Abstract
Endoscopic ultrasound (EUS) is a key modality for the evaluation of suspected pancreatic cystic neoplasms (PCNs), as the entire pancreatic gland can be demonstrated with high spatial resolution from the stomach and duodenum. Detailed information can be acquired about the internal contents of the cyst(s) [septum, capsule, mural nodules (MNs)], its relation with the main pancreatic duct (MPD), and any parenchymal changes in the underlying gland. PCNs comprise true cysts and pseudocysts. True cysts can be neoplastic or nonneoplastic. Here, we describe serous cystic neoplasm (SCN), mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasm (IPMN) as prototype neoplastic cysts, along with nonneoplastic lymphoepithelial cysts (LECs).
Keywords: Endoscopic ultrasound (EUS), intraductal papillary mucinous neoplasm (IPMN), mucinous cystic, serous cystic
INTRODUCTION
Pancreatic cystic neoplasms (PCNs) are being detected with increasing frequency, primarily as a result of increased physician awareness and widespread availability of cross-sectional imaging in concert with technological improvements.[1] PCNs comprise a potpourri of lesions types including intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN), serous cystic neoplasm (SCN), lymphoepithelial cyst (LEC), epidermoid cysts, and cystic degeneration of solid tumors. The most important issues that the endosonographer faces are ensuring appropriate patient treatment while avoiding overtreatment and undue patient anxiety, and selecting patients who would benefit from surgery.[2,3]
The diagnostic accuracy of EUS morphology to differentiate between cystic lesions of the pancreas ranges from 51% to 90%.[4] One study[5] found that 20% of resected PCNs are benign, even in a tertiary hospital, indicating the challenge involved in accurately diagnosing PCN.
Being less invasive as well as having imaging from anatomical proximity to the pancreas with high resolution renders EUS as an ideal tool for diagnosing PCN.[6] Recent improvements in EUS hardware and software have provided new options for image enhancement. Contrast-enhancement and elastography are novel modalities that might improve the detection and characterization of suspected lesions.[7,8] Here, we review the application of EUS morphology for the differentiation and follow-up of PCNs.
CLASSIFICATION
Figure 1 shows the classification of PCNs, which consist of true cysts covered with the epithelium and pseudocysts that are not.
Figure 1.
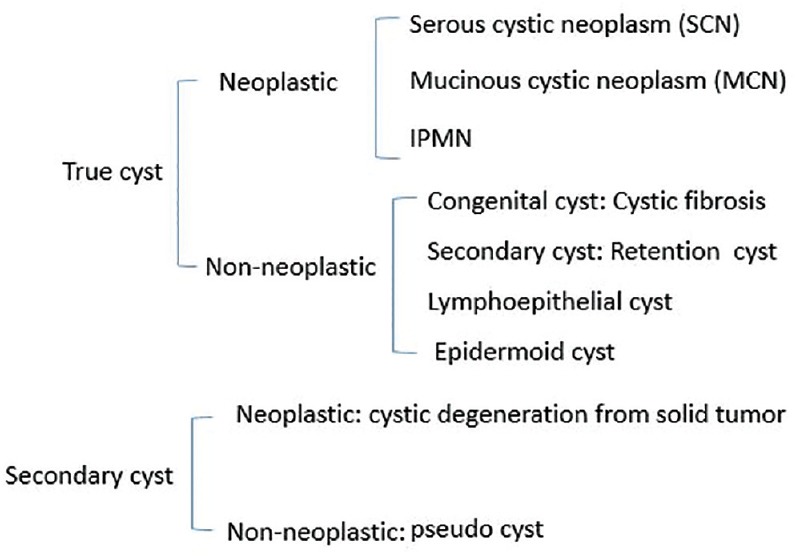
Classification of pancreatic cystic neoplasms
True cysts can be neoplastic or nonneoplastic. Neoplastic true cysts include SCN, MCN, and IPMN types of neoplasms. Nonneoplastic cysts comprise cystic fibrosis, and retention, lymphoepithelial, and epidermoid cysts.
Secondary cysts can be classified as cystic degeneration from solid tumors and nonneoplastic pseudocysts.
TRUE CYSTS — NEOPLASMS
Serous cystic neoplasms
Characteristics
SCNs are cystic tumors formed by glycogen-rich epithelial cells that produce serous fluid. The epithelial cells are cuboidal with clear cytoplasm.[9] One characteristic feature of SCN is a rich vascular formation referred to as a RecN-epithelial network located immediately below the epithelium.
SCNs have typical[1,10] honeycomb-like microcystic [Figures 2a and 3], or mixed macrocystic and microcystic morphology [Figures 2b and 4], with an aggregation of large- and small-cyst architectures. Morphologic variants include macrocystic type SCN with only larger cysts [Figure 2c],[1,10] and the solid type SCN [Figures 2d and 5][10] with macroscopically unrecognizable microcysts. Thus, SCN comprises neoplasms with various gross and microscopic findings. Around 10% of SCNs are unilocular without an obvious microcystic component.[11]
Figure 2.
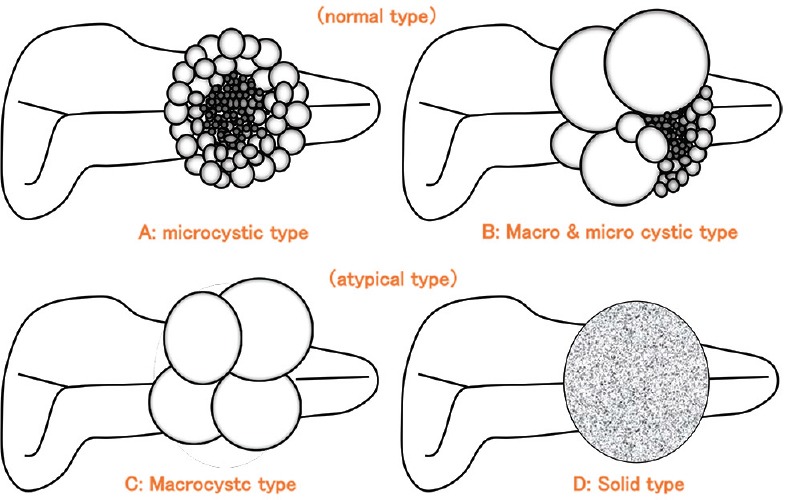
Classification of serous cystic neoplasms (SCNs)
Figure 3.
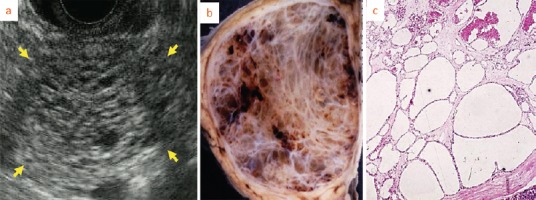
Microcystic type serous cystic neoplasms (SCNs). EUS image (arrow) shows honeycomb-like appearance caused microcyst accumulation
Figure 4.
Macrocystic type and microcystic type serous cystic neoplasms (SCNs)
Figure 5.
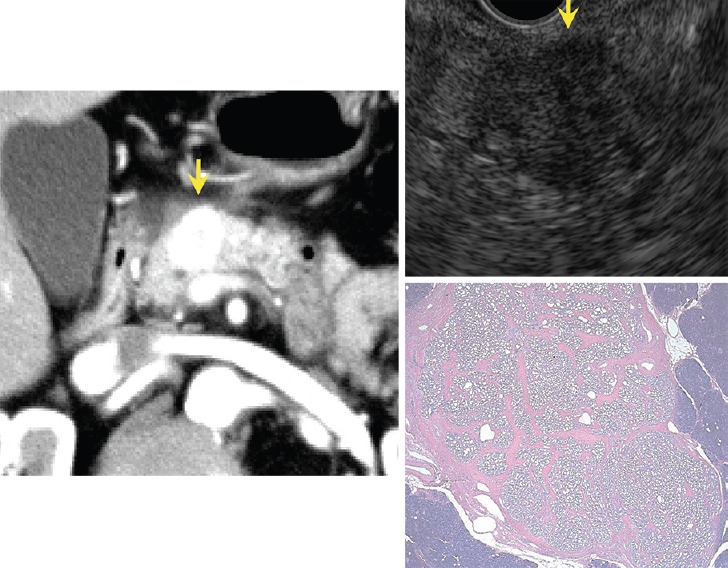
Solid type serous cystic neoplasm (SCN). Enhancement is intense on CT. Honeycomb-like appearance is undetectable by EUS but pathological microscopic findings show small microcystic appearance
Imaging features
A microcystic honeycomb structure is the typical structure of SCN. Low-resolution imaging modalities such as percutaneous ultrasound cannot define the internal microcystic structures, and only show a solid, echo-poor mass, unlike EUS.[12] Contrast-enhanced EUS can further clarify microcyst structures.[13] The septum and wall are usually thin, and typical microcystic SCN with calcification of a central fibrosis scar can be clearly visualized on EUS images.[14,15] The macrocystic type without a microcystic component requires differentiation from solid type SCN, MCN, or branch type IPMN. Solid type SCN needs to be differentiated from neuroendocrine neoplasms (NENs), which can be accomplished using EUS-FNA.[16,17]
Treatment strategies
Malignant pancreatic SCNs are very rare, as the malignant potential is thought to be only 1-2%.[12,18] Therefore, as a reasonable strategy for lesions that can be diagnosed as SCN by an imaging modality would be surveillance imaging.[1] Considering the possibility of rare malignant SCN, a cautious follow-up should be advised.
Mucinous cystic neoplasms
Characteristics
MCNs arise almost exclusively (>95%) in women (age range: 40-50 years), typically in the body and tail of the pancreas.[15,19] Estrogen receptor-positive and progesterone receptor-positive ovarian type stroma (OTS) is characteristic [Figure 6]. New data suggest that the proliferative effect of estrogen and progesterone hormones plays an important contributory role in the development of MCN, which is consistent with the female gender restriction of MCN.[20] MCN predominantly manifests as unilocular or multilocular cystic lesions, usually with mucinous contents. The epithelial lining of MCN usually takes the form of a single layer of cuboidal to columnar cells with minimal variation in nuclear size and shape. Unlike SCN, MCN can be malignant.[15]
Figure 6.
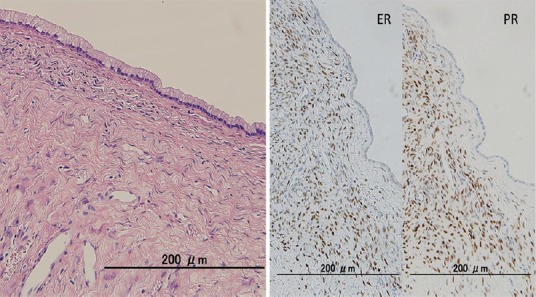
Ovarian type stroma (OTS) immediately below the epithelium detected by microscopy is estrogen-receptor and progesterone-receptor positive
Imaging features
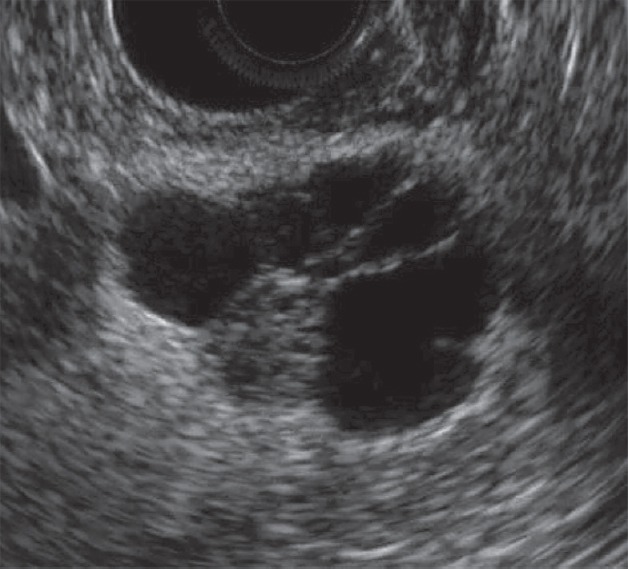
MCNs usually present as macrocystic lesions with a rounded morphology, an irregular septum, thick wall and complex content that can be particulate, viscous and dense due to mucin and hemorrhage [Figure 7]. Ovarian type stroma is a defining feature of MCN[17] that assumes the form of bland spindle cells forming a compact layer immediately beneath the epithelium. Cysts in cysts [Figure 8][21] appearance is characteristic of MCN, and EUS can detect even small cysts within cysts. MCN does not communicate with the pancreatic ductal system although a recent Japanese multicenter study[21] found that 18.1% of MCNs had a luminal connection with the pancreatic ductal system.
Figure 7.
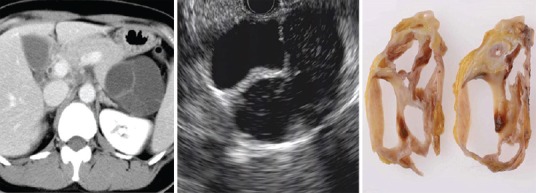
Image of MCN adenoma shows round macrocystic lesion with thickened wall and septum
Figure 8.
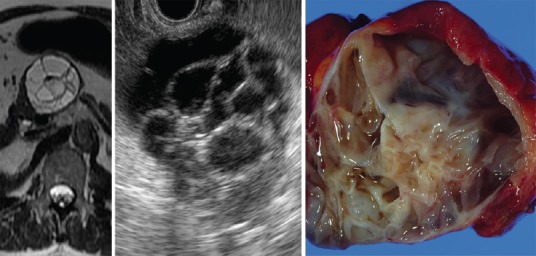
Image of MCN adenoma shows cysts within cysts
Although mural cysts are characteristic of MCN, they can be misdiagnosed by computed tomography (CT) as mural nodules (MNs). Thus, mural cysts determined by CT should be confirmed by EUS [Figure 9].
Figure 9.
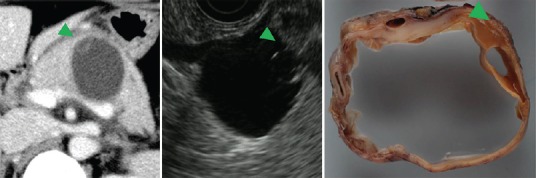
Mural cyst of MCN adenoma. Apparent mural nodule in CT image appearing as a mural cyst in EUS image is MCN
Because cyst cavities are independent and do not communicate with individual cysts in MCN, differences in the echogenicity of individual cysts contents can be visualized by EUS. Peripheral calcification along the thick wall has been found in 10-25% of the patients.
Treatment strategies
A Japanese multicenter study[21] found that the frequency of invasive and noninvasive carcinoma arising from MCN is 3.8% and 13.4%, respectively. Resection is considered the choice of treatment for most MCNs because of their malignant potential, when patients have low or acceptable operative risk. This aggressive approach is supported by the 2012 consensus guidelines of the International Association of Pancreatology.[22]
Intraductal papillary mucinous neoplasms
More IPMN cysts are being recognized as neoplasms. A large, retrospective study of resected pancreatic cystic lesions found that IPMNs were the most commonly resected types of cysts, accounting for about 2% of resections.[23] IPMNs are classified as main duct (MD)-IPMNs, branch duct (BD)-IPMNs arising from branches, or mixed IPMNs arising in both the MD and the side branches.
Not only do MD-IPMNs and BD-IPMNs morphologically differ, but they also have different histological gastric (70%), intestinal (20%), pancreatobiliary (<10%) and oncocytic (<5%) subtypes with varying degrees of risk of malignancy and aggressiveness.[24] The term “incipient IPMN” has been recently proposed to describe cysts between 0.5 cm and 1 cm in diameter, lined with a gastric type mucinous epithelium.[25]
Imaging features of branch duct and mixed type intraductal papillary mucinous neoplasm [Figure 10]
Figure 10.
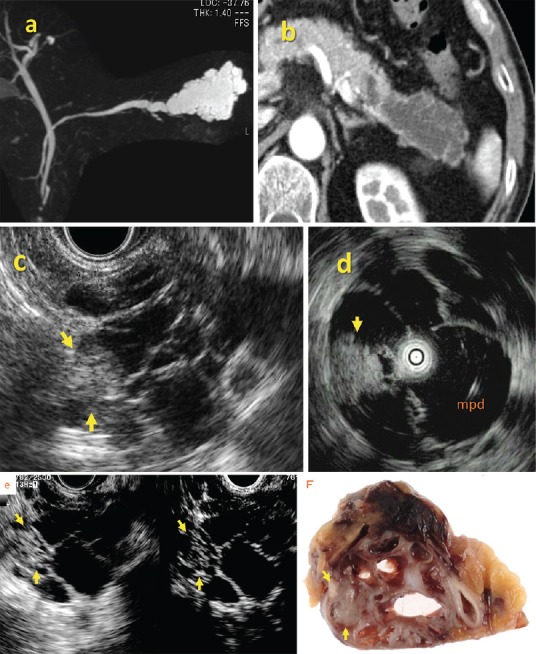
Branch duct (BD) type IPMN (noninvasive carcinoma). Hyperechoic mass (arrow) in dilated branch is evident on EUS and IDUS images. Contrast agents show blood flow signals in mass indicating mural nodules
A key feature of IPMN is dilation of a BD or MD due to proliferative papillary tumors, or large amounts of secreted intraductal mucin. Accordingly, the size of IPMN depends on the diameter of the dilated BD, MD, and MNs. The diameter of dilated ducts can be measured by either Multi-Detectors CT (MDCT) or magnetic resonance cholangiopancreatography (MRCP) but only EUS can accurately define the size of MNs. The presence of MNs is considered to be the most reliable indicator of whether an IPMN tumor is benign or malignant.[26,27,28] However, a cutoff diameter for differentiating benign from malignant nodules has been controversial, and ranges between 5 mm and 10 mm.
The revised international guidelines 2012[29] recommend that cysts with worrisome features should undergo a detailed evaluation by EUS. A notable change from previous guidelines is that side-branch dilation of ≥3 cm in BD-IPMN, which was previously an indication for surgery,[30] is now considered a worrisome feature. These lesions should be carefully assessed for MNs using EUS. In all of these situations, EUS is a key modality for the risk stratification and classification of IPMN lesions.
Protein plaques can be differentiated by their characteristic annular hyperechoic appearance with a low echoic central part [Figure 11], whereas mucin is difficult to discriminate from MNs by B-mode imaging. Caution is required in this regard because the misdiagnosis of mucin as a nodule will lead to overdiagnosis of malignancy. The use of ultrasound contrast agents such as Sonazoid® can rule out MNs if there is an absence of blood flow signals in the intracystic structures.[31]
Figure 11.
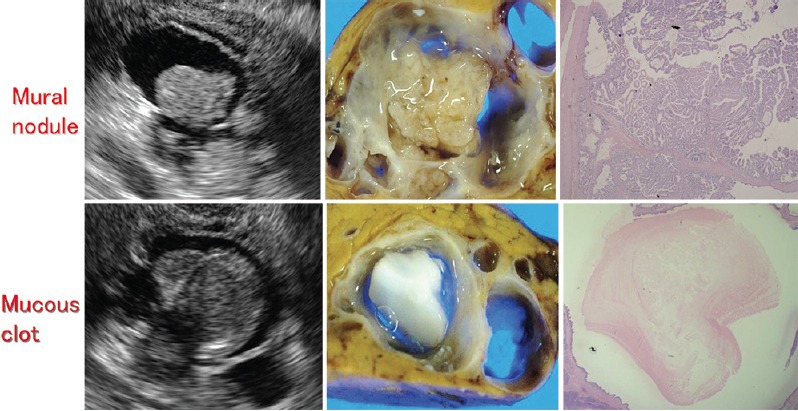
Upper and lower panels show mural nodule (adenoma) and mucous clot, respectively
Imaging features of main duct type intraductal papillary mucinous neoplasms [Figure 12]
Figure 12.
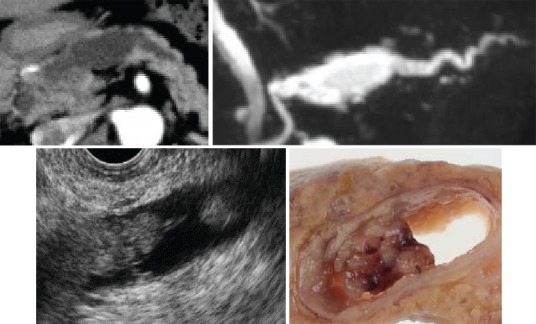
MD type IPMN (noninvasive carcinoma). Mural nodule is detectable inside MPD
MD IPMNs are defined by segmental or diffuse MD dilation to >5 mm, without BD dilation >5 mm. An MD diameter ≥10 mm is considered to be high-risk stigmata, according to the International Consensus Guidelines,[29] and resection is recommended in such situations. The entire pancreatic duct until the ampulla of Vater should be observed to rule out upstream ductal dilation due to chronic pancreatitis or obstruction by a pancreatic ductal adenocarcinoma (PDAC).
Large papillary projections in a dilated MD can be evaluated using CT or MRCP but EUS might be the most suitable modality for visualizing smaller nodules. MD IPMN has a tendency of superficial intraductal extension. Hence, accurate preoperative assessment of the longitudinal extent of the disease is important to decide whether pancreatectomy should be total or partial. Intraductal ultrasound (IDUS) and peroral pancreatoscopy (POPS) are other useful modalities for determining the extent of intraductal superficial lesions.
Protocol for follow up of patients with intraductal papillary mucinous neoplasm
When both high-risk stigmata and worrisome features are absent, MNs are undetectable by EUS, lesions are localized in the BD, and cytology findings of pancreatic juice are negative, the revised international guidelines specify a follow-up protocol using CT/magnetic resonance imaging (MRI) and EUS depending on whether a cyst is 1-2 cm or 2-3 cm in diameter.[29]
A large natural history study of BD-IPMN from Japan,[32] based on a nationwide survey, found an 18% rate of disease progression, and stable disease in 82% of the 349 patients without MNs at initial diagnosis, over a mean observation period of 3.7 years. The rate of IPMC occurrence in these patients was 2.5%.
The recently reported rates of PDAC concomitant with IPMN range from 2.0% to 9.3%. Hence, patients with IPMN should be regarded as being at a high risk of developing PDAC.
These observations highlight the importance not only of evaluating IPMN lesions but also of carefully observing the entire pancreas during follow-up EUS studies to avoid overlooking PDAC. Regular EUS evaluations can allow early detection of PDAC in such situations.
TRUE CYSTS — NONNEOPLASMS
Lymphoepithelial cysts [Figure 13]
Figure 13.
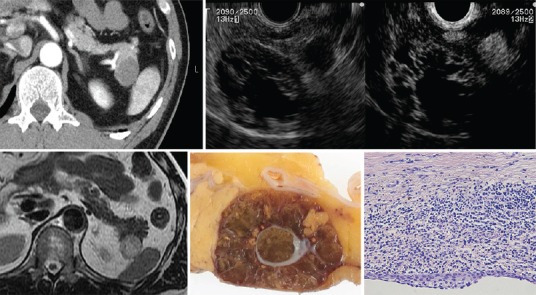
Lymphoepithelial cysts
Characteristics
LECs are rare, benign lesions characterized by mature squamous epithelium with surrounding nonneoplastic lymphoid elements.[33,34,35] They are more common in men, tend to occur predominantly as extrapancreatic lesions, and are well-demarcated from surrounding pancreatic parenchyma [Figure 13].[36,37,38]
Imaging features
LECs can be unilocular (40%) or multilocular (60%), well-circumscribed, and sharply distinct from the adjacent pancreas. They can resemble extrapancreatic lesions. The cyst wall is usually 1-3 mm thick, and the cysts contain cheesy keratinaceous debris. On EUS, LECs have a thin septum and wall, a clear margin, and contain viscous echogenic keratin.
Treatment strategy
Malignant transformation has not been described, and a conservative follow-up strategy is recommended.
Secondary cysts
Cystic degeneration of solid tumors
Solid pseudopapillary tumors,[39,40] cystic neuroendocrine neoplasms of the pancreas,[41,42,43,44] acinar cell neoplasms,[45] and pancreatic adenocarcinomas (adenosquamous and/or anaplastic carcinoma)[46] can occasionally appear as cystic masses.
SUMMARY
We have reviewed the key EUS features of pancreatic cystic lesions. B-mode EUS alone cannot be used to diagnose pancreatic cystic lesions and thus, contrast-enhanced ultrasound, CT, and MRI are required to supplement the EUS findings of such lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sakorafas GH, Smyrniotis V, Sarr MG. Pancreatic cystic neoplasms. 2015 [Google Scholar]
- 2.Hocke M, Cui XW, Domagk D, et al. Pancreatic cystic lesions: The value of contrast-enhanced endoscopic ultrasound to influence the clinical pathway. Endosc Ultrasound. 2014;3:123–30. doi: 10.4103/2303-9027.131040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennon AM, Wolfgang C. Cystic neoplasms of the pancreas. J Gastrointest Surg. 2013;17:645–53. doi: 10.1007/s11605-012-2072-6. [DOI] [PubMed] [Google Scholar]
- 4.Barresi L, Tarantino I, Granata A, et al. Pancreatic cystic lesions: How endoscopic ultrasound morphology and endoscopic ultrasound fine needle aspiration help unlock the diagnostic puzzle. World J Gastrointest Endosc. 2012;4:247–59. doi: 10.4253/wjge.v4.i6.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho CS, Russ AJ, Loeffler AG, et al. Preoperative classification of pancreatic cystic neoplasms: The clinical significance of diagnostic inaccuracy. Ann Surg Oncol. 2013;20:3112–9. doi: 10.1245/s10434-013-2986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakai Y, Isayama H, Itoi T, et al. Role of endoscopic ultrasonography in pancreatic cystic neoplasms: Where do we stand and where will we go? Dig Endosc. 2014;26:135–43. doi: 10.1111/den.12202. [DOI] [PubMed] [Google Scholar]
- 7.Teoh AY, Tang RS. Clinical evaluation of new diagnostic modalities of endoscopic ultrasound for pancreaticobiliary diseases. Dig Endosc. 2015;27(Suppl 1):55–9. doi: 10.1111/den.12444. [DOI] [PubMed] [Google Scholar]
- 8.Kitano M, Kamata K, Imai H, et al. Contrast-enhanced harmonic endoscopic ultrasonography for pancreatobiliary diseases. Dig Endosc. 2015;27(Suppl 1):60–7. doi: 10.1111/den.12454. [DOI] [PubMed] [Google Scholar]
- 9.Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: Tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–21. doi: 10.1097/01.sla.0000179651.21193.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hruban R, Pitman M, Klimstra D. Washington DC: American Registry of Pathology, Armed Forces Institute of Pathology; 2007. AFIP atlas of tumor pathology. Series 4 fascicle 6: Tumors of the Pancreas; p. 6. [Google Scholar]
- 11.Kubo H, Nakamura K, Itaba S, et al. Differential diagnosis of cystic tumors of the pancreas by endoscopic ultrasonography. Endoscopy. 2009;41:684–9. doi: 10.1055/s-0029-1214952. [DOI] [PubMed] [Google Scholar]
- 12.Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan Pancreas Society. Pancreas. 2012;41:380–7. doi: 10.1097/MPA.0b013e31822a27db. [DOI] [PubMed] [Google Scholar]
- 13.Robinson SM, Scott J, Oppong KW, et al. What to do for the incidental pancreatic cystic lesion? Surg Oncol. 2014;23:117–25. doi: 10.1016/j.suronc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Kucera JN, Kucera S, Perrin SD, et al. Cystic lesions of the pancreas: Radiologic-endosonographic correlation. Radiographics. 2012;32:E283–301. doi: 10.1148/rg.327125019. [DOI] [PubMed] [Google Scholar]
- 15.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, et al. Primary pancreatic cystic neoplasms revisited. Part I: Serous cystic neoplasms. Surg Oncol. 2011;20:e84–92. doi: 10.1016/j.suronc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Huang P, Staerkel G, Sneige N, et al. Fine-needle aspiration of pancreatic serous cystadenoma: Cytologic features and diagnostic pitfalls. Cancer. 2006;108:239–49. doi: 10.1002/cncr.21911. [DOI] [PubMed] [Google Scholar]
- 17.Nelsen EM, Buehler D, Soni AV, et al. Endoscopic ultrasound in the evaluation of pancreatic neoplasms-solid and cystic: A review. World J Gastrointest Endosc. 2015;7:318–27. doi: 10.4253/wjge.v7.i4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galanis C, Zamani A, Cameron JL, et al. Resected serous cystic neoplasms of the pancreas: A review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820–6. doi: 10.1007/s11605-007-0157-4. [DOI] [PubMed] [Google Scholar]
- 19.Acar M, Tatli S. Cystic tumors of the pancreas: A radiological perspective. Diagn Interv Radiol. 2011;17:143–9. doi: 10.4261/1305-3825.DIR.3254-09.1. [DOI] [PubMed] [Google Scholar]
- 20.Sano M, Driscoll DR, De Jesus-Monge WE, et al. Activated wnt signaling in stroma contributes to development of pancreatic mucinous cystic neoplasms. Gastroenterology. 2014;146:257–67. doi: 10.1053/j.gastro.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: A multi-institutional study of the Japan Pancreas Society. Pancreas. 2011;40:67–71. doi: 10.1097/MPA.0b013e3181f749d3. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152(Suppl 1):S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid RM, Siveke JT. Approach to cystic lesions of the pancreas. Wien Med Wochenschr. 2014;164:44–50. doi: 10.1007/s10354-013-0244-y. [DOI] [PubMed] [Google Scholar]
- 25.Adsay V, Mino-Kenudson M, Furukawa T, et al. Members of the Verona Consensus Meeting, 2013. Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001173. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu Y, Yamaue H, Maguchi H, et al. Validation of a nomogram for predicting the probability of carcinoma in patients with intraductal papillary mucinous neoplasm in 180 pancreatic resection patients at 3 high-volume centers. Pancreas. 2015;44:459–64. doi: 10.1097/MPA.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu Y, Yamaue H, Maguchi H, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: Analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas. 2013;42:883–8. doi: 10.1097/MPA.0b013e31827a7b84. [DOI] [PubMed] [Google Scholar]
- 28.Hijioka S, Shimizu Y, Mizuno N, et al. Can long-term follow-up strategies be determined using a nomogram-based prediction model of malignancy among intraductal papillary mucinous neoplasms of the pancreas? Pancreas. 2014;43:367–72. doi: 10.1097/MPA.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Chari S, Adsay V, et al. International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 31.Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: Differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628–34. doi: 10.1097/SLA.0b013e3181a189a8. [DOI] [PubMed] [Google Scholar]
- 32.Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: A multicenter study in Japan. Pancreas. 2011;40:364–70. doi: 10.1097/MPA.0b013e31820a5975. [DOI] [PubMed] [Google Scholar]
- 33.Terakawa H, Makino I, Nakagawara H, et al. Clinical and radiological feature of lymphoepithelial cyst of the pancreas. World J Gastroenterol. 2014;20:17247–53. doi: 10.3748/wjg.v20.i45.17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mege D, Grégoire E, Barbier L, et al. Lymphoepithelial cyst of the pancreas: An analysis of 117 patients. Pancreas. 2014;43:987–95. doi: 10.1097/MPA.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 35.Fujita K, Fujimoto M, Terajima H, et al. Education and imaging. Hepatology: Pancreatic lymphoepithelial cyst mimicking mucinous cystic neoplasm. J Gastroenterol Hepatol. 2015;30:235. doi: 10.1111/jgh.12829. [DOI] [PubMed] [Google Scholar]
- 36.Adsay NV, Hasteh F, Cheng JD, et al. Squamous-lined cysts of the pancreas: Lymphoepithelial cysts, dermoid cysts (teratomas), and accessory-splenic epidermoid cysts. Semin Diagn Pathol. 2000;17:56–65. [PubMed] [Google Scholar]
- 37.Procacci C, Megibow AJ. Imaging of the Pancreas: Cystic and Rare Tumors. Berlin: Springer-Verlag; 2003. p. 302. [Google Scholar]
- 38.Kim YS, Cho JH. Rare nonneoplastic cysts of pancreas. Clin Endosc. 2015;48:31–8. doi: 10.5946/ce.2015.48.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadiyala V, Lee LS. Endosonography in the diagnosis and management of pancreatic cysts. World J Gastrointest Endosc. 2015;7:213–23. doi: 10.4253/wjge.v7.i3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jana T, Shroff J, Bhutani MS. Pancreatic cystic neoplasms: Review of current knowledge, diagnostic challenges, and management options. J Carcinog. 2015;14:3. doi: 10.4103/1477-3163.153285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitra V, Nayar MK, Leeds JS, et al. Diagnostic performance of endoscopic ultrasound (EUS)/endoscopic ultrasound--fine needle aspiration (EUS-FNA) cytology in solid and cystic pancreatic neuroendocrine tumours. J Gastrointestin Liver Dis. 2015;24:69–75. doi: 10.15403/jgld.2014.1121.vmi. [DOI] [PubMed] [Google Scholar]
- 42.Pai M, Habib N, Senturk H, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–9. doi: 10.4240/wjgs.v7.i4.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koh YX, Chok AY, Zheng HL, et al. A systematic review and meta-analysis of the clinicopathologic characteristics of cystic versus solid pancreatic neuroendocrine neoplasms. Surgery. 2014;156:83–96 e2. doi: 10.1016/j.surg.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 44.D’Onofrio M, De Robertis R, Capelli P, et al. Uncommon presentations of common pancreatic neoplasms: A pictorial essay. Abdom Imaging. 2015;40:1629–44. doi: 10.1007/s00261-015-0388-x. [DOI] [PubMed] [Google Scholar]
- 45.Lee TY, Cheon YK, Shim CS. Clinical role of contrast-enhanced harmonic endoscopic ultrasound in differentiating solid lesions of the pancreas: A single-center experience in Korea. Gut Liver. 2013;7:599–604. doi: 10.5009/gnl.2013.7.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imaoka H, Shimizu Y, Mizuno N, et al. Ring-enhancement pattern on contrast-enhanced CT predicts adenosquamous carcinoma of the pancreas: A matched case-control study. Pancreatology. 2014;14:221–6. doi: 10.1016/j.pan.2014.02.005. [DOI] [PubMed] [Google Scholar]


