Abstract
A 75-year-old man suffering from opioid-refractory due to an advanced pancreatic adenocarcinoma was treated with endoscopic ultrasound (EUS)-guided celiac plexus neurolysis (CPN) combined to EUS-guided tumor ablation. No major complications were recorded during the procedure. In the days following the procedure, mild diarrhea and fever were the only minor complications experienced by the patient. Complete tumor devascularization was assessed by means of computed tomography (CT) 48 h after the procedure. The patient remained pain-free without need of opioid, and was treated only with paracetamol for 20 weeks. Our results were optimal in terms of pain relief and immediate tumor response (assessed by means of CT and tumor marker levels). The present case demonstrates that the combined approach (EUS-guided ethanol ablation and CPN) may be a valuable option in patients with pancreatic cancer. Randomized-controlled trials are needed to confirm this result.
Keywords: Ablation, celiac plexus neurolysis, echoendoscopy, pancreatic cancer
INTRODUCTION
Advanced pancreatic cancer commonly induces severe refractory pain. In such patients, endoscopic ultrasound (EUS)-guided celiac plexus neurolysis (CPN) has been reported to be effective in inducing pain relief. However, this result is often only suboptimal and transient. Furthermore, CPN has not been definitively confirmed as effective in improving patient survival.
CASE REPORT
A 75-year-old man suffering from opioid refractory pain (recorded as 9 according to visual analog scale (VAS)) due to an advanced stage IV histologically-proven pancreatic adenocarcinoma with liver metastases and celiac trunk infiltration not suitable for surgery, was referred to our center. EUS confirmed the nodular hypoechoic lesion of 40 × 38 × 33 mm in the pancreas [Figure 1]. Ca 19-9 level was 987.07 U/mL. As the tumor stage was beyond any possibility of surgical radical care and in order to relieve patient's symptoms, after informed consent was obtained, EUS-guided CPN combined to EUS-guided tumor ablation was arranged. Under sedation with propofol, EUS was conducted with a Pentax FG-36UA ultrasound endoscope (Pentax Europe Ltd, Hamburg, Germany) using a curved-array transducer. A 22 G needle (Cook Medical Inc, Bloomington, Indiana, USA) was introduced though the endoscope's working channel to inject 10 mL 2.0% lidocaine and 20 mL of ethylic alcohol 95% into the base of the celiac trunk at its origin from the aorta [central approach, Figure 2]. After performing CPN, 40 mL 75% of lesion volume of ethanol (concentration 95%) were directly injected into the tumor [Figure 3].
Figure 1.
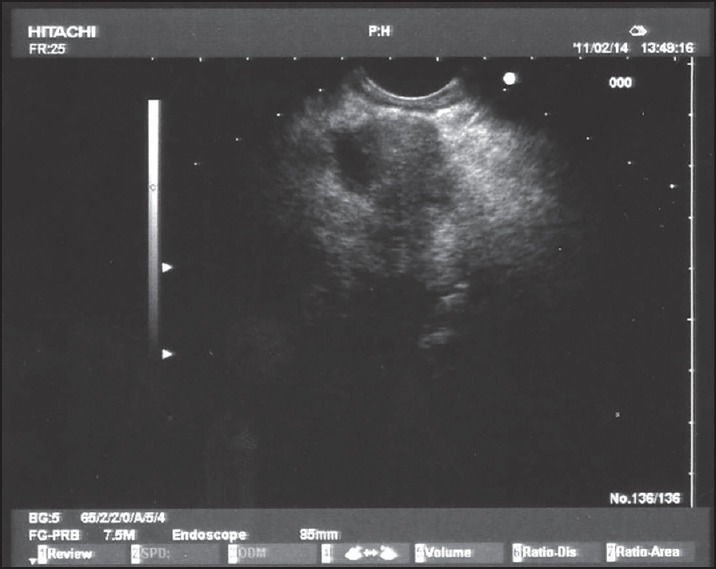
Tumoral nodule in the body of the pancreas
Figure 2.
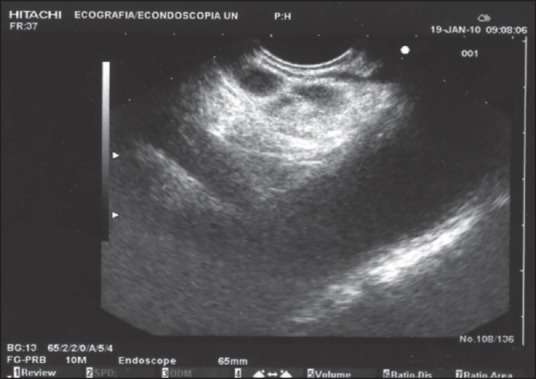
Celiac plexus neurolysis performed via the central approach
Figure 3.
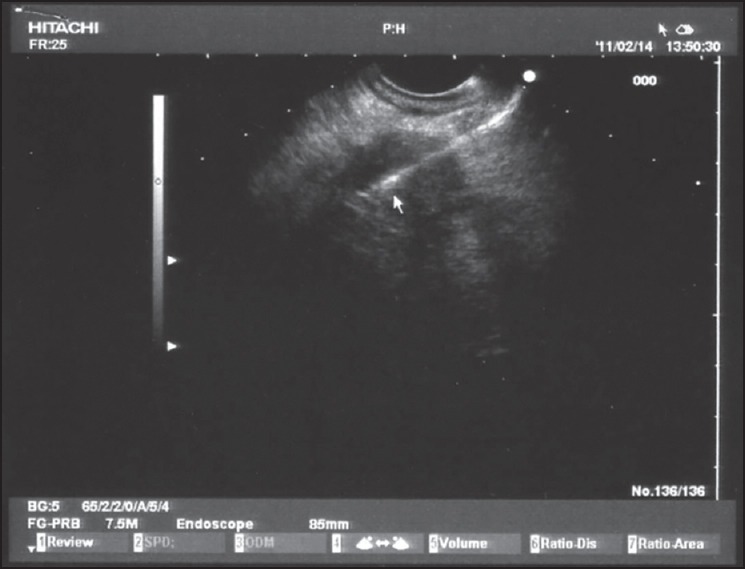
Ethanol ablation of the pancreatic tumor
No major complications were recorded during the procedure. In the days following the procedure, mild (grade 2) diarrhea and fever were the only minor complications experienced by the patient. Complete tumor devascularization was assessed by means of computed tomography (CT) 48 h after the procedure [Figure 4]. Ca 19-9 dropped down to 56.84 U/mL at 2 weeks after EUS.
Figure 4.
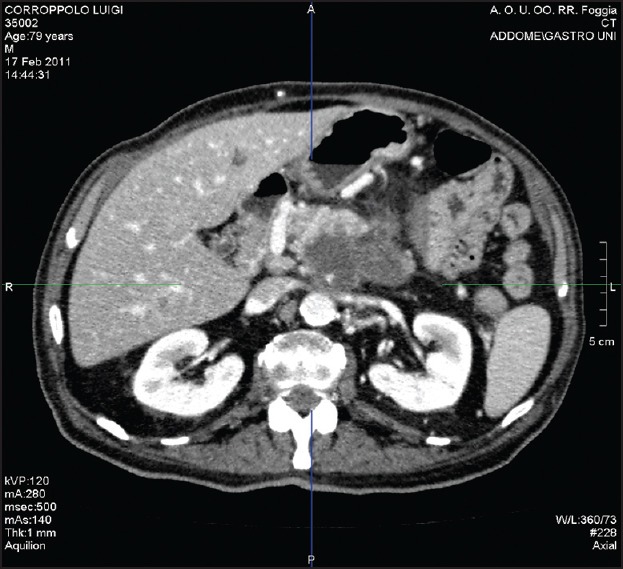
Tumor response assessed by means of computed tomography (CT) 48 h after the procedure
Complete pain relief, namely VAS 0,[1] was achieved 3 days after the endoscopic ablation. The patient remained pain-free without need of opioid, and was treated only with paracetamol for 20 weeks and afterwards experienced pain relief, (defined as pain within 30% of baseline)[1] until death occurred 30 weeks after the procedure.
DISCUSSION
Advanced pancreatic cancer commonly induces severe refractory pain. In such patients, where opioids result often ineffective, EUS-guided CPN has been reported to induce pain relief in 70-80% of cases.[2,3] However, most patients achieve only suboptimal and transient relief, probably due to technical failure or further nociceptive impulses which cannot be interrupted by the neurolysis. Furthermore, definitive data on its efficacy in improving survival is still lacking.
Therefore, it was decided to try a combined approach directed both to the celiac plexus and the neoplastic mass, in order to delay tumor progression and lengthen pain-free survival as well as overall survival.
The results were optimal in terms of pain relief and immediate tumor response (assessed by means of CT and tumor marker levels).
The present case demonstrates that the combined approach (EUS-guided ethanol ablation and CPN) may be a valuable option aimed at improving both prognosis and pain control in patients with pancreatic cancer. Randomized-controlled trials comparing EUS-CPN alone to CPN associated to tumor ablation are needed to confirm this result.
ACKNOWLEDGMENTS
The authors thank Mr. Graziano Di Pasquale and Mrs. Feliciana La Monaca for their expert nursing endoscopic assistance.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.LeBlanc JK, Al-Haddad M, McHenry L, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: One injection or two? Gastrointest Endosc. 2011;74:1300–7. doi: 10.1016/j.gie.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto H, Kitano M, Kamata K, et al. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle. Am J Gastroenterol. 2010;105:2599–606. doi: 10.1038/ajg.2010.339. [DOI] [PubMed] [Google Scholar]
- 3.Sahai AV, Lemelin V, Lam E, et al. Central vs. Bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: A comparative study of short-term effectiveness. Am J Gastroenterol. 2009;1042:326–9. doi: 10.1038/ajg.2008.64. [DOI] [PubMed] [Google Scholar]


