Abstract
Cystic lesions of the pancreas (CLPs) are increasingly diagnosed due to the growing utilization of cross-sectional imaging modalities. The differentiation between true cysts (epithelial tumors) and nonepithelial lesions (such as pseudocysts) relies on clinical and imaging characteristics, but more reliably obtained by endoscopic ultrasound (EUS) fine-needle aspiration. Due to their malignant potential, some of the true pancreatic cysts require further assessment and periodic follow-up. Therefore, it is important to establish a solid diagnosis at the time of detection of the various types of pancreatic cysts. Due to the limitations of cytology and biochemical markers in accurately classifying cyst pathology, the search for specific molecular markers associated with each type of cyst is ongoing. In this chapter, we will review some of the emerging molecular markers in pancreatic cystic fluid and their potential impact on endosonography and pancreatic cyst management.
Keywords: Deoxyribonucleic acid (DNA) mutations, intraductal papillary-mucinous neoplasm, micro-ribonucleic acids, molecular markers, mucinous cystic neoplasm, pancreatic cysts
INTRODUCTION
In recent years, the diagnosis of cystic lesions of the pancreas (CLPs) has increased dramatically due to the widespread use of cross-sectional radiologic imaging technologies.[1] According to radiological literature, the prevalence of CLPs on computed tomography (CT) and magnetic resonance imaging (MRI) is estimated to range between 2.4% and 14%.[2,3,4] Small CLPs of unclear clinical significance have been reported much more frequently (up to 39%) during screening of asymptomatic individuals with high-risk of pancreatic malignancy.[5] A recent population-based study placed the overall frequency of detecting malignancy in CLPs at 2.9% in patients surveyed for known pancreatic cysts, with an annual incidence of 0.4% per year.[6] Based on the presence of epithelial tissue, the World Health Organization (WHO) classifies CLPs into epithelial and nonepithelial lesions.[7] Inflammatory pancreatic fluid collections (pancreatitis-associated pseudocyst) are not considered true cysts due to the absence of epithelial component.
Mucinous CLPs are epithelial mucin-producing neoplasms, which are composed of two distinct groups: Intraductal papillary-mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs). Despite the fact that mucinous CLPs are considered premalignant, many of them remain indolent and do not exhibit an aggressive biological behavior. Because of this malignant potential; however, mucinous CLPs require baseline investigation to assess the risk of malignant transformation and follow-up at intervals. Furthermore, these tumors sometimes need surgical resection if already malignant at the time of diagnosis or strongly suspected to be so based on preoperative assessment. Therefore, it is necessary to distinguish between mucinous and non-mucinous CLPs prior to making final management recommendations.
Endoscopic ultrasound (EUS) has emerged over the last 2 decades as the diagnostic modality of choice to characterize CLPs and identify high-risk lesions that require surgical resection. High-risk EUS stigmata include presence of mural nodules, associated solid component, thick septations, and main duct dilation.[8] Fine needle aspiration (FNA) improves the accuracy of EUS imaging alone through the detection of mucinous epithelium and atypical cells.[9] However, the diagnostic utility of cytology is limited by its inadequate diagnostic yield, reported to be as low as 31%.[10] Cyst fluid tumor markers like carcinoembryonic antigen (CEA) have been used to differentiate potentially mucinous lesions. It has been noted that a CEA level of ≥192 ng/mL discriminates mucinous from non-mucinous pancreatic cysts with a sensitivity of 75%, specificity of 83%, and overall accuracy of 79%.[11] Nevertheless, CEA has a limited role in detecting malignancy or in predicting malignant progression.[12,13,14,15,16]
Recently, molecular markers have been suggested as an adjunct to cytology and CEA to accurately diagnose mucinous cysts and identify early malignancy within lesions. While most of the efforts in this field remain investigational, there have been advancements in the last decade that led to the adoption of some genetic assays in clinical practice. In this chapter, we will discuss the expanding role molecular markers play in the diagnosis of CLPs.
DEOXYRIBONUCLEIC ACID (DNA)-BASED CYST FLUID ASSAYS
K-ras and tumor suppressor genes mutations
Although numerous genetic alterations leading to pancreatic adenocarcinoma (PDAC) have been identified in the recent decade, the mutation in the K-ras oncogene remains the most extensively studied. Additional mutations in tumor suppressor genes (leading to what is referred to as loss of heterozygosity or allelic imbalance) can be found alone or in conjunction with K-ras mutations and accumulation of such genetic hits is believed to pave the way to carcinogenesis.[17,18]
K-ras has been evaluated as an adjunct to EUS-FNA to improve diagnostic yield in pancreatic solid lesions when cytology is inconclusive. In one of the largest series, Ogura et al.,[19] prospectively analyzed K-ras mutations in 394 pancreatic masses of various pathologies including PDACs and other benign lesions. When combined with cytohistopathology, the sensitivity, specificity, and diagnostic accuracy of this assay was 87%, 100%, and 89%, respectively. The addition of K-ras mutation analysis increased sensitivity and accuracy by 6% and 5%, respectively (P < 0.001). K-ras mutations were only found in 3% of non-pancreatic ductal adenocarcinoma masses. The addition of other somatic mutations of tumor suppressor genes like p53 and p16 to K-ras has been shown to increase the sensitivity of cancer detection to 100% in cases where FNA was inconclusive.[20] This was further confirmed by a meta-analysis including eight prospective studies and 931 patients that reported a pooled sensitivity of EUS-FNA for the diagnosis of PDAC of 81% and a specificity of 97%. K-ras gene analysis alone had sensitivity and specificity of 77% and 93%, respectively; while the combination of EUS-FNA plus K-ras mutation analysis increased the sensitivity to 89%, while retaining a high specificity of 92%. Overall, K-ras mutation testing applied to cases that were inconclusive by EUS-FNA reduced the false-negative rate by 56%, with a false-positive rate of 11%.[21]
This knowledge led to increased interest assessing similar changes in mucinous cystic lesions as they progress through various stages of dysplasia; and logically, mutations known in PDAC were investigated. It has been demonstrated that in malignant transformation of mucinous cysts,[22] K-ras mutations appear to occur early in the process.[23] In IPMN, this is reported to be a result of tumor suppressor gene inactivation, which is represented by loss of heterozygosity at 9p12 (p16) and 17 p13 (p53).[24] On the other hand, in benign serous cysts, Moore et al.,[25] described allelic losses on chromosome 10q in 50% and on chromosome 3p in 40% of cases, but reported complete absence of K-ras or p53 mutations in these tumors. Kim et al.,[26] found that one-third of MCNs were associated with K-ras mutations and further variable changes in tumor suppressor genes like p16 and p53, but were not observed in any serous cyst.
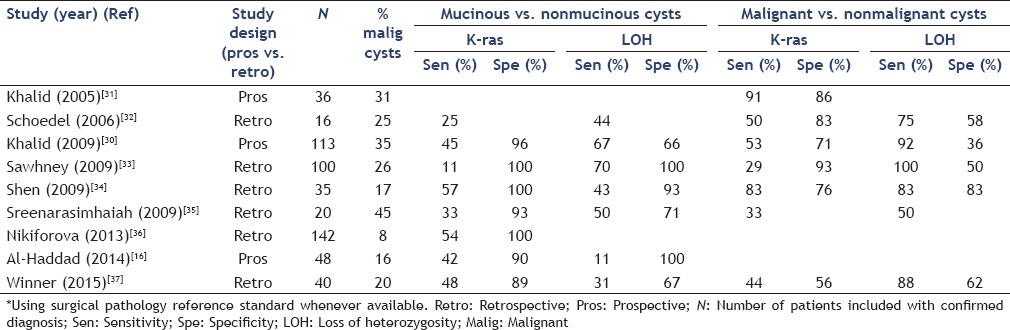
The use of the above markers has been evaluated in both pancreatic juice and cyst fluid.[27,28,29] In a multicenter, prospective study, Khalid et al.,[30] performed cyst fluid DNA analysis from EUS-FNA specimens in 124 patients with confirmatory surgical pathology or malignant cytology. Elevated amounts of cyst fluid DNA, high-amplitude K-ras mutations followed by specific allelic loss (loss of heterozygosity) were associated with maximum specificity for malignancy (96%). In all lesions, K-ras mutation provided 96% specificity for mucinous cysts. Several other studies have since demonstrated the utility of K-ras mutation as a highly specific marker for mucinous cysts particularly when cyst morphology and cytology were nondiagnostic [Table 1]. In a recent large series, Nikiforova et al., assessed the utilization of K-ras testing in 618 CLPs.[36] Surgical follow-up information was available for 142 (26%) patients and consisted of 53 K-ras-mutated and 89 K-ras-wild-type cysts. Overall, K-ras mutations had a specificity of 100%, but a sensitivity of 54% for mucinous differentiation. When stratified by cyst type, K-ras had a sensitivity of 67% and 14% for IPMNs and MCNs, respectively. The lower diagnostic performance of K-ras observed in MCNs could signify an alternative mutational pathway and certainly requires further investigation.
Table 1.
Studies assessing DNA-based markers for the diagnosis of benign and malignant mucinous lesions of the pancreas*
We have recently demonstrated the utility of DNA analysis of cystic fluid in a prospective study including CLPs lacking high risk morphological features.[16] Surgical resection was performed in 48 patients, confirming a mucinous pathology in 38 (79%). In this group, molecular analysis (including K-ras and multiple tumor suppressor gene mutations) had a sensitivity of 50% and a specificity of 80% in identifying mucinous lesions (accuracy of 56%). The combination of molecular analysis with cyst fluid CEA and cytology resulted in higher diagnostic performance for mucinous cysts than either one of its individual components, with a sensitivity, specificity, and accuracy of 74%, 70%, and 73%, respectively. In a multicenter retrospective study, the utilization of a commercially available DNA assay (RedPath Integrated Pathology, Pittsburgh, PA) was retrospectively assessed.[38] The lack of high risk morphological features and lack of mutations on cyst fluid analysis provided a 97% probability of benign follow-up for up to 7 years. The presence of multiple mutations was associated with a relative hazard ratio for malignant outcome of 50.2 and was more predictive of this outcome than the International Consensus Guidelines for mucinous pancreatic cysts management,[39] whose surgical resection criteria were associated with a hazard ratio of 9.
A recent systematic shed some light on the role molecular analysis play in the diagnosis of CLPs. This review included 12 studies totaling 1,115 patients, out of whom 362 had surgical pathology available. The sensitivity and specificity of cytology was 42% and 99%; while the sensitivity and specificity of K-ras was 39% and 95%, respectively. When the two were combined, the sensitivity and specificity were 71% and 88%, respectively.[40]
Since CEA continues to be considered the most useful biochemical marker for differentiating mucinous from non-mucinous pancreatic cysts,[11] several studies sought to compare the accuracy of CEA to DNA analysis. In a study of 100 patients with CLPs, only fair agreement between the two tests was found. CEA alone had the highest sensitivity (82%) compared to 11% for K-ras mutation and 70% for allelic imbalance.[33] Combining CEA with K-ras mutations achieved 100% sensitivity for the diagnosis of mucinous cyst in the same study. Another study reached the same conclusion where the combination of CEA and K-ras mutations correctly identified 94% of malignant/premalignant cysts.[41] The CEA and DNA analyses in these studies were considered complementary and together identified the vast majority of mucinous cysts included. In one study, patients with K-ras mutations were more likely to have atypia on cytology or pathology and higher CEA compared to wild-type K-ras.[42] Moreover, K-ras mutants were also more likely to be associated with two or more loss of heterozygosity mutations.
The utilization of DNA assays in clinical practice is evolving as further long-term performance data of such assays become available. One outcome-based study categorized cysts as non-indolent- and indolent-based on surgical pathology and clinical outcomes.[43] Fifty-one patients underwent pancreatic surgery (10 had malignant, 18 had mucinous, and 16 had benign cysts). Additional 63 patients were followed long-term and 13 patients died of pancreatic cancer. On multivariate regression analysis, the presence of cystic solid component, patient symptoms, cyst size >3 cm, and detection of K-ras mutations in cyst fluid were independently associated with a non-indolent course. Cyst fluid K-ras mutation was the only component of cyst fluid analysis associated with non-indolent behavior in this analysis. This behavior included the need to resect a mucinous cyst, cyst progression, development of malignancy, and death due to pancreatic cancer. Additionally, cysts with K-ras mutations were more likely to have atypia on cytology or pathology specimens.[43] As previously stated, other studies confirmed the association between aggressive biological behavior and K-ras mutations in IPMNs in particular, including progression to malignancy and referral to surgical resection.[42,44,45,46]
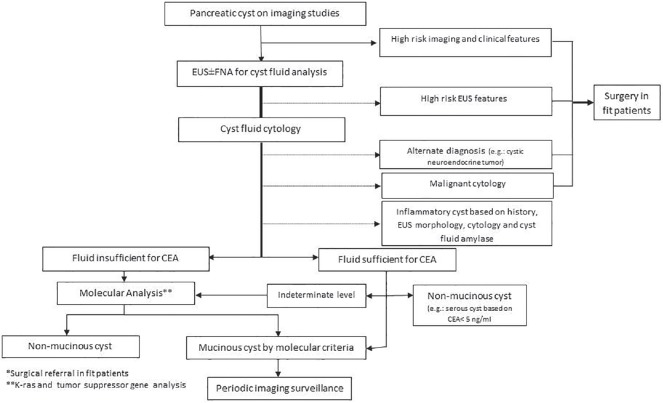
The above data strongly supports the use of K-ras mutations in pancreatic cyst fluid in patients with indeterminate or insufficient EUS-FNA cytology and/or cyst fluid CEA to diagnose mucinous cysts in clinical practice [Figure 1]. There is emerging data that the presence of this oncogene mutation could be a prognosticator for aggressive biological behavior. Many molecular labs associated with tertiary and referral institutions currently offer this assay in addition to one commercial assay (RedPath Integrated Pathology, Pittsburgh, PA). The amount of cyst fluid necessary for analysis is minimal-typically a fraction of a milliliter (few drops) making this a desirable assay when only a small amount of cyst fluid could be aspirated due to its high viscosity or small lesion size. In our practice, if the amount of fluid is too small for CEA assay (less than 0.5 mL); we typically provide small aliquot for a K-ras assay in addition to the cytology smear. If more than 0.5 mL of fluid is obtained, then we are typically able to allocate some fluid for all three main tests (CEA, K-ras, and cytology). Nevertheless, the cost of the molecular analysis remains one of the factors limiting its mass utilization, despite the fact that many insurers started to cover this test in the US. Finally, emerging literature suggest that genetic molecular changes occur frequently, and may not correlate with changes in cyst size, morphology, or CEA.[37,47] This makes interpretation of molecular assay findings on subsequent surveillance exams difficult, and therefore, in our practice, we tend not to repeat molecular analysis beyond the initial (index) exam.
Figure 1.
An algorithm for the management of pancreatic cystic lesions outlining the role of the currently available molecular assays in the diagnosis of such lesions. EUS: Endoscopic ultrasound; FNA: Fine needle aspiration, CEA: Carcinoembryonic antigen
GNAS
GNAS is an oncogene known to be mutated in pituitary and other uncommon types of tumors. Wu et al.,[48] initially reported on the prevalence of GNAS in 132 IPMNs — including both surgical specimens and fluid aspirated from IPMN — out of whom 66% were found to have a GNAS mutation and 81% were found to have a K-ras mutation. Interestingly, 96% of IPMNs had at least either one GNAS or K-ras mutation. A higher rate of GNAS mutations were found in more advanced and dysplastic IPMNs. A recent study evaluated the prevalence of GNAS mutations in small subcentimeter “incipient” pancreatic cysts that do not meet the cut-off for IPMN definition (1 cm). Mutational analysis revealed K-ras mutations in all 21 incipient IPMNs, whereas seven lesions (33%) in seven individual patients harbored GNAS mutations. The presence of GNAS mutations in those small “incipient” IPMNs suggests that a fraction of these cysts are in fact small IPMNs.
GNAS was assessed in another recent study by Lee et al., who reported on 68 resected CLPs of mucinous and non-mucinous pathology.[49] GNAS mutations were more common in IPMN compared to non-IPMN lesions (42 vs. 4%). No GNAS mutations were detected in PDAC and MCN, while two serous cystadenomas carried GNAS mutations. Double mutations with K-ras and GNAS were only present in IPMN.
Additionally, GNAS has been shown to be measurable in secretin-stimulated pancreatic juice. In a study where fluid was collected from the duodenum in 291 patients undergoing screening for pancreatic cysts/cancer,[50] 64% of patients with IPMNs were found to have a GNAS mutation contained within the pancreatic juice. No significant difference was found in the GNAS status of cyst fluid and the neoplastic grade or size of IPMNs. No control patients without IPMNs had GNAS mutations; and the presence of GNAS mutation at baseline was predictive of the development of new cysts at surveillance exam.
In summary, early data suggest that GNAS mutations are specific for IPMN and can be found concurrently with K-ras mutations. The association between degree of dysplasia and GNAS remains uncertain and requires further research to clarify.
NON-DNA CYST FLUID ASSAYS
Micro-ribonucleic acid (miRNA)
miRNAs are small, noncoding RNA molecules (18-25 nucleotides) involved in regulating gene expression at the post-transcriptional level. miRNAs appear to inhibit gene expression by either blocking protein translation or by degrading the mRNA.[51] miRNAs also appear to be involved in the induction of gene expression which occurs through binding to complementary regions in the promoter.[52] In the most recent database (miRBase21 release, http://microrna.sanger.ac.uk), over 28,000 mature miRNAs were identified in over 150 species, including 2,000 miRNAs in the human genome.
MicroRNAs are involved in cell signaling and are known to be upregulated and overexpressed in multiple types of tumors including pancreatic cancer, where differential expression of certain miRNAs was highly associated not only with the diagnosis of a malignant lesion but also the overall biological behavior.[53,54,55] In one study, cyst fluid aspirated from 40 pathologically diagnosed cyst specimens were analyzed for miRNA.[56] Three miRNAs (miR-21, miR-221, and miR-17-3p) were found to overexpressed in mucinous versus non-mucinous cysts at 7.0, 7.9, and 5.4-folds, respectively. Out of the three, miR-21 was found to perform the best with an area under the curve (AUC) of 0.89 giving a sensitivity of 80% and a specificity of 76% to diagnose a mucinous cyst. However, no miRNA was able to differentiate between the different types of mucinous lesions (IPMNs vs. MCNs).
The question of which miRNAs can be further pursued as makers of advanced dysplasia in mucinous CPLs remains the most pressing. A recent study[57] included 55 surgical IPMN specimens in addition to 65 cysts aspirated with EUS-FNA, where 750 different miRNA were analyzed. A total of 26 and 37 candidate miRNAs were identified from the surgical specimens and FNA fluid, respectively, that were differentially expressed between low- and high-grade IPMNs. Computational modeling yielded one with AUC of 1 with a sensitivity of 89% and a specificity of 100%. The most important miRNAs in the model were miR-24, miR-30-3p, miR-18a, miR-91a, and miR-342-3p. In a similar study, Lubezky et al.,[58] analyzed tissue from 55 surgically resected IPMNs and found 15miRNAs to be differentially expressed in benign vs. malignant IPMNs with miRNA levels increasing as the grade of the IPMN increased. Three miRNAs reached statistical significance in the analysis: miR-217, miR-708, and miR-10a. In another recent study using next generation sequencing, a wider spectrum of 13 miRNAs were found to be overexpressed and two were underexpressed in cyst fluid from resected IPMN lesions with invasive carcinoma.[59]
In conclusion, miRNAs are a very promising tool for identifying mucinous and dysplastic IPMN lesions. Despite the large number of human microRNAs identified to date, a few have been reliably associated with mucinous lesions (like miR-21) and more specific ones associated with malignancy or progression of dysplasia within benign lesions have yet to be confidently identified. Additionally, running microRNA assays remains costly and is currently conducted under mostly research protocols.
Miscellaneous cyst fluid markers
Cell-mediated and humoral immune responses have been studied in various cancers, including PDAC. Cytokine markers associated with Th1 and Th2 immune response in particular have been shown to discriminate pancreatic cancer from nonmalignant inflammatory and normal pancreatic tissue in both serum and pancreatic juice samples.[60,61] One study evaluated cytokines in cyst fluid from 40 patients undergoing resection for IPMNs including interleukin 1B (IL1B), IL2, IL4, IL5, IL8, IL10, IL12, and IL13.[62] IL1B was found to be overexpressed in high risk IPMNs (invasive carcinoma and high grade dysplasia) compared to low risk IPMNs on multivariate analysis. This was prospectively validated in 15 more surgically resected IPMN, and IL1B was associated with a positive predictive value of 71% for high risk IPMNs and negative predictive value of 75% for low risk IPMNs. Levels of cytokines, however, did not correlate with CEA levels or cytology.
Translational scientists continue to explore additional molecular markers with potential applications in pancreatic cysts. The list continues to grow to include proteomic studies assessing promising molecules such as olfactomedin-4[63] and Brg1 in addition to several mucinous glycoproteins.[64] Currently, markers remain in the exploration phase and will require validation before being adopted in clinical care.
In conclusion, the field of molecular testing of fluid in CPLs is expanding and is propelled forward by the deficiencies of cytology and CEA in accurately defining cyst pathology or future biological behavior. Of the various markers investigated recently, K-ras remains the most widely studied and utilized due to its specificity for mucinous lesions. Other biomarkers including microRNAs are very promising, but require further investigation.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–84. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 4.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–11. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Canto MI, Hruban RH, Fishman EK, et al. American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu BU, Sampath K, Berberian CE, et al. Prediction of malignancy in cystic neoplasms of the pancreas: A population-based cohort study. Am J Gastroenterol. 2014;109:121–9. doi: 10.1038/ajg.2013.334. [DOI] [PubMed] [Google Scholar]
- 7.Bosman FT. World Health Organization. International agency for research on cancer. WHO classification of tumours of the digestive system. In: Carneiro F, Hruban RH, Theise ND, editors. 4th ed. Vol. 3. Lyon: IARC Press; 2010. p. 417. [Google Scholar]
- 8.Al-Haddad M, El Hajj, II, Eloubeidi MA. Endoscopic ultrasound for the evaluation of cystic lesions of the pancreas. JOP. 2010;11:299–309. [PubMed] [Google Scholar]
- 9.Hong SK, Loren DE, Rogart JN, et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc. 2012;75:775–82. doi: 10.1016/j.gie.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 10.de Jong K, Poley JW, van Hooft JE, et al. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: Initial results from a prospective study. Endoscopy. 2011;43:585–90. doi: 10.1055/s-0030-1256440. [DOI] [PubMed] [Google Scholar]
- 11.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 13.Brugge WR. Should all pancreatic cystic lesions be resected? Cyst-fluid analysis in the differential diagnosis of pancreatic cystic lesions: A meta-analysis. Gastrointest Endosc. 2005;62:390–1. doi: 10.1016/j.gie.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: A prospective single-center experience. Gastrointest Endosc. 2006;64:697–702. doi: 10.1016/j.gie.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 15.Cizginer S, Turner B, Bilge AR, et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011;40:1024–8. doi: 10.1097/MPA.0b013e31821bd62f. [DOI] [PubMed] [Google Scholar]
- 16.Al-Haddad M, DeWitt J, Sherman S, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79–87. doi: 10.1016/j.gie.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Shin SH, Kim SC, Hong SM, et al. Genetic alterations of K-ras, p53, c-erbB-2, and DPC4 in pancreatic ductal adenocarcinoma and their correlation with patient survival. Pancreas. 2013;42:216–22. doi: 10.1097/MPA.0b013e31825b6ab0. [DOI] [PubMed] [Google Scholar]
- 18.Uemura K, Hiyama E, Murakami Y, et al. Comparative analysis of K-ras point mutation, telomerase activity, and p53 overexpression in pancreatic tumours. Oncol Rep. 2003;10:277–83. [PubMed] [Google Scholar]
- 19.Ogura T, Yamao K, Sawaki A, et al. Clinical impact of K-ras mutation analysis in EUS-guided FNA specimens from pancreatic masses. Gastrointest Endosc. 2012;75:769–74. doi: 10.1016/j.gie.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Salek C, Benesova L, Zavoral M, et al. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J Gastroenterol. 2007;13:3714–20. doi: 10.3748/wjg.v13.i27.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuccio L, Hassan C, Laterza L, et al. The role of K-ras gene mutation analysis in EUS-guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: A meta-analysis of prospective studies. Gastrointest Endosc. 2013;78:596–608. doi: 10.1016/j.gie.2013.04.162. [DOI] [PubMed] [Google Scholar]
- 22.Yan L, McFaul C, Howes N, et al. Molecular analysis to detect pancreatic ductal adenocarcinoma in high-risk groups. Gastroenterology. 2005;128:2124–30. doi: 10.1053/j.gastro.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Gerdes B, Wild A, Wittenberg J, et al. Tumor-suppressing pathways in cystic pancreatic tumors. Pancreas. 2003;26:42–8. doi: 10.1097/00006676-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Wada K, Takada T, Yasuda H, et al. Does “clonal progression” relate to the development of intraductal papillary mucinous tumors of the pancreas? J Gastrointest Surg. 2004;8:289–96. doi: 10.1016/j.gassur.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Moore PS, Zamboni G, Brighenti A, et al. Molecular characterization of pancreatic serous microcystic adenomas: Evidence for a tumor suppressor gene on chromosome 10q. Am J Pathol. 2001;158:317–21. doi: 10.1016/S0002-9440(10)63971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SG, Wu TT, Lee JH, et al. Comparison of epigenetic and genetic alterations in mucinous cystic neoplasm and serous microcystic adenoma of pancreas. Mod Pathol. 2003;16:1086–94. doi: 10.1097/01.MP.0000094088.37888.A6. [DOI] [PubMed] [Google Scholar]
- 27.Berthelemy P, Bouisson M, Escourrou J, et al. Identification of K-ras mutations in pancreatic juice in the early diagnosis of pancreatic cancer. Ann Intern Med. 1995;123:188–91. doi: 10.7326/0003-4819-123-3-199508010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Tada M, Teratani T, Komatsu Y, et al. Quantitative analysis of ras gene mutation in pancreatic juice for diagnosis of pancreatic adenocarcinoma. Dig Dis Sci. 1998;43:15–20. doi: 10.1023/a:1018803532543. [DOI] [PubMed] [Google Scholar]
- 29.Tateishi K, Tada M, Yamagata M, et al. High proportion of mutant K-ras gene in pancreatic juice of patients with pancreatic cystic lesions. Gut. 1999;45:737–40. doi: 10.1136/gut.45.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc. 2009;69:1095–102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Khalid A, McGrath KM, Zahid M, et al. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol. 2005;3:967–73. doi: 10.1016/s1542-3565(05)00409-x. [DOI] [PubMed] [Google Scholar]
- 32.Schoedel KE, Finkelstein SD, Ohori NP. K-Ras and microsatellite marker analysis of fine-needle aspirates from intraductal papillary mucinous neoplasms of the pancreas. Diagn Cytopathol. 2006;34:605–8. doi: 10.1002/dc.20511. [DOI] [PubMed] [Google Scholar]
- 33.Sawhney MS, Devarajan S, O’Farrel P, et al. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastrointest Endosc. 2009;69:1106–10. doi: 10.1016/j.gie.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Brugge WR, Dimaio CJ, et al. Molecular analysis of pancreatic cyst fluid: A comparative analysis with current practice of diagnosis. Cancer. 2009;117:217–27. doi: 10.1002/cncy.20027. [DOI] [PubMed] [Google Scholar]
- 35.Sreenarasimhaiah J, Lara LF, Jazrawi SF, et al. A comparative analysis of pancreas cyst fluid CEA and histology with DNA mutational analysis in the detection of mucin producing or malignant cysts. JOP. 2009;10:163–8. [PubMed] [Google Scholar]
- 36.Nikiforova MN, Khalid A, Fasanella KE, et al. Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: A clinical experience of 618 pancreatic cysts. Mod Pathol. 2013;26:1478–87. doi: 10.1038/modpathol.2013.91. [DOI] [PubMed] [Google Scholar]
- 37.Winner M, Sethi A, Poneros JM, et al. The role of molecular analysis in the diagnosis and surveillance of pancreatic cystic neoplasms. JOP. 2015;16:143–9. doi: 10.6092/1590-8577/2941. [DOI] [PubMed] [Google Scholar]
- 38.Al-Haddad MA, Kowalski T, Siddiqui A, et al. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy. 2015;47:136–42. doi: 10.1055/s-0034-1390742. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Gillis A, Cipollone I, Cousins G, et al. Does EUS-FNA molecular analysis carry additional value when compared to cytology in the diagnosis of pancreatic cystic neoplasm? A systematic review. HPB (Oxford) 2015;17:377–86. doi: 10.1111/hpb.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talar-Wojnarowska R, Pazurek M, Durko L, et al. A comparative analysis of K-ras mutation and carcinoembryonic antigen in pancreatic cyst fluid. Pancreatology. 2012;12:417–20. doi: 10.1016/j.pan.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Mertz H. K-ras mutations correlate with atypical cytology and elevated CEA levels in pancreatic cystic neoplasms. Dig Dis Sci. 2011;56:2197–201. doi: 10.1007/s10620-010-1556-z. [DOI] [PubMed] [Google Scholar]
- 43.Rockacy MJ, Zahid M, McGrath KM, et al. Association between KRAS mutation, detected in pancreatic cyst fluid, and long-term outcomes of patients. Clin Gastroenterol Hepatol. 2013;11:425–9. doi: 10.1016/j.cgh.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Lubezky N, Ben-Haim M, Marmor S, et al. High-throughput mutation profiling in intraductal papillary mucinous neoplasm (IPMN) J Gastrointest Surg. 2011;15:503–11. doi: 10.1007/s11605-010-1411-8. [DOI] [PubMed] [Google Scholar]
- 45.Mizuno O, Kawamoto H, Yamamoto N, et al. Single-pattern convergence of K-ras mutation correlates with surgical indication of intraductal papillary mucinous neoplasms. Pancreas. 2010;39:617–21. doi: 10.1097/MPA.0b013e3181c75d9b. [DOI] [PubMed] [Google Scholar]
- 46.Jang JY, Park YC, Song YS, et al. Increased K-ras mutation and expression of S100A4 and MUC2 protein in the malignant intraductal papillary mucinous tumor of the pancreas. J Hepatobiliary Pancreat Surg. 2009;16:668–74. doi: 10.1007/s00534-009-0105-7. [DOI] [PubMed] [Google Scholar]
- 47.DeWitt JM, Al-Haddad M, Sherman S, et al. Alterations in cyst fluid genetics following endoscopic ultrasound-guided pancreatic cyst ablation with ethanol and paclitaxel. Endoscopy. 2014;46:457–64. doi: 10.1055/s-0034-1365496. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee LS, Doyle LA, Houghton J, et al. Differential expression of GNAS and KRAS mutations in pancreatic cysts. JOP. 2014;15:581–6. doi: 10.6092/1590-8577/2432. [DOI] [PubMed] [Google Scholar]
- 50.Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013;62:1024–33. doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambros V, Bartel B, Bartel DP, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–9. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Place RF, Li LC, Pookot D, et al. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105:1608–13. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szafranska AE, Davison TS, John J, et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26:4442–52. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 54.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 55.Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–54. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryu JK, Matthaei H, Dal Molin M, et al. Elevated microRNA miR-21 levels in pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology. 2011;11:343–50. doi: 10.1159/000329183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthaei H, Wylie D, Lloyd MB, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–24. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lubezky N, Loewenstein S, Ben-Haim M, et al. MicroRNA expression signatures in intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2013;153:663–72. doi: 10.1016/j.surg.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Paris PL, Chen J, et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015;356:404–9. doi: 10.1016/j.canlet.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noh KW, Pungpapong S, Wallace MB, et al. Do cytokine concentrations in pancreatic juice predict the presence of pancreatic diseases? Clin Gastroenterol Hepatol. 2006;4:782–9. doi: 10.1016/j.cgh.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 61.Seicean A, Popa D, Mocan T, et al. Th1 and Th2 profiles in patients with pancreatic cancer compared with chronic pancreatitis. Pancreas. 2009;38:594–5. doi: 10.1097/MPA.0b013e31819313d0. [DOI] [PubMed] [Google Scholar]
- 62.Maker AV, Katabi N, Qin LX, et al. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2011;17:1502–8. doi: 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuoghi A, Farina A, Z’Graggen K, et al. Role of proteomics to differentiate between benign and potentially malignant pancreatic cysts. J Proteome Res. 2011;10:2664–70. doi: 10.1021/pr2000557. [DOI] [PubMed] [Google Scholar]
- 64.Maker AV, Katabi N, Gonen M, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2011;18:199–206. doi: 10.1245/s10434-010-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]