Abstract
Background and Objectives:
Pancreatic cystic neoplasms (PCNs) are being increasingly identified. Recent reports have described the utility of endoscopic ultrasound (EUS) in the characterization of PCNs. This study presents the diagnostic value of EUS in PCNs.
Materials and Methods:
A total of 108 patients (78 women and 30 men; average age, 50 years) who were confirmed pathologically to have PCNs were analyzed retrospectively. We analyzed the clinical characteristics of 108 patients and compared the diagnostic performance of computed tomography (CT), magnetic resonance imaging (MRI), and EUS with or without fine-needle aspiration (FNA).
Results:
EUS with or without FNA significantly increased the accuracy for diagnosing PCNs compared with CT (P = 0.002) and MRI (P = 0.006). According to the tumor size, the further analysis of these impacts was provided. EUS was superior to CT in the characterization of PCNs in small (< 3 cm) lesions (P = 0.003), similarly superior to MRI in large (>3 cm) lesions (P = 0.030). Furthermore, EUS is valuable for precisely characterizing internal structures, for example, septa (P = 0.004, compared with CT; P = 0.033, compared with MRI) and mural nodules (P = 0.028, compared with CT).
Conclusions:
In our study, EUS with or without FNA is the ideal tool for providing detailed imaging of PCNs and performed better than MRI and CT.
Keywords: Computed tomography, endoscopic ultrasound, magnetic resonance imaging, pancreatic cystic neoplasms
INTRODUCTION
Owing to the widespread use of cross-sectional imaging, pancreatic cystic neoplasms (PCNs) are now recognized more commonly by gastroenterologists.[1] Of all pancreatic cystic lesions, the prevalence of PCNs is reported to account for up to 60%.[2]
Concerning epithelial type, mucinous or nonmucinous, PCNs are classified by World Health Organization (WHO) into mucinous cystic neoplasm (MCN), serous cystic neoplasm (SCN), intraductal papillary mucinous neoplasm (IPMN), solid pseudopapillary neoplasm (SPN), cystic neuroendocrine neoplasm, ductal adenocarcinoma with cystic degeneration, and acinar-cell cystic neoplasm.[3]
Currently the main imaging methods to diagnose PCNs include computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS) with or without fine-needle aspiration (FNA). CT is often the predominant method for the characterization of cystic lesions based on its thin-section technique, enhanced or unenhanced, which can provide detailed information of the cyst's structure. MRI may be superior to CT because of its ability to determine whether there is involvement of the main pancreatic duct. However, in spite of the high quality of modern CT and MRI, the ability to distinguish neoplastic from nonneoplastic pancreatic cystic lesions remains imperfect. Recent reports have demonstrated the use of EUS in the diagnosis of PCNs, for its high spatial resolution which can describe the internal structures such as septa and mural nodules.[4,5] The detailed imaging presented by EUS provides morphologic criteria for differentiation between various subtypes of PCNs.[6] In addition, EUS with FNA also provides a way guiding the biopsy for suspicious lesions and analyzing the cytology and biochemistry of cystic fluid. As is reported, EUS is safe and well-tolerated with a complication rate of less than 1%.[4] With the help of the high quality of the images combined with the ability to direct FNA of cystic lesions, endoscopists can distinguish among benign, malignant, and inflammatory cystic lesions of the pancreas.[7]
In this study, we retrospectively analyzed the characterization of 108 patients with PCN who were finally confirmed pathologically. In addition, we compared the diagnostic performances of CT, MRI, and EUS with or without FNA in PCNs.
MATERIALS AND METHODS
From March 2003 to September 2013, 108 patients confirmed pathologically to have PCN at Nanjing Drum Tower Hospital were analyzed retrospectively. This study was approved by Nanjing Drum Tower Hospital Institutional Review Board.
A total of 108 patients had PCNs, with the following breakdown by type: SCN (n = 26, 24%), MCN (n = 46, 43%), IPMN (n = 11, 10%), and SPN (n = 25, 23%). All medical records were reviewed in detail (including sex, age, presenting symptoms, tumor markers, CT, MRI, EUS with or without FNA, type of surgery, and pathologic features).
In these 108 patients, most patients (n = 95, 88%) underwent CT and 64 patients (59.3%) performed MRI. There were 76 patients (70.4%) who underwent EUS and 24 patients (22.2%) subsequently performed FNA [Table 1]. Furthermore, 61 patients underwent both CT and EUS, 53 patients underwent both MRI and EUS, and 63 patients underwent both CT and MRI.
Table 1.
Accuracy of CT, MRI, and EUS of all the patients for diagnosing the PCNs
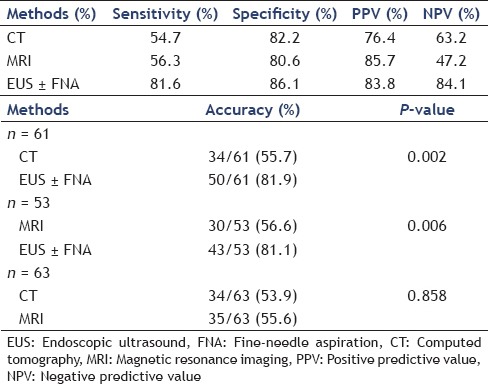
The sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV) of diagnosing PCNs in our patients with CT, MRI, and EUS with or without FNA for identifying PCNs were calculated. All statistics were performed using Statistical Package for Social Sciences (SPSS) software. Measurement data were shown with mean ± standard deviation. The Pearson chi-square test was applied to compare the difference of the data. P < 0.05 was considered statistically significant.
RESULTS
Patient demographics and clinicopathological features
The mean age of the 108 patients was 49.7 ± 15.7 years (ranging from 14 to 78 years). In these patients, there were 78 women (72.2%) and 30 men (27.8%). Of the 108 patients, 46 patients were identified incidentally because of unrelated indications. In our data; abdominal pain or dyspepsia (38%), abdominal mass (5.6%), pancreatitis (8.3%), and jaundice (1.8%) were the common clinical manifestations.
In our series, all cystic lesions were confirmed pathologically, except for three patients who only had EUS-guided fine-needle aspiration (FNA) for cytological and biochemical analysis. The size of PCNs ranged from 0.8 to 22.5 cm. The mean size of PCNs was 5.75 ± 3.93 cm. On pathology, the 108 patients with PCNs were divided into four grades: Adenoma (n = 82, 75.9%), borderline (n = 14, 13.0%), carcinoma in situ (n = 4, 3.7%), and invasive carcinoma (n = 8, 7.4%).
The characteristics of four major types of PCNs
Distinguishing among the four major types of PCNs is important. SCNs occurred mostly in women (14/26), and patients were nearly in their late 50s to early 60s (57.24 ± 9.22 years). Previous studies showed that they occurred most frequently in the body or the tail of the pancreas.[8] While in our series (26 patents with SCNs), the neoplasms in 14 patients occurred in the head of the pancreas and in seven patients occurred in the body or the tail of the pancreas with the overall mean size of 3.77 ± 1.61 cm.
MCNs more frequently occurred in women (37/46), with a peak incidence in the 50s (50.87 ± 14.7 years). In our series, MCNs mean size was 6.6 ± 4.49 cm. Furthermore, the body and the tail of the pancreas (n = 39, 84.8%) were predominantly affected.
IPMNs were diagnosed in the elderly, in their 60s and 70s (64.36 ± 8.64 years). In contrast, there was a slight male preponderance (7/11). They occurred frequently in the head of the pancreas (n = 7, 63.6%) and the mean size of IPMNs was 3.19 ± 1.51 cm.
The patients with SPNs occurred predominantly in young women (23/25). The mean age at diagnosis was 34.44 ± 12.62 years. The mean size of SPNs (7.38 ± 3.99 cm) was larger than others. Sometimes SPNs were analyzed to occur evenly throughout the pancreas.
Diagnostic imaging of PCNs
The sensitivity, specificity, PPV, and NPV of CT, MRI, and EUS with or without FNA of all the patients were shown in Table 1a.
Nowadays, CT generally serves as the main examination before surgery. The accuracy of CT and MRI for making the correct diagnosis in pancreatic cysts ranges from 40 to 60%.[9,10] However, the routine diagnostic methods for diagnosing PCNs were only modestly effective. In our series, the sensitivity of CT and MRI for diagnosing PCNs was 54.7 (52/95) and 56.3% (36/64), respectively [Table 1a]. The imaging of CT and MRI for making incorrect diagnosis in PCNs was summarized in Table 2.
Table 2.
Incorrect diagnosis made by CT, MRI, and EUS in our series
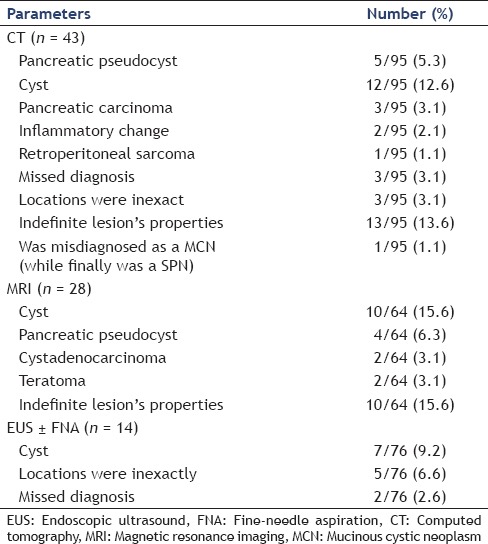
Recent advances in EUS have made it possible for more accurate diagnosis. In this study, the sensitivity of EUS with or without FNA was 81.6% (62/76) [Table 1a]. However, there were still 14 patients with incorrect diagnosis [Table 2].
Incremental diagnostic accuracy of EUS over CT and MRI
The sensitivity of these three diagnostic methods was significantly different between each other (P = 0.000). In our series, there were 61 patients underwent both CT and EUS. We then found that EUS with or without FNA (50/61) increased the accuracy for diagnosing PCNs compared with CT (34/61) (81.9 vs. 55.7%, P = 0.002). Similarly, in those 53 patients who underwent both MRI and EUS, EUS with or without FNA (43/53) was superior to MRI (30/53) in the characterization of PCNs (81.1 vs. 56.6%, P = 0.006). There was no significant difference between the ability of CT (34/63) and MRI (35/63) to correctly diagnose PCNs (53.9 vs. 55.6%, P = 0.858) in our data [Table 1b].
In general, tumor size would impact on the accuracy of methods in the characterization of PCNs. A perspective for further analysis of these impacts was provided. In our series, there were 15 patients who underwent both CT and EUS in small (< 3 cm) lesions, 46 patients in large (≥3 cm) lesions. We found that the EUS with or without FNA (12/15) was superior to CT (4/15) in the characterization of PCNs in small lesions (80.0 vs. 26.7%, P = 0.003). However, there was no significant difference between EUS (38/46) and CT (30/46) for diagnosing PCNs in large lesions (82.6 vs. 65.2%, P = 0.058) [Table 3]. Thirteen patients underwent both MRI and EUS in small lesions and 40 patients in large lesions. There was no significant difference between EUS (11/13) and MRI (7/13) in the characterization of PCNs for small lesions (84.6 vs. 53.8%, P = 0.084). Perhaps because of the sample size in our study was rather small. Furthermore, in these 13 patients, five patients were IPMN. MRI could determine whether there is involvement of the main pancreatic duct, nowadays MRCP is the optimal choice to diagnose the IPMN.[11] The accuracy of MRI for diagnosing these five IPMNs was 80% (4/5). In those large lesions, EUS with or without FNA (32/40) was superior to MRI (23/40) for diagnosing PCNs (80.0 vs. 57.5%, P = 0.030) [Table 3].
Table 3.
To evaluate the performance characteristics of EUS compared with CT and MRI in the PCNs
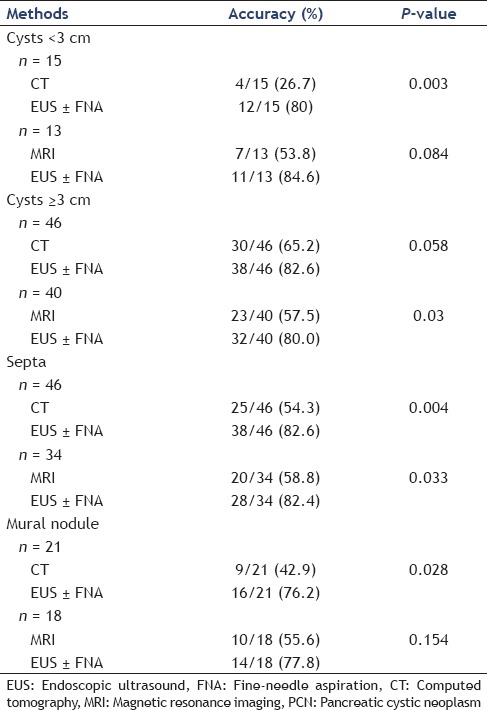
For its high spatial resolution, EUS could precisely show internal structures, for example, septa and mural nodules. In our series, septa were detected on 25 of 46 lesions by CT and 38 of 46 lesions by EUS (54.3 vs. 82.6%, P = 0.004). Likewise, septa were detected on 20 of 34 lesions by MRI and 28 of 34 lesions by EUS (58.8 vs. 82.4%, P = 0.033). Of the mural nodules, the EUS with or without FNA (16/21) was superior to CT (9/21) in the characterization of mural nodules in PCNs (76.2 vs. 42.9%, P = 0.028). However, there was no significant difference between EUS (14/18) and MRI (10/18) in the characterization of mural nodules in PCNs (77.8 vs. 55.6%, P = 0.154) [Table 3].
DISCUSSION
The clinical manifestation of the PCNs is usually atypical. Currently the diagnosis and management of the PCNs are common problems. Much emphasis is placed on the size and the morphology of the PCNs. It is necessary to distinguish mucinous from nonmucinous cysts and benign from malignant cysts[12,13], and important for doctors to decide which tumors require surgery and which may be managed expectantly.
For the diagnostic modalities of PCNs, the noninvasive diagnosis primarily depend on CT and MRI features, especially CT scan, which continues to be the most common diagnostic method. On CT images, parenchymal changes, cystic lesions, and a diffusely dilated pancreatic duct are the most common findings.[14] For the reason that MRCP is able to clearly present the ductal connection in patients, MRI is increasingly adopted to diagnose pancreatic cystic lesions. MRCP is the optimal choice to diagnose the IPMN and detect type and extent of disease.[11] However, CT and MRI cannot exactly analyze the characterization of the pancreatic cysts.[9,15] While, other than CT and MRI, EUS allows close and high-resolution imaging of pancreatic cystic lesions morphology. Even though the position of the lesion is usually known before the EUS examination, it is important to examine the entire pancreas, starting with the uncinate process and finishing with the tail.[16,17] Because of the ability in the characterization of main ductal communication, septa and mural nodules, as well as the ability to direct the analysis of cystic fluid for cytology and biochemistry, EUS is considered as an optimal imaging modality to diagnose pancreatic cystic lesions.[12,18]
On EUS imaging, there are various morphologic features for the subtypes of PCNs. The serous cystadenoma generally reveals a microcystic lesion in EUS imaging with little free fluid in the locules.[14] The typical imaging of EUS for SCN includes aggregates of some small fluid filled cavities (typically less than 5 mm in size) making a honeycomb appearance, which are separated by thin septa. However, MCNs are often thin-walled; septated cavities contain highly viscous clear fluid with diameter greater than 1-2 cm that may be difficult to aspirate. EUS demonstrates a dilation of main pancreatic duct with mural ductal nodules and intra-luminal filling defects in main-duct IPMN (MD-IPMN). Branch-duct IPMN (BD-IPMN) shows multiple pancreatic cystic lesions that communicate with the pancreatic duct.[1] SPNs present with various features and ranges from a total solid to a mixed solid and cystic mass. They are usually well-defined and present with hypoechoic masses.[19] Some morphological features on EUS imaging of the various subtypes of PCNs in our series are presented in Figure 1.
Figure 1.
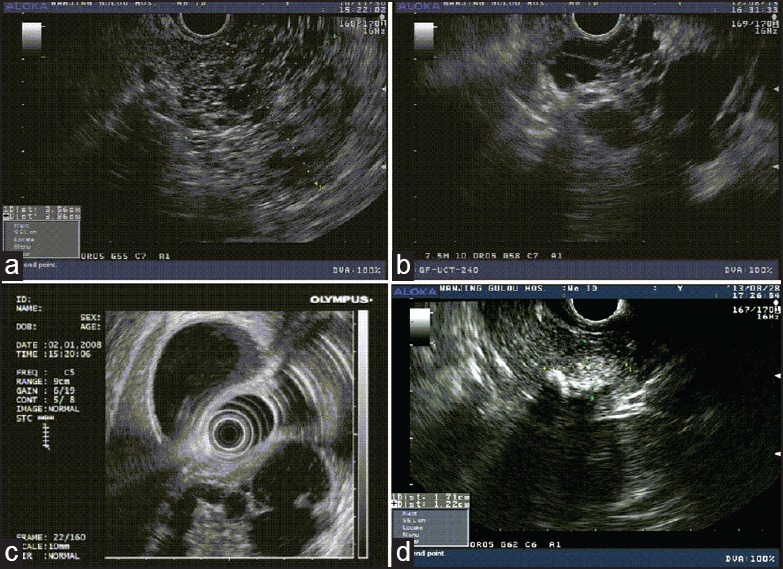
The morphologic features on EUS imaging of the various subtypes of PCNs. (a) EUS feature of microcystic serous cystadenoma with honeycombing appearance in the neck of the pancreas in a 29-yearold female patient with abdominal pain, which was approximately 3.6 × 3.9 cm. (b) EUS image of a MCN in a 76-year-old male patient with presence of septations and approximately 3.7 × 2.8 cm in the head of the pancreas. (c) EUS image of an IPMN in a 78-year-old male with the papillary form. The diameter of the main pancreatic duct was 2.0 cm which was located in the head of the pancreas. (d) EUS appearance of the SPN in a 35-year-old female patient which presented a mixture echo mass about 1.42 × 0.95 cm and presence of calcifications in the head of the pancreas. PCN = Pancreatic cystic neoplasm, EUS = Endoscopic ultrasound, MCN = Mucinous cystic neoplasm, IPMN = Intraductal papillary mucinous neoplasm, SPN = Solid pseudopapillary neoplasm
In this study, we described the value of EUS in the characterization of PCNs by retrospectively analyzing the patients in our hospital. On one hand, we analyzed the clinical characteristics of the 108 patients with PCNs. On the other hand, we compared the diagnostic performances of CT, MRI, and EUS with or without FNA in PCNs. In consequence, EUS was significantly more sensitive in accurately classifying a cyst as neoplasm than CT (P = 0.002) and MRI (P = 0.006). In addition, the EUS was superior to CT in the characterization of PCNs in small lesions (P = 0.003), similarly superior to MRI in large lesions (P = 0.030). Furthermore, EUS is valuable for precisely characterizing internal structures, for example, septa (P = 0.004, compared with CT and P = 0.033, compared with MRI) and mural nodules (P = 0.028 compared with CT). Then, in our 24 patients who underwent EUS then subsequently performed FNA, the accuracy of FNA alone was 58.3% (14/24) in concordance with other studies, which report that the accuracy is approximately 50%.[16]
In spite of our findings, a large prospective, multicenter, ultrasound study found that the accuracy of EUS examination alone for distinguishing mucinous from nonmucinous cystic lesions was only 51%.[16] A retrospective study compared MRI and EUS in the diagnosis of pancreatic cystic lesions[20] and yielded no significant difference between the ability of MRI and EUS to correctly classify pancreatic lesions as cystic or solid. Nevertheless, gastroenterologists prefer EUS because the biopsy and aspiration can be performed during the same examination which can increase diagnostic accuracy.[21] A retrospective study reported the incremental increase in diagnostic yield of EUS over CT and MRI for predicting the neoplastic cyst is 36 and 54%, respectively.[18]
Yet, there were also some limitations of this study. Our study was retrospective, leading to some inaccurate information, which could not be avoided. In addition, the sample size in our study was rather small. We hope to enlarge our database in the near future. Furthermore, we could not acquire interobserver agreement of images of CT, MRI, and EUS.
In conclusion, EUS with or without FNA plays a very important role in the characterization of PCNs which composed of a wide range of lesions from benign cysts to malignancies. In our study, EUS was superior to CT and MRI in accurately classifying a cyst as a neoplasm. More well-designed, prospective studies are needed to enhance our understanding of the accurate diagnostic value of EUS in PCNs.
ACKNOWLEDGMENT
This work was supported by a grant from the Key Project of Nanjing Medical Science and Technique Development Foundation (ZKX12019).
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Mohamadnejad M, Eloubeidi MA. Cystsic lesions of the pancreas. Arch Iran Med. 2013;16:233–9. [PubMed] [Google Scholar]
- 2.Basturk O, Coban I, Adsay NV. Pancreatic cysts: Pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423–38. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 3.Brugge WR, Lauwers GY, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 4.Scheiman JM. Management of cystic lesions of the pancreas. J Gastrointest Surg. 2008;12:405–7. doi: 10.1007/s11605-007-0350-5. [DOI] [PubMed] [Google Scholar]
- 5.Sahani DV, Kadavigere R, Saokar A, et al. Cystic pancreatic lesions: A simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–84. doi: 10.1148/rg.256045161. [DOI] [PubMed] [Google Scholar]
- 6.Brugge WR. The role of EUS in the diagnosis of cystic lesions of the pancreas. Gastrointest Endosc. 2000;52:S18–22. doi: 10.1067/mge.2000.110720. [DOI] [PubMed] [Google Scholar]
- 7.Al-Haddad M, El Hajj II, Eloubeidi MA. Endoscopic ultrasound for the evaluation of cystic lesions of the pancreas. JOP. 2010;11:299–309. [PubMed] [Google Scholar]
- 8.Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: Tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–9. doi: 10.1097/01.sla.0000179651.21193.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: Relative accuracy of CT and MRI. AJR Am J Roentgenol. 2007;189:648–56. doi: 10.2214/AJR.07.2365. [DOI] [PubMed] [Google Scholar]
- 10.Sainani NI, Saokar A, Deshpande V, et al. Comparative Performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009;193:722–31. doi: 10.2214/AJR.08.1253. [DOI] [PubMed] [Google Scholar]
- 11.Waters JA, Schmidt CM, Pinchot JW, et al. CT vs. MRCP: Optimal classification of IPMN type and extent. J Gastrointest Surg. 2008;12:101–9. doi: 10.1007/s11605-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 12.Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339–49. doi: 10.1111/j.1572-0241.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 13.Shen J, Brugge WR, Dimaio CJ, et al. Molecular analysis of pancreatic cyst fluid a comparative analysis with current practice of diagnosis. Cancer. 2009;117:217–27. doi: 10.1002/cncy.20027. [DOI] [PubMed] [Google Scholar]
- 14.Brugge WR. The use of EUS to diagnose cystic neoplasms of the pancreas. Gastrointest Endosc. 2009;69:S203–9. doi: 10.1016/j.gie.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Fisher WE, Hodges SE, Yagnik V, et al. Accuracy of CT in predicting malignant potential of cystic pancreatic neoplasms. HPB (Oxford) 2008;10:483–90. doi: 10.1080/13651820802291225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59–64. doi: 10.1067/mge.2003.298. [DOI] [PubMed] [Google Scholar]
- 18.Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas. 2013;42:717–21. doi: 10.1097/MPA.0b013e3182883a91. [DOI] [PubMed] [Google Scholar]
- 19.Jani N, Dewitt J, Eloubeidi M, et al. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: A multicenter experience. Endoscopy. 2008;40:200–3. doi: 10.1055/s-2007-995364. [DOI] [PubMed] [Google Scholar]
- 20.Kim YC, Choi JY, Chung YE, et al. Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. AJR Am J Roentgenol. 2010;195:947–52. doi: 10.2214/AJR.09.3985. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad NA, Kochman ML, Lewis JD, et al. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am J Gastroenterol. 2001;96:3295–300. doi: 10.1111/j.1572-0241.2001.05328.x. [DOI] [PubMed] [Google Scholar]


