Abstract
Objective
Adverse health outcomes related to exposure to endocrine disrupting chemicals (EDCs), including increased incidences of coronary heart disease, prostate and testicular cancers, and birth defects have been reported in firefighters or their offspring. We, therefore, measured the estrogenic and antiestrogenic activity of extracts of used firefighter gear to assess exposure to these agents.
Methods
Extracts and known chemical contaminants were examined for estrogenicity and antiestrogenicity in yeast cells expressing the estrogen receptor.
Results
Most extracts of used gear, and phthalate diesters detectable on this gear displayed strong antiestrogenic effects. Notably, new glove and hood extracts showed significant estrogenic activity.
Conclusions
Overall, our data suggest that firefighters are exposed to both estrogenic and antiestrogenic agents, possibly phthalates that may lead to health risks observed in this occupation as a result of perturbation of hormone homeostasis.
Introduction
Firefighting is one of the most hazardous occupations1, yet remains one of the least studied. Firefighters are continually exposed to chemicals in the vapor state during fire suppression, firefighters are continually exposed to chemicals in the vapor state, absorbed on smoke particulates, from deposits left on the skin, and indirectly from contaminated clothing. Previous reports have shown common components of deposits of this nature on firefighter gloves and hoods are phthalate diesters.1,2 These chemicals are used as plasticizers in a variety of synthetic polymers, including polyvinyl chloride, encompassing a large variety of products ranging from shower curtains, wire sheathing, toys, lubricants, and to building materials.3 They are highly lipophilic and are easily absorbed transdermally in humans and experimental animal models.4-6 Transdermal absorption of lipophilic chemicals increases dramatically with increased temperature and exercise,6 suggesting that this may be a critical route for firefighter exposure. Currently, there is a pronounced concern regarding potential health effects of phthalate diesters, which are classified as endocrine disrupting chemicals (EDCs), agents that have been shown to mimic or disrupt estrogenic and other hormone activities.7 Reported effects of exposure to EDCs are increased incidence of coronary heart disease, certain types of cancer, and reproductive defects in offspring,8 possibly via toxicity toward sperm cells. 9-11
Epidemiological studies suggest that firefighting is associated with an increase of cancer in several tissues, including the liver, bladder, prostate, thyroid, testis and cervix.12, 13 Estrogen regulates various male and female functions including reproductive development and function. Perturbation in hormone-dependent tissues such as the prostate, testis, and cervix may lead to carcinogenesis. A disturbance in pro-oxidant-antioxidant balance is suggested to be a contributing factor to prostate cancer.14 The commonly used phthalate diester plasticizer di (2-ethylhexyl) phthalate (DEHP) has been shown to be genotoxic to a human prostate cancer cell line, LNCaP, by a mechanism involving pro-oxidant and oxidant activity.15 Reports describing an increase in congenital defects in the offspring of firefighters,16 and developmental defects in response to endocrine disruptors in experimental animals,17 further suggesting that adverse health effects reported in firefighter offspring may result from phthalate exposure.
Hormones initiate their downstream function via binding to nuclear receptors,18 and the majority of these functions involve interactions with a specific DNA element to turn on gene transcriptions. Once a hormone ligand is bound to the receptor, it is then able to bind to a specific response element. In the case of estrogens, estrogen receptor (ER) recognizes estrogen response element (ERE) and transactivates downstream gene transcription, maintaining all the estrogen-dependent functions. Any chemicals that are able to bind to or interfere with the ER and disrupt transactivation are therefore potentially endocrine disruptors.
Estrogen screening assays have been in common use throughout the recent past. One method involves using mammalian cell lines, specifically breast cancer cell lines MCF-7 and ZR-75, to determine the effects of commercially available phthalate diesters on cell proliferation. 18 The throughput of this type of cell-based assay is limited, however, and its sensitivity for E2 is only around the nanomolar range. Another method to screen for estrogenic activity uses yeast cells genetically engineered to produce the enzyme β -galactosidase in response to estrogen treatment. Estrogen concentrations are measured by the absorbance change resulting from cleavage of chlorophenol red-β-D-galactopyranoside by this enzyme. 19 While being sensitive enough to detect estrogen activity at the picomolar range18, the assay has one major drawback, which needs about 4 to 6 days to complete18. This severely limits the throughput of this assay.
Information regarding EDC exposure of firefighters is extremely limited. In this study, we investigated whether extracts of firefighting gear have estrogen-disrupting activity. We applied a newly developed yeast estrogen screening assay, which is more robust and highly sensitive to estrogenic or antiestrogenic activities in extracts of firefighter gear.
METHODS
Chemicals
17β -Estradiol (E2), dimethyl sulfoxide (DMSO), butylbenzyl Phthalate (BBP), bis(2-ethylhexyl) phthalate (DEHP) and di(n-octyl) phthalate (DnOP) were all purchased from Sigma-Aldrich (St. Louis, MO). The Beta-Glo® Reagent was purchased from Promega (Madison, WI).
Sample Collection
The Cincinnati Fire Department generously donated firefighter gloves and hoods. Two types of clothing were gathered: clean gloves and hoods that were never used prior to analysis, and gloves and hoods that had been used for at least 8 weeks without being washed prior to analysis.
An 8 cm. square of material was cut out of the center of the gloves’ palms. A 5 cm. square section was cut from the rear flap of each hood.
Sample Extraction
Each section of the material was washed with 40ml of methylene chloride for 30 minutes to extract organic compounds and the extract then evaporated to dryness and dissolved in 0.5 ml DMSO for analysis.
Determination of estrogenic activity by yeast estrogen screening assay (YES)
To determine estrogenic activity, human ERalpha was expressed in yeast cells containing the ER expression plasmid Yep-ER under the control of the yeast triose dehydrogenase promoter, and the reporter plasmid Yep-ERE, containing the vitellogenin estrogen response element (ERE) driven by the yeast CYC1 (iso-1-cytochrome C) promoter. Both Yep-ER and Yep-ERE vectors were gifts from Dr. Paul Mak at University of Massachusetts Medical School. The ERE was fused to the E. coli lac-Z gene which encodes the enzyme β -galactosidase. The protease-deficient yeast strain BJ2168 was transformed through a standard protocol14 with the Yep-ERE and the ER expression plasmids to generate estrogen responsive yeast cells. The ER yeast strain was cultured in synthetic dropout (SD) medium (Clontech, Mountain View, CA) and agar with tryptophan and uracil.
The LacZ gene responsible for encoding β -galactosidase was located downstream of the ERE, which was activated by a ligand-bound ER. Chemicals that mimic estrogen interacted with the ER, facilitating the transcription of β-galactosidase. This enzyme catalyzes the cleavage of D-luciferin from β -galactopyranosyl-luciferin. D-luciferin serves as substrate for luciferase to produce a luminescent signal which is proportional to the amount of β-galactosidase generated by the yeast cells. The enzyme luciferase and β-galactopyranosyl-luciferin were obtained via Beta-Glo® Reagent, Luminescence was measured with a Victor2 Multi-label Counter (PerkinElmer, Waltham, MA).
Determination of Estrogenicity of the Smoke-Derived Compounds
Yeast cells were spread onto SD agar plates and incubated at 30°C until individual colonies appeared. A 1mm diameter colony was selected and placed into 3mL of the appropriate medium which was then incubated at 30°C overnight with shaking. The next day the cells were vortexed and 100μl of the culture was added to 9.9mL of fresh appropriate medium, giving a 1:100 dilution. This prevented yeast cell growth from becoming saturated, and allowed the cells to continue dividing.
Cells were then pipetted onto a 96-well plate, 99μL per well and the absorbance of each well measured at 562nM in order to make sure each well has similar amount of cells. Extracts were mixed thoroughly by vortexing, briefly centrifuged and clear samples inoculated into wells at a 1:100 ratio. Each assay was done in triplicate. Then yeast was incubated inside a sealed box with a small water reservoir to avoid evaporation, at 30°C for 24 hours.
Following incubation the cells were mixed with a pipette and 5μL placed into another 96-well plate. 45μL of double distilled water was added to the treated yeast cells to give a 1:10 ratio. 50μL of Beta-Glo® Reagent was then added to each well and the plate was gently agitated with a Bell Dancer (Stovall Life Science, Peosta, IA) for an hour. Luminescence signal was then measured.
The activity of 1nM 17β-estradiol (E2) was measured in each assay as a positive control. In order to determine the antiestrogenicity of the extracts or phthalate diesters, they were co-incubated with 1nM E2. These assays contained phthalate diesters at concentrations of 1μM and 10μM. These concentrations were found to have discrete estrogenic activity19.
In order to ensure the test samples did not affect yeast growth, absorbance at 562nm was monitored at three different time points during experiments. The first absorbance reading was taken after the mixture of yeast cells and appropriate SD medium was transferred to the 96-well plate. This reading was to ensure that a uniform number of yeast cells were allocated evenly in each well before commencement of the assay. Next, an absorbance reading was taken immediately after the yeast cells were treated. This was used to determine if the extracts or phthalate diesters interfered with the absorbance reading. The last absorbance reading was taken after the 24-hour incubation period. This absorbance was used to determine whether the samples cause any change in yeast cell proliferation or even cell death. When assaying the samples with color, the number of yeast cells per well was counted using hematocyometer instead of measuring the absorbance. The sample coloration did not significantly the amount of luminescence measured (data not shown).
Statistical analysis
The data were expressed as mean ± standard error (SEM). Statistical significance of differences among treatment groups was determined by one-way or two-way analysis of variance and covariance (ANOVA), whenever appropriate, and Tukey test using GraphPad Prism (GraphPad, La Jolla, CA). A P value < 0.05 was considered statistically significant.
RESULTS
Validation of the YES assay
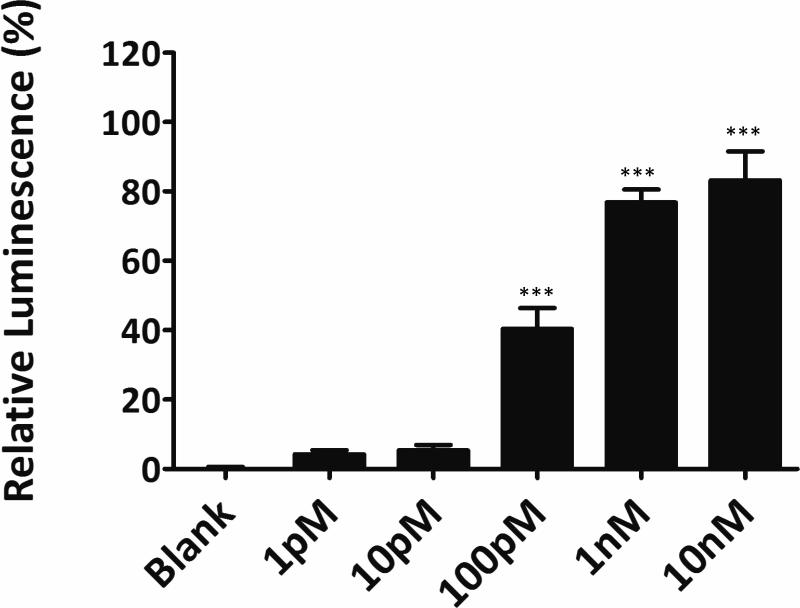
The yeast assay was extremely sensitive to concentrations of 17β-estradiol, significantly down to 100pM (Figure 1) and showed a level of sensitivity significantly higher to most mammalian cell based assays, down to the subnanomolar range.18, 19 It's sensitivity was comparable with other published yeast assay18. Yeast-based assay therefore was applied to assess the estrogenic and antiestrogenic activity of extracts and contaminants of firefighter gear.
Figure 1.
Determination of the effect of different concentrations of 17β -Estradiol using YES assay. Final concentration of E2 was calculated based on the total volume. One-way ANOVA was performed to compare the mean of different groups. *** p<0.0001 when compared with blank.
DMSO activity
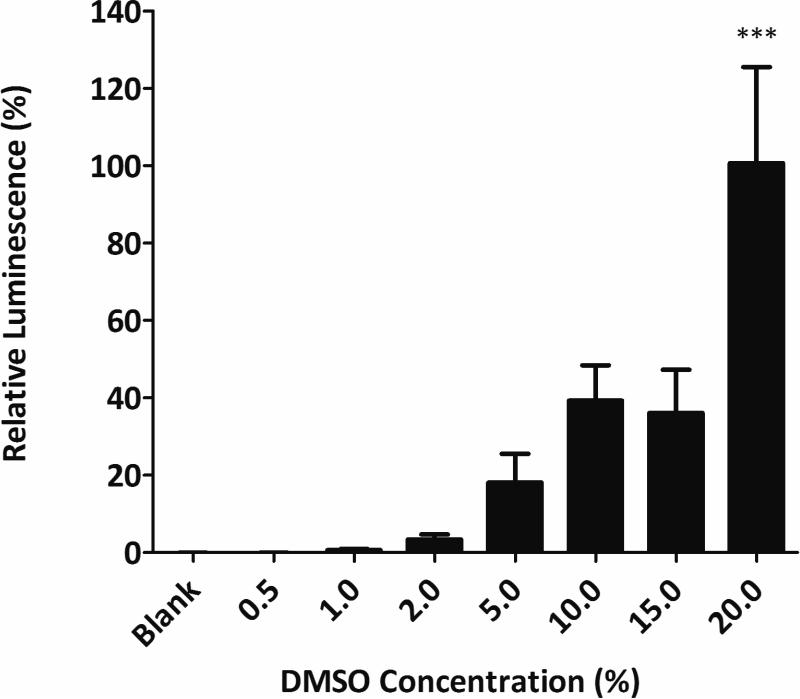
DMSO was used as the solvent for the agents or extracts examined in this study; therefore its activity alone was determined. Concentrations up to 15% had no statistically significant effect, whereas 20% DMSO showed significant estrogenic activity (p<0.0001) when compared with the blank (Figure 2). Subsequent assays were contained <1% DMSO and therefore had minimal effect on the measured fluorescence.
Figure 2.
Determination of the effect of DMSO using YES assay. Percent of DMSO used as total volume. One-way ANOVA was performed to compare the mean of different groups. *** p<0.0001 when compared with blank.
Activity of Phthalate diesters
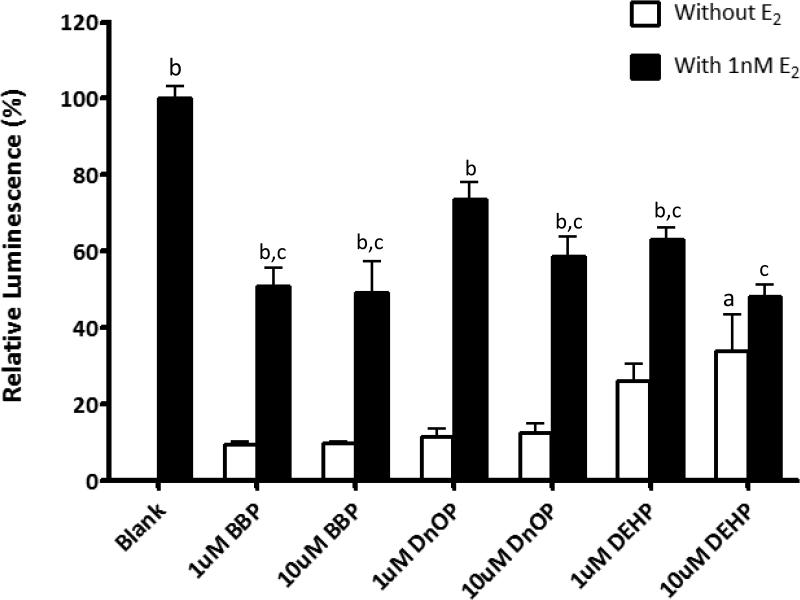
Three phthalate diesters BBP, DnOP, and DEHP showed low estrogenic activity in the YES assay at concentrations of 1 or 10uM (Figure 3). DEHP at higher concentration (10uM) showed significant estrogenic activity (p<0.05) when compared with the blank and the other two phthalates (BBP and DnOP). There is no significant difference among the three phthalate diesters in terms of estrogenic activity. However, when the assays contained both phthalate diester and 1nM E2, estrogenic activity with almost all phthalates (except 1uM DnOP) was significantly suppressed (p<0.01) when compared with that of 1nM E2 alone. These results indicated that even though BBP, DnOP, and DEHP do not have strong estrogenic activity, they act as significant antiestrogens or ER antagonists.
Figure 3.
Determination of estrogenic and antiestrogenicity activity of phthalate diesters BBP, DnOP, and DEHP using YES assay. Total volume concentrations used for 24 hour treatment with E2, BBP, DNoP, and DEHP. “Blank” was setup with 1% DMSO and 1nM E2 was added in “blank” as a positive control. Two-way ANOVA was performed to compare the mean of different groups. “a” indicates p<0.05 when compared with the blank without E2; “b” indicates p<0.01 when compared with their own phthalate without E2. “c” indicates p<0.05 when compared with the blank with E2.
Estrogenic activity of extracts of new and soiled firefighter gloves
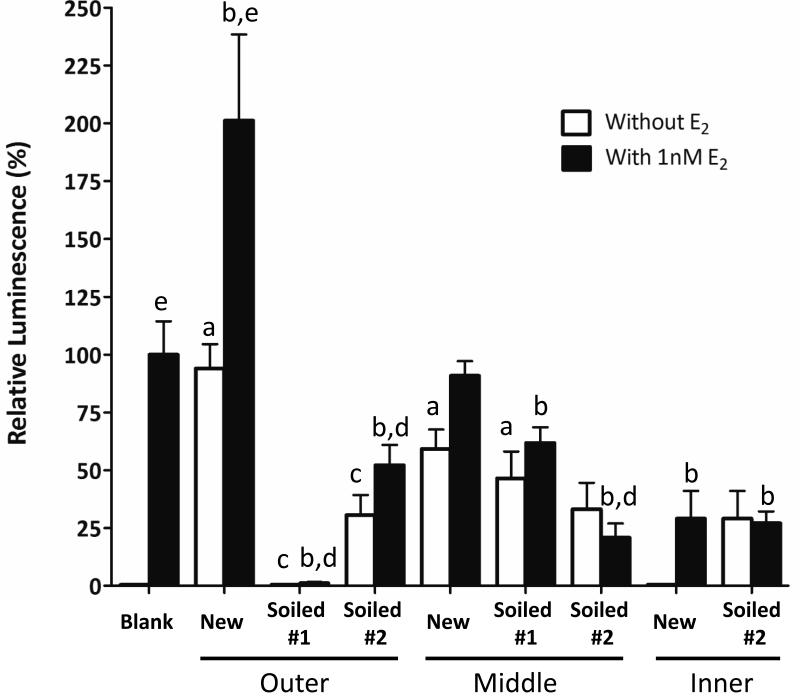
Three different layers (outer, middle and inner) of new and soiled firefighter gloves were analyzed in this study. Significant estrogenic activity (p<0.01) was measured in the outer layer of the new gloves (Figure 4). When compared with the new glove, the estrogenic activity detected in the outer layer was significantly dropped (p<0.005) after the gloves (soiled #1 and #2) were used for at least 8 weeks without being washed. When 1nM E2 was co-incubated with the samples from outer glove layer, only the new one showed significant increase (p<0.005) in estrogenic activity when compared with their respective control without E2. In contrast, addition of E2 did not significantly augment the activity in both soiled outer samples (soiled #1 and #2) when compared with their respective control (without E2). However, the activity detected in both soiled samples was still significantly (p<0.01) lower than the 1nM E2 blank, suggesting the presence of antiestrogen chemicals in the soiled gloves.
Figure 4.
Determination of estrogenic and antiestrogenicity activity of the extracts from new and soiled firefighter gloves using YES assay. Extracts were evaporated to dryness and dissolved in 0.5 ml DMSO. 1μL of extract was used as treatment for 24 hours. “Blank” was setup with 1% DMSO and 1nM E2 was added in “blank” as a positive control. One-way ANOVA was performed to compare the mean of different groups. “a” indicates p<0.01 when compared with the blank without E2; “b” indicates p<0.05 when compared with blank with E2 control; “c” indicates p<0.005 when compared with new glove type (outer, middle and inner) without E2; “d” indicates p<0.005 when compared with new glove type (outer, middle and inner) with E2.; “e” indicates p<0.005 when compared with their respective no E2 control.
For the middle layer, samples from new and soiled #1 glove were estrogenic as the activity was significantly (p<0.01) higher than the blank without E2 (Figure 4). No significant difference was found between new and soiled samples in term of estrogenic activity. However, antiestrogen activity was significantly (p<0.05) detected in the both soiled samples when they were co-treated with E2.
For the inner layer, no significant estrogenic activity was found either in new or soiled gloves. Nevertheless, significant antiestrogenic activity (p<0.05) was detected in all inner glove samples regardless of whether the gloves have been used or not.
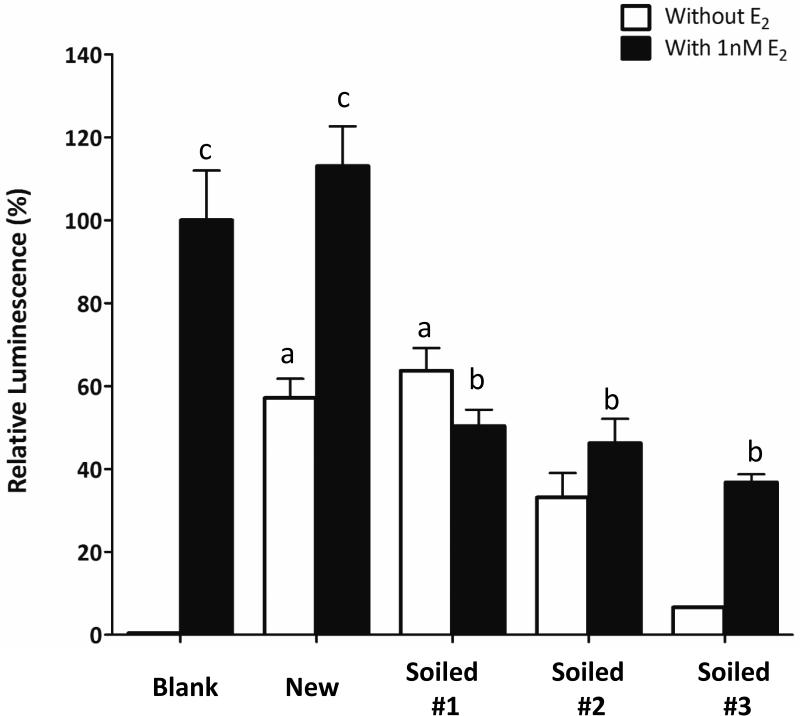
Estrogenic activity of extracts of new and soiled firefighter hoods
With regard to firefighter hoods, samples from new and one of the soiled one (soiled #1) showed significant amount of estrogenic activity when compared with blank control (without E2) (Figure 5). When 1nM E2 was added, all soiled samples showed significant decrease (p<0.01) in activity when compared with the E2 only control, suggesting some antiestrogenic chemicals were deposited onto the hood during firefighting.
Figure 5.
Determination of estrogenic and antiestrogenicity activity of the extracts of new and used hoods using YES assay. Extracts were evaporated to dryness and dissolved in 0.5 ml DMSO. 1μL of extract was used for treatment for 24 hours. “Blank” was setup with 1% DMSO and 1nM E2 was added in “blank” as a positive control. One-way ANOVA was performed to compare the mean of different groups. “a” indicates p<0.001 when compared with the blank without E2; “b” indicates p<0.001 when compared with the blank with E2; “c” indicates p<0.001 when compared with their own control without E2.
Overall, the data suggest that clean firefighter equipment, especially in new hood, outer and middle gloves, contains mostly estrogenic chemicals and antiestrogenic chemicals like phthalates, are likely to be deposited to the gear during firefighting.
DISCUSSION
Firefighters are constantly exposed to a mixture of hazardous agents, including a few that have been found to have endocrine disrupting activity.1 This prompted us to develop a YES assay that would have high throughput while able to detect burning-derived agents found on firefighters’ gloves and hoods at nanomolar, and even picomolar, concentrations. In this study, we were successful in detecting significant amounts of EDCs in extracts from firefighter gear.
Our improved assay has shown to be high throughput and more sensitive method. In a previous study designed to measure estrogenic activity of phthalate esters in vitro, an incubation period between 4 and 6 days was recorded for yeast cultures. 19 A total of 48hrs from yeast culture to luminescence reading was also required. In contrast, our new assay only takes less than 48hrs to complete. By decreasing the total incubation period, we have limited the possibility that the medium would be evaporated which would cause a higher concentration of β-galactosidase and lead to inaccurate luminescence readings. Contrasting other yeast assay, we were able to show estrogenic activity caused by DEHP suggesting that our assay is more sensitive to chemicals with estrogenic properties19. The previous yeast estrogen screening assay did not measure yeast growth during incubation. This is significant in that certain chemicals may induce or inhibit yeast growth. By measuring the concentration of yeast, we were able to modify the concentrations of the extracts.
The inhibition of estrogenic activity by individual phthalate diesters and extracts we observed could be explained by competition between these agents and estradiol for the estrogen receptor, but also by alteration of the helix-12 region on the receptor. This could lead to the inhibition of binding of a co-activator to the estrogen receptor.16, 17 Without the co-activator the estrogen receptor would be unable to bind to the estrogen response element. Either mechanism would ultimately result in inhibition of the production of β-galactosidase.
Extracts from new equipment that had not been worn displayed mostly estrogenic properties. The extracts from a new hood and new middle layer of a glove showed moderate levels of estrogenic activity. When paired with E2 the activity increased marginally, demonstrating estrogenic competition (Figure 4.). The outer layer of a glove showed comparable estrogenic activity to 1nM E2 and when co-incubated with 1nM E2 the estrogenic activity doubled, suggesting some chemicals coated on the new outer layer are potent estrogens. In contrast, the inner layer of a new glove displayed little to no estrogenic effect and when combined with 1nM E2, there was a small increase in estrogenic activity, demonstrating an antagonist effect. This variation among the different layers and pieces of firefighter gear could be caused by different flame retardants, which are estrogenic in nature.20
Soiled firefighter gear displayed both low estrogenic and strong antiestrogenic properties. Extracts that expressed low estrogenic activity demonstrated estrogenic competition when paired with 1nM E2. In contrast, the extracts that expressed strong antiestrogenic properties inhibited most of the effect of E2 (Figure 4 & 5). The variability of these results suggests that, in addition to flame retardants, firefighters are exposed to different EDCs and varying quantities, which could be dependent on different exposures from firefighting scenes.
Unpredictable exposure can occur from different fires such as an industrial fire that contains more plastic materials compared with a residential fire. Furthermore, exposure to these EDCs may arise as a result of dermal contact with gear before or after a fire event, or via inhalation during overhaul, the phase where firefighters handle burnt and smoldering materials to find hidden fires and potentially combustible materials. During this period, self-contained breathing apparatus is commonly not used.21 This could increase the risk of exposing to unidentified harmful chemicals or EDCs.
Further identification of the specific chemicals present is required. Among these agents are phthalate diesters, which are major contaminants of soiled firefighter gear1 and have been shown to have endocrine disrupting activity.7-10 Previous studies have indicated that firefighters are exposed to a complex mixture of EDCs including fire retardants on the gear.22 This information may be constructive in designing future gear and protocols for firefighters to minimize exposure to estrogenic and antiestrogenic chemicals.
Estrogen plays a major role in both the male and female reproductive systems, perturbation of estrogen homeostasis has further been identified as a contributing factor in the progression of cancer of various types and developmental disorders.7-11 Perturbation of estrogen activity has been implicated in prostate and testicular cancers,23, 24 the most prevalent cancers reported in firefighters,13 and it has been observed that when prostate and breast cancer cell lines are treated with estrogens and xenoestrogens cell proliferation increases dramatically.14,19 Increased xenoestrogen and antiestrogen exposure could therefore increase the risk of developing cancer in hormone sensitive tissues of those types. Reduction of estrogen levels in male human volunteers has also been reported to decrease sexual function and increase body mass.25
In summary, we have found that firefighter equipment, specifically gloves and hoods, are impregnated with potent endocrine disrupting agents, with both estrogenic and antiestrogenic activity. Perturbation of hormone homeostasis could therefore occur with prolonged exposure ultimately leading to carcinogenesis, especially in hormone-sensitive tissues, or to other hormone - mediated adverse health outcomes.
Acknowledgments
This research study was partially supported by the National Institute for Occupational Safety and Health Pilot Research Project Training Program Education and Research Center Grant at University of Cincinnati (#T42/OH008732-06) as well as the National Institute of Environmental Health Sciences sponsored Center for Environmental Genetics at University of Cincinnati (ES006096). We thank Dr. Sarah To for her careful and critical reading of our manuscript.
Footnotes
Authors declare no conflicts of interest for this work.
References
- 1.Alexander BM, Baxter CS. Plasticizer contamination of firefighter personal protective clothing – a potential factor in increased health risks in firefighters. J Occup Environmental Hygiene. 2014 doi: 10.1080/15459624.2013.877142. 10.1080/15459624.2013.877142. [DOI] [PubMed] [Google Scholar]
- 2.Sanders JM, Bucher JR, Peckham JC, Kissling GE, Hejtmancik MR, Chhabra RS. Carcinogenesis studies of cresols in rats and mice. Toxicology. 2009;257:33–9. doi: 10.1016/j.tox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittassek M, Koch HM, Angerer J, Brüning T. Assessing exposure to phthalates – The human biomonitoring approach. Mol. Nutr. Food. Res. 2011;55:7–31. doi: 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- 4.Roberts MS, Anderson RA, Swarbrick J. Permeability of human epidermis to phenolic compounds. J Pharm Pharmacol. 1977;29:677–683. doi: 10.1111/j.2042-7158.1977.tb11434.x. [DOI] [PubMed] [Google Scholar]
- 5.Frasch HF, Zang LY, Barbero AM, Anderson SE. In vitro dermal penetration of 4-chloro-3-methylphenol from commercial metal working fluid and aqueous vehicles. J Toxicol Environmental Health A. 2010;73:1394–405. doi: 10.1080/15287394.2010.497444. [DOI] [PubMed] [Google Scholar]
- 6.Danon A, Ben-Shimon S, Ben-Zvi Z. Effect of exercise and heat exposure on percutaneous absorption of methyl salicylate. Eur J Clin Pharmacol. 1986;31:49–52. doi: 10.1007/BF00870985. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen S, Boberg J, Axelstad M, et al. Low-dose perinatal exposure to di(2-ethylhexyl)phthalate induces anti-androgenic effect in male rats. Reprod Toxicol. 2010;30:313–21. doi: 10.1016/j.reprotox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Lam JC, 4th, Chapin RE, Teague K, Lawton AA, Reel JR. Reproductive effects of four phthalic acid esters in the mouse. Toxicol Appl Pharmacol. 1987;88:255–69. doi: 10.1016/0041-008x(87)90011-1. [DOI] [PubMed] [Google Scholar]
- 9.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–91. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 10.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Human Reproduction. 2007;22:688–95. doi: 10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- 11.Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int J Androl. 2008;31:112–7. doi: 10.1111/j.1365-2605.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma F, Fleming LE, Lee DJ, Trapido E, Gerace TA. Cancer incidence in florida professional firefighters, 1981 to 1999. J Occup Environmental Med. 2006;48:883–890. doi: 10.1097/01.jom.0000235862.12518.04. [DOI] [PubMed] [Google Scholar]
- 13.LeMasters GK, Genaidy AM, Succop P, et al. Cancer risk among firefighters: a review of meta-analysis of 32 studies. J Occup Environmental Med. 2006;48:1189–1202. doi: 10.1097/01.jom.0000246229.68697.90. [DOI] [PubMed] [Google Scholar]
- 14.Oberley TD, Zhong W, Szweda LI, Oberley LW. Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate. 2000;44:144–155. doi: 10.1002/1097-0045(20000701)44:2<144::aid-pros7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Erkekoğlu Pınar, Rachidi Walid, De Rosa Viviana, et al. Protective effect of selenium supplementation on the genotoxicity of di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate treatment in LNCaP cells. Free Radical Bio & Med. 2010;49:559–566. doi: 10.1016/j.freeradbiomed.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Olshan AF, Teschke K, Baird PA. Birth defects among offspring of firemen. Am J Epidemiol. 1990;131:312–321. doi: 10.1093/oxfordjournals.aje.a115500. [DOI] [PubMed] [Google Scholar]
- 17.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 18.Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Env Hlth Perspectives. 1997;98:802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TS, Jung KK, Kim SS, Kang IH, Baek JH, Nam HS, Hong SK, Lee BM, Hong JT, Oh KW, Kim HS, Han SY, Kang TS. Effects of in utero exposure to di (n-butyl) phthalate on development of male reproductive tracts in Sprague-Dawley rats. J Toxicol Environ Health A. 2010;73:1544–59. doi: 10.1080/15287394.2010.511579. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Quan, Lu Meiya, Dong Xiaowu, et al. Potential estrogenic effects of phosphorus-containing flame retardants. Env Sci & Tec. 2014;48(12):6995–7001. doi: 10.1021/es5007862. [DOI] [PubMed] [Google Scholar]
- 21.Bolstad-Johnson DM, Burgess JL, Crutchfield CD, Storment S, Gerkin R, Wilson JR. Characterization of firefighter exposures during fire overhaul. Am Indust Assoc Hyg J. 2000;61:636–641. doi: 10.1080/15298660008984572. [DOI] [PubMed] [Google Scholar]
- 21.Shaw SD, Berger ML, Harris JH, Yun SH, Wu Q, Liao C, Blum A, Stefani A, Kannan K. Persistent organic pollutants including polychlorinated and polybrominated dibenzo-p-dioxins and dibenzofurans in firefighters from Northern California. Chemosphere. 2013;91:1386–94. doi: 10.1016/j.chemosphere.2012.12.070. [DOI] [PubMed] [Google Scholar]
- 22.Ho SM, Leung YK, Chung I. Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer. Ann N Y Acad Sci. 2006;1089:177–93. doi: 10.1196/annals.1386.005. [DOI] [PubMed] [Google Scholar]
- 23.Giannandrea F, Paoli D, Figà-Talamanca I, Lombardo F, Lenzi A, Gandini L. Effect of endogenous and exogenous hormones on testicular cancer: the epidemiological evidence. Int J Dev Biol. 2013;57:255–63. doi: 10.1387/ijdb.130015fg. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–20. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]