Abstract
Neurological melioidosis is a rare condition, as less than 30 cases have been reported in the last 50 years. We present a case of neurological melioidosis, presenting with a cerebral abscess in a returning traveler from an endemic area. While traveling in Cambodia on holiday, the patient was admitted to local hospital for pneumonia. Her condition improved after antimicrobial treatment, and she returned to Norway when discharged. The patient had several contacts with the health care system after returning to Norway, due to recurrent fever and deterioration. Short-term antimicrobial treatment was given with temporary improvement in her condition. Eventually she developed stroke-like symptoms, and a cerebral abscess was found. Cultures from the abscess were positive for Burkholderia pseudomallei and the treatment was adjusted accordingly.
Keywords: Burkholderia pseudomallei, Neurological melioidosis, Cerebral abscess, Treatment
Introduction
Melioidosis is a disease caused by the pathogen Burkholderia pseudomallei and the classical endemic regions are Southeast Asia and territory of Northern Australia [1]. Outside these regions the disease is uncommon, but is seen in returning travelers from endemic areas. B. pseudomallei is a facultative Gram-negative bacillus found in soil and surface water in the endemic regions. Acquisition of the bacterium occurs mainly by inhalation of contaminated dust or percutaneous inoculation of contaminated soil and the majority of the cases occur in the rainy season [1]. Traditional risk factors for more severe disease are excessive alcohol consumption, diabetes, and chronic renal and lung disease [2]. The microbe is also defined as a Category B bioterrorism agent due to its infectious potential and high virulence [1]. The most common presentation of melioidosis is pneumonia, but a variety of clinical presentations are described, including neurological melioidosis. Accounting for approximately 4% of all cases of melioidosis, neurological melioidosis has a high mortality rate of approximately 25% [3].
Case report
On holiday in Cambodia, during the rainy season, a 26-year-old previously healthy woman and her boyfriend rented 4-wheel all-terrain vehicles (ATVs) and went on a trip into more rural areas. The woman rode behind her boyfriend in the trails, and his bike made whirls of dust in front of her. Six days later she developed a high fever and respiratory symptoms and was admitted to the local hospital. Blood samples showed erythrocyte sedimentation rate (ESR) 28 mm, C-reactive protein (CRP) 306 mg/L, white blood cell (WBC) count 23 × 103/mm3, neutrophils 18 × 103/mm3, hemoglobin 12.7 g/dL, normal platelets and creatinine. Initial chest X-ray showed a patchy opacity in her left lung, and she was diagnosed with pneumonia. Blood cultures, urine, sputum, and pleural fluid cultures were all negative. Her condition improved after 10 days of ceftazidime intravenously, and she returned to Norway.
Two days after returning, she was admitted to hospital with recurrent pneumonia. During this hospital stay, she reported a tingling sensation and numbness in the skin on the left side of her face and body. These symptoms were not further investigated. She was dismissed from hospital after one week of ceftazidime treatment.
Two days later she was admitted to Oslo University Hospital with fever, headache, left sided hemiparesis and localized seizures in the left side of her face and left arm. Blood samples showed ESR 28 mm, hemoglobin 12.4 g/dL, CRP 11.5 mg/L, WBC count 20 × 103/mm3 and neutrophils 17 × 103/mm3. Computer tomography (CT) and magnetic resonance imaging (MRI) scans of the brain showed a lesion in the right frontal lobe, tentatively diagnosed as a glioma or an abscess (Fig. 1). Supplementary CT body scans did not reveal any other infection sites. Brain biopsy and diagnostic abscess drainage was performed. From the biopsy material a non-fermentative Gram-negative bacillus was isolated, and given her history, B. pseudomallei was suspected. To avoid laboratory-acquired infections the laboratory personnel were informed of the possible contagious microbe and further laboratory work was done within Biosafety Level 3 facilities. Initial identification of the microbe was done from cultured colonies by using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). The results suggested B. thailandiensis, a non-pathogenic species, genetically closely related to B. pseudomallei. Finally 16 rRNA PCR gene sequencing from the abscess material confirmed B. pseudomallei.
Fig. 1.
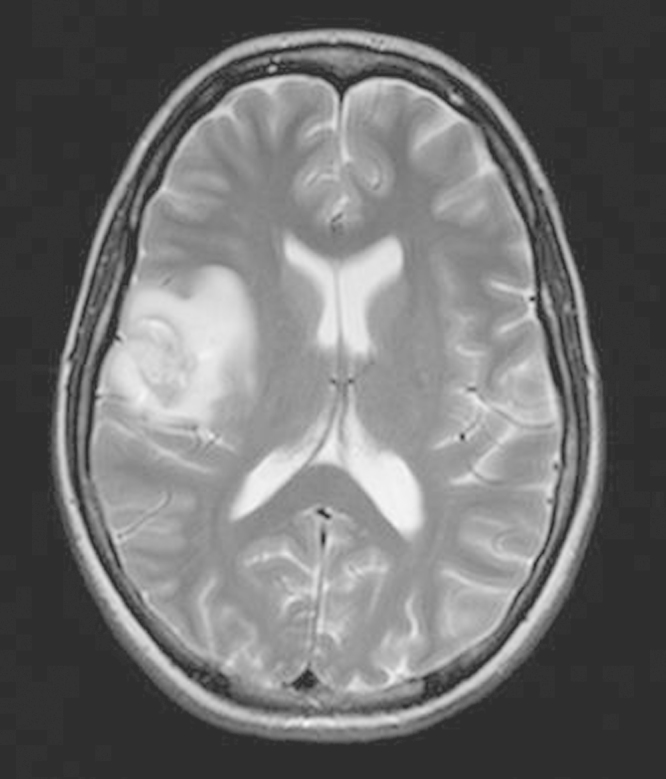
Initial MRI brain scan.
Shortly after the brain biopsy, on ceftazidime treatment (2 grams three times daily), the patient deteriorated. Susceptibility testing of the B .pseudomallei isolate, however, showed no resistance to ceftazidime which was increased to four times daily and sulfamethoxazole/trimethoprim (SXT) was added with subsequent improvement. The patient, who weighed 52 kg, received 4 weeks of treatment with ceftazidime 2 g four times daily and SXT (2400 mg/480 mg daily). Due to subsequent bone marrow suppression and rash, SXT was switched to oral amoxicillin/clavulanate (3500 mg/875 mg daily) and doxycycline (200 mg daily) for a total of 6 months. After 12 months of follow-up no relapse had occurred, MRI scans showed marked improvement (Fig. 2) and the patient completely recovered without neurological sequelae.
Fig. 2.
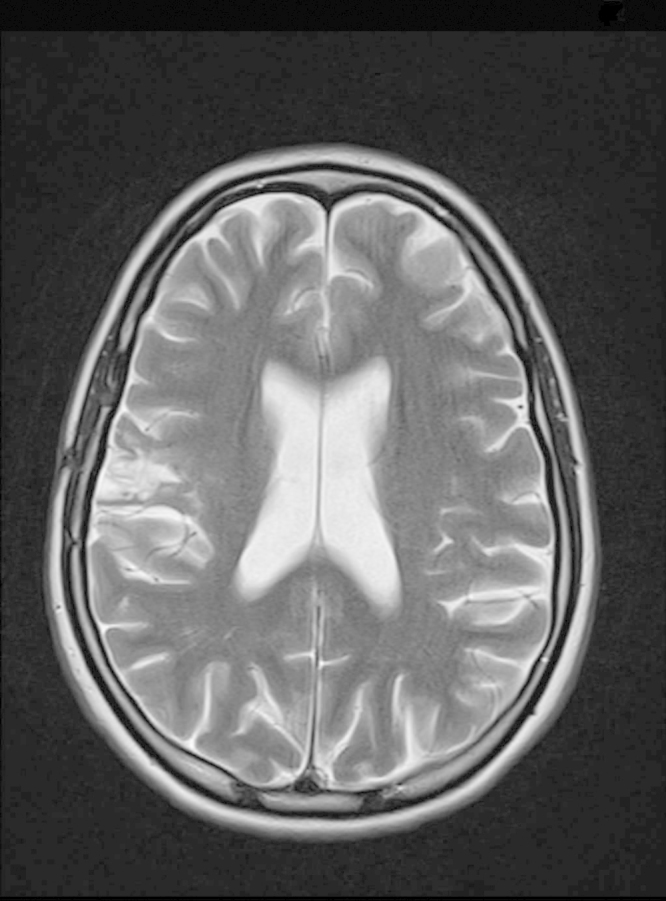
MRI brain scan after 10 months of follow-up.
Discussion
To our knowledge, no previous cases of neurological melioidosis from Cambodia have been published. Few clinicians have encountered the condition in travelers returning from endemic regions [4], [5]. Only 2 previously reported cases of traditional melioidosis in Norway have been published [6]. Diagnosing neurological melioidosis, far from endemic regions, is challenging, due to lack of clinical experience, low rate of detection and diagnostic challenges. Our patient had no traditional risk factors, except potentially exposure to B. pseudomallei while riding the ATV motorbikes. Two Australian publications found that 25–42% of their patients with neurological melioidosis had no risk factors [7], [8]. Neurological melioidosis might have a different risk profile than ordinary melioidosis, and may not trigger the clinical suspicion of the disease. Culture of B. pseudomallei from any clinical specimen remains the diagnostic gold standard. Cerebrospinal fluid (CSF) has shown a low detection rate [8], and searching for other sites of infection is essential [9]. In general, fast, accurate and safe microbe identification is always important, but especially as B. pseudomallei and B. mallei, both are classified as Category B bioterrorism agents [1]. All clinical and microbiological experience with these microbes is useful, due to its effective bioterror potential. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) has the potential of rapid and reliable identification of pathogens, including B. pseudomallei [10]. The availability of a database containing the validated reference spectra limited the possibility of accurate species identification in our case. 16 rRNA PCR gene sequencing was the most accurate identification method in our setting.
The pathogenesis of cerebral melioidosis has been debated. At present, there is no evidence of specific CNS invasive strains [7]. Our patient had no signs of sinusitis as a possible link to neurological melioidosis, as seen in other cases [3].
Applying case defining guidelines recently published by Cheng et al. [11], our patient had an inhalational melioidosis, with a probable primary focus in the left lung and secondary dissemination to CNS. It has been suggested that macro abscess formation can occur, particularly in untreated or insufficiently treated cases and this feature carries a poor prognosis [12]. B. pseudomallei can disseminate rapidly, and the initial delay in diagnosing and adequately treating the condition may have contributed to the development of neurological melioidosis in our patient.
The best antimicrobial treatment for cerebral melioidosis is still being discussed. Currently recommended initial therapy is intravenous ceftazidime or meropenem, alone or with SXT [3], [8], [13]. After returning to Norway she received initial treatment according to treatment recommendations with intravenous ceftazidime and SXT for 4 weeks. However, the eradication therapy consisted of amoxicillin 3.5 g/day and clavulanic acid 875 mg/day given in an eight hourly regime, combined with doxycycline 200 mg daily for six months. The concentration of amoxicillin–clavulanic acid in CSF is <10% relative to blood, which falls below the MIC for B. pseudomallei. Accordingly, amoxicillin–clavulanic acid is not recommended for eradication therapy when CNS manifestations [14]. Pharmacokinetic and pharmacodynamic studies in plasma concentration conclude that by oral eradication, an eight hourly dosing interval of 1000 mg amoxicillin and 250 mg clavulanic acid is sub-optimal [15], and a six hourly regimen should be considered.
To conclude, this case report demonstrates a successful outcome in a patient with neurological melioidosis, despite challenges in diagnosing the disease and using eradication therapy alternative to that recommended. It highlights the clinical and diagnostic challenges encountered and shows the importance of awareness in clinicians and laboratory personal of this disease in non-endemic areas, in returning travelers or as a consequence of bioterror.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Cheng A.C., Currie B.J. Melioidosis: epidemiology, pathophysiology and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currie B.J., Ward L., Cheng A. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin Prospective study. PLoS Negl Dis. 2010;4:11. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chadwick D.R., Ang B., Sitoh Y.Y., Lee C.C. Cerebral melioidosis in Singapore: a review of 5 cases. Trans R Soc Trop Med Hyg. 2002;96:72–76. doi: 10.1016/s0035-9203(02)90248-8. [DOI] [PubMed] [Google Scholar]
- 4.Vestal M.L., Wong E.B., Milner D., Gormley W.B., Dunn I.F. Cerebral melioidosis for the first time in the western hemisphere. J Neurosurg. 2013;119(6):1591–1595. doi: 10.3171/2013.5.JNS12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peetermans W.E., Van Wijngaerden E., Van Eldere J. Melioidosis brain and lung abscess after travel to Sri Lanka. Clin Infect Dis. 1999;28:921–922. doi: 10.1086/517247. [DOI] [PubMed] [Google Scholar]
- 6.Hesstvedt L., Wilhelmsen M., Mengshoel A.G., Dyrhol Riise A.M. Two Norwegian patients with melioidosis presenting with bacteraemia and splenic and prostatic abscesses. J Travel Med. 2011;18:418–421. doi: 10.1111/j.1708-8305.2011.00550.x. [DOI] [PubMed] [Google Scholar]
- 7.Currie B.J., Fisher D.A., Howard D.M., Burrow J.N.C. Neurological melioidosis. Acta Tropica. 2000;74:145–151. doi: 10.1016/s0001-706x(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 8.Deuble M., Aquilina C., Norton R. Neurologic melioidosis. Am J Trop Med Hyg. 2013;89:535–539. doi: 10.4269/ajtmh.12-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limmathurotsakul D., Chaowagul W., Wongsrikaew A., Day N.P., Peacock S.J. Variable presentation of neurological melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2007;77:118–120. [PubMed] [Google Scholar]
- 10.Karger A., Stock R., Ziller M., Elschner M.C., Bettin B., Melzer F. Rapid identification of Burkholderia mallei and Burkholderia pseudomallei by intact cell matrix-assisted laser desorption/ionisation mass spectrometric typing. BMC Microbiol. 2012;12(October):229. doi: 10.1186/1471-2180-12-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng A.C., Currie B.J., Dance D., Funnell S.G.P., Limmathurotsakul D., Simpson A.J.H. Clinical definitions of melioidosis. Am J Trop Med Hyg. 2013;88:411–413. doi: 10.4269/ajtmh.12-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergin P., Boyes L., Sage M. Australas Radiol. 2005;49:79–83. doi: 10.1111/j.1440-1673.2005.01404.x. [DOI] [PubMed] [Google Scholar]
- 13.Kandasamy Y., Norton R. Pediatric melioidosis in north Queensland, Australia. J Ped Child Health. 2008;44:706–708. doi: 10.1111/j.1440-1754.2008.01410.x. [DOI] [PubMed] [Google Scholar]
- 14.Bakken J.S., Bruun J.N., Gaustad P., Tasker T.C. Penetration of amoxicillin and potassium clavulanate into cerebrospinal fluid of patients with inflamed meninges. J Antimicrob Agents Chemother. 1986;30:481–484. doi: 10.1128/aac.30.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chierakul W., Wangboonskul J., Singtoroj T., Pongtavornpinyo W., Short J., Baharjan B. Pharmacokinetic and pharmacodynamic assessment of co-amoxiclav in the treatment of melioidosis. J Antimicrob Chemother. 2006;58:1215–1220. doi: 10.1093/jac/dkl389. [DOI] [PubMed] [Google Scholar]


