Abstract
A large epidemic of Chikungunya fever currently affects the Caribbean, Central and South America. Despite a high number of reported cases, little is known on the occurrence of severe clinical complications. We describe four Venezuelan patients with a severe and/or lethal course who exhibit unusual manifestations of the disease.
Case 1 describes a 75 year-old man with rapid onset of septic shock and multi-organ failure. Cases 2 and 3 describe two patients with rapid aggressive clinical course who developed shock, severe purpuric lesions and a distinct area large of necrosis in the nasal region. Case 4 depicts a splenectomized woman with shock, generalized purpuric lesions, bullous dermatosis and acronecrosis of an upper limb.
Chikungunya fever in the Western hemisphere may also associate with atypical and severe manifestations. Some patients experience a life-threatening, aggressive clinical course, with rapid deterioration and death due to multisystem failure.
Keywords: Chikungunya fever, Severe, Hemorrhagic fever, Western hemisphere
Introduction
Chikungunya is a mosquito-borne virus (family Togaviridae, genus Alphavirus), first identified more than 60 years ago in modern-day Tanzania, which is responsible for outbreaks of acute febrile polyarthralgia. It is transmitted primarily by Aedes aegypti and Ae. albopictus mosquitoes [1]. Epidemics have been described in Africa, the Middle East, Europe, India, Southeast Asia, and most recently in the Americas, where large numbers of cases of disease have been reported from more than 20 countries in the Caribbean and Central and South America [1], [2].
Three genotypes of Chikungunya virus (CHIKV), called West African, East/Central/South African (ECSA), and Asian, have been defined [3]. Sequence analysis revealed that the virus circulating in the Caribbean is phylogenetically related to Asian genotype strains recently circulating in Indonesia, China, and the Philippines [4].
Although the disease is not generally considered life-threatening and prior to 2005 severe clinical forms of the infection were rarely reported, the Réunion island outbreak revealed new and serious forms never described before [5]. Little clinical information exists on the occurrence of severe and/or lethal infections during the current epidemic of Chikungunya fever (CHIKF) in the Americas. Here, we describe a series of four severely ill adult Venezuelan patients seen in a short period of time. Our report is intended to elicit clinicians’ awareness of the atypical and severe forms of the disease in our region, as a way to potentially improve the outcomes of such life-threatening conditions.
Case reports
Case 1
A 75 year old patient with no known past medical illnesses developed chills and fever accompanied by generalized arthralgia and bilateral swelling in the hands and feet interphalangeal joints, ankles and knees. He also experienced a marked loss of appetite with decreased urinary volume. After 3 days, he was seen in an emergency room with the diagnosis of febrile exanthem probably caused Chikungunya virus, severe sepsis with multi-organ failure and severe metabolic acidosis.
On admission, he was tachycardic and febrile. Positive physical findings included a dry oral mucosa and a generalized erythematous macular rash which was more intense and confluent on feet (Fig. 1), axillary folds and forearms. Bilateral inflammatory edema was evident in the knee, ankle and interphalangeal joints with marked functional limitation of the latter. Laboratory results revealed marked leukocytosis with neutrophilia, elevation of C reactive protein, elevated procalcitonin, and abnormal renal function tests (Table 1).
Fig. 1.
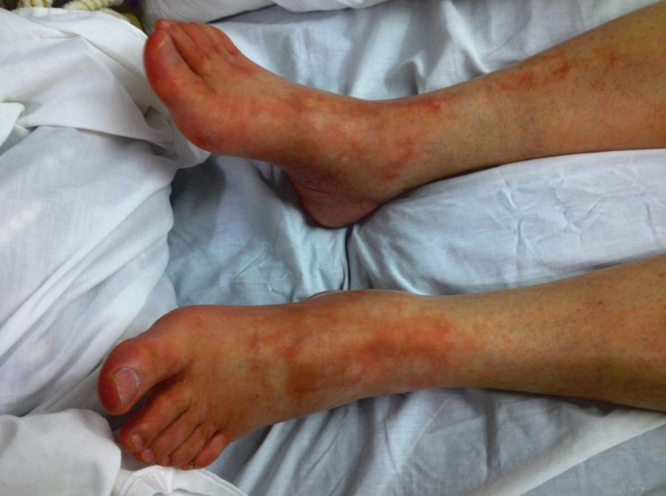
Bilateral, erythematous poorly-delineated plaques of different shapes and sizes, surrounding small areas of normal skin are seen in both feet and ankles. Lesions are more confluent over the toes and interdigital folds. Interphalangeal and metatarsophalangeal joints are diffusely swollen.
Table 1.
Summary of relevant laboratory results on admission, of four adult Venezuelan patients with severe CHIKF.
| BP (Hg mm3) | WBC × 109 L–1 (%PMNs) | Hgb (g/dL) | Hct (%) | Platelets × 109 L–1 | BUN (mg/dL) | Creatinine (mg/dL) | |
|---|---|---|---|---|---|---|---|
| Case 1 | 100/60 | 21,400 (96%) | 14.3 | 43.3 | 155 | 88 | 5.6 |
| Case 2 | 100/70 | 30,120 (91%) | 8.7 | 28.6 | 177 | 27 | 2.1 |
| Case 3 | 115/65 | 15,000 (85%) | 13.0 | 42.0 | 50 | 48 | 3.9 |
| Case 4 | 105/60 | 22,300 (97%) | 11.1 | 33.7 | 44 | 41 | 1.87 |
| CPK | Albumin | AST | ALT | Total bilirubin | AP (U/L) | PT | PTT |
|---|---|---|---|---|---|---|---|
| 413 | 0.9 | 414 | 104 | 4.6 | 870 | 13 | 30 |
| 236 | 2.3 | 73 | 27 | 0.8 | 108 | 14.1 | 35 |
| 1062 | 2.5 | 176 | 147 | 1.8 | 578 | 22 | Incoagulable |
| 3726 | 2.3 | 939 | 531 | 2.3 | 129 | 13 | 30 |
His clinical status deteriorated over 48 h with the onset of generalized soft tissue edema, distinctive palpebral and facial swelling (Fig. 2), multiple bullae, hemodynamic instability, hepatic cholestasis, atrial fibrillation with rapid ventricular response, metabolic acidosis, and oliguric acute kidney failure. He was transferred to the ICU, where his condition continued to worsen, further manifesting a septic shock syndrome with progressive multiple organ dysfunction. He died 24 h later due to cardiorespiratory arrest, in association with unresponsive ventricular fibrillation. A postmortem liver biopsy was obtained which revealed moderate acute hepatitis, hepatocyte necrosis with apoptosis, macrovesicular fat metamorphosis and diffuse edema. No granulomas were observed.
Fig. 2.
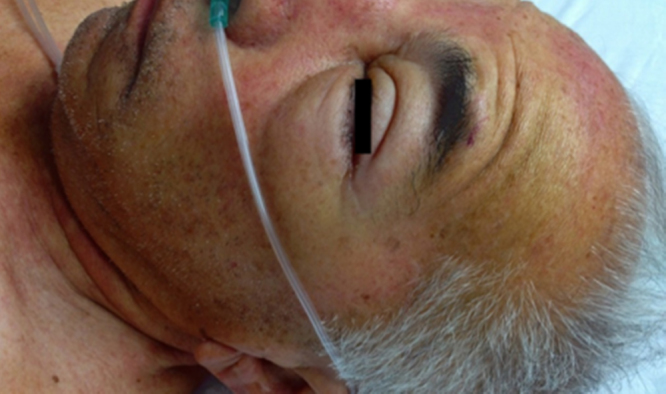
Diffuse facial edema and erythema with marked bilateral palpebral swelling.
Repeated blood, urine and respiratory cultures failed to identify bacterial pathogens. Serum samples for Chikungunya virus were positive by conventional and real time PCR and negative for dengue virus. A dengue IgM serology was also negative. Leptospirosis, malaria, typhoid fever, meningococcemia, and other severe bacterial, viral and parasitic diseases prevalent locally were ruled out.
This patient represents the first documented lethal case of CHIKF in Venezuela.
Case 2
A 53 year old female with a recent diagnosis of iron deficiency anemia presented with a 3 day history of fever, chills and rigors. She also complained of swelling of her face and extremities as well as severe, disabling arthralgias affecting all of the interphalangeal joints of the hands as well as the wrists, elbows and knees. On admission, the patient had fever, appeared ill, and the physical examination was most remarkable for a maculopapular erythematous rash affecting the four extremities and an anasarca-like appearance. Relevant laboratory results on admission (Table 1) included severe leukocytosis with mild thrombocytopenia, increased prothrombin time, hyperglycemia, hypoalbuminemia, elevated BUN and creatinine, LDH, AST, ALT, CK-MB, CRP and procalcitonin levels.
Over 48 h the patient developed acute respiratory distress, requiring intubation and ventilatory support. Severe oliguric renal failure, DIC and hemodynamic instability also ensued. A conspicuous, sharply delineated and necrotic skin lesion over the entire nasal region appeared (Fig. 3). Her eyelids were mildly swollen with numerous petechiae. The rash also became petechial affecting on the trunk, and distal areas of upper and lower limbs. The patient died 24 h later.
Fig. 3.
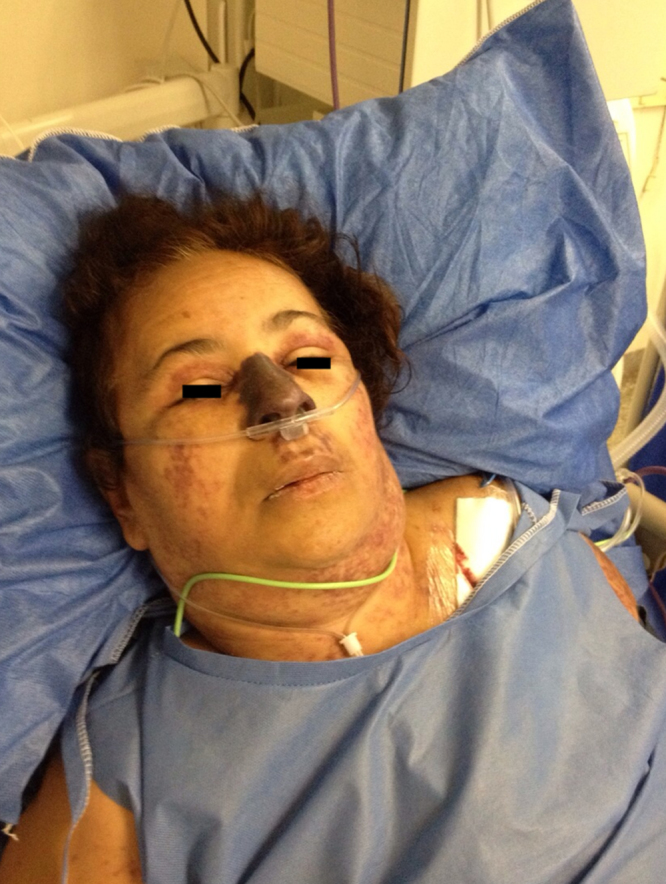
Diffuse facial edema with multiple confluent ecchymoses on the eyelids, cheeks and upper neck. A sharply delineated necrotic lesion covers the patient's entire nasal region.
Multiple blood cultures were negative. Serum samples were reported positive for Chikungunya virus by conventional and real time PCR and negative for dengue virus. A dengue IgM serology was also negative. Other common and locally important bacterial, viral and parasitic pathogens were also excluded.
Case 3
A 65 year old male with known history of hypertension treated with amlodipine and valsartan experienced 4 days of fever, malaise, generalized arthralgias and arthritis of all the arm and leg joints. 24 h prior to admission he noticed dark urine and a violaceous skin tone to his feet.
Upon arrival to the ER, the patient appeared severely ill, and was tachycardic, tachypneic and hypotensive. He exhibited mucosal and cutaneous pallor, livedo reticularis and generalized ecchymoses. His hands were swollen, hot, and extremely painful, exhibiting an intense violaceous erythematous rash (Fig. 4). Distinct, rapidly confluent cyanotic areas covered the philtral area, nostrils and later the entire nasal region (Fig. 5). Multiple and rapidly expanding bullae were noted on the upper and lower limbs. Laboratory results (Table 1) revealed respiratory alkalosis, impaired renal function, moderate leukocytosis with neutrophilia, thrombocytopenia, prolonged PTT, hypoalbuminemia as well as elevated LDH, ALT and procalcitonin serum levels. The patient was placed on norepinephrine, given bicarbonate IV infusions and volume expanders, and transferred to the ICU, where he was intubated and placed on mechanical ventilation. Six hours later, he suffered irreversible cardiac arrest and expired.
Fig. 4.
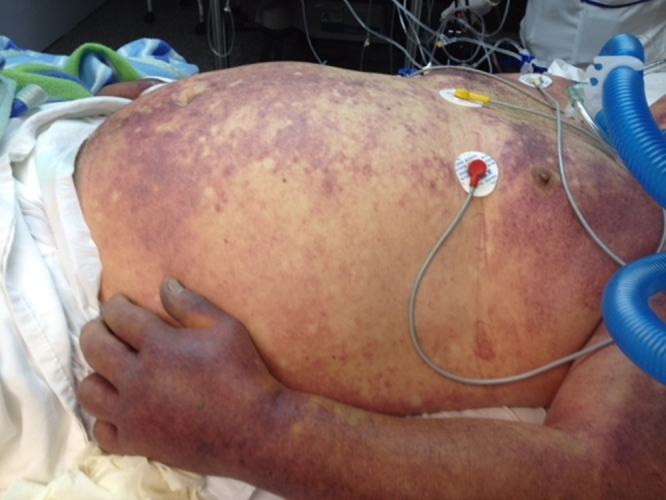
Generalized confluent ecchymoses are seen over the trunk of the patient. Extended uniform erythematous violaceous plaques involve the upper limbs and are more intense on distal areas. A diffuse inflammatory swelling of the right hand and the interphalangeal and metacarpophalangeal joints is visible.
Fig. 5.
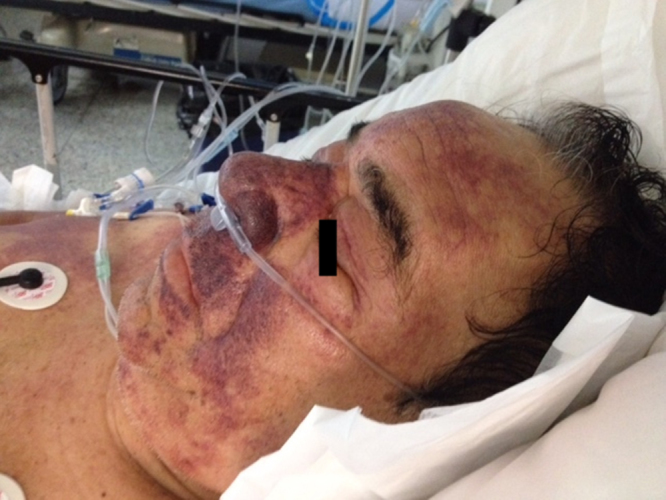
Ecchymotic plaques cover most of the face of the patient, being more conspicuous and confluent on his cheeks, philtral area and nostrils.
Multiple blood cultures were negative. Serum samples were positive for Chikungunya virus by conventional and real time PCR and negative for dengue virus. A dengue IgM serology was also negative.
Case 4
A 32 year old female suddenly experienced malaise, myalgias, arthralgias, headache and fever that improved with the use of acetaminophen. She had underwent splenectomy 14 years before due to Hodgkin's lymphoma and had been immunized against Streptococcus pneumonae and Neisseria meningitidis. The following day, she was feeling worse and was evaluated at a medical center feeling worse and found to be hypotensive and tachycardic. Laboratory results (Table 1) showed thrombocytopenia, leukopenia, and increased serum levels of ALT, BUN, creatinine and procalcitonin. She was admitted to the ICU, remaining hemodynamically instable. Multiple confluent purpuric lesions were noticed to rapidly develop on her face and limbs.
24 h later she was transferred to a larger medical facility. Upon arrival, she was conscious but appeared severely ill and was tachycardic, hypotensive and tachypneic. Large generalized purpuric lesions were evident on her limbs, face and oropharynx, as well as acrocyanosis in both hands and feet with localized necrosis of finger tips (Fig. 6). The tip of the nose and surrounding skin were necrotic. Chest X-rays and an echocardiogram were normal. Empiric antimicrobial therapy was started with meropenem and vancomycin and she was given a pulse dose of methylprednisolone.
Fig. 6.
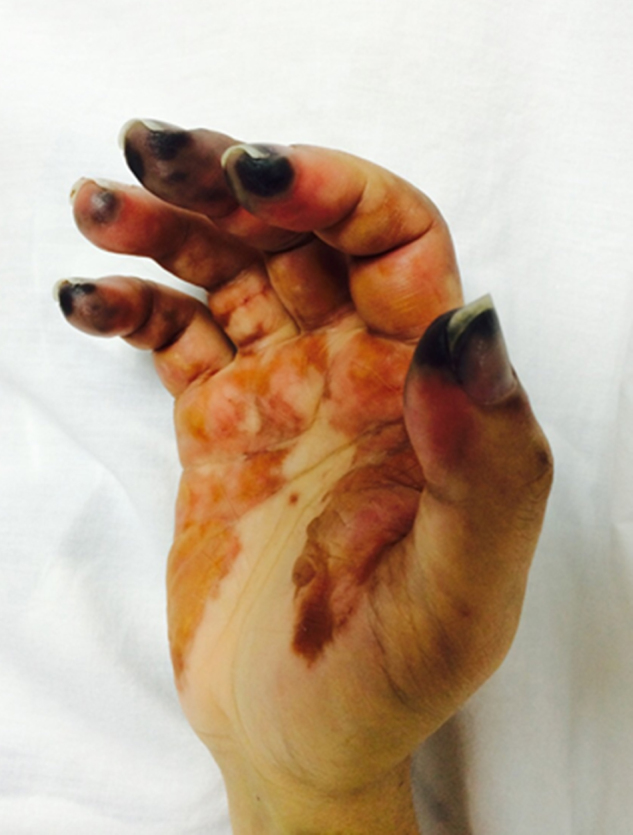
Diffuse inflammatory swelling of the right hand. Confluent ecchymoses of palm and fingers, as well as necrosis of finger tips, are evident.
Her condition continued to steadily deteriorate, requiring intubation and ventilator support. Rapidly expanding bullae developed on both upper and lower limbs (Fig. 7). Bilateral basal pulmonary infiltrates were seen on the chest X-ray and a repeat echocardiogram revealed global hypokinesia with a low ventricular ejection fraction. Nevertheless, after 72 h she began to improve gradually, and was discharged after 12 days.
Fig. 7.
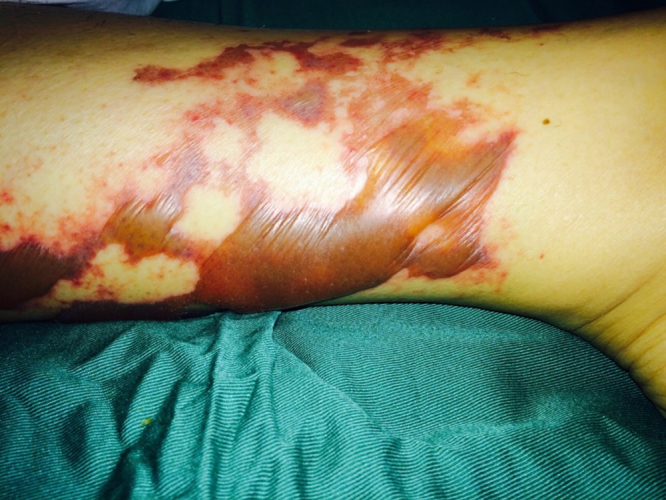
Characteristic bullae filled with abundant serous fluid, along with confluent ecchymotic plaques on patient's arm.
Serial blood cultures and bacterial antigen tests were negative. Conventional and real time PCR from serum samples were positive for CHIKV and negative for dengue virus. A dengue IgM serology was also negative. Other common and locally important viral and parasitic pathogens were also excluded by serology.
Discussion
Typical presentation of CHIKF includes abrupt onset of fever, arthralgias, and occasionally a maculopapular rash. Polyarticular arthritis and tenosynovitis can occur and may result in excruciating joint pain which can last for months to years. Indeed, the virus and illness name is derived from the Swahili word that means “to walk bent over” [6]. In the past, rare instances of hepatitis, myocarditis, hemorrhagic manifestations, and meningitis or encephalitis were described [6].
Recent outbreaks of CHIKF involving large number of patients, such as the 2005 epidemic of the French island territory of Réunion which affected 34% of all inhabitants, have resulted in more detailed descriptions of clinical manifestations including rare or previously unknown complications. These include neurologic syndromes (meningitis, encephalitis, and Guillain-Barré syndrome); neuro-ophthalmologic findings such as retrobulbar neuritis; cardiac complications (pericarditis and myocarditis); and maternal–fetal transmission resulting in abortion and congenital illness which all have been reported recently in association with CHIKV infection, suggesting that these severe presentations may be more common than previously considered [2], [5].
The proportion of atypical cases in all CHIKF patients in the Réunion experience was 0.3% with an overall case-fatality rate of 10.7%. Case fatality rate (CFR) reached 29%, however, when only severe cases were considered. The incidence of atypical cases, severe cases, and CFR increased with age. Indeed, the lethality rate was more than 5 times higher in patients aged ≥65 years as compared to those aged <45 years [5].
CHIKV infection seems to be responsible for severe clinical presentations, not only in elderly patients or patients at high risk, but also in younger patients with an unremarkable medical history. The mortality of severe cases may as high as 48% and CHIKV has been strongly suspected to have neurologic, hepatic, and myocardial tropism, with dramatic complications [5], [7]. Severe clinical forms are associated with the presence of several underlying medical conditions in about 90% of the cases. Pre-existing respiratory or cardiovascular diseases and hypertension have been recognized as risk factors of developing severe illness [5].
Until now, lethal cases of CHIKF have been almost exclusively associated with infections by the ECSA genotype, a clade of which was responsible for the large epidemics on islands in the Indian Ocean and the Indian subcontinent, from where most atypical and severe cases have been reported [1], [2], [5], [7]. However, as of November 26, 2014, according to the Pan American Health Organization, at least 149 deaths out of 747,317 have been attributed to CHIKF in the Caribbean region during the current ongoing epidemic. Case fatality rates from Martinique and Guadaloupe islands have been 0.97 and 0.73 per 1000, respectively [8].
Although no epidemiological data on the morbidity and mortality of the Venezuelan outbreak has yet been provided by the health authorities, the authors are aware of at least 20 additional, virologically documented, lethal cases in the country. Moreover, estimates of the approximate total number of cases of CHIKF, based on the number of unexplained acute febrile illness episodes seen at the outpatient clinics of the Ministry of Health, since June 2014, when the first authochonous patients were confirmed, are above 1,900,000 cases [9]. Therefore, it is plausible that the recognized deaths are only a small fraction of the real number.
It may be tempting to attribute the occurrence of an unexpected number of severe and lethal cases to a change in the virulence of the virus, the findings in a recent report characterizing the whole genome of the 2013 CHIKV detected on St. Martin Island [6] and the phylogenetic tree generated from sequencing data of CHIKV from British Virgin Islands in 2014 [10] show no significant differences with the Asian genotype being closely related to strains recently isolated in China and the Philippines. The partial sequencing of the virus obtained from our four patients and several other local severe and/or lethal cases, have not shown any evidence of mutations that could associate with increased virulence (Zoila Moros, personal communication). This finding supports that a single homogeneous CHIKV strain of the Asian genotype was recently introduced into the Caribbean and is currently moving throughout the region [4].
Our four patients developed clinical manifestations of severe CHIKF. The unique necrotic cutaneous nasal lesion observed in three of the cases, to our knowledge, has not been observed before in patients with CHIKF and will be discussed in detail elsewhere.
In countries where both diseases are endemic the differential diagnosis between severe CHIKF and severe dengue may be a challenge. A characteristic finding of the latter, not observed in any of the discussed patients, is the occurrence of massive capillary leakage and hemoconcentration. Moreover, dengue virus infection was ruled out in all the cases by both specific serology and PCR.
In countries experiencing epidemics of CHIKF clinicians must be familiar with the possibility of occurrence of atypical and severe cases of the disease, as such knowledge may help to improve its future management and strengthen the strategy of mitigation.
References
- 1.Morrison T.E. Reemergence of chikungunya virus. J Virol. 2014;88:11644–11647. doi: 10.1128/JVI.01432-14. Epub 2014 July 30. PMID: 25078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers A.M., Logue C.H. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 3.Powers A.M., Brault A.C., Tesh R.B., Weaver S.C. Re-emergence of chikungunya and o’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 4.Leparc-Goffart I., Nougairede A., Cassadou S., Prat C., de Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 5.Economopoulou A., Dominguez M., Helynck B., Sissoko D., Wichmann O., Quenel P. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Réunion. Epidemiol Infect. 2009;137:534–541. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- 6.Pialoux G., Gaüzère B.A., Jauréguiberry S., Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 7.Lemant J., Boisson V., Winer A., Thibault L., André H., Tixier F. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med. 2008;36:2536–2541. doi: 10.1097/CCM.0b013e318183f2d2. PMID: 18679124. [DOI] [PubMed] [Google Scholar]
- 8.PAHO http://www.paho.org/hq/index.php?option=com_topics&view=article&id=343&Itemid=40931Data
- 9.Boletin Epidemiológico Semanal. Venezuelan Ministry of Health http://www.mpps.gob.ve/index.php?option=com_phocadownload&view=category&id=43:ano2014&Itemid=915
- 10.Lanciotti R.S., Valadere A.M. Transcontinental movement of Asian genotype chikungunya virus. Emerg Infect Dis. 2014;20:1400–1402. doi: 10.3201/eid2008.140268. [DOI] [PMC free article] [PubMed] [Google Scholar]


