–1461. doi: 10.1111/j.1349-7006.2011.01986.x. [DOI] [PubMed] [Google Scholar]
- 32.Church S, Jensen S, Anthony P, Restifo N, Fox B. Tumor-specific CD4+ T cells maintain effector memory tumor-specific CD8+ T cells. Euro J Immun. 2014;44:69–79. doi: 10.1002/eji.201343718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Bree GJ, Daniels H, Schilfgaarde M, Jansen HM, Qut TA, vanLier RA, Jonkers RE. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis. 2007;195:1718–1725. doi: 10.1086/517612. [DOI] [PubMed] [Google Scholar]