Summary
Objective
To derive a multivariable diagnostic model for symptomatic midfoot osteoarthritis (OA).
Methods
Information on potential risk factors and clinical manifestations of symptomatic midfoot OA was collected using a health survey and standardised clinical examination of a population-based sample of 274 adults aged ≥50 years with midfoot pain. Following univariable analysis, random intercept multi-level logistic regression modelling that accounted for clustered data was used to identify the presence of midfoot OA independently scored on plain radiographs (dorso-plantar and lateral views), and defined as a score of ≥2 for osteophytes or joint space narrowing in at least one of four joints (first and second cuneometatarsal, navicular-first cuneiform and talonavicular joints). Model performance was summarised using the calibration slope and area under the curve (AUC). Internal validation and sensitivity analyses explored model over-fitting and certain assumptions.
Results
Compared to persons with midfoot pain only, symptomatic midfoot OA was associated with measures of static foot posture and range-of-motion at subtalar and ankle joints. Arch Index was the only retained clinical variable in a model containing age, gender and body mass index. The final model was poorly calibrated (calibration slope, 0.64, 95% CI: 0.39, 0.89) and discrimination was fair-to-poor (AUC, 0.64, 95% CI: 0.58, 0.70). Final model sensitivity and specificity were 29.9% (95% CI: 22.7, 38.0) and 87.5% (95% CI: 82.9, 91.3), respectively. Bootstrapping revealed the model to be over-optimistic and performance was not improved using continuous predictors.
Conclusions
Brief clinical assessments provided only marginal information for identifying the presence of radiographic midfoot OA among community-dwelling persons with midfoot pain.
Keywords: Midfoot, Pain, Osteoarthritis, Diagnosis, Primary care
Introduction
Foot pain is a common symptom in the general population, affecting an estimated 24% of community-dwelling older adults1, and is frequently encountered in primary care2, 3, 4. Osteoarthritis (OA) is likely to be one underlying cause of foot pain. Among adults aged 50 years and over, 17% have been estimated to have symptomatic radiographic foot OA5, however, the basis for clinically diagnosing foot OA in symptomatic individuals is far from clear.
At the knee, where more research has been undertaken, the European League Against Rheumatism (EULAR) guidelines recommend the clinical diagnosis of knee OA, and highlighted the particular risk factors, clinical history and physical examination findings likely to be most informative6. However the ability to discriminate subtypes, for example patellofemoral OA, may be limited7.
At the foot, diagnostic research is currently restricted to the first metatarsophalangeal joint (MTPJ)8. We have recently shown that polyarticular midfoot dominant OA may constitute a distinct subtype of foot OA9 and that symptomatic midfoot OA affects approximately 12% of adults aged 50 years and over, with most people reporting foot-related disability and recently utilising primary health care for foot pain10. Although often present in primary care, the ability to provide targeted treatment for the functional consequences of midfoot OA may be limited by the challenges of clinical diagnosis11.
Our aim was therefore to derive a clinically practicable multivariable diagnostic model for symptomatic midfoot OA among community-dwelling persons with midfoot pain.
Methods
Study population
Data were collected via a population-based health survey and research assessment clinic as part of the Clinical Assessment Study of the Foot (CASF)5, 12. The health survey gathered information on general health, foot-specific features, demographic and socio-economic characteristics. The research assessment clinic collected physical examination data using brief clinical assessments and plain radiography. Inclusion criteria for the present analysis were: adults aged ≥50 years who were registered with one of four general practices in North Staffordshire, United Kingdom, and who responded to a health survey, provided consent to further contact, consent to participate in a research assessment clinic and had midfoot pain in the last month. Based on self-reported shading on either dorsal or plantar views of a foot manikin in the health survey, midfoot pain was ascertained using a pre-defined regional marking template (© The University of Manchester 2000. All rights reserved)13, 14.
Individuals with non-specific inflammatory arthritis, rheumatoid arthritis or psoriatic arthritis, as indicated by primary care and local hospital medical record review, or on an X-ray report by a consultant musculoskeletal radiologist, were excluded from the analyses. Ethical approval was obtained from Coventry Research Ethics Committee (REC reference number: 10/H1210/5).
Data collection
Research assessment clinic attenders underwent standardised clinical interview and physical examination performed by one of seven trained research therapists (four physiotherapists, three podiatrists). Assessors had between 1 and 35 years of post-qualification experience, reflecting the broad range of expertise found in clinical practice, and were required to satisfy pre-study training requirements and undergo quality control sessions during the study12.
During the same research assessment clinic, plain radiographs were taken of both feet from weight-bearing dorso-plantar and lateral projections. All clinical assessors were blind to participants’ radiographic images and outcomes. The presence of midfoot OA was defined as a score of two or more for osteophytes or joint space narrowing at the first or second cuneometatarsal, navicular-first cuneiform or talonavicular joints on either dorso-plantar or lateral views. The included joints represent the medial midfoot region and were selected as the joints of the lateral midfoot were not included in the radiographic foot atlas as they could not be as reliably evaluated15. Radiographs were scored using a published atlas and scoring system15 by a single experienced reader (MM) who was blind to all clinical assessment outcomes. The radiographs of 60 participants were selected at random and were rescored 8 weeks later by MM and independently scored by HBM. Intra-rater reliability for the presence of midfoot OA in each foot was found to be excellent (mean unweighted κ = 0.90; 95% confidence interval (CI): 0.74, 0.99, mean percentage agreement = 95%) and inter-rater reliability was fair (mean unweighted κ = 0.32; 95% CI: 0.19, 0.45, mean percentage agreement = 63%).
Reference standard for symptomatic midfoot OA
Symptomatic midfoot OA was confirmed using the atlas by Menz et al.15 and defined as the co-occurrence in the same foot of midfoot pain (ascertained from self-reported shading on a foot manikin as defined above) and the presence of radiographic OA (as defined above).
Selected predictor variables
A total of 16 predictor variables were selected from both health survey and research assessment clinic data. These were selected based on three criteria: (i) known risk factors for symptomatic OA at other joint sites, or (ii) have a mechanically-driven putative link to symptomatic midfoot OA, and (iii) be clinically practicable in primary care consultations. In meeting these criteria, three variables were identified and selected as recognised independent risk factors for OA (age, gender and body mass index (BMI))16. Age and gender were ascertained from the health survey and BMI was calculated from measured height and weight. Following pre-study consensus work with a multidisciplinary team of practicing clinicians, we selected static brief clinical assessments that could detect observable deficits, which will have direct implications for both static and dynamic loading of the midfoot. These included the following:
Static foot posture
-
i)
Arch Index: ratio of middle third area to the whole foot area, excluding toes, calculated from carbon footprints taken in relaxed bipedal standing. Higher Arch Index ratios indicate lower arch17, 18.
-
ii)
Foot Posture Index: 6-item assessment performed in relaxed bipedal standing. A summative score (range, −12 to +12) classified feet as supinated, normal or pronated19.
-
iii)
Navicular height: height of the navicular tuberosity from the floor in relaxed bipedal standing, measured in millimetres with a ruler, and normalised for foot size by dividing by foot length20.
Range of motion (ROM)
-
iv)
First MTPJ dorsiflexion ROM: maximum passive hallux extension, measured in degrees using a goniometer in non-weight-bearing with the ankle in a relaxed position and the first ray allowed to freely plantarflex21.
-
v)
Subtalar joint inversion/eversion ROM: maximum passive ROM measured in degrees with a goniometer in non-weight-bearing22.
-
vi)
Ankle dorsiflexion ROM, with the knee flexed/extended: active ROM measured in degrees with an inclinometer during a weight-bearing lunge test23, 24.
Palpation and observation
-
vii)
Midfoot exostosis: palpable presence or absence of bony prominence on the dorsum of the foot in non-weight-bearing.
-
viii)
Plantar tenderness: palpable presence or absence of point tenderness at plantar fascia-calcaneal insertion25 and middle portion of plantar surface26 in non-weight-bearing.
-
ix)
Lesser toe deformity: palpable presence or absence of deformities, in one or more lesser toes, including mallet, hammer and claw toe in non-weight-bearing and retracted toe observed in standing27.
-
x)
Hallux valgus: ascertained using five line drawings of the foot progressing in severity (15° increments) using a validated self-report instrument and dichotomised present or absent definition (three most severe vs two least severe)28.
For Arch Index, navicular height, 1st MTPJ dorsiflexion, subtalar inversion/eversion and ankle dorsiflexion with the knee flexed/extended, intra-class correlation coefficients (ICC) previously reported for intra-rater reliability range from 0.82–0.9917,20, 21, 22, 23, 24, with the Foot Posture Index being slightly lower (0.61)20. Inter-rater reliability ICC have been documented for subtalar inversion/eversion (0.73 and 0.62, respectively)22 and ankle dorsiflexion with the knee flexed/extended (0.97 and 0.92, respectively)23, 24. For the dichotomised hallux valgus definition, unweighted kappa scores were 0.83 for intra-rater and 0.55 for inter-rater reliability28.
Statistical analysis
All feet with midfoot pain were entered into the analysis. All continuous variables were screened to check appropriate range values and to identify any apparent outliers29. Where possible, dichotomised or categorised cut-offs applied to continuous variables were based on previous evidence. Across all feet, navicular height was divided into tertiles on the variable distribution to produce categories consistent with the Arch Index, and the subtalar and ankle ROM variables were dichotomised on the median, as no suitable prior information was identified. As the proportion of missing data for each predictor variable was <5%, multiple imputation was considered unnecessary.
The data had a non-hierarchical structure with feet nested within person and were analysed using a random intercept multi-level logistic regression model30. Each predictor variable was individually entered into the model with presence of symptomatic midfoot OA as the outcome. Significant independent predictor variables (P < 0.25 from likelihood ratio tests31) were then simultaneously entered into the model with age, gender and BMI force-entered, and manual backward elimination of variables (P = 0.05) performed. The final model was refitted using data from participants with no missing predictor variable data. Predicted risks were calculated on the estimated variable effects and the intercept for each foot. The proportion of the sample that could be correctly classified (ruled-in as having symptomatic midfoot OA) or correctly classified as midfoot pain (ruled-out for symptomatic midfoot OA) was determined by imposing a practical cut-off of 50%. Subsequently, sensitivity and specificity with 95% CIs were calculated for the overall final model.
Model performance was assessed with the calibration slope and area under the curve (AUC). Ideally a calibration slope with a value of 1 indicates the predicted and observed risks are the same30, and an AUC value ≥0.8 indicates “excellent” discrimination31. Model performance was then compared with a model containing age, gender and BMI only.
The internal validity of the final derived model and the performance measures were evaluated using 1000 bias-corrected bootstrap samples with replacement resampling on clusters, i.e., at the person level32. This is an important step in checking the degree of statistical overfitting and therefore over-optimism in the model's discriminative ability33. Using the bias-corrected bootstrap model, sensitivity and specificity were re-estimated.
Although dichotomising or categorising continuous predictors arguably assists clinical interpretability, it has been criticised for resulting in a loss of information and poorly fitting models34. We therefore re-ran the model-fitting procedures with all continuous predictor variables in their original form. The six-items of the Foot Posture Index that generate a summative score were Rasch-transformed into a single interval score, previously shown to improve internal construct validity35. All analyses were conducted using STATA V.13.0 (Stata Corporation, Texas, USA).
Results
Study participants
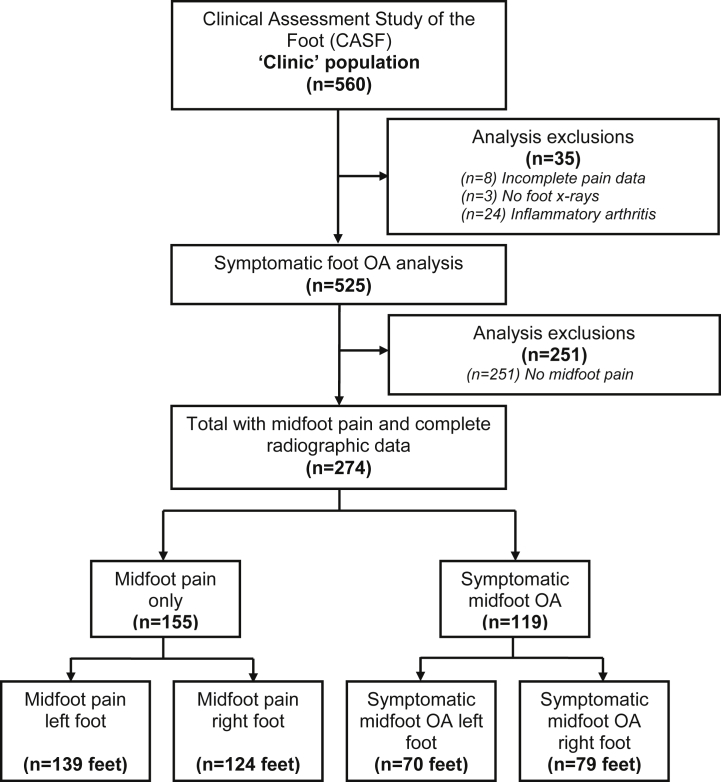
Of the 560 participants who attended the research assessment clinic between June 2010 and September 2011, 525 were potentially eligible for this analysis following the exclusion of individuals with incomplete pain data (n = 8), absent radiographic data (n = 3) and inflammatory arthritis (n = 24). This left 525 participants with foot pain and radiographic data, of whom 274 participants had both midfoot pain and complete radiographic data. Of these participants, 155 (57%) had midfoot pain only and 119 (43%) had symptomatic midfoot OA. From this sample of individuals, there were 263 feet with midfoot pain only and 149 with symptomatic midfoot OA (Fig. 1). Mean age (±SD) was 65.0 (8.6) years (age range 50–87), and 54% were female.
Fig. 1.
Flowchart of clinic attenders into analysis.
All clinical values for each predictor variable appeared appropriate and no data distributions were unduly influenced by outliers.
Diagnostic model
Of the 16 selected predictor variables, 10 were associated with the outcome (P < 0.25 from likelihood ratio tests) (Table I). These were age, BMI, Arch Index, Foot Posture Index, navicular height, subtalar inversion, ankle dorsiflexion with the knee flexed, midfoot exostosis, plantar fascia insertion tenderness and lesser toe deformity. Although gender was not statistically significant (P = 0.28), this was also a retained force-entered variable, due to previously established and consistent links with OA.
Table I.
Descriptive characteristics and univariable analysis for the occurrence of symptomatic midfoot OA
| Predictor variable (categorisation) | Total | Midfoot pain | Symptomatic midfoot OA | Multi-level logistic regression Midfoot pain vs symptomatic midfoot OA P* |
|---|---|---|---|---|
| People | (n = 274) | (n = 155) | (n = 119) | |
| Demographics | ||||
| Age (years) | ||||
| 50–64 | 142 (52) | 92 (59) | 50 (42) | |
| 65–74 | 89 (32) | 48 (31) | 41 (34) | |
| 75+ | 43 (16) | 15 (10) | 28 (24) | 0.0145 |
| Gender | ||||
| Male | 125 (46) | 73 (47) | 52 (44) | |
| Female | 149 (54) | 82 (53) | 67 (56) | 0.2751 |
| Body composition | ||||
| Body mass index | ||||
| Non-obese (<30 kg/m2) | 134 (50) | 85 (56) | 49 (42) | |
| Obese (≥30 kg/m2) | 136 (50) | 67 (44) | 69 (58) | 0.0069 |
| Feet | (n = 412) | (n = 263) | (n = 149) | |
| Static foot posture | ||||
| Arch Index (ratio) | ||||
| High arch | 57 (14) | 42 (16) | 15 (10) | |
| Normal | 265 (64) | 178 (68) | 87 (58) | |
| Low arch | 89 (22) | 42 (16) | 47 (32) | 0.0013 |
| Foot Posture Index (−12 to +12) | ||||
| Supinated (<0) | 34 (8) | 26 (10) | 8 (5) | |
| Normal (0–5) | 212 (52) | 132 (50) | 80 (54) | |
| Pronated (≥6) | 165 (40) | 105 (40) | 60 (41) | 0.1861 |
| Navicular height (ratio) | ||||
| High (0.18–0.29) | 136 (33) | 92 (35) | 44 (30) | |
| Normal (0.16–0.18) | 136 (33) | 95 (37) | 41 (28) | |
| Low (0.06–0.16) | 137 (34) | 73 (28) | 64 (43) | 0.0161 |
| Range of motion | ||||
| 1st MTPJ (degrees) dorsiflexion | ||||
| Low (<64) | 197 (48) | 123 (47) | 74 (50) | |
| High (≥64) | 215 (52) | 140 (53) | 75 (50) | 0.4242 |
| Subtalar joint (degrees) Inversion | ||||
| Low (2–25) | 215 (52) | 130 (49) | 85 (58) | |
| High (26–50) | 195 (48) | 133 (51) | 62 (42) | 0.0858 |
| Eversion | ||||
| Low (0–11) | 215 (52) | 136 (52) | 79 (54) | |
| High (12–55) | 195 (48) | 127 (48) | 68 (46) | 0.7425 |
| Ankle dorsiflexion (degrees) | ||||
| Knee flexed | ||||
| Low (55–78 from 0) | 191 (47) | 106 (41) | 85 (59) | |
| High (28–54 from 0) | 212 (53) | 153 (59) | 59 (41) | 0.0069 |
| Knee extended | ||||
| Low (64–89 from 0) | 201 (50) | 125 (48) | 76 (52) | |
| High (35–63 from 0) | 204 (50) | 134 (52) | 70 (48) | 0.3978 |
| Palpation/Observation | ||||
| Midfoot exostosis | ||||
| Absent | 141 (34) | 78 (30) | 63 (42) | |
| Present | 271 (66) | 185 (70) | 86 (58) | 0.0139 |
| PF insertion tenderness | ||||
| Absent | 322 (78) | 202 (77) | 120 (81) | |
| Present | 89 (22) | 60 (23) | 29 (19) | 0.2405 |
| PF midsole tenderness | ||||
| Absent | 194 (47) | 128 (49) | 66 (45) | |
| Present | 217 (53) | 135 (51) | 82 (55) | 0.9655 |
| Lesser toe deformity | ||||
| Absent | 147 (36) | 102 (39) | 45 (30) | |
| Present | 263 (64) | 160 (61) | 103 (70) | 0.0773 |
| Hallux valgus | ||||
| Absent | 263 (64) | 169 (64) | 94 (64) | |
| Present | 148 (36) | 94 (36) | 54 (36) | 0.6799 |
MTPJ, metatarsophalangeal joint; PF, plantar fascia.
P values are for the likelihood ratio test, with significance set at 0.25.
Manual backward selection was performed on 262 participants with complete data on all the included predictor variables and produced a final model with six parameters from four variables. These included the three force-entered variables (age, gender and BMI) and Arch Index. The final model was refitted to 269 participants with complete data on the retained predictor variables (Table II).
Table II.
Multivariable multi-level logistic regression model for symptomatic midfoot OA
| Predictor variable | Total | Symptomatic midfoot OA | Multi-level logistic regression Midfoot pain vs symptomatic midfoot OA |
|
|---|---|---|---|---|
| β (95% CI) | OR (95% CI) | |||
| People | (n = 269) | (n = 118) | ||
| Age (years) | ||||
| 50–64 | 137 (51) | 49 (42) | 1 | 1 |
| 65–74 | 89 (33) | 41 (35) | 0.49 (−0.31, 1.28) | 1.63 (0.73, 3.61) |
| 75+ | 43 (16) | 28 (24) | 1.16 (0.12, 2.20) | 3.19 (1.13, 9.05) |
| Gender | ||||
| Male | 121 (45) | 52 (44) | 1 | 1 |
| Female | 148 (55) | 66 (56) | 0.14 (−0.57, 0.85) | 1.15 (0.56, 2.35) |
| Body mass index | ||||
| Non-obese | ||||
| (<30 kg/m2) | 133 (49) | 49 (42) | 1 | 1 |
| Obese | ||||
| (≥30 kg/m2) | 136 (51) | 69 (58) | 0.71 (−0.04, 1.46) | 2.03 (0.96, 4.29) |
| Feet | (n = 404) | (n = 147) | ||
| Arch Index | ||||
| Normal (0.21–0.28) | 262 (65) | 85 (58) | 1 | 1 |
| High arch (<0.21) | 55 (14) | 15 (10) | −0.19 (−1.21, 0.83) | 0.82 (0.30, 2.28) |
| Low arch (>0.28) | 87 (22) | 47 (32) | 1.18 (0.31, 2.05) | 3.25 (1.36, 7.76) |
| Constant | −1.91 (−2.78, −1.03) | |||
β, beta coefficient; OR, odds ratio.
The model fit was poor for the observed data (calibration slope, 0.64, 95% CI: 0.39, 0.89). Although Arch Index was marginally informative when added to age, gender and BMI, discrimination remained fair-to-poor (AUC, 0.64, 95% CI: 0.58, 0.70 vs 0.62, 95% CI: 0.57, 0.68). For the overall model, sensitivity was 29.9% (95% CI: 22.7, 38.0) and specificity was 87.5% (95% CI: 82.9, 91.3).
Comparison of the beta coefficients and odds ratios for the final derived model (Table II) and the same estimates following bias-corrected bootstrapping indicated the model to be over-optimistic (data not shown). Overall bias-corrected model sensitivity was 25.9% (95% CI: 19.0, 33.7) and specificity was 89.9% (95% CI: 85.5, 93.3).
Sensitivity analyses
Repeating the modelling with variables in their original continuous form, did not identify any additional predictors, and overall model performance was effectively unchanged (calibration slope, 0.61, 95% CI: 0.38, 0.85; AUC, 0.66, 95% CI: 0.60, 0.71; sensitivity, 53.2%, 95% CI: 41.5, 64.7; specificity, 67.6%, 95% CI: 62.2, 72.6) (data not shown).
Discussion
Our study found that in a population-based sample of adults aged 50 years and older with midfoot pain, brief clinical assessments added little to age, gender and BMI in the discrimination of individuals with underlying midfoot OA on plain radiographs from those without these structural changes. Although several physical examination variables were associated with symptomatic midfoot OA, these were often either too weakly associated to be included in a diagnostic model (Foot Posture Index, subtalar inversion, plantar fascia insertion tenderness and lesser toe deformity) or lacked strong association after adjusting for age (navicular height) or combinations of age, gender, BMI and Arch Index (ankle dorsiflexion with the knee flexed and midfoot exostosis). The retained Arch Index predictor, indicating a more pronated foot posture among those with symptomatic midfoot OA, would appear to be biologically plausible and is consistent with earlier observations36, 37. In isolation, the Arch Index appeared to be a potentially useful predictor of symptomatic midfoot OA.
Although the low overall bias-corrected sensitivity (25.9%) is accompanied by a high specificity (89.9%), considered together with an AUC of 0.64, the final model remains only fair-to-poor at discriminating between people with and without symptomatic midfoot OA.
Accurate clinical diagnosis of symptomatic OA compared to plain radiographs has been mixed at other joint sites including the knee7, 38, 39, hip40,41, and hand42. Despite this, the clinical diagnosis of OA has been recommended in previous guidelines6, 43. At the foot, a diagnostic model developed to predict the presence of radiographic OA at the first MTPJ in adults with first MTPJ pain reported better performance than the present model (AUC, 0.87, 95% CI: 0.80, 0.93)8. Better discrimination may be explained by the more anatomically specific assessment of the first MTPJ used in the Zammit et al.8 study, compared to the broader foot examination we used to identify radiographic OA in the midfoot complex.
Strengths of this study are the population-based sample and standardised quality-controlled protocol for the collection of clinical and radiographic data. Despite this, there are a number of methodological issues that may explain the fair-to-poor performance of the model. First, the selected predictors may lack discriminatory ability. Even if measured perfectly, these clinical assessments may not be very strongly associated with the presence/absence of radiographic OA. For example, if they are causes of midfoot OA, they may be relatively weak causes, or if they are manifestations of midfoot OA, they may provide relatively weak signals. The strength of univariable association required for adequate discrimination is very high44. Given the complex pathogenesis and structure/pain associations in OA, discrimination from any one single measure is unlikely, which supports the need to evaluate multivariable clinical assessment models. The present model examined 16 predictor variables, however soft tissue assessments such as posterior tibial tendon dysfunction or local swelling and tenderness were not considered. It is possible that our model could be improved by adding more clinical predictors or other diagnostic markers45, 46.
Second, random and systematic errors in the clinical assessment measurements may also influence our findings. All assessors undertook protocol training and quality control monitoring, and we also chose clinical assessments previously shown to be reliable. However, we did not formally evaluate the reliability of clinical assessments within this study.
Third, symptomatic midfoot OA in an individual joint was defined as ≥2 for osteophytes or joint space narrowing using the scoring system established by Menz et al.15. With nearly half (43%) of the 274 eligible participants comprising the study sample having radiographic midfoot OA, this underscores the very high prevalence among older adults that report midfoot pain. Of the 263 feet with midfoot pain but classed as ‘no midfoot radiographic OA’, 248 (94%) had a score of one. Whilst grade one radiographic changes did not meet our threshold for symptomatic midfoot OA, it may be that disease manifestations and variations in structural appearance between grade one and two are too subtle to be clinically discernible. Recent work on knee OA has shown that grade one is a strong predictor of future grade two47. This suggests that grade one may have been a more suitable cut-off. Since it is not possible to know from this sample what the prevalence of grade one midfoot changes may be in an asymptomatic population, a question for future research is whether midfoot pain alone in adults aged 50 years and over without inflammatory arthritis provides adequate grounds for ‘ruling in’ symptomatic midfoot OA.
By assembling the sample from a cohort of individuals with foot pain in the last 12 months, it is possible that participants may have had concurrent symptoms elsewhere in their foot. Restricting analysis to individuals with foot pain only in the midfoot region was not possible due to small numbers. A sensitivity analysis, where univariable analyses for all predictor variables (excluding the force-entered variables: age, gender and BMI) was repeated after excluding 33 individuals with symptomatic first MTPJ OA (defined as co-occurring pain and radiographic change as defined above), indicated that 14 of the 16 observed associations had similar magnitude and precision that would not have statistically significantly altered the model (data not shown). Although the four selected joints can be reliably scored and used to represent midfoot OA, this present analysis pertains only to the identification of radiographic OA in the medial midfoot. Whilst clinically the occurrence of OA in the lateral midfoot is understood to be rare by comparison48, osteoarthritic changes in other midfoot joints could also contribute to symptoms in both midfoot pain and symptomatic midfoot OA groups. Furthermore, an alternative reference standard such as magnetic resonance imaging (MRI) or ultrasound may have generated different results and future studies could consider comparing the use of other imaging modalities for the foot.
Finally, misclassification may have arisen in the musculoskeletal midfoot pain domain. Narrowing this domain to exclude those with prevalent conditions such as diabetes, peripheral vascular disease or gout may help in being able to diagnose symptomatic midfoot OA, but this would also limit the generalizability of such insights as multimorbidity is often quite high in this age group. Of the 274 participants in this sample, 19% and 37% had self-reported diabetes and peripheral vascular disease respectively. Only 5% had a primary care consultation for gout within 18 months either side of research clinic attendance.
The population-based recruitment for this study meant that although the spectrum of severity across the sample is likely to be mild, this has relevance for primary care. Furthermore, although a physical examination may be of limited value for discriminating the presence or absence of symptomatic midfoot OA, brief clinical assessments may be better used to identify abnormal structural and postural presentations that could inform more targeted treatments.
In summary, this study did not allow development of a clinically practicable diagnostic model for symptomatic midfoot OA. Person-level information including age, gender and BMI provided only marginal diagnostic information and only very minor additional improvements in model performance were achieved with brief clinical assessment information. Before primary care clinicians can be confident that the diagnosis of symptomatic midfoot OA necessitates the use of X-ray, future research should examine whether these or other, more anatomically-specific, clinical assessments can show better discrimination in other samples, using alternative modelling techniques, or compared to other imaging modalities such as MRI and ultrasound.
Contributions
MJT, ER, GP, AM and HBM conceived the study. MJT, ER, GP and AM designed the study. MJT, ER and MM were responsible for data acquisition. Analysis was undertaken by MJT and TR. All authors interpreted data, drafted or revised the article critically for important intellectual content, and approved the final version of the manuscript.
Funding
This work was funded by an Arthritis Research UK Programme Grant (18174) and service support through West Midlands North CLRN. The study funders had no role in the study design; data collection, analysis or interpretation; in the writing of the paper; or in the decision to submit the paper for publication. MJT was supported by West Midlands Strategic Health Authority through a Nursing, Midwifery, and Allied Health Professionals Doctoral Research Training Fellowship (NMAHP/RTF/10/02). HBM is currently a National Health and Medical Research Council of Australia Senior Research Fellow (ID: 1020925).
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
We would like to thank the administrative, health informatics and research nurse teams of Keele University's Arthritis Research UK Primary Care Centre, the staff of the participating general practices and the Haywood Hospital, particularly Dr Jackie Saklatvala, Carole Jackson and the radiographers at the Department of Radiology. We would like to acknowledge the contributions of Linda Hargreaves, Gillian Levey, Liz Mason, Dr Jennifer Pearson, Julie Taylor and Dr Laurence Wood to data collection. We would also like to thank Adam Garrow and the University of Manchester for permission to use the foot manikin (© The University of Manchester 2000. All rights reserved).
Contributor Information
M.J. Thomas, Email: m.thomas@keele.ac.uk.
E. Roddy, Email: e.roddy@keele.ac.uk.
T. Rathod, Email: t.rathod@keele.ac.uk.
M. Marshall, Email: m.marshall@keele.ac.uk.
A. Moore, Email: a.j.moore@bristol.ac.uk.
H.B. Menz, Email: h.menz@latrobe.edu.au.
G. Peat, Email: g.m.peat@keele.ac.uk.
References
- 1.Thomas M.J., Roddy E., Zhang W., Menz H.B., Hannan M.T., Peat G.M. The population prevalence of foot and ankle pain in middle and old age: a systematic review. Pain. 2011;152:2870–2880. doi: 10.1016/j.pain.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Rekola K.E., Keinänen-Kiukaanniemi S., Takala J. Use of primary health services in sparsely populated country districts by patients with musculoskeletal symptoms: consultations with a physician. J Epidemiol Community Health. 1993;47:153–157. doi: 10.1136/jech.47.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mäntyeslkä P., Kumpusalo E., Ahonen R., Kumpusalo A., Kauhanen J., Viinamäki H. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001;89:175–180. doi: 10.1016/s0304-3959(00)00361-4. [DOI] [PubMed] [Google Scholar]
- 4.Jordan K.P., Kadam U.T., Hayward R., Porcheret M., Young C., Croft P. Annual consultation prevalence of regional musculoskeletal problems in primary care: an observational study. BMC Musculoskelet Disord. 2010;11:144. doi: 10.1186/1471-2474-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roddy E., Thomas M.J., Marshall M., Rathod T., Myers M., Menz H.B. The population prevalence of symptomatic radiographic foot osteoarthritis in community-dwelling older adults: cross-sectional findings from the Clinical Assessment Study of the Foot. Ann Rheum Dis. 2015;74:156–163. doi: 10.1136/annrheumdis-2013-203804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W., Doherty M., Peat G., Bierma-Zeinstra S.M.A., Arden N.K., Bresnihan B. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- 7.Peat G., Duncan R.C., Wood L.R.J., Thomas E., Muller S. Clinical features of symptomatic patellofemoral joint osteoarthritis. Arthritis Res Ther. 2012;14:R63. doi: 10.1186/ar3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zammit G.V., Munteanu S.E., Menz H.B. Development of a diagnostic rule for identifying radiographic osteoarthritis in people with first metatarsophalangeal joint pain. Osteoarthritis Cartilage. 2011;19:939–945. doi: 10.1016/j.joca.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Rathod T., Marshall M., Thomas M.J., Myers M., Menz H.B., Thomas E. Patterns of joint involvement in foot osteoarthritis: findings from the Clinical Assessment Study of the Foot. Osteoarthritis Cartilage. 2014;22(Supplement):S206. [Google Scholar]
- 10.Thomas M., Peat G., Rathod T., Moore A., Menz H.B., Roddy E. The epidemiology of midfoot pain and symptomatic midfoot osteoarthritis: cross-sectional findings from the Clinical Assessment Study of the Foot. Rheumatology (Oxford) 2014;53(Suppl 1):i129–i130. [Google Scholar]
- 11.Thomas M.J., Moore A., Roddy E., Peat G. “Somebody to say ‘come on we can sort this’”: a qualitative study of primary care consultation among older adults with symptomatic foot osteoarthritis. Arthritis Care Res. 2013;65:2051–2055. doi: 10.1002/acr.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roddy E., Myers H., Thomas M.J., Marshall M., D'Cruz D., Menz H.B. The clinical assessment study of the foot (CASF): study protocol for a prospective observational study of foot pain and foot osteoarthritis in the general population. J Foot Ankle Res. 2011;4:22. doi: 10.1186/1757-1146-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrow A.P., Silman A.J., Macfarlane G.J. The Cheshire Foot Pain and Disability Survey: a population survey assessing prevalence and associations. Pain. 2004;110:378–384. doi: 10.1016/j.pain.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Chatterton B.D., Muller S., Thomas M.J., Menz H.B., Rome K., Roddy E. Inter and intra-rater repeatability of the scoring of foot pain drawings. J Foot Ankle Res. 2013;6:44. doi: 10.1186/1757-1146-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menz H.B., Munteanu S.E., Landorf K.B., Zammit G.V., Cicuttini F.M. Radiographic classification of osteoarthritis in commonly affected joints of the foot. Osteoarthritis Cartilage. 2007;15:1333–1338. doi: 10.1016/j.joca.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Neogi T., Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39:1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavanagh P.R., Rodgers M.M. The arch index: a useful measure from footprints. J Biomech. 1987;20:547–551. doi: 10.1016/0021-9290(87)90255-7. [DOI] [PubMed] [Google Scholar]
- 18.Menz H.B., Fotoohabadi M.R., Wee E., Spink M.J. Visual categorisation of the arch index: a simplified measure of foot posture in older people. J Foot Ankle Res. 2012;5:10. doi: 10.1186/1757-1146-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redmond A.C., Crosbie J., Ouvrier R.A. Development and validation of a novel rating system for scoring standing foot posture: the Foot Posture Index. Clin Biomech. 2006;21:89–98. doi: 10.1016/j.clinbiomech.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Menz H.B., Munteanu S.E. Validity of 3 clinical techniques for the measurement of static foot posture in older people. J Orthop Sports Phys Ther. 2005;35:479–486. doi: 10.2519/jospt.2005.35.8.479. [DOI] [PubMed] [Google Scholar]
- 21.Hopson M.M., McPoil T.G., Cornwall M.W. Motion of the first metatarsophalangeal joint. Reliability and validity of four measurement techniques. J Am Podiatr Med Assoc. 1995;85:198–204. doi: 10.7547/87507315-85-4-198. [DOI] [PubMed] [Google Scholar]
- 22.Menadue C., Raymond J., Kilbreath S.L., Refshauge K.M., Adams R. Reliability of two goniometric methods of measuring active inversion and eversion range of motion at the ankle. BMC Musculoskelet Disord. 2006;7:60. doi: 10.1186/1471-2474-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennell K.L., Talbot R.C., Wajswelner H., Techovanich W., Kelly D.H., Hall A.J. Intra-rater and inter-rater reliability of a weight-bearing lunge measure of ankle dorsiflexion. Aust J Physiother. 1998;44:175–180. doi: 10.1016/s0004-9514(14)60377-9. [DOI] [PubMed] [Google Scholar]
- 24.Munteanu S.E., Strawhorn A.B., Landorf K.B., Bird A.R., Murley G.S. A weightbearing technique for the measurement of ankle joint dorsiflexion with the knee extended is reliable. J Sci Med Sport. 2009;12:54–59. doi: 10.1016/j.jsams.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Buchbinder R. Plantar fasciitis. N Engl J Med. 2004;350:2159–2166. doi: 10.1056/NEJMcp032745. [DOI] [PubMed] [Google Scholar]
- 26.McPoil T.G., Martin R.L., Cornwall M.W., Wukich D.K., Irrgang J.J., Godges J.J. Heel pain – plantar fasciitis: clinical practice guidelines linked to the international classification of function, disability, and health from the orthopaedic section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2008;38:A1–A18. doi: 10.2519/jospt.2008.0302. [DOI] [PubMed] [Google Scholar]
- 27.Menz H.B. Churchill Livingstone Elsevier; Edinburgh: 2008. Foot Problems in Older People. Assessment and Management. [Google Scholar]
- 28.Roddy E., Zhang W., Doherty M. Validation of a self-report instrument for assessment of hallux valgus. Osteoarthritis Cartilage. 2007;15:1008–1012. doi: 10.1016/j.joca.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Altman D.G. Chapman & Hall; London: 1991. Practical Statistics for Medical Research. [Google Scholar]
- 30.Bouwmeester W., Twisk J.W.R., Kappen T.H., van Klei W.A., Moons K.G., Vergouwe Y. Prediction models for clustered data: comparison of a random intercept and standard regression model. BMC Med Res Methodol. 2013;13:19. doi: 10.1186/1471-2288-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosmer D.W., Lemeshow S. 2nd edn. John Wiley & Sons, Inc; New York: 2000. Applied Logistic Regression. [Google Scholar]
- 32.Bouwmeester W., Moons K.G., Kappen T.H., van Klei W.A., Twisk J.W., Eijkemans M.J. Internal validation of risk models in clustered data: a comparison of bootstrap schemes. Am J Epidemiol. 2013;177:1209–1217. doi: 10.1093/aje/kws396. [DOI] [PubMed] [Google Scholar]
- 33.Steyerberg E.W., Harrell F.E., Jr., Borsboom G.J., Eijkemans M.J., Vergouwe Y., Habbema J.D. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 34.Royston P., Altman D.G., Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 35.Keenan A.M., Redmond A.C., Horton M., Conaghan P.G., Tennant A. The Foot Posture Index: Rasch analysis of a novel, foot-specific outcome measure. Arch Phys Med Rehabil. 2007;88:88–93. doi: 10.1016/j.apmr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Menz H.B., Munteanu S.E., Zammit G.V., Landorf K.B. Foot structure and function in older people with radiographic osteoarthritis of the medial midfoot. Osteoarthritis Cartilage. 2010;18:317–322. doi: 10.1016/j.joca.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Rao S., Baumhauer J.F., Nawoczenski D.A. Is barefoot regional plantar loading related to self-reported foot pain in patients with midfoot osteoarthritis. Osteoarthritis Cartilage. 2011;19:1019–1025. doi: 10.1016/j.joca.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Claessens A.A.M.C., Schouten J.S.A.G., van den Ouweland F.A., Valkenburg H.A. Do clinical findings associate with radiographic osteoarthritis of the knee? Ann Rheum Dis. 1990;49:771–774. doi: 10.1136/ard.49.10.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peat G., Thomas E., Duncan R., Wood L., Wilkie R., Hill J. Estimating the probability of radiographic osteoarthritis in the older patient with knee pain. Arthritis Rheum. 2007;57:794–802. doi: 10.1002/art.22785. [DOI] [PubMed] [Google Scholar]
- 40.Birrell F., Croft P., Cooper C., Hosie G., Macfarlane G., Silman A. Predicting radiographic hip osteoarthritis from range of movement. Rheumatology (Oxford) 2001;40:506–512. doi: 10.1093/rheumatology/40.5.506. [DOI] [PubMed] [Google Scholar]
- 41.Bierma-Zeinstra S.M.A., Oster J.D., Bernsen R.M.D., Verhaar J.A.N., Ginai A.Z., Bohnen A.M. Joint space narrowing and relationship with symptoms and signs in adults consulting for hip pain in primary care. J Rheumatol. 2002;29:1713–1718. [PubMed] [Google Scholar]
- 42.Marshall M., van der Windt D., Nicholls E., Myers H., Dziedzic K. Radiographic thumb osteoarthritis: frequency, patterns and associations with pain and clinical assessment findings in a community-dwelling population. Rheumatology (Oxford) 2011;50:735–739. doi: 10.1093/rheumatology/keq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W., Doherty M., Leeb B.F., Alekseeva L., Arden N.K., Bijlsma J.W. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68:8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 44.Pepe M.S., Janes H., Longton G., Leisenring W., Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 45.Pencina M.J., D'Agostino R.B., Sr., D'Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 46.Sundström J., Byberg L., Gedeborg R., Michaëlsson K., Berglund L. Useful tests of usefulness of new risk factors: tools for assessing reclassification and discrimination. Scand J Public Health. 2011;39:439–441. doi: 10.1177/1403494810396556. [DOI] [PubMed] [Google Scholar]
- 47.Kerkhof H.J., Bierma-Zeinstra S.M., Arden N.K., Metrustry S., Castano-Betancourt M., Hart D.J. Prediction model for knee osteoarthritis incidence, including clinical, genetic and biochemical risk factors. Ann Rheum Dis. 2014;73:2116–2121. doi: 10.1136/annrheumdis-2013-203620. [DOI] [PubMed] [Google Scholar]
- 48.Russell D.F., Ferdinand R.D. Review of the evidence: surgical management of 4th and 5th tarsometatarsal joint osteoarthritis. Foot Ankle Surg. 2013;19:207–211. doi: 10.1016/j.fas.2013.06.002. [DOI] [PubMed] [Google Scholar]