Abstract
Background:
The treatment of pancreatic cancer represents a major objective in clinical research, as it still remains the fourth leading cause of cancer deaths among men and women, with approximately 6% of all cancer-related deaths.
Materials and Methods:
We studied the assessment of an endoscopic ultrasound (EUS)-guided radiofrequency ablation (RFA) probe through a 19G needle in order to achieve a desirable necrosis area in the pancreas. Radiofrequency ablation of the head of the pancreas was performed on 10 Yorkshire pigs with a weight between 25 kg and 35 kg and a length of 40-70 cm. Using an EUS-guided RFA experimental probe, we ablated an area of 2-3 cm width. The biological samples were harvested after 3 days and 5 days and necropsy was performed 1 week after the procedure.
Results:
All pigs showed no significant change regarding their behavior and no signs of complication was encountered. Blood analysis revealed increased values of amylase, alkaline phosphatase, and gamma-glutamyl transpeptidase on the 3rd day but a decrease on the 5th day. After necropsy and isolation of the pancreas, the ablated area was easily found, describing a solid necrosis. The pathological examination revealed a coagulative necrosis area with minimal invasion and inflammatory tissue at about 2 cm surrounding the lesion.
Conclusion:
EUS-RFA is a feasible technique and might represent a promising therapy for the future treatment of pancreatic cancer. However, further studies are necessary to investigate EUS-guided RFA as an option for palliation in pancreatic cancer until it can be successfully used in human patients.
Keywords: Endoscopic ultrasound (EUS), pancreas, radiofrequency ablation (RFA)
INTRODUCTION
The treatment of pancreatic cancer represents a major objective in clinical research as it still remains the fourth leading cause of cancer deaths among men and women and accounts for approximately 6% of all cancer-related deaths.[1] The prognosis is extremely poor, with an overall survival rate of 25% and 6% after 1 year and 5 years, respectively. Surgery remains the primary option in treating pancreatic cancer patients although it is not always possible, as by the time of diagnosis distant metastasis are already present in about 50% of the patients.[2]
However, new therapeutic methods are willing to be introduced either as a palliative or curative options, including radiofrequency ablation (RFA), cryotherapy, photodynamic therapy, and brachytherapy. RFA produces a high level of heat within the tumor, leading in a final stage to tumor necrosis, replacing the dead tumor cells with scar tissue, which shrinks over time.[3,4] Although RFA has been performed through a percutaneous approach or during laparotomy, an endoscopic ultrasound (EUS) approach may be more effective for improved real-time imaging and assessment of deeply located pancreatic tumors, while minimizing the damage of surrounding tissue and nearby structures.
The main objective of the present study was to assess the feasibility and safety of EUS-guided RFA of the pancreas using an experimental survival porcine model. The secondary aim included the pathological assessment (size, shape, location, etc.) of the EUS-guided RFA ablation zone, based on standardized settings of the RFA procedure.
MATERIALS AND METHODS
Ten Yorkshire pigs with a weight between 25 kg and 35 kg and length of range 40-70 cm were used based on the written approval of the Ethics Committee of the University of Medicine and Pharmacy Craiova (UMFCV), Romania, and according to the European legislation regarding animal rights. All animals were maintained in special locations at the animal facility of the UMFCV, with a controlled temperature of 20-22°C, in separate cages and were subjected to fasting for solids and liquids for 24 hours and 6 hours respectively prior to the procedure. Premedication was injected in the posterior thigh and consisted of ketamine 20 mg/kgc (Intervet, Bucharest, Romania), xylazine 2 mg/kgc (Bioveta Romania S.R.L., Cluj-Napoca, Romania), and athropine 0.015 mg/kgc (Biofarm SA, Bucuresti, Romania). An 18G or 20G peripheral venous catheter (WellcathPlus™, Wellmed, Noida, Uttar Pradesh, India) was placed on the marginal vein of the ear (vena auricularis caudalis). Anesthesia was maintained with propofol (Fresenius Kabi, Brasov, Romania) 0 mg/kgc and 5 mg/kgc continuously, fentanyl (Gedeon Richter România S.A, Târgu Mureş, Romania) 3 μg/kgc, and pavulone (pancuronium bromide, N.V. Organon, Oss, Netherland) 0 mg/kgc and 1 mg/kgc. Also, antibiotic prophylaxis was administered using ceftriaxone (Sandoz, Kundl, Austria) 1 g. During the intervention, the animals were under electrocardiographic and peripheral capillary oxygen saturation (SpO2) monitoring.
The RFA procedure was performed using the RITA Medical System 1,500 RF radiofrequency generator from Angiodynamics (New York, USA). A transduodenal approach was performed using a linear endoscopic ultrasound scope (GFUCT140-AL5, Olympus, Tokyo, Japan) with a large interventional channel, coupled with the corresponding EvisExera system (Olympus, Tokyo, Japan) and an AlokaProSound5500 US system (Hitachi-Aloka, Tokyo, Japan). All the equipment was dedicated for animal use. The linear EUS scope was guided immediately below the gastroesophageal junction until a good visualization of the head of the pancreas was obtained [Figure 2]. An experimental RFA probe [Emcision ltd, London, United Kingdom (0.33 mm, 0.013″)] [Figure 1], with a working length of 200 cm was inserted through a 19G EUS-fine-needle aspiration (FNA) needle (Olympus, Tokyo, Japan). The head of the pancreas underwent four sessions of consecutive ablations with 4-6 mm of the catheter exposed at 5 W, 10 W, 15 W, and 20 W for 120 s each [Figure 3]. The ablation area, with a length of 2-3 cm, was monitored after the procedure using contrast-enhanced (SonoVue™-Bracco, Milan, Lombardy, Italy) power Doppler EUS. No immediate complications were recorded. Recovery from anesthesia was carried out either spontaneously or by antagonizing the analgesic, the neuromuscular blocker, and/or the benzodiazepines.
Figure 2.
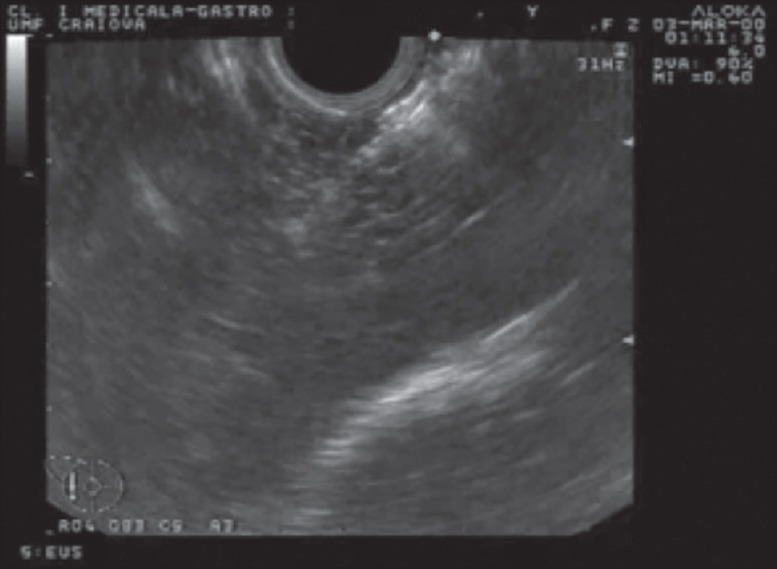
EUS image with the radiofrequency ablation (RFA) probe prior ablation
Figure 1.
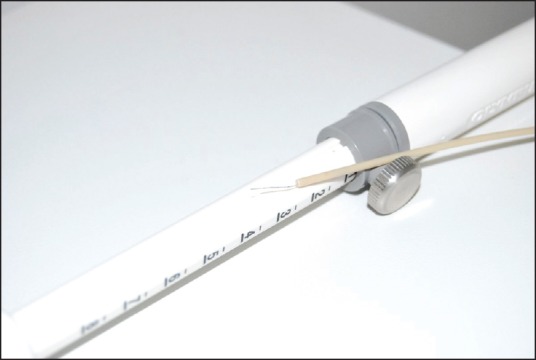
Habib™ RF DUO 13 (0.33 mm, 0.013”), with a working length of 200 cm was inserted through a 19G endoscopic ultrasound-fine-needle aspiration (EUS-FNA) needle (Olympus, Tokyo, Japan)
Figure 3.
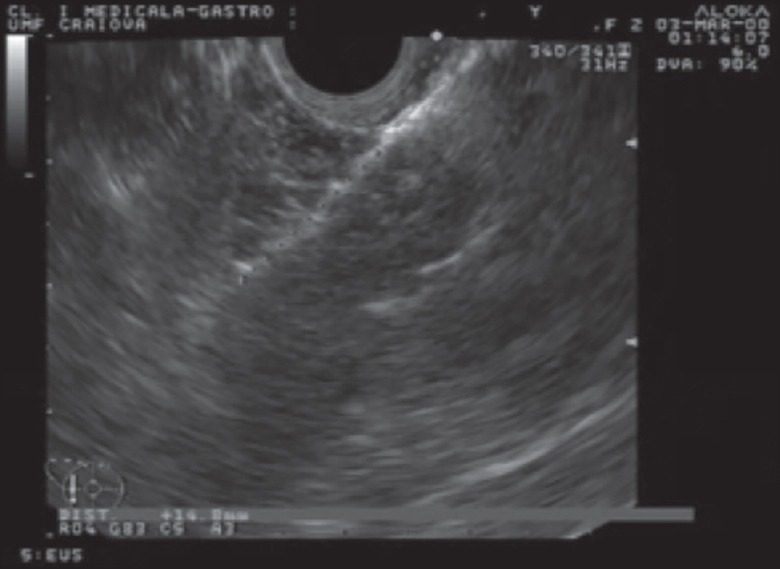
EUS image with the RFA probe after ablation
Biological samples including total bilirubin, transaminases [aspartate transaminase (AST) and alanine transaminase (ALT)], gama-glutamyl transpeptidase (GGT), alkaline phosphatase (AP), and amylase were harvested before the procedure as well as 3 days and 5 days after intervention. One week later, the pigs were euthanized. Necropsy was immediately performed, and the pancreas was surgically excised and transported in special sealed boxes with formalin for pathological examination. The macroscopic aspect of the pancreas was observed as well as the relationship with the nearby organs (gastric wall/intestinal wall, possible collections or adherences). Histological sections were taken at every 5 mm from the ablation site and the surrounding area (two section cranially and another two sections distally at 2-3 cm from the ablation zone). The 4 μm histologic sections were stained with hematoxylin and eosin (H&E) and Masson's trichrome (Bioptica, Albedo, Bucharest, Romania, Code 21-010802IC).
RESULTS
All the pigs showed no significant changes regarding their behavior and no signs of complications were encountered. There were no problems in identifying the pancreas of the pigs using EUS even though its position when compared to humans was a bit higher on the posterior wall of the stomach. The RFA probe inserted through the working channel of the EUS scope was clearly visualized under EUS real-time imaging. The head of the pancreas was targeted and it underwent four consecutive ablation procedures, increasing the voltage with 5 W during each procedure. A hyperechogenic, elliptic lesion appeared surrounding the inserted RFA probe, with a median diameter of 2.65 cm and an interquartile range (IQR) of 0.5 cm. No immediate complications were noted.
There was no behavioral change or weight change during the follow-up week. Blood analysis revealed increased values of amylase, AP, and GGT on the 3rd day. However, their levels started to decrease within the 5th day after the procedure. No variations were found in liver transaminases or bilirubin.
Necropsy was performed a week after the EUS-guided RFA procedures. The pancreas was successfully identified and extracted during laparotomy [Figure 4]. No collections, adhesions, no injury of major vessels or nearby organs were observed in 8 of the pigs. A moderate level of ascites (100 ml of clear liquid) was noticed in 1 yucatan pig, while another presented an inflammatory reaction of the stomach wall, retroperitoneal fibrosis and adhesions on the bowel wall which were probably caused by incomplete penetration of the gastric wall. Because mucosa of the gastric wall is thicker than humans, this probably was a iatrogenic complication. When slicing the pancreas, on the part located near the gastric wall, a lesion (2 × 3 cm in size), with a whitish peripheral rim of 15 to 20 mm, of pseudocystic structure with liquid inside was revealed [Figure 5].
Figure 4.
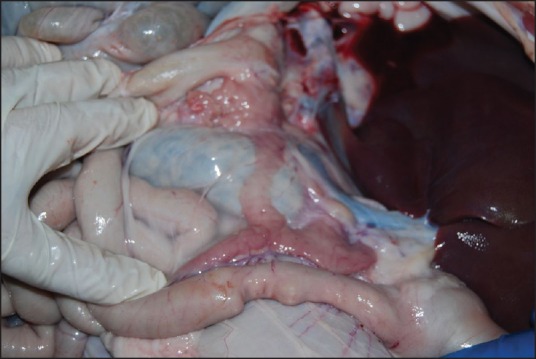
Exposure of the pancreas during necropsy
Figure 5.
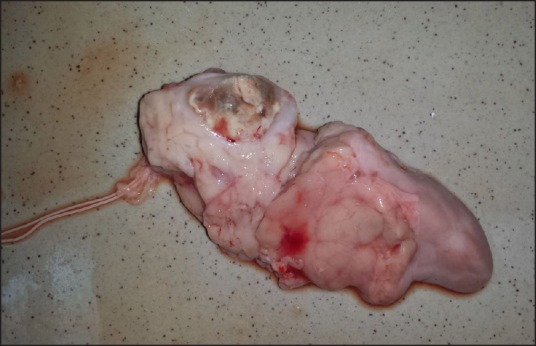
Ablation area in the pancreas
Histopathologic examination of the necrotic pancreatic specimens harvested from all the studied pigs showed focal lesions, with a center ablation area of structured (coagulation) necrosis of glandular parenchyma [Figure 6a], fatty tissue necrosis with limestone precipitations, and recent thrombosis of blood vessels. An inflammatory demarcation lizereum with fibrinous exudate and neutrophils was observed [Figure 6b]. On the periphery, a granulation tissue was formed with neoformation vessels and mononuclear inflammatory infiltrate [Figure 6c], which further maturated to a thick collagen tissue [Figure 6d]. All examined samples had no evidence of focal pancreatitis at 2-3 cm distance from the lesions.
Figure 6.
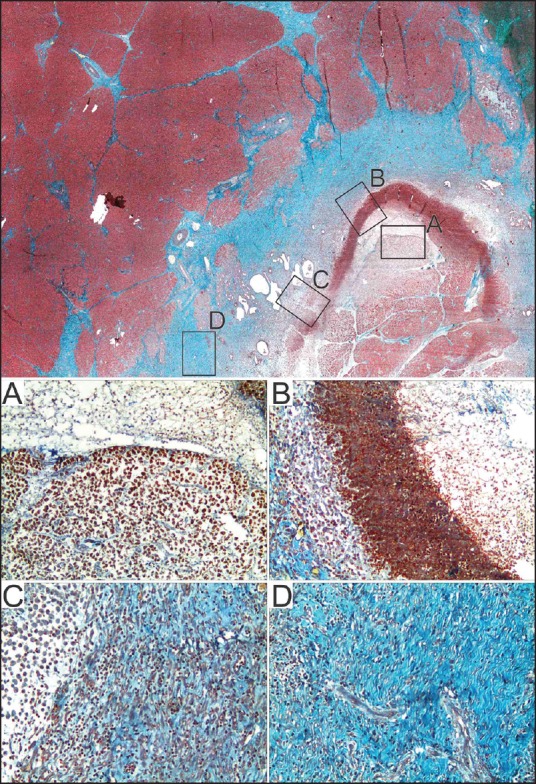
Pathological scan of the entire ablation area: central necrosis area (a) surrounded by lizereum inflammatory demarcation (b, fibrinous exudate + neutrophils) followed by a granulation tissue (c), which changes to the periphery into a collagen dense tissue (d) separating it from adjacent normal parenchyma. Col Tricromic Masson, X4 (for all detailed images A-D, the magnification was ×100)
DISCUSSIONS
Although progress has been made in the early detection of other gastrointestinal tract malignancies, the prognosis of pancreatic cancer has not improved, as the 5-year survival rate still does not exceed 5%.[5,6] EUS remains one of the most accurate methods in visualizing the pancreas and therefore, detecting pancreatic masses.[7,8] EUS offers the opportunity of visualizing deeply located lesions, including small lesions less than 2 cm, as well as a good display of their relations with the surrounding structures, exceeding both computed tomography (CT) and magnetic resonance imaging (MRI).[9] However, what truly differentiates it from other imaging techniques is the ability of obtaining specimens for pathological diagnosis by using EUS FNA.[10] EUS-FNA was first introduced for pancreatic cancer in 1991[11,12] and had a major impact on the patient's diagnosis, turning out to be the “backbone” for the use of therapeutic EUS procedures such as cryoablation, photodynamic therapy, brachytherapy, and RFA. With a wide use in treating focal malignancies,[13] RFA seems to arouse a high interest in treating pancreatic cancer so far.
When comparing to liver tumors, which are usually surrounded by normal parenchyma, ablating a part of the pancreas might prove to be rather challenging because of its complex anatomy. The pancreatic head, surrounded by different structures and in a close relation to the common bile duct as well as major blood vessels (splenic and mesenteric vessels as well as the portal vein) may limit the indications of RFA to locally advanced nonmetastatic pancreatic cancer. Additionally, the pancreatic tissue, which is biologically very thermosensitive when subjected to high levels of heat will cause an inflammatory response followed by edema, fibrotic changes, and in a final stage, cystic transformation.[14]
Our study has demonstrated that EUS-guided RFA on a normal porcine pancreas is certainly feasible and a well-tolerated technique, with no mortality or morbidity 1 week after the procedure and with no major complications encountered. The first evaluation of EUS-RFA in an animal model was performed in 1999 by Goldberg et al.,[15] using a commercial RF needle and successfully obtaining a 1-cm wide area of necrosis, with only one of the pigs developing pancreatitis. Several more studies have been performed showing that this therapeutic method is technically successful, with good correlations between the area of ablation and the histological findings,[14] or with few cases of pancreatitis developed.[16] Nevertheless, some authors highlighted that for a better evaluation, a longer follow-up period of time as well as a larger number of experimental pigs is necessary.[17]
Table 1.
Laboratory tests before and after pancreatic RFA

Under EUS-guidance, we performed four ablations of the head of the pancreas. We started with 5 W and 120 s and increased with 5 W more after each procedure. Focusing on the idea that complications are directly proportional with the ablation exposure time and the ablative power, we tried to emphasize that exposing the pancreatic tissue to consecutive increased ablation procedures may be more effective with fewer side effects. Studies so far showed that recent complications such as, burn of the gastric wall, hemorrhage, and fistulas were due to improper placement of the electrode and long exposure of high current.[16,18,19,20] We encountered only one burn in the gastric wall with minor bleeding in our second pig. As later complications after performing necropsy, no collections or adhesions and no injury of major vessels or nearby organs were observed; only one pig presented almost 100 mL of clear liquid within the peritoneum.
In spite of all that, the 10 pigs developed a pancreatic inflammatory reaction characterized by high levels of pancreatic enzymes while no evidence of abnormal animal behavior was observed. Furthermore, during the follow-up, a considerable decrease was noticed in their levels. Regarding the histology aspects after ablation, we have only found areas of focal coagulative necrosis delimited by an inflammatory response but without any inflammatory response at 2-3 cm around the lesion. In contrast to the other authors,[15,16] our EUS-guided RFA technique was devoid of complications; no pancreatitis reaction was observed adjacent to the ablation zones. Thus, the technique appears to be well-tolerated and may be effective in the treatment of unresectable pancreatic tumors considering the dimensions of the induced ablation zone that were close to those found by Kim et al.[17]
CONCLUSION
EUS-RFA might represent a promising therapy for the future treatment of pancreatic cancer, with several studies so far on experimental animals. The technique seems feasible but a good follow-up is still necessary after the procedure due to the inflammatory pancreatic response. Further studies are necessary to investigate EUS-guided RFA as an option for palliation in pancreatic cancer until it can be successfully used in human patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This article was financed by the Partnership program in priority areas - PN II, implemented with support from National Authority of Scientific Research (ANCS), CNDI - UEFISCDI, project nr. 2011-3.1-0252 (NANO-ABLATION).
REFERENCES
- 1.Atlanta: American Cancer Society; 2010. American Cancer Society. Cancer Facts and Figures 2010. [Google Scholar]
- 2.Singh SM, Longmire WP, Jr, Reber HA. Surgical palliation for pancreatic cancer. The UCLA experience. Ann Surg. 1990;212:132–9. doi: 10.1097/00000658-199008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: Results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Onofrio M, Barbi E, Girelli R, et al. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: An overview. World J Gastroenterol. 2010;16:3478–83. doi: 10.3748/wjg.v16.i28.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: A comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–61. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 8.Rösch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–52. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Cui Z, Hu Z. Role of endoscopic ultrasound in treatment of pancreatic cancer. Endosc Ultrasound. 2013;2:181–9. doi: 10.4103/2303-9027.121238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamantis A, Magiorkinis E, Koutselini H. Fine-needle aspiration (FNA) biopsy: Historical aspects. Folia Histochem Cytobiol. 2009;47:191–7. doi: 10.2478/v10042-009-0027-x. [DOI] [PubMed] [Google Scholar]
- 11.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 12.Vilmann P, Săftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy: Equipment and technique. J Gastroenterol Hepatol. 2006;21:1646–55. doi: 10.1111/j.1440-1746.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- 13.Feng K, Ma KS. Value of radiofrequencyablation in the treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:5987–98. doi: 10.3748/wjg.v20.i20.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrara S, Arcidiacono PG, Albarello L, et al. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: A preliminary study. Endoscopy. 2008;40:321–6. doi: 10.1055/s-2007-995595. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg SN, Mallery S, Gazelle GS, et al. EUS-guided radiofrequency ablation in the pancreas: Results in a porcine model. Gastrointest Endosc. 1999;50:392–401. doi: 10.1053/ge.1999.v50.98847. [DOI] [PubMed] [Google Scholar]
- 16.Gaidhane M, Smith I, Ellen K, et al. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) of the pancreas in a porcine model. Gastroenterol Res Pract 2012. 2012 doi: 10.1155/2012/431451. 431451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Seo DW, Hassanuddin A, et al. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc. 2012;76:1039–43. doi: 10.1016/j.gie.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Matsui Y, Nakagawa A, Kamiyama Y, et al. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14–20. doi: 10.1097/00006676-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Casadei R, Ricci C, Pezzilli R, et al. A prospective study on radiofrequency ablation locally advanced pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2010;9:306–11. [PubMed] [Google Scholar]
- 20.Tang Z, Wu YL, Fang HQ, et al. Treatment of unresectable pancreatic carcinoma by radiofrequency ablation with ‘cool-tip needle’: Report of 18 cases. Zhonghua Yi Xue Za Zhi. 2008;88:391–4. [PubMed] [Google Scholar]


