Abstract
Bladder cancer rarely presents clinically with a myelophthisic picture from diffuse bone marrow infiltration especially in the absence of detectable skeletal metastases. A 75-year old man presented with newly diagnosed urothelial cell carcinoma of the bladder. Pathology from transurethral resection of bladder tumor demonstrated muscle-invasive disease. Pre-therapy imaging including CT abdomen/pelvis, CXR and bone scan demonstrated liver lesions concerning for metastatic disease but no skeletal metastases. Labs were notable for isolated thrombocytopenia, hypercalcemia and acute kidney injury prompting hospitalization. Hematologic work-up including bone marrow aspiration and biopsy revealed diffuse infiltration of the bone marrow by urothelial cancer. The case illustrates the importance of fully investigating otherwise unexplained clinical findings in patients with clinically localized urothelial cancer prior to curative intent surgery.
Keywords: Urothelial cancer, Bone marrow metastases, Thrombocytopenia
Introduction
Skeletal metastases occur in 32–47% of patients in ante-mortem and post-mortem series of metastatic urothelial cancer.1 Bone metastases in bladder cancer are often osteolytic. Thrombocytopenia in cancer patients is often therapy related though other coexisting etiologies should be considered including other medications, viral infections, DIC and immune thrombocytopenia (ITP). Myelophthisis is an uncommon cause of thrombocytopenia in metastatic epithelial cancer wherein diffuse bone marrow infiltration by neoplastic cells results in replacement of hematopoietic elements in the marrow. Myelophthisis is characterized by a leukoerythroblastic picture in the peripheral smear with prominent immature leukocytes, nucleated erythrocytes, teardrop cells (dacryocytes) and giant platelets.
Clinical presentation
A 75 year old Caucasian man presented to his urologist for evaluation of recent hematuria. Cystoscopy demonstrated a sessile right lateral bladder wall tumor with some diverticular formation around it without exophytic component. Pathology revealed noninvasive low-grade papillary urothelial carcinoma. Urine cytology demonstrated atypical urothelial cells. CT abdomen and pelvis showed a 4.9 × 2.9 × 3.3 cm right postero-lateral intramural bladder mass at the inferior edge of a bladder diverticulum without evidence of metastatic disease. Repeat TURBT showed a high-grade urothelial carcinoma invading the muscularis propria. No angiolymphatic invasion or divergent differentiation was identified.
Past medical history included hypertension, right bundle branch block, obstructive sleep apnea, gastroesophageal reflux, benign prostatic hypertrophy and remote history of hepatitis A. His medication list included Enalapril, Tamsulosin, Diltiazem, MS Contin, Norco and Docusate as needed. He had not recently received antibiotics, heparin or non-steroidals. Family history was notable only for a mother with colon cancer in her 80s. He did not use tobacco, alcohol or illicit drugs.
Complete blood count revealed platelets of 119,000 k/mm3 (decreased from 210,000 k/mm3 3 weeks earlier), white blood cell count 7900/mm3 and hemoglobin 14.7 g/dL. Basic metabolic panel and liver function tests were within normal limits.
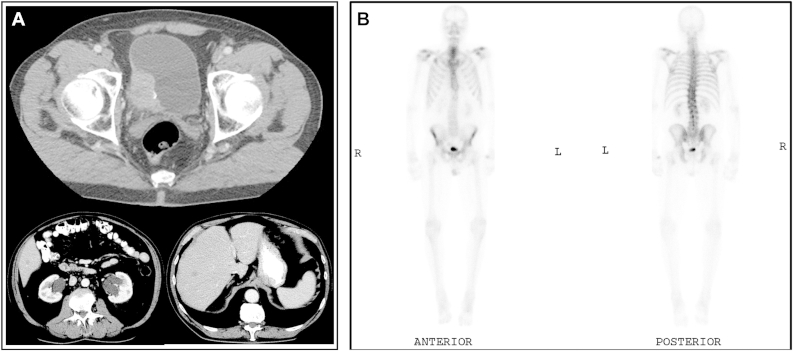
When due to begin chemotherapy 12 days later, progressive thrombocytopenia with platelets of 60,000 k/mm3 was noted. White blood cell count was 10,800/mm3 (68% neutrophils, 16% lymphocytes, 9% monocytes, 4% myelocytes and 2% metamyelocytes). Hemoglobin was normal. Calcium was 12.3 mg/dL, Creatinine 1.4 mg/dL, BUN 30; AST 87 IU/L, ALT 47 IU/L and alkaline phosphatase 148 IU/L. CT abdomen and pelvis demonstrated 4 new right hepatic lobe lesions ranging from 0.7 to 1.3 cm concerning for metastatic disease (Fig. 1A). Bone scan demonstrated mild increased likely post-traumatic uptake in the right lateral second rib (Fig. 1B). Chest X-ray was negative for any acute abnormality.
Figure 1.
A. Axial imaging of the abdomen showing large right postero-lateral wall bladder tumor and hypodense liver lesions. B. Whole body bone scan showing mild radioisotope uptake in the lateral right second rib and no other areas of abnormal bony uptake.
Hypercalcemia and acute kidney injury resolved with hydration and IV bisphosphonate. Repeat white blood cell count was 10,700/mm3, hemoglobin 13.2 g/dL, and platelets 54,000 k/mm3. DIC work up was negative with PT 11.9, INR 1.2, PTT 31.8 and fibrinogen 507 mg/dL. Serum protein electrophoresis and urine protein electrophoresis demonstrated glomerular proteinuria. Hepatitis A, B, and C and HIV serologies were negative. Vitamin B12, folic acid, TSH, serum immunoglobulins and haptoglobin were normal.
Peripheral blood smear revealed mild anisocytosis, normal white blood cells, no schistocytes, spherocytes or dacrocytes, and normal-appearing but decreased number of platelets. There were occasional giant platelets. An abdominal ultrasound demonstrated bladder mass and multiple hypoechoic 1 cm right hepatic lobe masses. The spleen was 11.4 cm. There was concern for autoimmune thrombocytopenia (ITP) versus infiltration of the marrow by metastatic urothelial carcinoma or less likely a primary bone marrow disorder. He was empirically started on prednisone 1 mg/kg for possibility of ITP without improvement. Histopathologic evaluation of the liver biopsy was consistent with metastatic high-grade urothelial carcinoma (Fig. 2).
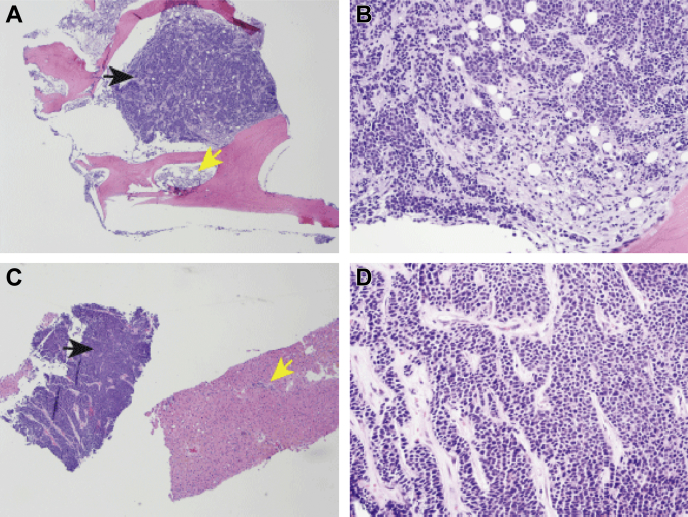
Figure 2.
Histopathology of urothelial carcinoma in bone marrow biopsy (A, B) and liver (C, D). Solid nests and trabeculae of tumor cells indicated by black arrows are adjacent to residual normal bone and residual normal liver indicated by yellow arrows (A, C). The tumor cells have pleomorphic and hyperchromatic nuclei with minimal amount of cytoplasm and demonstrate similar morphological features in metastatic cancer within bone (B) and liver (D). A and C, 40×. B and D, 200×.
Bone marrow aspiration and biopsy from the iliac crest was then performed. Bone marrow was almost completely replaced by diffuse sheets and nests of atypical epithelial cells with high nuclear/cytoplasmic ratio, small nucleoli and numerous mitoses (Fig. 2). The morphology of the epithelial cells present was identical to that seen in the prior liver biopsy with the metastatic foci. Only a small 2 mm focus of residual hematopoietic elements was seen. Erythroid elements showed full spectrum of maturation with no evidence of dysplasia. Myeloid lineage showed slight left shift in maturation but no morphologic evidence of dysplasia. Megakaryocytes were decreased without atypia. Immunohistochemistry revealed neoplastic epithelial cells diffusely and strongly positive for cytokeratin cocktail CAM5.2 and AE1/3, focally for CK7 and did not stain for CK5/6, p63, chromogranin A, synaptophysin, TTF1, CD45 or TdT. CD45 stained only the residual hematopoietic elements.
We analyzed the TURBT specimen by next generation sequencing (liver biopsy FFPE tissue was submitted but was inadequate) for a targeted gene panel with capture DNA and RNASeq (Paradigm, Ann Arbor, MI). Macro-dissection was performed and resulted in an increase in tumor content from an estimated 35% to approximately 60%. The Cancer Diagnostic (PCDx) Report revealed an EGFR c.2367C>T mutation which was considered as silent and not actionable (the finding is however 2 bases away from an actionable finding). No actionable gains in copy number variation were seen. Messenger RNA over-expression was noted for BRCA1, TUBB3, CAIX, survivin, TOPO2a, TS, PARP1, BAX, TP, MET, FABP5, EZH2 and LRP6. Of note, elevations of BRCA1, TUBB3, CAIX, survivin, PARP1 and EZH2 suggest but not determine minimal single agent activity for platinum agents (level II-3 for BRCA1 and level II-3 for survivin), vinca alkaloids, taxanes, and bevacizumab. High TOPO2a suggests clinical benefit for anthracyclines. Usually, an elevated TP (thymidine phosphorylase) indicates clinical benefit for fluoropyrimidines; however, this is annulled by high TS (thymidylate synthase), survivin and CAIX.
The patient received eight weekly infusions of paclitaxel but passed away from pneumonia and sepsis. His platelet count declined further without indication of clinical benefit from chemotherapy.
Discussion
To our knowledge, this is an extremely unusual clinical presentation of urothelial cell carcinoma of the bladder diffusely infiltrating the bone marrow causing isolated thrombocytopenia. Surreptitious infiltration of the bone marrow may not be detected on routine axial imaging.
Cytopenias from diffuse bone marrow involvement by urothelial cancer complicate the administration of optimal chemotherapy as in this patient's clinical course. We suspect that the unusual pattern of metastasis could reflect an unusual biological variant of urothelial cancer. The case also illustrates the shortcomings of currently available commercial targeted next generation sequencing assays for clinical use that choose a gene panel for analysis. The detection of actionable genomic aberrations in the tumor is only about 25–30%. The Cancer Genome Atlas Research Network (TCGA) project and institutional studies have uncovered multiple molecular genomic abnormalities in urothelial cancer providing an exceptional framework to inform therapeutic approaches.2
Conflict of interest
There is no conflict of interest for any of the authors.
Footnotes
Funding support: AA is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 2KL2TR000434. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Shinagare A.B., Ramaiya N.H., Jagannathan J.P. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol. 2011;196:117–122. doi: 10.2214/AJR.10.5036. [DOI] [PubMed] [Google Scholar]
- 2.Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]