Abstract
Pancreatic cysts are being encountered frequently because of rapid advances in radiologic technology and an increased cross-sectional imaging demand. Management of pancreatic cystic tumors is challenging because most of them are asymptomatic; they are potentially malignant, and surgery has substantial morbidity and mortality. Endoscopic ultrasound (EUS)-guided fine-needle aspiration of pancreatic cystic tumors is accepted as a minimally invasive technique, which also enables injection of ablative agents into cysts under EUS guidance. In this review, the basic procedural steps and technical considerations of cyst ablation and their clinical issues including safety, feasibility, and therapeutic outcome will be summarized.
Keywords: Cyst ablation, endoscopic ultrasound (EUS), ethanol lavage, paclitaxel injection, pancreatic cystic tumor
INTRODUCTION
The prevalence of asymptomatic pancreatic cyst was approximately 24.3% in the autopsies of elderly Japanese patients.[1] The trend of increased discovery of pancreatic cystic tumors is particularly important because these tumors are composed of a wide range of lesions ranging from benign to malignant.[2] The revised Sendai guidelines for the management of pancreatic cysts suggest operation for mucinous cystic neoplasms (MCNs), main duct intraductal papillary mucinous neoplasms, and branch duct intraductal papillary mucinous neoplasms with high-risk features.[3] Surveillance is recommended for mucinous cysts that do not meet the above surgical indications. However, patients with advanced comorbid conditions or of an elderly age are considered at high risk for surgery, especially if pancreaticoduodenectomy is contemplated for cystic tumors of the head of the pancreas. Moreover, patients tend to be reluctant to undergo a pancreatic resection in the absence of definite evidence of malignancy. Given the perioperative morbidity and mortality risk of pancreatic resection, an individualized treatment strategy based on a risk-benefit analysis[4] is essential, and a minimally invasive alternative modality is required. Recent research has focused on the promise of endoscopic ultrasound (EUS)-guided ethanol ablation as a safe and effective therapeutic modality for pancreatic cystic tumors. In this review, the technical issues and treatment outcomes of EUS-guided cyst ablation will be summarized.
TECHNIQUE OF ENDOSCOPIC ULTRASOUND-GUIDED ABLATION OF PANCREATIC CYST
EUS imaging is the prerequisite step in evaluating the characteristics of pancreatic cystic tumors. Anatomic and morphologic features of pancreatic cysts are evaluated as follows: Location, number, size, and characteristic features (septation, wall thickness, mural nodule, calcifications, communication with pancreatic duct, dilatation of pancreatic duct).[5] EUS-guided fine-needle aspiration is performed with a 22-gauge or 19-gauge needle. The cysts are evacuated until they almost completely collapse. The amount of aspirated fluid, its color, clarity, and viscosity are recorded, and samples are sent to check amylase and carcinoembryonic antigen (CEA) concentration and for cytological examination.[5] Ablative agent is injected into the cyst using an equal volume of originally aspirated fluid. The main concept of ablation is the lavage of the cyst, repeated injection, and aspiration of ablative agent in the cystic cavity for 3-5 min.[5] Instead of lavage, retention (a single injection of ethanol and removal after 3-5 min) could be another option according to the characteristics of the cystic lesion. At the end of the lavage session, the ethanol and fluid mixture in the cyst is completely evacuated. In selected cases, paclitaxel is injected into the cyst (equal volume of originally aspirated fluid from the cyst) and is retained in the cyst without removal. We prefer using a 22-gauge needle to 19-gauge or 25 gauge needle, since a 19-gauge needle is prone to leakage of ablative agent and a 25-gauge needle is not appropriate for aspiration of viscous cystic fluid and injection of viscous paclitaxel.
CHOICE OF ABLATIVE AGENTS
To date, ethanol (80-99%) and paclitaxel have been investigated as ablative agents in pancreatic cysts.
A commonly used ablative agent is ethanol owing to its cost-effectiveness, ready availability, and rapid ablative effect. The low viscosity of ethanol permits repeated filling and emptying of the cyst. The concept of ablation of the pancreatic cyst is based on the experience of cyst ablation in the liver, kidney, and thyroid gland.[6] Ethanol injection is believed to induce cell membrane breakdown, rapid protein degradation, and vascular blockage.[7,8]
Paclitaxel is a chemotherapeutic agent used in the treatment of ovarian cancer, breast cancer, and lung cancer.[9] Its mechanism of action seems to involve inhibition of the disassembly of microtubules during cell division and the induction of apoptosis.[9] Cremophor EL, a polyoxyethylated castor oil vehicle of paclitaxel, forms a product of a hydrophobic and viscous character, exerting a durable ablative effect in the cyst with a low risk of leakage.[10] The highly viscous paclitaxel solution should be diluted 1:1 in 0.9% normal saline and be prepared in a final dose concentration of 3 mg/mL paclitaxel before injection.[11] Alternative formulations of paclitaxel, using a less viscous delivery vehicle, (e.g., polymeric micelle) can be injected without dilution.[11] In contrast to ethanol, paclitaxel needs to be retained in the cystic cavity to induce apoptosis and tumor cell death. Removing paclitaxel is difficult because of high viscosity.
OUTCOMES OF CLINICAL TRIALS
Clinical trials of endoscopic ultrasound-guided cyst ablation are summarized in Table 1
Table 1.
Clinical trials of EUS-guided ethanol ablation for cystic tumor of pancreas
An initial pilot study involving 25 patients with presumed mucinous cysts evaluated the efficacy and safety of EUS-guided ethanol lavage.[12] During follow-up of 6-12 months, 8 out of 23 patients had complete resolution. All septated cysts persisted during follow-up after ethanol ablation. Histologic evidence of epithelial ablation was observed in five patients who underwent resection. No complications were reported.[12]
A prospective double-blinded randomized control trial was conducted involving 42 patients who underwent EUS-guided ablation using 80% ethanol or saline lavage of cysts.[13] Ethanol lavage resulted in a greater decrease in cyst size than did saline.[13] The overall cyst ablation was 33.3%. Complication rates were similar in both groups, with one instance of complication of acute pancreatitis in the ethanol lavage group.
To enhance the effect of ethanol ablation therapy, Oh et al. performed EUS-guided injection and lavage of ethanol followed by injection of paclitaxel into pancreatic cystic tumors.[15] An initial pilot study found that complete resolution of pancreatic cystic tumors was observed in 11 out of 14 (79%) patients after treatment.[15] A minor complication including hyperamylasemia and abdominal pain was found in only one patient. The authors suggested that ethanol lavage with paclitaxel injection was a safe and effective method for treating pancreatic cystic tumors.[15] A subsequent study involving 52 patients who underwent EUS-guided ethanol lavage with paclitaxel injection for pancreatic cysts was conducted.[11] Forty-three patients were followed up for a median period of 22 months, and four patients underwent surgery for persistent cysts.[11] The histopathological extent of epithelial ablation for those who underwent surgery ranged from 0% to 100% [Figure 1]. Ultimately, complete response was achieved in 29 (62%) patients, partial response in 6 patients, and persistent cysts in 12 patients.[11] The small cyst volume was the only independent predictor associated with complete resolution of the cyst.[11] Complications were transient fever (n = 1), abdominal discomfort (n = 1), pericystic spillage (n = 1), pancreatitis (n = 1), and splenic vein obliteration (n = 1).[11]
Figure 1.
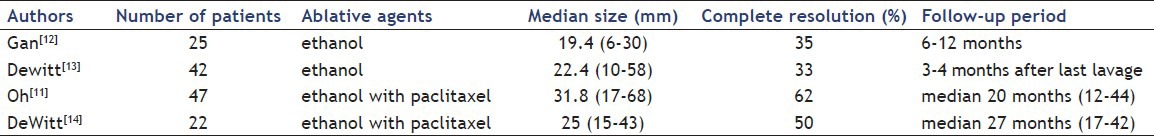
Extent of histopathological changes after ablation of pancreatic cystic lesion:[16] (a) Atrophied epithelia with fibrosis (b) Transitional zone (c) Remnant mucinous epithelia
DiMaio et al. analyzed the effectiveness of multiple EUS-guided ethanol lavage sessions for the ablation of pancreatic cystic tumors.[5] The outcome demonstrated that multiple ethanol lavage sessions resulted in a greater decrease in the size compared with one ethanol lavage treatment. Complete resolution of the cyst was not seen in any patient after the first EUS-guided ethanol lavage but it was achieved in 38% of patients (5 out of 13) who underwent two sequential ethanol lavage treatments.[5]
A recent study evaluated the changes in pancreatic cystic fluid DNA following EUS-guided ablation with ethanol and paclitaxel.[14] Cystic fluid analysis after ablation revealed the elimination of all baseline mutations in eight out of 11 patients. The post-ablation image demonstrated complete response in 10 (50%) patients, partial response in 5 (25%) patients, and persistent cyst in 5 (25%) patients. These data suggested that EUS-guided ablation with ethanol and paclitaxel may possibly eliminate mutant DNA in neoplastic pancreatic cysts.[14]
Questions remain unanswered despite the results of the clinical trials described above. First, the risk of overtreatment of benign cysts is inevitable in the absence of pretreatment pathologic confirmation. Differential diagnosis of pancreatic cysts is still challenging despite an extensive workup, and inclusion criteria of current clinical trials were mainly based on the cross-sectional imaging. Second, pathologic confirmation of cysts is not possible without surgical resection; current evidence cannot suggest the preventive role of ablative therapy in premalignant lesion. Third, complete response on imaging study does not mean that an eradication of tumor and regular follow-up are required even after the initial response of ablative therapy. Fourth, the results of long-term follow-up are essential to figure out the possibility of preventing cancer development.
SPECIAL CONSIDERATIONS FOR ABLATION
The baseline cyst size may be predictive of clinical success. Oh et al. reported that an initial cyst size of less than 35 mm was predictive of complete resolution after ablation.[11] However, DeWitt et al. found that a size of less than 25 mm did not predict resolution after ethanol ablation.[17]
The ideal morphology for ablation is a unilocular cyst. However, the presence of multilocularity is not considered a contraindication to treatment. From the viewpoint of cyst morphology, cysts that have a few locules (up to 5-6) are preferably indicated because the presence of multiple septa in cysts may prevent the delivery of the ablative agent into all locules.[18,19] In a septated cyst, ablation may be performed initially in one locule of the cyst. Thereafter, the needle sequentially penetrates each septum and ablation is continued until all the locules are covered. Polycystic serous cystadenomas are not considered to be an indication for ablation due to the presence of as many as hundreds of small cysts, which hinder uniform distribution of the ablative agent.[18] EUS-guided ethanol ablation should only be considered in selected cases. An experienced endoscopist should consider before treatment whether the morphology favors a safe and effective ablation of the entire cyst epithelia.
The permanence of the cyst ablative effect is a major concern. Successful cyst ablation achieved for an initial short-term period may not guarantee a long-term resolution and the preventive effect against premalignant lesion.[16] Oh et al. reported in their clinical trial of 47 patients who underwent EUS-guided ethanol lavage with paclitaxel injection that complete response was achieved in 29 (62%) patients over a median follow-up period of 21.7 months.[11] Two MCNs that showed a good initial response showed regrowth at 12 months.[11] Even radiologic evidence may not ensure the histologic evidence of cyst resolution. Close monitoring is mandatory even after complete radiologic resolution although an individualized surveillance policy may be acceptable based on risk factors.[11]
To enhance the therapeutic efficacy of ablation, multiple EUS-guided ethanol lavage sessions can be tried [Figure 2].[19] Dimaio et al. found that multiple lavage sessions resulted in a significantly greater decrease in the size of the cyst compared with only one treatment session.[5] Tailored intervention based on the internal cyst morphology is required to improve the treatment efficacy, and a booster ablation should be considered when complete resolution is predicted initially and an additional ablation session can be safely performed.[19]
Figure 2.
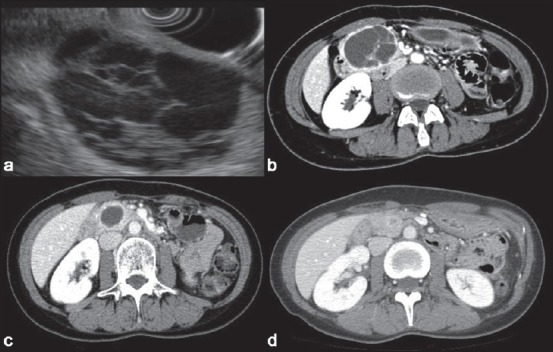
Endoscopic ultrasonography (EUS) and computed tomography (CT) of a septated pancreatic cyst.[19] A 5-cm septated cyst was noted in the head of the pancreas on (a) EUS and (b) CT (c). A follow-up CT 24 months after the first ethanol lavage showed a marked decrease in size but the cyst still persisted. The patient underwent a second session of ethanol lavage (d). A follow-up CT scan 2 years after the second lavage demonstrated cyst resolution, demonstrating sustained treatment response
SAFETY ISSUES
Regardless of the ablative method, safety and response to treatment are important issues. Acute pancreatitis is the major concern in performing ethanol injection into the pancreatic cysts. A plausible mechanism for pancreatitis may relate to a direct cytotoxic effect on the ductal epithelium, the activation of zymogen, and/or pancreatic ductal hypertension due to direct injection of the ethanol.[5] Previous studies reported pancreatitis in 2% to 10%, abdominal pain in 2% to 20%, and intracystic bleeding in 2% of the patients.[11,12,13,14] Unintentional injection of an ablative agent into the pancreatic parenchyma might increase the risk of pancreatitis.[11]
Thrombosis in the portal venous system adjacent to the cyst has been reported in two patients after ethanol lavage and paclitaxel injection [Figure 3].[11,20] Evidence of extensive inflammation around the cysts is found as wall calcification and fibrosis in the resected specimen.[20] Moreover, pericystic leakage of ablative agents may induce the extension of inflammation into the adjacent vascular structure.[20] When a cyst is located close to the portal venous system, the volume of injected ethanol should be calculated thoroughly and extensive lavage is contraindicated to prevent ethanol leakage and the local spread of inflammation.[20]
Figure 3.
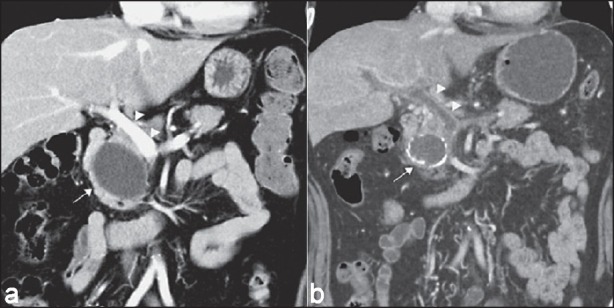
Portal vein thrombosis after EUS-guided pancreatic cyst ablation.[20] (a) Initial CT scan: A 5.2 cm cyst (arrow) located adjacent to the main portal vein (arrow heads) at the head of the pancreas. (b) Follow-up CT scan after second cyst ablation: Cyst (arrow) with rim calcification and portal vein thrombosis (arrow heads)
To further enhance the ablative effect, the chemotherapeutic agent, paclitaxel, is injected into the cyst. Although a low dose of the chemotherapeutic agent is locally injected, theoretical concerns have been raised about the possibility of adverse systemic events. Oh et al. demonstrated that plasma paclitaxel concentration after local injection of paclitaxel was so low that it was nearly undetectable and rarely caused systemic toxicity.[21]
PROPOSED INDICATIONS AND FUTURE PERSPECTIVES
Careful patient selection is needed to avoid unnecessary procedures, and to tailor interventions to maximize therapeutic efficacy. Indications proposed for EUS-guided cyst ablation include:
A benign mucinous cystic tumor or serous cystic tumor,
Cysts that are morphologically indeterminate,
A cystic tumor greater than 2 cm and less than 5 cm in diameter,
A unilocular or oligolocular cyst, with six or fewer locules,
Cyst without communication with the main pancreatic duct, and
Patients who are reluctant to opt for surgery or who possess high perioperative risk.
EUS-assisted ethanol ablation of a pancreatic cystic tumor is considered to be a relatively safe and potentially effective procedure. When a pancreatic cystic tumor is completely resolved, the surveillance strategy may be modified and may no longer even be necessary if regrowth is not observed over a certain period of time. Further studies are warranted to determine the long-term outcomes of ethanol ablation of pancreatic cystic neoplasms. However, technical difficulty and vigorous effort during the procedure are the obstacles of widespread use of this technique. EUS-guided ablation therapy needs a fine control of endoscope and stable positioning of the needle into the cyst during lavage. A dedicated device and novel ablative agent should be developed to improve technical feasibility. EUS-guided ablation therapy might offer an alternative to the traditional surgical approach in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kimura W, Nagai H, Kuroda A, et al. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 2.Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: A review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–78. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Fernández-del Castillo C, Adsay V, et al. ; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 5.DiMaio CJ, DeWitt JM, Brugge WR. Ablation of pancreatic cystic lesions: The use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas. 2011;40:664–8. doi: 10.1097/MPA.0b013e3182128d06. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Seo DW, Paik WH, et al. Ethanol lavage of huge hepatic cysts by using EUS guidance and a percutaneous approach. Gastrointest Endosc. 2014;80:1014–21. doi: 10.1016/j.gie.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Bean WJ, Rodan BA. Hepatic cysts: Treatment with alcohol. AJR Am J Roentgenol. 1985;144:237–41. doi: 10.2214/ajr.144.2.237. [DOI] [PubMed] [Google Scholar]
- 8.Gelczer RK, Charboneau JW, Hussain S, et al. Complications of percutaneous ethanol ablation. J Ultrasound Med. 1998;17:531–3. doi: 10.7863/jum.1998.17.8.531. [DOI] [PubMed] [Google Scholar]
- 9.Ho KY, Brugge WR EUS 2008 Working Group. EUS 2008 Working Group document: Evaluation of EUS-guided pancreatic-cyst ablation. Gastrointest Endosc. 2009;69(Suppl):S22–7. doi: 10.1016/j.gie.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 10.Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–14. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 11.Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–9. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Gan SI, Thompson CC, Lauwers GY, et al. Ethanol lavage of pancreatic cystic lesions: Initial pilot study. Gastrointest Endosc. 2005;61:746–52. doi: 10.1016/s0016-5107(05)00320-2. [DOI] [PubMed] [Google Scholar]
- 13.DeWitt J, McGreevy K, Schmidt CM, et al. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: A randomized, double-blind study. Gastrointest Endosc. 2009;70:710–23. doi: 10.1016/j.gie.2009.03.1173. [DOI] [PubMed] [Google Scholar]
- 14.DeWitt JM, Al-Haddad M, Sherman S, et al. Alterations in cyst fluid genetics following endoscopic ultrasound-guided pancreatic cyst ablation with ethanol and paclitaxel. Endoscopy. 2014;46:457–64. doi: 10.1055/s-0034-1365496. [DOI] [PubMed] [Google Scholar]
- 15.Oh HC, Seo DW, Lee TY, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636–42. doi: 10.1016/j.gie.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Oh HC, Brugge WR. EUS-guided pancreatic cyst ablation: A critical review (with video) Gastrointest Endosc. 2013;77:526–33. doi: 10.1016/j.gie.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 17.DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862–6. doi: 10.1016/j.gie.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 18.Oh HC, Seo DW, Kim SC, et al. Septated cystic tumors of the pancreas: Is it possible to treat them by endoscopic ultrasonography-guided intervention? Scand J Gastroenterol. 2009;44:242–7. doi: 10.1080/00365520802495537. [DOI] [PubMed] [Google Scholar]
- 19.Oh HC, Seo DW, Song TJ. Resolution of a septated pancreatic cyst by booster endoscopic ultrasonography-guided ablation. J Dig Dis. 2011;12:497–9. doi: 10.1111/j.1751-2980.2011.00538.x. [DOI] [PubMed] [Google Scholar]
- 20.Oh HC, Seo DW, Kim SC. Portal vein thrombosis after EUS-guided pancreatic cyst ablation. Dig Dis Sci. 2012;57:1965–7. doi: 10.1007/s10620-012-2103-x. [DOI] [PubMed] [Google Scholar]
- 21.Oh HC, Kang H, Brugge WR. Cyst fluid amylase and CEA levels in the differential diagnosis of pancreatic cysts: A single-center experience with histologically proven cysts. Dig Dis Sci. 2014;59:3111–6. doi: 10.1007/s10620-014-3254-8. [DOI] [PubMed] [Google Scholar]


