Abstract
The effective anti-tumorigenic potential of non-steroidal anti-inflammatory drugs (NSAIDs) and eicosonoid (EP; EP1–4) receptor antagonists prompted us to test their efficacy in Kaposi's sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV) related lymphomas. Our study demonstrated that (1) EP1–4 receptor protein levels vary among the various non-Hodgkin’s lymphoma (NHL) cell lines tested (BCBL-1:KSHV+/EBV−;BC-3: KSHV+/EBV−; Akata/EBV+: KSHV−/EBV+; and JSC-1 cells: KSHV+/EBV+ cells); (2) 5.0 µM of EP1 antagonist (SC-51322) had a significant anti-proliferative effect on BCBL-1, BC-3, Akata/EBV+, and JSC-1 cells; (3) 50.0 µM of EP2 antagonist (AH6809) was required to induce a significant anti-proliferative effect on BCBL-1, Akata/EBV+, and JSC-1 cells; (4) 5.0 µM of EP4 antagonist (GW 627368X) had a significant anti-proliferative effect on BC-3, Akata/EBV+, and JSC-1 cells; (5) COX-2 selective inhibitor celecoxib (5.0µM) had significant anti-proliferative effects on BCBL-1, BC-3, Akata/EBV+, and JSC-1 cells; and (6) a combination of 1.0µM each of celecoxib, SC-51322 and GW 627368X could potentiate the pro-apoptotic properties of celecoxib or vice-versa. Overall, our studies identified the synergistic anti-proliferative effect of NSAIDs and EP receptor blockers on KSHV and EBV related B cell malignancies.
Keywords: cyclooxygenase-2, COX-2 inhibitors, celecoxib, PGE2, EP receptors, EP receptor antagonists, KSHV latency, KSHV lymphomas, EBV lymphomas, non-Hodgkin’s lymphomas, AIDS linked lymphomas
INTRODUCTION
Malignant lymphomas account for 3–4% of all human malignancies.1, 2 Several infectious agents are known to cause aggressive malignant lymphomas such as lymphotropic herpes viruses, KSHV and Epstein-Barr virus (EBV).2 EBV and KSHV are the etiological agents underlying aggressive non-Hodgkin lymphomas (NHL) such as Burkitt’s lymphoma (BL), and primary effusion lymphoma (PEL) and multicentric Castleman’s disease (MCD), respectively.3–7 Interestingly, KSHV and EBV related lymphomas are often associated with HIV/AIDS or in the context of immune deficiency such as organ transplantation.3–7 The pathogenesis underlying KSHV and EBV related lymphomas is proposed to be the result of mutually dependent interactions between viral proteins, angiogenic factors, and proinflammatory mechanisms that under the conditions of immune suppression overcome host defenses against uncontrolled cell growth.4, 7 In recent years, several studies have suggested the role of a proinflammatory tumor microenvironment as a pivotal player in KSHV and EBV related oncogenesis.4 Consequently, drugs targeting viral infection and proinflammatory mechanisms are extensively studied to develop a disease specific treatment against KSHV and EBV associated lymphomas.4, 8–16
Studies using non-steroidal anti-inflammatory drugs (NSAIDs) have demonstrated their anti-cancer potential due to the blockade of major angiogenic stress response protein cyclooxygenase-2 (COX-2).17–30 COX-2 and PGE2 are proposed to play a crucial role in the pathogenesis underlying several cancers.31 The tumorigenic properties of COX-2 are attributed to its metabolite prostaglandin E2 (PGE2) that exerts its effect through eicosonoid (EP) receptors belonging to the seven-transmembrane rhodopsin family of G protein coupled receptors (GPCRs) designated as EP1, EP2, EP3, and EP4.32 The signaling pathways downstream of the EP receptors consist of canonical G-protein coupled signal molecules such as Ca2+, cAMP, PI3K, cell survival pathways and transcription regulators (AKT, ERK, NFκB, and β-catenin).32–38 Several recent studies suggested that EP receptors could be linked to major pathways involved in cancer such as angiogenesis, apoptosis, cell proliferation, metastasis, and immune suppression.32–38 The link between EP receptors and tumorigenesis has also revealed the possibility of using highly specific EP receptor antagonists, such as SC-51322 (EP1 antagonist), AH6809 (EP2 antagonist), and AH23848 (EP4 antagonist), as anti-cancer drugs.32–38 Taken together, these studies have indicated that EP receptors are vital in COX-2/PGE2 mediated inflammation and oncogenesis.
Although the anti-cancer effects of COX-2 inhibitors in treating NHLs are well studied, the chemotherapeutic potential of EP receptor antagonists is still unexplored in any lymphoma. In addition, the potential of creating a synergistic chemotherapeutic effect on cancer cells by the simultaneous blockade of COX-2 and EP receptors is also unexamined. Interestingly, our results demonstrate the anti-proliferative effects of EP1, EP2, and EP4 antagonists and the COX-2 inhibitor celecoxib on KSHV+/EBV− (BCBL-1 and BC-3), KSHV−/EBV+ (Akata/EBV+), and KSHV+/EBV+ (JSC-1) cell lines. EP1 and EP4 receptor blockade and COX-2 inhibition by celecoxib also induced apoptosis in BCBL-1 and Akata/EBV+ cells but not KSHV−/EBV− cell lines BJAB and Akata/EBV−. Combining EP1 and EP4 antagonists with celecoxib at concentrations that were not anti-proliferative, however, drastically induced apoptosis in BCBL-1 and Akata/EBV+ cells.
METHODS
Cell cultures
Body cavity B cell lymphoma (BCBL-1; KSHV+/EBV−), Akata/EBV+ (KSHV−/EBV+), Akata/EBV− (KSHV−/EBV−), BC-3 (KSHV+/EBV−), JSC-1 (KSHV+/EBV+), and BJAB (KSHV−/EBV−) cells were cultured in RPMI 1640 (Gibco BRL, Grand Island, New York) medium with 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, Utah), 2mM L-glutamine (Gibco BRL), and penicillin/streptomycin (Gibco BRL). Human microvascular dermal endothelial cells were cultured as described before.39 All cell lines were tested for mycoplasma contamination prior to be included in the experiments using MycoAlert™ PLUS mycoplasma detection Kit (Lonza Walkersville, Maryland) as per the manufacturer's instructions. It is a selective biochemical test that exploits the activity of mycoplasmal enzymes which are found in all six of the main mycoplasma cell culture contaminants and the vast majority of 180 mycoplasma species, but are not present in eukaryotic cells.
Reagents
Celecoxib was from Sigma, St. Louis, Mo. DAPI (4,6-diamidino-2-phenylindole) and rabbit-cleaved-caspase 3 antibody were from Invitrogen, Carlsbad, CA. GW 627368X (EP4 antagonist: 4-(4,9-diethoxy-1,3-dihydro-1-oxo-2H-benz[f]isoindol-2-yl)-N-(phenylsulfonyl benzenacetamide)), and AH6809 (EP2 antagonist: 6-isopropoxy-9-oxoxanthene-2-carboxylic acid) were purchased from Cayman Chemical, Ann Arbor, MI. SC-51322 (EP1 antagonist) was purchased from Enzo Life Sciences, Plymouth Meeting, PA.
Antibodies
Mouse monoclonal antibodies against human COX-2 and rabbit polyclonal antibodies against EP1, EP2, EP3, and EP4 receptors were purchased from Cayman Chemicals, Ann Arbor, MI. Mouse tubulin antibody was from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Rabbit-cleaved-caspase 3 antibody was from Invitrogen, Carlsbad, CA. Conjugates of anti mouse/rabbit-alkaline phosphatase and anti-mouse/rabbit-horseradish peroxidase were from Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD. Alexa-488/594-coupled anti-mouse antibody was from Molecular Probes, Eugene, OR.
Fluorescence activated cell sorting (FACS)
NHL cells after indicated treatments were permeabilized with permeabilization buffer (BD Biosciences, San Jose, CA), washed with permeabilization/wash buffer (BD Biosciences), blocked with 3% BSA (Sigma), and incubated with cleaved-caspase 3 antibody for 1h. In experiments with HMVEC-d cells, cells were serum starved for 24h, infected with KSHV (20 DNA copies per cell) for 2h or left uninfected (UN), washed with PBS, and supplemented with serum free DMEM throughout the experiment. At 24h, 48h, and 72h post-infection, cells were collected, permeabilized with permeabilization buffer, washed with permeabilization/wash buffer (BD Biosciences), blocked with 3% BSA, and incubated with EP1, EP2, EP3 or EP4 antibodies for 1h. The cells were washed again with permeabilization/wash buffer and stained with Alexa 488 secondary antibody and total protein levels were then measured with a FACSCalibur flow cytometer (Becton Dickinson, Bedford, MA). 10,000 cells were analyzed by gating all samples by forward and side scatter to eliminate dead cells. The data was analyzed with CellQuest Pro software (Becton Dickinson) at the Rosalind Franklin University of Medicine and Science (RFUMS) flow cytometry core facility.
Western blotting
Total cell lysates prepared from respective cells were resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gels. The resolved proteins transferred to nitrocellulose membranes were immunoblotted with the indicated primary antibodies. α-tubulin was used as loading control. Species-specific horseradish peroxidase secondary antibodies were used for detection. Immunoreactive bands were developed by Pierce Super Signal West Pico or Femto reagents (Pierce, Rockford, IL). The bands were scanned and quantitated with ImageQuant software following standard protocols.39
Real-time reverse transcription PCR (RT-PCR)
5×105 of BCBL-1, BC-3, Akata/EBV+, and BJAB cells were seeded with a combination of 1.0 µM each of celecoxib, SC- 51322, and GW 627368X and were replenished with the drugs every 12h. RNA samples were collected at 8h and 24h to examine the gene expression levels of Bcl-2-associated death promoter (BAD), B-cell lymphoma-2/Bcl–associated × protein (BAX), Bcl-10, Bcl-2, Bcl-xL, BH3 interacting-domain death agonist (BID), cellular inhibitor of apoptosis-1/2 (cIAP-1 and cIAP-2), Fas-Associated protein with Death Domain or FAS antigen or TNFRSF6 (FADD), p53, p21, Survivin, tumor necrosis factor (TNF), and X-linked inhibitor of apoptosis protein (XIAP) transcripts were detected by real time RT-PCR as described before.39. Normalization was done with respect to 18s. Primer sequences used for various genes were 18s (5′- AACCCGTTGAACCCCATT-3′ and 5′- CCATCCAATCGGTAGTAGCG-3′), BAD (5′- TGGATGACCTCGATGATGAAG-3′ and 5′-GCACAGCAACGCAGATGCG-3′), BAX (5′- TCCCCCCGAGAGGTCTTTT-3′ and 5′-CGGCCCCAGTTGAAGTTG-3′), Bcl-10 (5′- CAGGCTGGTCTTGAACT-3′ and 5′-GCTCGTGTCTGTAATCC-3′), Bcl-2 (5′- GAGGAGCTCTTCAGGGACGG-3′ and 5′-GGTGCCGGTTCAGGTACTCA-3′), Bcl-xL (5′- TCCTTGTCTACGCTTTCCACG-3′ and 5′-GGTCGCATTGTGGCCTTT- 3′), BID (5′- GTGAACCAGGAGTGAGTCGG-3′ and 5′-GACATCACGGAGCAAGGAC-3′), cIAP-1 (5′- CCAGCCTGCCCTCAAACCCTCT-3′ and 5′-GGGTCATCTCCGGGTTCCCAAC-3′), cIAP-2 (5′-GACAGGAGTTCATCCGTCAAGTTC-3′ and 5′-CCTGGGCTGTCTGATGTGG-3′), FADD (5′-TGCAGAAGATGTAGATTGTGTGATGA-3′ and 5′- GGGTCCGGGTGCAGTTTATT-3′), p53 (5′-TAACAGTTCCTGCATGGGCGGC-3′ and 5′- AGGACAGGCACAAACACGCACC-3′), p21 (5′-GAGGCCGGGATGAGTTGGGAGGAG-3′ and 5′-CAGCCGGCGTTTGGAGTGGTAGAA-3′), Survivin (5′- GCCCAGTGTTTCTTCTGCTT-3′ and 5′-CCGGACGAATGCTTTTTATG-3′), TNF (5′- TGGCCCAGGCAGTCAGA-3′ and 5′-GGTTTGCTACAACATGGGCTACA-3′), XIAP (5′- GAGAAGATGACTTTTAACAGTTTTGA-3′ and 5′- TTTTTTGCTTGAAAGTAATGACTGTGT-3′). Statistics (t-test) were calculated with respect to 8h gene expression for each time point. Error bars represent mean ± SD (*) p<0.05. Upregulation or downregulation in gene expression are indicated in Table1.
Table 1.
Effect of concurrent targeting of COX-2 and EP1/EP4 receptors with 1.0µM each of celecoxib, SC-51322, and GW 627368X on various classes of NHL genes
| Akata/EBV+ (KSHV−/EBV+) |
BCBL-1 (KSHV+/EBV−) |
BC-3 (KSHV+/EBV−) |
BJAB (KSHV−/EBV−) |
|||||
|---|---|---|---|---|---|---|---|---|
| 8h | 24h | 8h | 24h | 8h | 24h | 8h | 24h | |
| Pro-apoptotic genes | ||||||||
| TNF | ⬆ | ⬆ | ⬆ | ⬆ | • | • | • | • |
| FADD | ⬆ | ⬆ | ⬆ | ⬆ | • | • | • | • |
| BID | ⬆ | ⬆ | ⬆ | ⬆ | • | • | • | • |
| BAD | ⬆ | ⬆ | ⬆ | ⬆ | • | • | • | • |
| BAX | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ✖ | ✖ | ✖ |
| p53 | ⬆ | ⬆ | ✖ | ⬆ | ⬆ | ⬆ | ✖ | ✖ |
| p21 | ✖ | ⬆ | ✖ | ⬆ | ⬆ | ⬆ | ✖ | ✖ |
| Anti-apoptotic genes | ||||||||
| CIAP-1 | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ |
| CIAP-2 | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ |
| Survivin | ⬇ | ⬇ | ⬇ | ⬇ | ✖ | ⬇ | ✖ | ⬇ |
| Bcl-2 | ⬇ | ⬇ | ⬇ | ⬇ | ✖ | ⬇ | ✖ | ✖ |
| Bcl-xL | ⬇ | ⬇ | ⬇ | ⬇ | ✖ | ✖ | ✖ | ✖ |
| Bcl-10 | ⬇ | ⬇ | ⬇ | ⬇ | • | • | • | • |
| XIAP | ✖ | ⬇ | ⬇ | ⬇ | • | • | • | • |
(⬆) Increase (⬇) Decrease (✖) No significant change (•) Undetermined
Measurement of PGE2
Secreted amounts of PGE2 were measured using a PGE2 ELISA kit as per the manufacturer’s guidelines (R & D systems, Minneapolis, MN).
Immunofluorescence
Cells, after acetone fixation to glass slides, were washed with PBS and treated with the indicated primary antibodies, washed with PBS, followed by Alexa 488 secondary antibody treatment. After being washed with PBS, the cells were mounted with antifade reagent containing DAPI. The fluorescence-positive cells were observed under a fluorescence microscope equipped with a Nikon Metamorph digital imaging system.
Cell proliferation and viability assays
5×105 of respective cells were seeded with respective drugs at the indicated concentrations and the number of proliferating and viable cells as examined by measuring their metabolically active mitochondria (an index of cell proliferation) based on a colorimetric assay (ATCC, Manassas, VA) as per the manufacturer's instructions at the indicated time points. Fold cell proliferation and cell death was calculated with respect to 0h for each treatment. A student’s t-test was employed (p<0.01) for all data analysis.
Cytotoxicity assay
Doses and time of incubation with drugs were chosen based on preliminary cytotoxicity assays. Supernatants of Body cavity B cell lymphoma (BCBL-1; KSHV+/EBV−), Akata/EBV+ (KSHV−/EBV+), Akata/EBV− (KSHV−/EBV−), BC-3 (KSHV+/EBV−), JSC-1 (KSHV+/EBV+), and BJAB (KSHV−/EBV−) cells treated with various drugs (celecoxib, GW 627368X, AH6809, and SC-51322) were collected at different time points (1d, 2d, 3d, 4d, 5d) to assess cellular toxicity using a cytotoxic assay kit (Promega, Madison, WI) as described in our previous manuscripts.39, 40
Real time PCR Array
1× 106 BCBL-1 and Akata/EBV+ cells were seeded with a combination of 1.0 µM each of celecoxib, GW 627368X, AH6809, and SC-51322 or left untreated and were replenished with the drugs every 12h for treated samples. RNA was collected at 8h and 24h post treatment, and was used to profile 84 key genes involved in lymphoma pathogenesis using RT2 profiler lymphoma PCR array as per manufacturer’s guidelines (SABiosciences, Valencia, CA).
RESULTS
EP1–4 receptor protein levels and PGE2 secretion vary between different NHL cell lines
Previous studies have reported the upregulation of COX-2 in NHL.17 Therefore, we examined the levels of EP1–4 receptors in KSHV−/EBV− (BJAB and Akata/EBV−), KSHV+/EBV− (BCBL-1 and BC-3), KSHV−/EBV+ (Akata/EBV+), and KSHV+/EBV+ (JSC-1) cell lines (Fig. 1a). Our results indicate that the protein levels of EP receptors varied between the cell types considerably and within the same cell type (Fig. 1a). In parallel experiments, we also demonstrated the presence of PGE2 secretion in the supernatants of BJAB (56pg/ml), Akata/EBV− (198pg/ml), BCBL-1 (274pg/ml), BC-3 (284pg/ml), Akata/EBV+ (313pg/ml), and JSC-1 (390pg/ml) cells (Fig. 1b). Immunofluorescence studies further confirmed the presence of EP1–4 receptors in all cell lines tested with a mainly cytoplasmic distribution (Fig. 1c).
Figure 1. EP receptors in non-Hodgkin lymphoma cell lines and de novo KSHV infected HMVEC-d cells.
(a) Total lysates from 5×105 BJAB, Akata/EBV−, BCBL-1, BC-3, Akata/EBV+, and JSC-1 cells were immunoblotted for EP1, EP2, EP3, and EP4. Data represents three independent experiments. Tubulin was used as loading control. (b) In parallel experiments, supernatants from the indicated cells were collected to measure the amount of PGE2 secreted by each cell line. (c) The indicated cell lines were stained for EP1, EP2, EP3, and EP4 receptors by immunofluorescence. (d) Mean fluorescent intensity (MFI) of EP1, EP2, EP3, and EP4 receptors in HMVEC-d cells infected with KSHV measured by FACS. Indicated are the MFI for the respective receptor at each time point. The data is representative of three independent experiments.
KSHV infection upregulates EP receptors in primary HMVEC-d cells
Previous studies have clearly described the role of the COX-2/PGE2 pathway in the KSHV latency program.39–42 Therefore, we next examined the effect of de novo KSHV infection on EP1–4 receptor levels in primary HMVEC-d cells by measuring the mean fluorescent intensity (MFI) of each receptor, post infection by FACS. The MFI for EP1, EP2, and EP3 receptors per cell increased at 24h to 53.4, 112.8, and 413 and at 48h to 57.4, 135.2, and 419 from 45.2, 115.7, and 347, respectively (Fig. 1d). The MFI for EP4 receptor increased to 254.3 at 24h from 188.7 (untreated) and decreased to 131.3 and 99.3 at 48h and 72h p.i., respectively (Fig. 1d). At 72h p.i., the MFI for EP1, EP2, and EP3 receptors per cell decreased to 40.2, 96.3, and 263 compared to untreated cells, respectively (Fig. 1d). Overall, these results indicate that KSHV infection regulates EP1–4 receptor levels.
EP1, EP2, and EP4 antagonists downregulated KSHV+ and EBV+ cell proliferation in culture
Our earlier studies have strongly indicated the role of COX-2 and EP receptors on the KSHV latency program.39–41, 42, 43, 44 The anti-prolilferative effects of EP receptor blockers have also been reported in other tumor model systems32–38 but never studied in KSHV related cancers. We first examined the effect of EP1 antagonist (SC-51322), EP2 antagonist (AH6809), and EP4 antagonist (GW 627368X) on human NHL cell lines BCBL-1 (KSHV+/EBV−), BC-3 (KSHV+/EBV−), Akata/EBV+ (KSHV−/EBV+), and JSC-1 (KSHV+/EBV+).
The EP1 antagonist (SC-51322) at 5.0µM induced significant proliferation arrest and cell death at day 5 post-treatment on BCBL-1 (Fig. 2a–b), BC-3 (Fig. 2c–d), and BJAB (Fig. 2i–j) cells. The drug at 5.0µM significantly downregulated cell proliferation and induced cell death at day 3 and sustained the effect on day 5 for Akata/EBV+ (Fig. 2e–f) and JSC-1 (Fig. 2g–h) cells. At 50.0µM concentration, SC-51322 induced proliferation arrest and cell death at day 2 for BCBL-1 (Fig. 2b), BC-3 (Fig. 2c–d), and JSC-1 cells (Fig. 2g–h), at day 1 for Akata/EBV+ (Fig. 2e–f) and BJAB (Fig. 2i–j) cells and was sustained until day 5. SC-51322 (0.5µM) induced significant cell death at day 5 in BCBL-1 (Fig. 2b) and BC-3 (Fig. 2d) cells, although we did not see a significant effect on cell proliferation.
Figure 2. Effects of SC-51322 on NHL cell lines.
5×105 BCBL-1 (a–b), BC-3 (c–d), Akata/EBV+ (e–f), JSC-1 (g–h), and BJAB (i–j) cells were seeded with the indicated concentrations of SC-51322 and were replenished with the drug every 12h for 5 days. The cells were not replenished with fresh media. Cell proliferation was measured by MTT assay at day 1 (1d), day 2 (2d), day 3 (3d), and day 5 (5d). The fold absorbance was calculated with respect to the untreated (UN) control before treatments. Statistics (t-test) were calculated with respect to untreated for each time point. Error bars represent mean ± SD.
The EP2 antagonist (AH6809) did not have any significant effect on the cell proliferation of BCBL-1, BC-3, Akata/EBV+, JSC-1, and BJAB cells at 0.5µM and 5.0µM concentrations (Fig. 3a, 3c, 3e, 3g, and 3i). However, AH6809 (0.5µM and 5.0µM) induced significant cell death at day 5 in BCBL-1 (Fig. 3b) and BC-3 cells (Fig. 3d). However, at 50.0µM, AH6809 induced significant proliferation arrest and cell death at day 3 for BCBL-1 (Fig. 3a–b), and JSC-1 cells (Fig. 3g–h), at day 2 for Akata/EBV+ cells (Fig. 3e–f) and was sustained until day 5 with no significant effect on BC-3 cells (Fig. 3c–d). 50.0µM AH6809 also induced significant cell death at day 3 in BJAB cells and sustained it with no significant effect on cell proliferation (Fig. 3i–j).
Figure 3. Effects of AH6809 on NHL cell lines.
5×105 BCBL-1 (a–b), BC-3 (c–d), Akata/EBV+ (e–f), JSC-1 (g–h), and BJAB (i–j) cells were seeded with the indicated concentrations of AH6809 and were replenished with the drug every 12h for 5 days. The cells were not replenished with fresh media. Cell proliferation was measured by MTT assay at day 1 (1d), day 2 (2d), day 3 (3d), and day 5 (5d). The fold absorbance was calculated with respect to the untreated (UN) control before treatments. Statistics (t-test) were calculated with respect to untreated for each time point. Error bars represent mean ± SD.
EP4 antagonist (GW 627368X) at 5.0µM induced significant proliferation arrest and cell death at day 5 in BC-3 (Fig. 4–d), Akata/EBV+ (Fig. 4e–f), and JSC-1 cells (Fig. 4g–h) with no significant effect on BCBL-1 (Fig. 4a) and BJAB (Fig. 4i) cell proliferation. However, GW 627368X (0.5µM) induced significant cell death at day 5 in BCBL-1 cells (Fig. 3b). At 50.0µM, GW 627368X downregulated cell proliferation and induced cell death significantly at day 2 for BC-3 (Fig. 4c–d), Akata/EBV+ (Fig. 4e–f), and JSC-1 cells (Fig. 4g–h), at day 1 for BJAB cells (Fig. 4i–j), at day 3 for BCBL-1 cells (Fig. 4a–b) and sustained it till day 5. At 0.5µM concentration, GW 627368X had no significant effect on cell proliferation and cell death in any of the cell lines (Fig. 4a–j).
Figure 4. Effects of GW 627268X on NHL cell lines.
5×105 BCBL-1 (a–b), BC-3 (c–d), Akata/EBV+ (e–f), JSC-1 (g–h), and BJAB (i–j) cells were seeded with the indicated concentrations of GW 627268X and were replenished with the drug every 12h for 5 days. The cells were not replenished with fresh media. Cell proliferation was measured by MTT assay at day 1 (1d), day 2 (2d), day 3 (3d), and day 5 (5d). The fold absorbance was calculated with respect to the untreated (UN) control before treatments. Statistics (t-test) were calculated with respect to untreated for each time point. Error bars represent mean ± SD.
COX-2 inhibitor celecoxib downregulates the proliferation of KSHV+ and EBV+ cells in culture
Previous reports have demonstrated the anti-proliferative effects of celecoxib at concentrations greater than 10µM on NHL cells45, 46 and the role of COX-2 in the KSHV latency program.39 Therefore, as a control for our cell proliferation studies we next examined whether we can replicate the effects of celecoxib on BCBL-1 (KSHV+/EBV), BC-3 (KSHV+/EBV), Akata/EBV+ (KSHV−/EBV+), JSC-1 (KSHV+/EBV+), and BJAB (KSHV−/EBV−). Celecoxib at 5.0µM concentration induced significant proliferation arrest and cell death in BCBL-1 (Fig. 5a–b), BC-3 (Fig. 5c–d), and JSC-1 cells (Fig. 5g–h) at 5 days post-treatment and at days 3 and 5 in Akata/EBV+ cells (Fig. 5e–f). There was no significant effect on any of the cell lines at 0.5µM (Fig. 5a–j). However, celecoxib (0.5µM and 5.0µM) induced significant cell death at day 5 in BCBL-1 cells (Fig. 5b). In contrast, at 50.0µM celecoxib induced sustained proliferation arrest and cell death in BCBL-1 cells (Fig. 5a–b) at day 2 and at day 1 in BC-3 (Fig. 5c–d), Akata/EBV+ (Fig. 5e–f), and JSC-1 (Fig. 5i–j) cells.
Figure 5. Effects of celecoxib on NHL cell lines.
5×105 BCBL-1 (a–b), BC-3 (c–d), Akata/EBV+ (e–f), JSC-1 (g–h), and BJAB (i–j) cells were seeded with the indicated concentrations of celecoxib and were replenished with the drug every 12h for 5 days. The cells were not replenished with fresh media. Cell proliferation was measured by MTT assay at day 1 (1d), day 2 (2d), day 3 (3d), and day 5 (5d). The fold absorbance was calculated with respect to the untreated (UN) control before treatments. Statistics (t-test) were calculated with respect to untreated for each time point. Error bars represent mean ± SD.
Celecoxib, SC-51322, and GW 627368X induces apoptosis in BCBL-1, BC-3, Akata/EBV+, and JSC-1 cells
We next examined whether the sustained anti-proliferative effects we observed with celecoxib, SC-51322, and GW 627368X were due to the induction of apoptosis by measuring the levels of cleaved-caspase 3. To investigate whether the anti-growth effects we observed were due to a specific anti-KSHV or anti-EBV effect or general cytotoxic effect, we selected 5.0µM, the lowest concentration of celecoxib, SC-51322, and GW 627368X that had a significant anti-proliferative effect and used KSHV/EBV− cell lines Akata/EBV− and BJAB cells as control cell lines. BCBL-1, Akata/EBV+, Akata/EBV−, and BJAB were seeded with 5.0µM celecoxib, SC-51322, and GW 627368X and supplemented with the drug every 12h for 5 days and the levels of cleaved-caspase 3 were compared between untreated and day 5 cells.
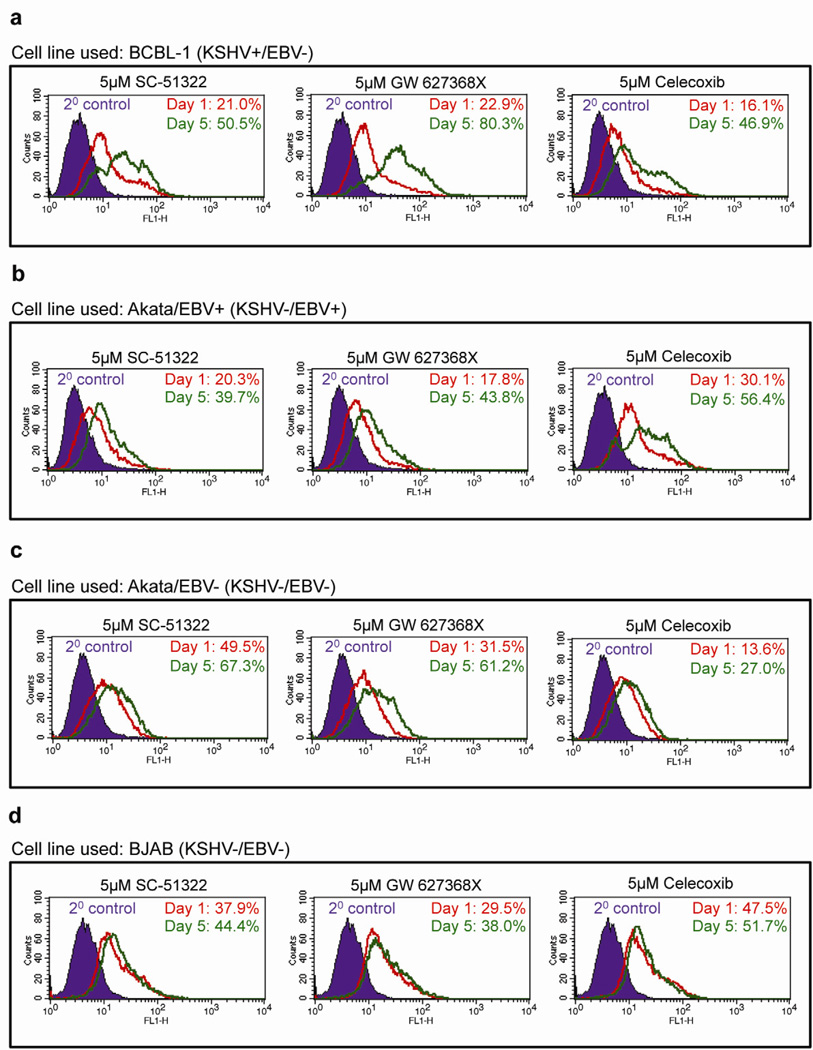
The COX-2 inhibitor celecoxib at 5.0µM, increased the percentage of cells positive for cleaved-caspase 3 at day 5 compared to day 1 in BCBL-1 (Fig. 6a) and Akata/EBV+ (Fig. 6b) cells, increased the most dramatically from 16.1% to 46.9% and 30.1% to 56.4%, respectively with a modest effect on Akata/EBV− (Fig. 6c) and BJAB (Fig. 6d) cells from 13.6% to 27.0% and 47.5% to 51.7%, respectively. Similarly, 5.0µM of EP1 antagonist SC-51322 also induced apoptosis with an increase in the percent of cleaved-caspase 3 positive cells at day 5 compared to day 1 in BCBL-1 (Fig. 6a) and Akata/EBV+ (Fig. 6b) cells, from 21.0% to 50.5% and 20.3% to 39.7% with a modest effect on Akata/EBV− (Fig. 6c) and BJAB (Fig. 6d) cells from 49.5% to 67.3% and 37.9% to 44.4%, respectively. 5.0µM of EP4 anatagonist GW 627368X had the most pro-apoptotic effect with an increase in the percent of cleaved-caspase 3 positive cells at day 5 compared to day 1 in BCBL-1 (Fig. 6a) and Akata/EBV+ (Fig. 6b) cells, from 22.9% to 80.3% and 17.8% to 43.8% with a modest effect on Akata/EBV− (Fig. 6c) and BJAB (Fig. 6d) cells from 31.5% to 61.2% and 29.5% to 38.0%, respectively.
Figure 6. Effect of celecoxib, SC-51322, and GW 627368X on the apoptotic marker cleaved-caspase-3 in BCBL-1, Akata/EBV+, Akata/EBV−, and BJAB cells.
(a–b) 5×105 BCBL-1 (a), Akata/EBV+ (b), Akata/EBV− (c), and BJAB (d) cells were seeded with celecoxib (5 µM), SC-51322 (5 µM) or GW 627368X (5 µM) and were replenished with the drug every 12h for 5 days. Samples were collected at days 1 and day 5 to examine the levels of the apoptotic marker cleaved-caspase 3 by FACS. The data is representative of two independent experiments.
Combinations of celecoxib, SC-51322, and GW 627368X potentiate the pro-apoptotic effects on BCBL-1 and Akata/EBV+ cells
PGE2 is the tumorigenic metabolite of COX-2 that exerts its effects through EP receptors.32 Therefore, we next examined whether treating BCBL-1 and Akata/EBV+ cells with a combination of 1.0µM each of SC-51322, GW 627368X, and celecoxib could induce additive pro-apoptotic effects. Although, we did not observe any additive effects with the combination of SC-51322 (5.0µM) and GW 627368X (5.0µM), compared to their individual pro-apoptotic effects, we observed an increase in the percent of cleaved-caspase 3 positive cells at day 5 compared to day 1 for BCBL-1 and Akata/EBV+ cells from 51.7% to 74.2% and 13.2% to 42.1%, respectively (Fig. 7a). We next investigated whether combining 1.0µM celecoxib with 1.0µM of SC-51322 and GW 627368X can potentiate the anti-proliferative effect of celecoxib or vice-versa. Although, 5.0µM of celecoxib, SC-51322 or GW 627368X was the least concentration required to induce proliferation arrest, the combination of celecoxib, SC-51322, and GW 627368X at 1.0µM increased the percent of cleaved-caspase 3 positive cells for BCBL-1 and Akata/EBV+ from 39.5% to 68.5% and 28.2% to 42.5%, respectively (Fig. 7b).
Figure 7. Effect of combinations of celecoxib, SC-51322, and GW 627368X on KSHV+/EBV− and KSHV−/EBV+ cells.
(a–b) 5×105 BCBL-1 and Akata/EBV+ cells were seeded with a combination of 5.0 µM each of SC-51322 and GW 627368X (a) or a combination of 1.0 µM each of celecoxib, SC-51322, and GW 627368X (b) and were replenished with the drugs every 12h for 5 days. Samples were collected at days 1 and 5 to examine the levels of the apoptotic marker cleaved-caspase 3 by FACS. The data is representative of two independent experiments.
We next examined whether combinations of celecoxib, SC-51322, and GW 627368X at 1.0µM each affected the expression of various pro-apoptotic and anti-apoptotic genes. In Akata/EBV+ cells, concurrent targeting of COX-2 and EP1/EP4 antagonists at 8h and 24h post treatment significantly upregulated pro-apoptotic genes TNF, FADD, BID, BAD, and BAX and significantly downregulated anti-apoptotic genes cIAP-1, cIAP-2, Survivin, Bcl-2, Bcl-xL, Bcl-10, and XIAP with no significant change in p21 (8h) and XIAP (8h) (Table 1). At 8h and 24h post-treatment, concurrent targeting of COX-2 and EP1/EP4 antagonists significantly upregulated pro-apoptotic genes TNF, FADD, BID, BAD, BAX, p53, and significantly downregulated anti-apoptotic genes Bcl-10, Bcl-2, Bcl-xL, cIAP-1, cIAP-2, and Survivin in BCBL-1 cells with no significant change in p21 (8h) and p53 (8h) (Table 1). In BC-3 cells, significant upregulation of pro-apoptotic genes BAX, p53, and p21 and significant downregulation of anti-apoptotic genes cIAP-1 and cIAP-2 was seen at 8h (Table 1) post-treatment with celecoxib, SC-51322, and GW 627368X treatments. At 24h, celecoxib and EP1/4 receptor antagonist combination treatment in BC-3 cells significantly upregulated p53, p21 and downregulated cIAP-1, cIAP-2, Survivin, and Bcl-2 (Table 1). With the exception of the significant downregulation of general cell survival molecules cIAP-1 and cIAP-2 at 8h and 24h and Survivin at 24h, concurrent targeting of COX-2 and EP1/EP4 antagonists had no significant effect on any of the apoptotic genes examined in BJAB cells (Table 1).
Concurrent targeting of COX-2 and EP1/EP4 receptors markedly alters the lymphoma gene expression profile of BCBL-1 and Akata/EBV+ cells
BCBL-1 and Akata/EBV+ cells are B cells transformed by latent infections of KSHV and EBV with overlapping gene expression profiles compared to other NHLs such as follicular lymphoma (FL) and diffused large B cell lymphoma (DLBCL).2–5, 7 Considering apoptosis pathway induction with concurrent inhibition of COX-2 and EP1/EP4 receptors (Table 1), we next examined the gene expression profile of key genes involved in lymphoma pathogenesis including lymphoma survival, immune and cell cycle regulation, cell adhesion, PI3K/Akt signaling, inflammation, gene fusion, differential methylation of promoters, and DNA repair, and also screened for key lymphoma therapeutic targets. BCBL-1 and Akata/EBV+ cells were seeded with a combination of 1.0 µM each of celecoxib, SC-51322, and GW 627368X and fold regulation for untreated samples at 24h and treated samples at 8h and 24h was calculated with respect to untreated samples at 8h and are listed in supplement tables 1–3 (BCBL-1) and supplement tables 4–6 (Akata/EBV+) with respective p-values (p<0.05 for significance). Clustergrams and dendrograms indicating co-regulated genes across groups (untreated samples at 8h and 24h and treated samples at 8h and 24h) for BCBL-1 and Akata/EBV+ cells are represented in supplemental figures 1–2. Drug treatments did not have a significant effect on any of the genes at 24h post treatment for BCBL-1 (Supplemental table 1–3) and Akata/EBV+ cells (Supplemental table 10–12).
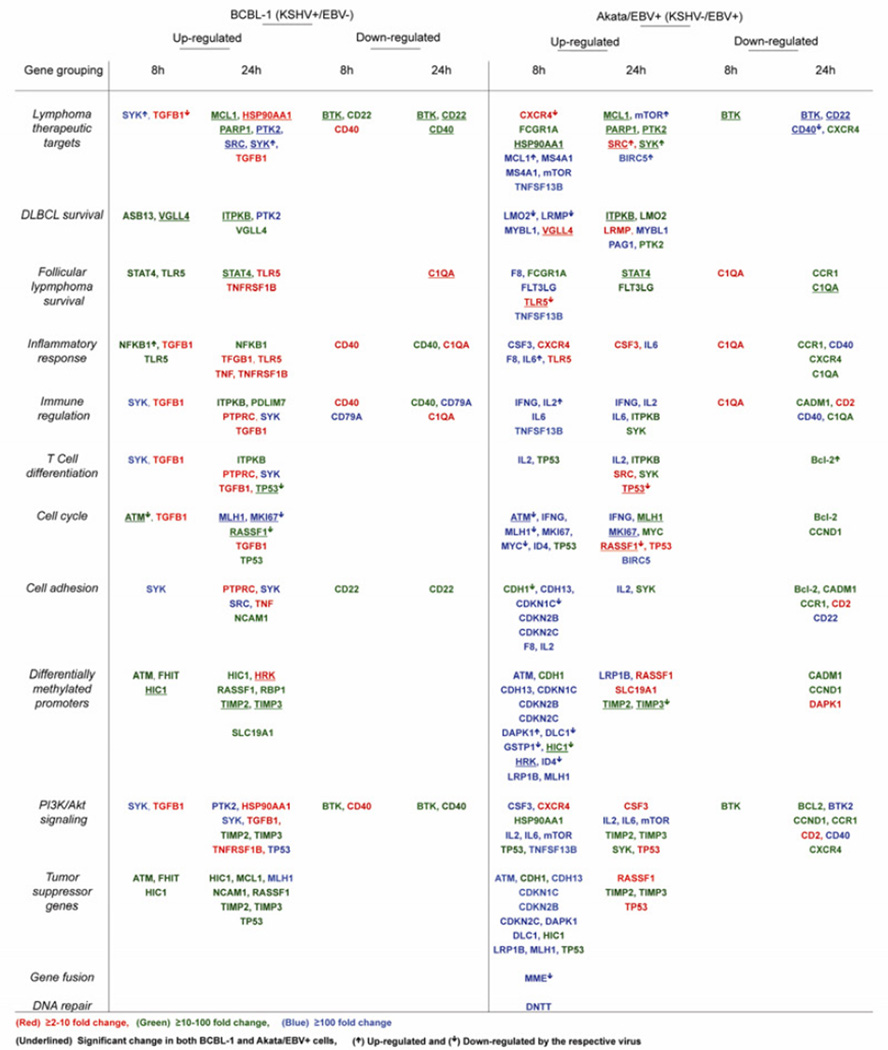
Table 2 lists the respective genes significantly (p<0.05) upregulated or downregulated at the indicated time points for BCBL-1 and Akata/EBV+ cells. Various genes were classified based on functionality using SA Biosciences pathway source references (Table 1). Genes significantly upregulated or downregulated upon concurrent inhibition of COX-2 and EP1/EP4 receptors in BCBL-1 or Akata/EBV+ cells are categorized based on fold regulation into three groups: a) ≥2–10 fold (shown in red), b) ≥10–100 fold (shown in green), and c) ≥100 fold (shown in blue) in Table 2.
Table 2.
Effect of concurrent targeting of COX-2 and EP1/EP4 receptors with 1.0µM each of celecoxib, SC-51322. and GW 627368X on various classes of NHL genes.
We observed ≥10–100 fold induction of lymphoma therapeutic target genes (SYK, PTK2, SRC), DLBCL survival (PTK2), cell adhesion (SYK, SRC), cell cycle (MK167, MLH1) and T cell differentiation (SYK), PI3K/AKT signaling (PTK2, TP53, SYK), and tumor suppressor pathway (MLH1) genes in BCBL-1 cells at the time points indicated in Table 2. ≥10–100 fold induction was observed in the expression of lymphoma therapeutic target genes (MCL1, PARP1), DLBCL survival (ASB13, VGLL4, ITPKB), follicular lymphoma survival (TLR5, STAT4), inflammatory response (NFKB1, TLR5), immune regulation (ITPKB, PDLIM7), T cell differentiation (ITPKB, TP53), cell cycle (RASSF1), cell adhesion (NCAM1), differentially methylated promoters (ATM, FHIT, HIC1, RASSF1, RBP1, TIMP2, TIMP3, SLC19A1), PI3K/AKT signaling (TIMP2, TIMP3), and tumor suppressor pathway (ATM, FHIT, HIC1, MCL1, NCAM1, RASSF1, TIMP2, TIMP3, TP53) genes in BCBL-1 cells at the time points indicated in Table 2. We observed ≥2–10 fold induction in the expression of lymphoma therapeutic target genes (TGFB1, HSP90AA1), follicular lymphoma survival (TLR5, TNFRSF1B), inflammatory response (TGFB1, TNF, TLR5, TNFRSF1B), immune regulation and T cell differentiation (TGFB1, PTPRC), cell cycle (TGFB1), cell adhesion (PTPRC, TNF), differentially methylated promoters (HRK), and PI3K/AKT signaling (HSP90AA1, TGFB1, TNFRSF1B) genes in BCBL-1 cells at the time points as indicated in Table 2. Celecoxib and EP1/EP4 receptor antagonist treatment downregulated genes of multiple pathways including lymphoma therapeutic targets (BTK, CD20, and CD40), follicular lymphoma survival (C1QA), inflammatory response (CD40, C1QA), cell adhesion (CD22), and PI3K/AKT signaling (BTK, CD40) at multiple folds at the time points indicated in Table 2.
Similar to BCBL-1 cells, we observed ≥10–100 fold induction of lymphoma therapeutic target genes (MCL1, MS4A1, mTOR, TNFSF13B, BIRC5), DLBCL survival (LMO2, LRMP, MYBL1, PAG1), follicular lymphoma survival (F8, FLT3LG, TNFSF13B), inflammatory response (CSF3, F8, IL6), immune regulation (IFNG, IL2, IL6, TNFSF13B), T cell differentiation (IL2), cell cycle (ATM, IFNG, MLH1, MK167, MYC, ID4, BIRC5), cell adhesion (CDH13, CDKN1C, CDKN2B, CDLN2C, F8, IL2), differentially methylated promoters (ATM, CDH13, CDKN1C, CDKN2B, CDKN2C, DAPK1, DLC1, GSTP1, HRK, ID4, LRP1B, MLH1, LRP1B), PI3K/AKT signaling (CSF3, IL2, IL6, mTOR, TNFSF13B), tumor suppressor pathway (ATM, CDH13, CDKN1C, CDKN2B, CDKN2C, DAPK1, DLC1, LRP1B, MLH1), gene fusion (MME), and DNA repair (DNTT) genes in Akata/EBV+ cells at the time points as indicated in Table 2.
≥10–100 fold induction was observed in the expression of lymphoma therapeutic target genes (FCGR1A, HSP90AA1, MCL1, PTK2, PARP1, SYK), DLBCL survival (ITPKB, LMO2, PTK2), follicular lymphoma survival (FCGR1A, STAT4, FLT3LG), immune regulation (ITPKB, SYK), T cell differentiation (TP53, ITPKB, SYK), cell cycle (TP53, MLH1, MYC), cell adhesion (CDH1, SYK), differentially methylated promoters (CDH1, HIC, TIMP2, TIMP3), PI3K/AKT signaling (HSP90AA1, TP53, TIMP2, TIMP3, SYK), and tumor suppressor pathway (CDH1, HIC1, TIMP2, TIMP3) genes in Akata/EBV+ cells at the time points indicated in Table 2. We found ≥2–10 fold induction in the expression of lymphoma therapeutic target genes (CXCR4, SRC), DLBCL survival (VGLL4, LRMP), follicular lymphoma survival (TLR5), inflammatory response (CXCR4, TLR5, CSF3), T cell differentiation (SRC, TP53), cell cycle (RASSF1, TP53), differentially methylated promoters (RASSF1, SLC19A1), PI3K/AKT signaling (CXCR4, CSF3, TP53), and tumor suppressor pathway (RASSF1) genes in Akata/EBV+ cells at the time points indicated in Table 2. COXIB and EP1/EP4 receptor antagonist treatment downregulated genes of multiple pathways including, lymphoma therapeutic targets (BTK, CD22, CD40, CXCR4), follicular lymphoma survival (C1QA, CCR1), inflammatory response (C1QA, CADM1, CD2, CD40), T cell differentiation (Bcl-2), cell cycle (Bcl-2, CCND1), cell adhesion (Bcl-2, CADM1, CCR1, CD2, CD22), differentially methylated promoters (CADM1, CCND1,DAPK1), and PI3K/AKT signaling (Bcl-2, BTK2, CCND1, CCR1, CD2, CD40,CXCR4) in Akata/EBV+ cells at multiple folds at the time points indicated in Table 2.
DISCUSSION
KSHV and EBV associated lymphomas are characterized by fully transformed B cells exhibiting multiple aspects of oncogenesis such as downregulation of immune screening of cancerous cells, downregulation of pro-apoptotic mechanisms, and bypass of cell cycle check points, upregulation of metastatic and proliferative mechanisms and creation of a proinflammatory microenvironment.3–7 The proinflammatory tumor microenvironment is enriched with several inflammatory cytokines (ICs), growth factors (GFs), and angiogenic factors (AFs) that constantly nourish a condition of chronic reactive inflammatory hyperplasia that plays an important role in driving the pathogenesis of aggressive lymphomas.3–7 COX-2, its oncogenic metabolite PGE2, and PGE2 receptors, namely, EP receptors and associated signaling, forms one of most crucial pathways that regulate inflammation. Many viruses such as HTLV-1, HCV, and herpes viruses including CMV, EBV, and KSHV, have evolved mechanisms to regulate the COX-2/PGE2/EP receptor pathway for creating a proinflammatory tumor microenvironment that provides them with a survival advantage.47, 48 Previous studies from our laboratory have demonstrated the role of EP receptors in the KSHV, latency program and the chemotherapeutic potential of NSAIDs in treating KSHV associated lymphomas.41 This prompted us to examine the therapeutic potential of EP receptor antagonists in treating KSHV and EBV related lymphomas and consequently examining whether combining EP receptor blockers with NSAIDs mutually potentiate their anti-proliferative effects.
Several cell lines such as BC-1, BC-3, BCBL-1, HBL-6 and JSC-1 have been established from primary effusion lymphoma tumors.4,7 BCBL-1 and BC-3 cells carry only the KSHV genome whereas multiple genome copies of KSHV and related EBV exist in BC-1, HBL-6 and JSC-1 cells.6,7 The Akata/EBV+ cell line is an EBV producing Burkitt’s lymphoma cell line that harbors the EBV episome and a Burkitt’s type chromosome translocation, t(8q−; 14q+).49 However, the BJAB and Akata/EBV− cell lines possess the t(8q−; 14q+) translocation but not the EBV episome.50 In our study, we chose KSHV+/EBV− (BCBL-1 and BC-3), KSHV−/EBV+ (Akata/EBV+), KSHV+/EBV+ (JSC-1), and KSHV−/EBV− (BJAB and Akata/EBV−) cell lines to cover the entire spectrum of KSHV and EBV associated lymphomas.
In the first part of our work, we screened for the specific EP receptor antagonist and the lowest concentration that had a specific effect on KSHV+/EBV− (BCBL-1 and BC-3), KSHV− /EBV+ (Akata/EBV+), and KSHV+/EBV+ (JSC-1) cell lines. EP receptor antagonists are reversible receptor blockers and therefore we supplemented the drug every 12h. The EC50 of SC-51322 (EP1 antagonist), AH 6809 (EP2 antagonist), and GW 627368X (EP4 antagonist) are 7.75 µM, 50 µM, and 10 µM, respectively.51–53 We used an exponential range of 0.5 µM, 5.0 µM, and 50.0 µM for all three EP receptor antagonists to delineate between a specific EP receptor mediated anti-proliferative effect and a non-EP receptor mediated anti-growth effect. The lowest concentration of SC-51322, and GW 627368X that had a significant sustained anti-proliferative effect on BCBL-1, BC-3, Akata/EBV+, and JSC-1 was 5.0 µM. In comparison, 50.0 µM AH6809 was required to induce a significant anti-proliferative effect. The anti-proliferative effects of SC-51322 and GW 627368X were initiated at earlier time points at 50.0 µM compared to 5.0 µM indicating the initiation of a non-EP receptor mediated anti-proliferative effect in addition to EP receptor blockade. Furthermore, at higher concentrations, EP receptor antagonists are also shown to block thromboxane receptors as well, which might also have contributed to their anti-growth effect.
Our decision to use celecoxib as the COX-2 inhibitor was due to its specificity for blocking COX-2 with 30 fold biochemical selectivity for COX-2 compared to COX-1.54, 55 Celecoxib is also known to induce anti-proliferative effects through non-COX-2 blockade mediated anti-proliferative effects at concentrations greater than 10.0 µM.55 Therefore, our data strongly suggests that the anti-proliferative effects we observed with 5.0 µM celecoxib were specifically due to an anti-COX-2 mediated effect and the dependency of BCBL-1 and Akata/EBV+ cells on COX-2 mediated cell survival mechanisms. This is further supported by our previous work where the COX-2 inhibitor nimesulide was selectively more pro-apoptotic to BCBL-1 compared to BJAB due to upregulation of the COX-2/PGE2 pathway in BCBL-1 cells.40
In the second part of our study, we examined whether the anti-proliferation arrest we observed is due to the induction of apoptosis by measuring the levels of the apoptotic marker cleaved-caspase 3. We did not use AH6809 in our apoptotic studies because the drug did not induce significant proliferation arrest in BCBL-1, BC-3, and JSC-1 cells at earlier time points except for Akata/EBV+ cells. We chose BCBL-1 and Akata/EBV+ cells with BJAB and Akata/EBV− cells as control cell lines. SC-51322, GW 627368X, and celecoxib at 5.0 µM were able to induce a modest level of apoptosis in BJAB cells compared to BCBL-1 and Akata/EBV+ cell lines. Our data on BCBL-1 cells is further supported by our earlier work that demonstrated the selective anti-proliferative effect of the COX-2 inhibitor nimesulide on BCBL-1 cells compared to BJAB due to the piracy of the COX-2/PGE2 receptor pathway for the maintenance of the KSHV latency program.41 Nimesulide downregulated the KSHV latency program resulting in the initiation of p53 mediated cell cycle check points and apoptosis in BCBL-1 cells.40 Our earlier studies have also demonstrated the utilization of EP receptors by KSHV infection for maintenance of the latency program.41 Similarly, in Akata/EBV+ cells, EBV might be utilizing the COX-2/PGE2/EP receptor pathway for latency and survival related mechanisms.
The levels of PGE2 secreted by BCBL-1, BC-3, Akata/EBV+, JSC-1, and Akata/EBV cells were much higher than BJAB cells. Therefore, our data indicating the increase in susceptibility of Akata/EBV− cell lines compared to BJAB to the pro-apoptotic effects of SC-51322, GW 627368X, and celecoxib could be due to elevation of PGE2 secretion and therefore activation of EP receptors, although neither cell line harbors the EBV episome. Previous report4 have demonstrated the accumulation of multiple oncogenic mutations in BL cell lines in culture that once harbored the EBV episome, which might be responsible for the activation of the COX-2/PGE2/EP receptor pathway in Akata/EBV− cell lines.
Our earlier reports have illustrated the activation of COX-2 gene expression, and subsequent increased protein levels and PGE2 secretion in de novo infected primary endothelial cells.42 Further studies have strongly indicated the utilization of the COX-2/PGE2/EP receptor pathway by the KSHV latency program.39–42 Therefore, our data demonstrating the elevation of EP receptors by de novo KSHV infection in HMVEC-d cells suggests that primary KSHV infection induces EP receptors as well. However, at day 3 p.i., the levels of EP receptors were reduced to base line levels. Our earlier reports have also shown that the initial surge in COX-2 induction and PGE2 secretion is a process regulated by KSHV infection and a self-sustained positive feedback loop through EP receptors. Therefore, after establishment of the sustained activation of COX-2 and PGE2 secretion, the individual activation of EP receptors might be a process mediated by the ligand PGE2 rather than a receptor level driven process. Although, an identical process cannot be generalized in the NHL cell lines we used due to the variable levels of EP receptors, the susceptibility of the Akata/EBV− cell line to the pro-apoptotic effects of SC-51322, GW 627368X, and celecoxib with PGE2 levels comparable to KSHV+ and EBV+ cell lines further supports our data. Similar work needs to be done with de novo EBV infection of primary endothelial cells.56
One of the major issues associated with NSAIDs is their non-specific side effects such GI toxicity from the inhibition of COX-1.32 Although highly specific COX-2 inhibitors such as COXIBs were introduced to resolve this, they were associated with an increased risk of thrombotic and cardiovascular events due to the absence of aspirin-like platelet aggregation inhibiting properties.32, 57 Although the chemotherapeutic effects of celecoxib are reported in several studies, they were due to non-specific effects from blocking cell survival kinases such as PI3K/Akt at concentrations greater than their EC50s.46 Therefore, we proposed the simultaneous blockade of COX-2 and EP receptors at concentrations closer to their EC50s to obtain the desired anti-COX-2/PGE2/EP receptor mediated chemotherapeutic effect without the non-specific anti-growth effect and associated side effects of NSAIDs. The anti-cancer potency of concurrent targeting of COX-2 and EP1/EP4 receptors is demonstrated by the induction of the apoptotic pathway and upregulation of the expression of several tumor suppressor genes in BCBL-1 and Akata/EBV+ cells such as ataxia telangiectasia mutated (ATM), fragile histidine triad gene (FHIT), hypermethylated in cancer 1 (HIC1), myeloid cell leukemia sequence 1 (MCL1), MutL homolog 1 (MLH1), ras association (RalGDS/AF-6) domain family member 1 (RASSF1), neural cell adhesion molecule 1 (NCAM1), TIMP2/3, tumor protein p53 (TP53), E-cadherin (CDH1), cadherin 13 (CDH13), death-associated protein kinase 1 (DAPK1), deleted in liver cancer 1 (DLC1), cyclin-dependent kinase inhibitor 1C (CDN1C), cyclin-dependent kinase inhibitor 2B (CDKN2B), cyclin-dependent kinase inhibitor 2C (CDKN2C), and low density lipoprotein receptor-related protein 1B (LRP1B).58–71 Several genes upregulated in our PCR based gene array study have also been shown to be downregulated by either indicated viral infections such as chemokine (C-X-C motif) receptor 4 (CXCR4), LIM domain only 2 (LMO2), v-myc myelocytomatosis viral oncogene homolog (MYC), toll-like receptor 5 (TLR5), and inhibitor of DNA binding 4 (ID4) by EBV72–75 Genes otherwise downregulated by KSHV protein LANA-1 (TGFB1), and vIRF1 (ATM) were upregulated upon celecoxib, EP1 antagonist SC-51322, and EP4 antagonist GW 627368X treatment76, 77 We observed induction in the expression of LMP1 downregulated genes (TGFB1, CXCR4, LRMP, TP53, RASSF1, and TIMP3) and EBNA2 downregulated gene (MME) were induced upon combination of COX-2 inhibitor and EP/EP4 receptor antagonist treatment72, 73, 78–81 Similarly, expression of genes downregulated by EBV infection mediated methylation such as MLH1, CDH1, DLC1, CDKN1C, glutathione S-transferase pi 1 (GSTP1), HIC1, and RASSF1 were also significantly upregulated in Akata/EBV+ cells treated with COX-2 inhibitor celecoxib, EP1 antagonist SC-51322, and EP4 antagonist GW 627368X.81–90 Expression of several genes induced by concurrent COX-2 and EP1/EP4 receptor inhibition has also been shown to be activated by either KSHV and EBV infections such as SRC, NFκB, mTOR, NFκB1, IL-2, IL-6, and BIRC5 (EBV) or by respective viral proteins such as SRC and SYK which are activated by KSHV proteins K1, K15, vGPCR and by EBV proteins LMP1 and LMP2A, and mTOR activation by EBV LMP1.90–103 The significance of such counter-intuitive results needs to be further delineated and it might very well be that the level of expression of these genes might be the deciding factor for survival versus cell death in BCBL-1 and Akata/EBV+ cells. The hijacking of the pro-survival effect of TNF via the TRAF pathway versus the pro-apoptotic effect by KSHV protein vFLIP is strongly suggestive of the existence of such mechanisms.104 FHIT and CSF3 are genes proposed to be undetectable in PEL and BL.105–107 However, FHIT and CSF3 were significantly upregulated in BCBL-1 and Akata/EBV+ cells, respectively indicating the reversal of mechanisms that downregulates FHIT and CSF3 by the concurrent inhibition of COX-2 and EP1/EP4 receptors. Viral mimicry and hijacking of host proteins are common themes in KSHV and EBV mediated tumor pathogenesis such as the role of KSHV-Bcl-2 and EBV-BHRFI in modulating Bcl-2 and MCL-1 mediated anti-apoptotic mechanisms and the role of EBV-LMP1 in regulating CD40 functions and inducing Btk mediated cell survival mechanisms.108–111 The downregulation of CD40, Btk, Bcl-2, Bcl-xL, Bcl-10 and MCL-1 along with the upregulation of BID, BAD, and Bax by concurrent COX-2 and EP1/EP4 receptor inhibition in BCBL-1 and Akata/EBV+ cells is strongly suggestive of the inhibition of the anti-apoptotic and pro-survival mechanisms initiated by viral proteins. In our studies with BCBL-1 and Akata/EBV+, TLR5 was significantly upregulated by concurrent COX-2 and EP1/EP4 receptor inhibition. KSHV genome copy number has been shown to be downregulated by TLR5 activation and EBV infection has been proposed to downregulate TLR5 signaling and therefore our data is strongly suggestive of the activation of host innate immune responses, which otherwise are subverted by viral infection.112,113 The upregulation of PARP1 in BCBL-1 cells and studies indicating the role of PARP1 in KSHV genome maintenance and replication plus blockade of the lytic cycle through poly-(ADP-ribosyl)-ation of LANA-1 and O-GlcNAcylation of KSHV-RTA raises the question of whether the lytic cyle is activated by COX-2 and EP receptor inhibition.114–116
Overall, our gene array based data further augment our proposal that the anti-cancer effects of concurrent targeting of COX-2 and EP1/EP4 receptors is due to the simultaneous inhibition of viral and non-viral mediated tumorigenic mechanisms acting at multiple levels such as viral-host protein interactions, host and viral gene expression through regulation of epigenetic mechanisms such as methylation, host signaling, immune system activation, pro-inflammatory and cell survival processes. The novelty of our study lies in the exploration of PGE2 receptor antagonists and COX-2 specific inhibitor in the lowest concentration in lymphoma cells expressing COX-2 and PGE2 receptors. Though there are some reports indicating the role of combination therapy in colorectal carcinogenesis,19,20 liver cancer,117 skin cancer,118 esophageal cancer,119 breast cancer,120 and non-small cell lung cancer (NSCLC)121 but has never been tested in any lymphoma. Our study has translational significance as it explored the combination of safe and more effective EP receptor antagonists and COXIB which could hit multiple targets simultaneously. Our study tested this novel hypothesis and reported that combination of the COX-2 inhibitor celecoxib, EP1 antagonist SC-51322, and EP4 antagonist GW 627368X induced apoptosis at concentrations below the lowest concentration required to induce proliferation than when used alone. Studies to understand the relevance of COX-2 and EP receptors in viral latency and chemotherapeutic potential of these combination therapies in non-invasive and invasive PEL-SCID xenograft and KS xenograft tumors in murine models are currently ongoing in our lab. PEL and KS models will pave the way for identifying a unique arena of drug targets for treating KSHV linked B and endothelial cell malignancies. As the expectation of developing a "magic bullet" for curing virus linked neoplasia is low, designing better treatment modalities such as additive therapies might be effective and will provide promising targets at multiple levels.
Supplementary Material
Supplemental Figure 1. Clustergrams performing non-supervised hierarchical clustering of the entire dataset to display a heat map with dendrograms indicating co-regulated genes across BCBL-1 cells treated with 1.0 µM each of celecoxib, SC-51322, and GW 627368X at 8h and 24h and untreated samples at 8h and 24h.
Supplemental Figure 2. Clustergrams performing non-supervised hierarchical clustering of the entire dataset to display a heat map with dendrograms indicating co-regulated genes across Akata/EBV+ cells treated with 1.0 µM each of celecoxib, SC-51322, and GW 627368X at 8h and 24h and untreated samples at 8h and 24h.
SPECULATIONS.
Our studies for the first time demonstrate the chemotherapeutic potential of EP1 and EP4 receptor antagonists and the synergistic pro-apoptotic effect of simultaneous blockade of COX-2 and EP1/EP4 receptors in treating KSHV+ and EBV+ NHL cell lines. Although our study was based on in vitro experiments using human cell lines, currently work is underway to test our hypothesis in animal models and to delineate the molecular and pathological mechanisms mediated by EP receptors in KSHV and EBV associated NHLs. Considering the role of inflammation in NHLs, we also predict that our model is applicable in developing a novel treatment modality in other NHLs as well.
ACKNOWLEDGEMENTS
This study was supported in part by Sigma Xi Grant-in-aid grant G2009101428 to AGP, and Public Health Service grant NIH CA075911, CA168472, R56 AI091767, and RFUMS H. M. Bligh Cancer Research Fund to BC, CA128560 and RFUMS startup Fund to NSW. We thank Keith Philibert and Rita Levine (late) for critically reading the manuscript, and help in FACS analyses at the RFUMS FACS/cell sorter core facility, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- COX-2
cyclooxygenase-2
- PGE2
prostaglandin E2
- NSAIDs
non-steroidal anti-inflammatory drugs
- EP
eicosonoid
- GPCRs
G protein coupled receptors
- PEL
primary effusion lymphoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read the journal’s policy on conflicts of interest.
REFERENCES
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the epidemiological evidence. J Intern Med. 2008;264:537–548. doi: 10.1111/j.1365-2796.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 3.Ablashi DV, Chatlynne LG, Whitman JE, Jr, Cesarman E. Spectrum of Kaposi's sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin Microbiol Rev. 2002;15:439–464. doi: 10.1128/CMR.15.3.439-464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone A, Cesarman E, Spina M, Gloghini A, Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113:1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 5.Carbone A, Gloghini A. HHV-8-associated lymphoma: state-of-the-art review. Acta Haematol. 2007;117:129–131. doi: 10.1159/000097459. [DOI] [PubMed] [Google Scholar]
- 6.Ganem D. KSHV and Kaposi's sarcoma: the end of the beginning? Cell. 1997;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 7.Ganem D. KSHV infection and the pathogenesis of Kaposi's sarcoma. Annu Rev Pathol. 2006;1:273–296. doi: 10.1146/annurev.pathol.1.110304.100133. [DOI] [PubMed] [Google Scholar]
- 8.Klass CM, Offermann MK. Targeting human herpesvirus-8 for treatment of Kaposi's sarcoma and primary effusion lymphoma. Curr Opin Oncol. 2005;17:447–455. doi: 10.1097/01.cco.0000172823.01190.6c. [DOI] [PubMed] [Google Scholar]
- 9.Curreli F, Friedman-Kien AE, Flore O. Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J Clin Invest. 2005;115:642–652. doi: 10.1172/JCI23334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An J, Sun Y, Fisher M, Rettig MB. Antitumor effects of bortezomib (PS-341) on primary effusion lymphomas. Leukemia. 2004;18:1699–1704. doi: 10.1038/sj.leu.2403460. [DOI] [PubMed] [Google Scholar]
- 11.Toomey NL, Deyev VV, Wood C, et al. Induction of a TRAIL-mediated suicide program by interferon alpha in primary effusion lymphoma. Oncogene. 2001;20:7029–7040. doi: 10.1038/sj.onc.1204895. [DOI] [PubMed] [Google Scholar]
- 12.Sarek G, Kurki S, Enback J, et al. Reactivation of the p53 pathway as a treatment modality for KSHV-induced lymphomas. J Clin Invest. 2007;117:1019–1028. doi: 10.1172/JCI30945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hocqueloux L, Agbalika F, Oksenhendler E, Molina JM. Long-term remission of an AIDS-related primary effusion lymphoma with antiviral therapy. Aids. 2001;15(2):280–282. doi: 10.1097/00002030-200101260-00023. [DOI] [PubMed] [Google Scholar]
- 14.Luppi M, Trovato R, Barozzi P, et al. Treatment of herpesvirus associated primary effusion lymphoma with intracavity cidofovir. Leukemia. 2005;19:473–476. doi: 10.1038/sj.leu.2403646. [DOI] [PubMed] [Google Scholar]
- 15.Halfdanarson TR, Markovic SN, Kalokhe U, Luppi M. A non-chemotherapy treatment of a primary effusion lymphoma: durable remission after intracavitary cidofovir in HIV negative PEL refractory to chemotherapy. Ann Oncol. 2006;17:1849–1850. doi: 10.1093/annonc/mdl139. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt AP, Bhende PM, Sin SH, Roy D, Dittmer DP, Damania B. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood. 2010;115:4455–4463. doi: 10.1182/blood-2009-10-251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard MP, Bancos S, Sime PJ, Phipps RP. Targeting cyclooxygenase-2 in hematological malignancies: rationale and promise. Curr Pharm Des. 2008;14:2051–2060. doi: 10.2174/138161208785294654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakhle YS. COX-2 and cancer: a new approach to an old problem. Br J Pharmacol. 2001;134:1137–1150. doi: 10.1038/sj.bjp.0704365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hull MA. Cyclooxygenase-2: how good is it as a target for cancer chemoprevention? Eur J Cancer. 2005;41:1854–1863. doi: 10.1016/j.ejca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Molecular cancer therapeutics. 2004;3:1031–1039. [PubMed] [Google Scholar]
- 21.Jones MK, Szabo IL, Kawanaka H, Husain SS, Tarnawski AS. von Hippel Lindau tumor suppressor and HIF-1alpha: new targets of NSAIDs inhibition of hypoxia-induced angiogenesis. Faseb J. 2002;16:264–266. doi: 10.1096/fj.01-0589fje. [DOI] [PubMed] [Google Scholar]
- 22.Jones MK, Wang H, Peskar BM, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 23.Kaur Saini M, Kaur J, Sharma P, Nath Sanyal S. Chemopreventive response of diclofenac, a non-steroidal anti-inflammatory drug in experimental carcinogenesis. Nutr Hosp. 2009;24:717–723. [PubMed] [Google Scholar]
- 24.Chen WT, Hung WC, Kang WY, et al. Overexpression of cyclooxygenase-2 in urothelial carcinoma in conjunction with tumor-associated-macrophage infiltration, hypoxia-inducible factor-1alpha expression, and tumor angiogenesis. Apmis. 2009;117:176–184. doi: 10.1111/j.1600-0463.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Nakamura S, Shibata K, et al. Etodolac inhibits EBER expression and induces Bcl-2-regulated apoptosis in Burkitt's lymphoma cells. Eur J Haematol. 2005;75:212–220. doi: 10.1111/j.1600-0609.2005.00498.x. [DOI] [PubMed] [Google Scholar]
- 26.He H, Xia HH, Wang JD, et al. Inhibition of human telomerase reverse transcriptase by nonsteroidal antiinflammatory drugs in colon carcinoma. Cancer. 2006;106:1243–1249. doi: 10.1002/cncr.21694. [DOI] [PubMed] [Google Scholar]
- 27.He Q, Luo X, Jin W, et al. Celecoxib and a novel COX-2 inhibitor ON09310 upregulate death receptor 5 expression via GADD153/CHOP. Oncogene. 2008;27:2656–2660. doi: 10.1038/sj.onc.1210894. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Li R, Pan Y, et al. High-throughput screen of protein expression levels induced by cyclooxygenase-2 during influenza a virus infection. Clin Chim Acta. 2011;412:1081–1085. doi: 10.1016/j.cca.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Li JY, Wang XZ, Chen FL, Yu JP, Luo HS. Nimesulide inhibits proliferation via induction of apoptosis and cell cycle arrest in human gastric adenocarcinoma cell line. World J Gastroenterol. 2003;9:915–920. doi: 10.3748/wjg.v9.i5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladetto M, Vallet S, Trojan A, et al. Cyclooxygenase-2 (COX-2) is frequently expressed in multiple myeloma and is an independent predictor of poor outcome. Blood. 2005;105:4784–4791. doi: 10.1182/blood-2004-11-4201. [DOI] [PubMed] [Google Scholar]
- 31.Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM. Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 2004;215:1–20. doi: 10.1016/j.canlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 34.Fulton AM, Ma X, Kundu N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer research. 2006;66:9794–9797. doi: 10.1158/0008-5472.CAN-06-2067. [DOI] [PubMed] [Google Scholar]
- 35.Majima M, Amano H, Hayashi I. Prostanoid receptor signaling relevant to tumor growth and angiogenesis. Trends Pharmacol Sci. 2003;24:524–529. doi: 10.1016/j.tips.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Piazuelo E, Jimenez P, Strunk M, et al. Effects of selective PGE2 receptor antagonists in esophageal adenocarcinoma cells derived from Barrett's esophagus. Prostaglandins Other Lipid Mediat. 2006;81:150–161. doi: 10.1016/j.prostaglandins.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Wu K, Bai F, et al. Celecoxib could reverse the hypoxia-induced Angiopoietin-2 upregulation in gastric cancer. Cancer Lett. 2006;242:20–27. doi: 10.1016/j.canlet.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Sharma-Walia N, Paul AG, Bottero V, et al. Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion. PLoS Pathog. 2010;6:e1000777. doi: 10.1371/journal.ppat.1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paul AG, Sharma-Walia N, Chandran B. Targeting KSHV/HHV-8 latency with COX-2 selective inhibitor nimesulide: a potential chemotherapeutic modality for primary effusion lymphoma. PLoS One. 2011;6:e24379. doi: 10.1371/journal.pone.0024379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.George Paul A, Sharma-Walia N, Kerur N, White C, Chandran B. Piracy of prostaglandin E2/EP receptor-mediated signaling by Kaposi's sarcoma-associated herpes virus (HHV-8) for latency gene expression: strategy of a successful pathogen. Cancer research. 2010;70:3697–3708. doi: 10.1158/0008-5472.CAN-09-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma-Walia N, Raghu H, Sadagopan S, et al. Cyclooxygenase 2 induced by Kaposi's sarcoma-associated herpesvirus early during in vitro infection of target cells plays a role in the maintenance of latent viral gene expression. J Virol. 2006;80:6534–6552. doi: 10.1128/JVI.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma-Walia N, Paul AG, Patel K, Chandran K, Ahmad W, Chandran B. Nfat and Creb Regulate Kaposi's Sarcoma Associated Herpes Virus (Kshv) Induced Cyclooxygenase-2 (Cox-2) J Virol. 2010;84:12733–12753. doi: 10.1128/JVI.01065-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma-Walia N, Patel K, Chandran K, Marginean A, Bottero V, Kerur N, George-Paul A. COX-2/PGE2: molecular ambassadors of Kaposi's sarcoma-associated herpes virus oncoprotein-vFLIP. Oncogenesis. 2012;1:e5. doi: 10.1038/oncsis.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bock JM, Menon SG, Sinclair LL, et al. Celecoxib toxicity is cell cycle phase specific. Cancer research. 2007;67:3801–3808. doi: 10.1158/0008-5472.CAN-06-3780. [DOI] [PubMed] [Google Scholar]
- 46.Schonthal AH. Direct non-cyclooxygenase-2 targets of celecoxib and their potential relevance for cancer therapy. Br J Cancer. 2007;97:1465–1468. doi: 10.1038/sj.bjc.6604049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynolds AE, Enquist LW. Biological interactions between herpesviruses and cyclooxygenase enzymes. Rev Med Virol. 2006;16:393–403. doi: 10.1002/rmv.519. [DOI] [PubMed] [Google Scholar]
- 48.Shelby BD, Nelson A, Morris C. Gamma-herpesvirus neoplasia: a growing role for COX-2. Microsc Res Tech. 2005;68:120–129. doi: 10.1002/jemt.20226. [DOI] [PubMed] [Google Scholar]
- 49.Takada K, Horinouchi K, Ono Y, et al. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991;5:147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto N, Takizawa T, Iwanaga Y, Shimizu N, Yamamoto N. Malignant transformation of B lymphoma cell line BJAB by Epstein-Barr virus-encoded small RNAs. FEBS Lett. 2000;484:153–158. doi: 10.1016/s0014-5793(00)02145-1. [DOI] [PubMed] [Google Scholar]
- 51.Keery RJ, Lumley P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br J Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson RJ, Giblin GM, Roomans S, et al. GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl] acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006;148:326–339. doi: 10.1038/sj.bjp.0706726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foudi N, Kotelevets L, Louedec L, et al. Vasorelaxation induced by prostaglandin E2 in human pulmonary vein: role of the EP4 receptor subtype. Br J Pharmacol. 2008;154:1631–1639. doi: 10.1038/bjp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi S, Klotz U. Clinical use and pharmacological properties of selective COX-2 inhibitors. Eur J Clin Pharmacol. 2008;64:233–252. doi: 10.1007/s00228-007-0400-7. [DOI] [PubMed] [Google Scholar]
- 55.Kardosh A, Wang W, Uddin J, et al. Dimethyl-celecoxib (DMC), a derivative of celecoxib that lacks cyclooxygenase-2-inhibitory function, potently mimics the anti-tumor effects of celecoxib on Burkitt's lymphoma in vitro and in vivo. Cancer Biol Ther. 2005;4:571–582. doi: 10.4161/cbt.4.5.1699. [DOI] [PubMed] [Google Scholar]
- 56.Jones K, Rivera C, Sgadari C, et al. Infection of human endothelial cells with Epstein-Barr virus. J Exp Med. 1995;182:1213–1221. doi: 10.1084/jem.182.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. Jama. 2006;296:1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 58.Sarita Rajender P, Ramasree D, Bhargavi K, Vasavi M, Uma V. Selective inhibition of proteins regulating CDK/cyclin complexes: strategy against cancer--a review. J Recept Signal Transduct Res. 2010;30:206–213. doi: 10.3109/10799893.2010.488649. [DOI] [PubMed] [Google Scholar]
- 59.Smith DI, Zhu Y, McAvoy S, Kuhn R. Common fragile sites, extremely large genes, neural development and cancer. Cancer Lett. 2006;232:48–57. doi: 10.1016/j.canlet.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 60.Martin J, St-Pierre MV, Dufour JF. Hit proteins, mitochondria and cancer. Biochim Biophys Acta. 2011;1807:626–632. doi: 10.1016/j.bbabio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12:233. doi: 10.1186/gb-2011-12-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao YC, Lo SH. Deleted in liver cancer-1 (DLC-1): a tumor suppressor not just for liver. Int J Biochem Cell Biol. 2008;40:843–847. doi: 10.1016/j.biocel.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 64.Zhao C, Bu X. Promoter methylation of tumor-related genes in gastric carcinogenesis. Histol Histopathol. 2012;27:1271–1282. doi: 10.14670/HH-27.1271. [DOI] [PubMed] [Google Scholar]
- 65.Overmeyer JH, Maltese WA. Death pathways triggered by activated Ras in cancer cells. Front Biosci. 2011;16:1693–1713. doi: 10.2741/3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534–543. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esteller M. Profiling aberrant DNA methylation in hematologic neoplasms: a view from the tip of the iceberg. Clin Immunol. 2003;109:80–88. doi: 10.1016/s1521-6616(03)00208-0. [DOI] [PubMed] [Google Scholar]
- 68.Dehennaut V, Leprince D. Implication of HIC1 (Hypermethylated In Cancer 1) in the DNA damage response. Bull Cancer. 2009;96:E66–E72. doi: 10.1684/bdc.2009.0959. [DOI] [PubMed] [Google Scholar]
- 69.Szymanska K, Hainaut P. TP53 and mutations in human cancer. Acta Biochim Pol. 2003;50:231–238. [PubMed] [Google Scholar]
- 70.Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia. 2002;16:444–454. doi: 10.1038/sj.leu.2402416. [DOI] [PubMed] [Google Scholar]
- 71.Paredes J, Figueiredo J, Albergaria A, et al. Epithelial E- and P-cadherins: role and clinical significance in cancer. Biochim Biophys Acta. 2012;1826:297–311. doi: 10.1016/j.bbcan.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Ehlin-Henriksson B, Mowafi F, Klein G, Nilsson A. Epstein-Barr virus infection negatively impacts the CXCR4-dependent migration of tonsillar B cells. Immunology. 2006;117:379–385. doi: 10.1111/j.1365-2567.2005.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan J, Cahir-McFarland E, Zhao B, Kieff E. Virus and cell RNAs expressed during Epstein-Barr virus replication. J Virol. 2006;80:2548–2565. doi: 10.1128/JVI.80.5.2548-2565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruf IK, Rhyne PW, Yang H, et al. Epstein-barr virus regulates c-MYC, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol Cell Biol. 1999;19:1651–1660. doi: 10.1128/mcb.19.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruf IK, Rhyne PW, Yang H, et al. EBV regulates c-MYC, apoptosis, and tumorigenicity in Burkitt's lymphoma. Curr Top Microbiol Immunol. 2001;258:153–160. doi: 10.1007/978-3-642-56515-1_10. [DOI] [PubMed] [Google Scholar]
- 76.Di Bartolo DL, Cannon M, Liu YF, et al. KSHV LANA inhibits TGF-beta signaling through epigenetic silencing of the TGF-beta type II receptor. Blood. 2008;111:4731–4740. doi: 10.1182/blood-2007-09-110544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin YC, Nakamura H, Liang X, et al. Inhibition of the ATM/p53 signal transduction pathway by Kaposi's sarcoma-associated herpesvirus interferon regulatory factor 1. J Virol. 2006;80:2257–2266. doi: 10.1128/JVI.80.5.2257-2266.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fries KL, Miller WE, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kenney JL, Guinness ME, Reiss M, Lacy J. Antisense to the Epstein- Barr virus (EBV)-encoded latent membrane protein 1 (LMP-1) sensitizes EBV-immortalized B cells to transforming growth factor-beta and chemotherapeutic agents. Int J Cancer. 2001;91:89–98. doi: 10.1002/1097-0215(20010101)91:1<89::aid-ijc1015>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 80.Polack A, Hortnagel K, Pajic A, et al. c-myc activation renders proliferation of Epstein-Barr virus (EBV)-transformed cells independent of EBV nuclear antigen 2 and latent membrane protein 1. Proc Natl Acad Sci U S A. 1996;93:10411–10416. doi: 10.1073/pnas.93.19.10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Man C, Rosa J, Lee LT, et al. Latent membrane protein 1 suppresses RASSF1A expression, disrupts microtubule structures and induces chromosomal aberrations in human epithelial cells. Oncogene. 2007;26:3069–3080. doi: 10.1038/sj.onc.1210106. [DOI] [PubMed] [Google Scholar]
- 82.Matsusaka K, Kaneda A, Nagae G, et al. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer research. 2011;71(23):7187–7197. doi: 10.1158/0008-5472.CAN-11-1349. [DOI] [PubMed] [Google Scholar]
- 83.Kang GH, Lee S, Kim WH, et al. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002;160:787–794. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan JL, Jones RJ, Kenney SC, et al. Epstein-Barr virus-specific methylation of human genes in gastric cancer cells. Infect Agent Cancer. 2010;5:27. doi: 10.1186/1750-9378-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee DC, Chua DT, Wei WI, Sham JS, Lau AS. Induction of matrix metalloproteinases by Epstein-Barr virus latent membrane protein 1 isolated from nasopharyngeal carcinoma. Biomed Pharmacother. 2007;61:520–526. doi: 10.1016/j.biopha.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 86.Kim J, Lee HS, Bae SI, Lee YM, Kim WH. Silencing and CpG island methylation of GSTP1 is rare in ordinary gastric carcinomas but common in Epstein-Barr virus-associated gastric carcinomas. Anticancer Res. 2005;25:4013–4019. [PubMed] [Google Scholar]
- 87.Zhou Q, Olivo M, Lye KY, Moore S, Sharma A, Chowbay B. Enhancing the therapeutic responsiveness of photodynamic therapy with the antiangiogenic agents SU5416 and SU6668 in murine nasopharyngeal carcinoma models. Cancer Chemother Pharmacol. 2005;56(6):569–577. doi: 10.1007/s00280-005-1017-0. [DOI] [PubMed] [Google Scholar]
- 88.Hutajulu SH, Indrasari SR, Indrawati LP, et al. Epigenetic markers for early detection of nasopharyngeal carcinoma in a high risk population. Mol Cancer. 2011;10:48. doi: 10.1186/1476-4598-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seng TJ, Low JS, Li H, et al. The major 8p22 tumor suppressor DLC1 is frequently silenced by methylation in both endemic and sporadic nasopharyngeal, esophageal, and cervical carcinomas, and inhibits tumor cell colony formation. Oncogene. 2007;26:934–944. doi: 10.1038/sj.onc.1209839. [DOI] [PubMed] [Google Scholar]
- 90.Dai Y, Tang Y, He F, et al. Screening and functional analysis of differentially expressed genes in EBV-transformed lymphoblasts. Virol J. 2012;9:77. doi: 10.1186/1743-422X-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shan B, Morris CA, Zhuo Y, Shelby BD, Levy DR, Lasky JA. Activation of proMMP-2 and Src by HHV8 vGPCR in human pulmonary arterial endothelial cells. J Mol Cell Cardiol. 2007;42:517–525. doi: 10.1016/j.yjmcc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Chandran B. Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells. J Virol. 2010;84:2188–2199. doi: 10.1128/JVI.01334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Klein RD. Prostaglandin E2 induces vascular endothelial growth factor secretion in prostate cancer cells through EP2 receptor-mediated cAMP pathway. Mol Carcinog. 2007;46:912–923. doi: 10.1002/mc.20320. [DOI] [PubMed] [Google Scholar]
- 94.Hatton O, Lambert SL, Krams SM, Martinez OM. Src kinase and Syk activation initiate PI3K signaling by a chimeric latent membrane protein 1 in Epstein-Barr virus (EBV)+ B cell lymphomas. PLoS One. 2012;7:e42610. doi: 10.1371/journal.pone.0042610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fotheringham JA, Coalson NE, Raab-Traub N. Epstein-Barr virus latent membrane protein-2A induces ITAM/Syk- and Akt-dependent epithelial migration through alphav-integrin membrane translocation. J Virol. 2012;86:10308–10320. doi: 10.1128/JVI.00853-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Oliveira DE, Ballon G, Cesarman E. NF-kappaB signaling modulation by EBV and KSHV. Trends Microbiol. 2010;18:248–257. doi: 10.1016/j.tim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Flanagan AM, Letai A. BH3 domains define selective inhibitory interactions with BHRF-1 and KSHV BCL-2. Cell Death Differ. 2008;15:580–588. doi: 10.1038/sj.cdd.4402292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pietrek M, Brinkmann MM, Glowacka I, et al. Role of the Kaposi's sarcoma-associated herpesvirus K15 SH3 binding site in inflammatory signaling and B-cell activation. J Virol. 2010;84:8231–8240. doi: 10.1128/JVI.01696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee BS, Lee SH, Feng P, Chang H, Cho NH, Jung JU. Characterization of the Kaposi's sarcoma-associated herpesvirus K1 signalosome. J Virol. 2005;79:12173–12184. doi: 10.1128/JVI.79.19.12173-12184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yokoi T, Miyawaki T, Yachie A, Kato K, Kasahara Y, Taniguchi N. Epstein-Barr virus-immortalized B cells produce IL-6 as an autocrine growth factor. Immunology. 1990;70:100–105. [PMC free article] [PubMed] [Google Scholar]
- 101.Todd SC, Tsoukas CD. EBV induces proliferation of immature human thymocytes in an IL-2-mediated response. J Immunol. 1996;156:4217–4223. [PubMed] [Google Scholar]
- 102.Burkhardt AL, Bolen JB, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen W, Hilton IB, Staudt MR, Burd CE, Dittmer DP. Distinct p53, p53:LANA, and LANA complexes in Kaposi's Sarcoma--associated Herpesvirus Lymphomas. J Virol. 2010;84:3898–3908. doi: 10.1128/JVI.01321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guasparri I, Wu H, Cesarman E. The KSHV oncoprotein vFLIP contains a TRAF-interacting motif and requires TRAF2 and TRAF3 for signalling. EMBO Rep. 2006;7:114–119. doi: 10.1038/sj.embor.7400580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tomeczkowski J, Yakisan E, Wieland B, Reiter A, Welte K, Sykora KW. Absence of G-CSF receptors and absent response to G-CSF in childhood Burkitt's lymphoma and B-ALL cells. Br J Haematol. 1995;89:771–779. doi: 10.1111/j.1365-2141.1995.tb08414.x. [DOI] [PubMed] [Google Scholar]
- 106.Roy D, Sin SH, Damania B, Dittmer DP. Tumor suppressor genes FHIT and WWOX are deleted in primary effusion lymphoma (PEL) cell lines. Blood. 2011;118:e32–e39. doi: 10.1182/blood-2010-12-323659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toujani S, Dessen P, Ithzar N, et al. High resolution genome-wide analysis of chromosomal alterations in Burkitt's lymphoma. PLoS One. 2009;4:e7089. doi: 10.1371/journal.pone.0007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uchida J, Yasui T, Takaoka-Shichijo Y, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- 109.Rastelli J, Homig-Holzel C, Seagal J, et al. LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class-switch recombination to IgG1. Blood. 2008;111:1448–1455. doi: 10.1182/blood-2007-10-117655. [DOI] [PubMed] [Google Scholar]
- 110.Bellows DS, Howell M, Pearson C, Hazlewood SA, Hardwick JM. Epstein-Barr virus BALF1 is a BCL-2-like antagonist of the herpesvirus antiapoptotic BCL-2 proteins. J Virol. 2002;76:2469–2479. doi: 10.1128/jvi.76.5.2469-2479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marshall WL, Yim C, Gustafson E, et al. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J Virol. 1999;73:5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gregory SM, West JA, Dillon PJ, Hilscher C, Dittmer DP, Damania B. Toll-like receptor signaling controls reactivation of KSHV from latency. Proc Natl Acad Sci U S A. 2009;106:11725–11730. doi: 10.1073/pnas.0905316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fathallah I, Parroche P, Gruffat H, et al. EBV latent membrane protein 1 is a negative regulator of TLR9. J Immunol. 2010;185:6439–6447. doi: 10.4049/jimmunol.0903459. [DOI] [PubMed] [Google Scholar]
- 114.Ohsaki E, Ueda K, Sakakibara S, Do E, Yada K, Yamanishi K. Poly(ADP-ribose) polymerase 1 binds to Kaposi's sarcoma-associated herpesvirus (KSHV) terminal repeat sequence and modulates KSHV replication in latency. J Virol. 2004;78:9936–9946. doi: 10.1128/JVI.78.18.9936-9946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ko YC, Tsai WH, Wang PW, et al. Suppressive regulation of KSHV RTA with O-GlcNAcylation. J Biomed Sci. 2012;19:12. doi: 10.1186/1423-0127-19-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu F, Tsai K, Chen HS, et al. Identification of host-chromosome binding sites and candidate gene targets for Kaposi's sarcoma-associated herpesvirus LANA. J Virol. 2012;86:5752–5762. doi: 10.1128/JVI.07216-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cusimano A, Fodera D, Lampiasi N, et al. Prostaglandin E2 receptors and COX enzymes in human hepatocellular carcinoma: role in the regulation of cell growth. Annals of the New York Academy of Sciences. 2009;1155:300–308. doi: 10.1111/j.1749-6632.2009.03701.x. [DOI] [PubMed] [Google Scholar]
- 118.Tober KL, Wilgus TA, Kusewitt DF, Thomas-Ahner JM, Maruyama T, Oberyszyn TM. Importance of the EP(1) receptor in cutaneous UVB-induced inflammation and tumor development. The Journal of investigative dermatology. 2006;126:205–211. doi: 10.1038/sj.jid.5700014. [DOI] [PubMed] [Google Scholar]
- 119.Gupta RA, DuBois RN. Cyclooxygenase-2 inhibitor therapy for the prevention of esophageal adenocarcinoma in Barrett's esophagus. Journal of the National Cancer Institute. 2002;94:406–407. doi: 10.1093/jnci/94.6.406. [DOI] [PubMed] [Google Scholar]
- 120.Xin X, Majumder M, Girish GV, Mohindra V, Maruyama T, Lala PK. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Laboratory investigation; a journal of technical methods and pathology. 2012;92:1115–1128. doi: 10.1038/labinvest.2012.90. [DOI] [PubMed] [Google Scholar]
- 121.Singh T, Sharma SD, Katiyar SK. Grape proanthocyanidins induce apoptosis by loss of mitochondrial membrane potential of human non-small cell lung cancer cells in vitro and in vivo. PLoS One. 2011;6:e27444. doi: 10.1371/journal.pone.0027444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Clustergrams performing non-supervised hierarchical clustering of the entire dataset to display a heat map with dendrograms indicating co-regulated genes across BCBL-1 cells treated with 1.0 µM each of celecoxib, SC-51322, and GW 627368X at 8h and 24h and untreated samples at 8h and 24h.
Supplemental Figure 2. Clustergrams performing non-supervised hierarchical clustering of the entire dataset to display a heat map with dendrograms indicating co-regulated genes across Akata/EBV+ cells treated with 1.0 µM each of celecoxib, SC-51322, and GW 627368X at 8h and 24h and untreated samples at 8h and 24h.