Abstract
A 26-year-old woman with gross hematuria was seen in a previous hospital. Magnetic resonance imaging (MRI) showed a tumor at the dome of the urinary bladder with invasion outside of the bladder wall. The patient underwent transurethral resection of the bladder tumor (TUR-BT). From the result of the pathological examination, the tumor was suggested to be carcinosarcoma of the bladder. The patient was then referred to our hospital for treatment. We performed radical cystectomy and ileal conduit diversion. Pathological examination of the excised specimen revealed an inflammatory myofibroblastic tumor as the basis for immunostaining of anaplastic lymphoma kinase (ALK).
Keywords: Inflammatory myofibroblastic tumor, ALK, Bladder tumor, FISH
Abbreviations: ALK, anaplastic lymphoma kinase; MRI, Magnetic resonance imaging; H&E, hematoxylin & eosin; IMT, inflammatory myofibroblastic tumor; TUR-BT, transurethral resection of the bladder tumor
Introduction
Inflammatory myofibroblastic tumor (IMT) is a distinctive neoplasm composed of myofibroblastic and fibroblastic spindle cells accompanied by an inflammatory infiltration of plasma cells, lymphocytes, and/or eosinophils. Because of its histologic features, it can be difficult to distinguish from other sarcomatoid tumors such as urothelial carcinoma sarcomatoid variant and leiomyosarcoma. We report a case of IMT, who underwent radial cystectomy at the diagnosis of carcinosarcoma of the bladder. ALK (anaplastic lymphoma kinase) gene translocation or ALK protein expression in IMT has been reported. We discuss the usefulness of ALK immunostaining and molecular biological analysis of the ALK gene in the differential diagnosis of IMT.
Case presentation
A 26-year-old woman presented to another hospital with gross hematuria. Computed tomography of the abdomen and pelvis revealed a 33-mm enhancing mass arising from the dome of the urinary bladder. Dynamic MRI of the bladder revealed an early enhancing lobulated bladder tumor infiltrating to the outside of the bladder wall (Fig. 1). Cystoscopy revealed a solitary non-papillary tumor with surrounding edema. The patient presented with severe anemia due to hematuria, and transurethral resection of the bladder tumor (TUR-BT) and coagulation were promptly performed. The pathological findings of the TUR-BT specimen showed spindle cell proliferation with enlarged nuclei, and immunostaining showed that the tumor was positive for both cytokeratin AE1/AE3 and vimentin, and the rate of MIB-1 positivity was 40%. The tumor was suggested to be a urothelial carcinoma sarcomatoid variant of the bladder. Thereafter, the patient was referred to our hospital for further treatment.
Figure 1.
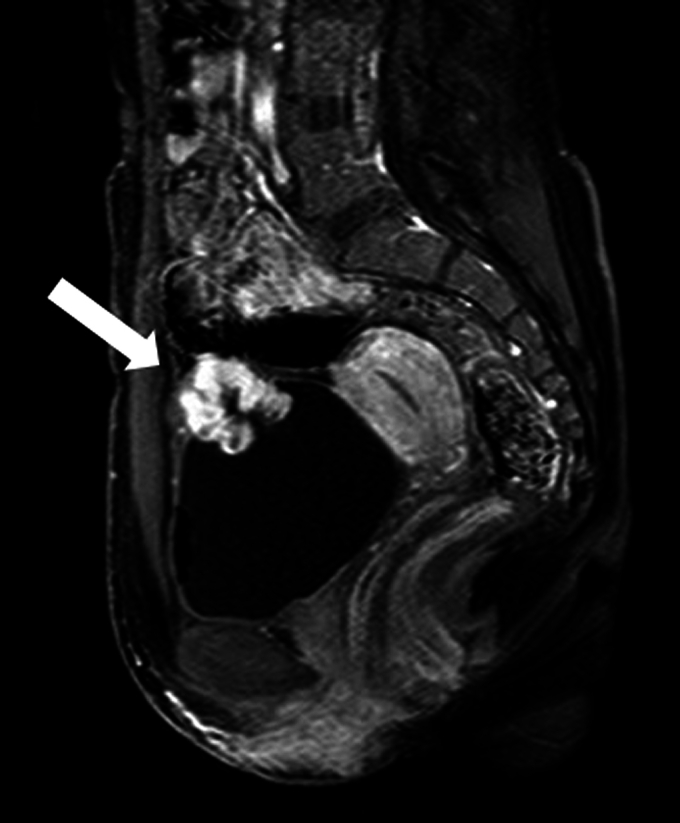
Sagittal contrast-enhanced MRI of the urinary bladder revealed early enhancement of a lobulated bladder tumor on the dome infiltrating to the outside of the bladder wall.
After we reviewed the pathological examination of the TUR-BT specimen, we diagnosed the patient as having carcinosarcoma of the bladder (cT3aN0M0). Because this was a high-grade carcinoma, we performed radical cystectomy and ileal conduit diversion with the emphasis on radical surgery. The pathological examination of excised specimen showed proliferation of spindle cells expanding mainly into the bladder musculature accompanied by inflammatory cell infiltrate (Fig. 2). Immunostaining showed that the tumor cells were positive for ALK-1, vimentin, and smooth muscle actin. Cytokeratin AE1/AE3 staining was diffusely positive, and p63 staining was uniformly negative. From the histomorphology and immunophenotype, the tumor was definitively diagnosed as IMT of the bladder different from the original diagnosis made following TUR-BT. To date, the patient has been followed for 10 months with no evidence of recurrence.
Figure 2.
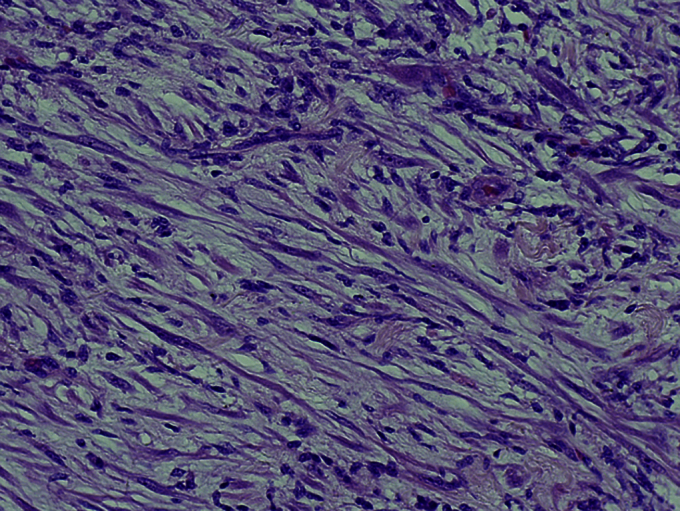
Histopathological findings revealed spindle cells and nuclear atypia (H&E staining, ×200).
Discussion
IMT is a rare neoplasm that occurs primarily in soft tissue and viscera. It is classified as intermediate (rarely metastasizing) in the WHO classification of soft tissue tumors. Approximately 25% of extrapulmonary IMTs recur, and metastasis occurs in <2% of cases. IMT occurs most frequently in the mesentery, omentum, retroperitoneum, pelvis, and abdominal soft tissue in 73% of cases.1 Its occurrence in the urinary bladder is unusual. A recent review article showed that IMT of the urinary bladder occurs at a mean age of 38.9 years with a predilection for females (51.7%).2 The most common presentation is hematuria followed by dysuria, urinary frequency, and other symptoms. Characteristic pathological findings include the presence of spindle cells, myofibroblastic and fibroblastic cells, and inflammatory cells consisting mainly of plasma cells and lymphocytes. IMT may be difficult to distinguish pathologically from urothelial carcinoma sarcomatoid variant and leiomyosarcoma because of its morphology.
According to a research article on the discrepancy in histopathological diagnosis of soft tissue tumors, following retrospective pathological review of patients referred with soft tissue lesions to a specialist in soft tissue sarcoma, either major or minor discrepancies between the referring and final diagnosis were present in 28.2% of cases. Additional immunohistochemical and molecular and molecular cytogenetic tests contributed to a change in the final diagnosis in 30.6% of all discrepant cases.3
In the present case, there was a diagnostic discrepancy between the TUR-BT specimen and the excised specimen. The deciding factor in changing the diagnosis was made in accordance with the immunohistochemical finding of ALK-1. At first, the spindle cells with swollen nuclei looked like carcinosarcoma in the TUR-BT specimen, but on examination of the excised specimen, although there were some enlarged nuclei with obvious nucleoli, there were no tumor cells with an epithelial morphology. After we noted the spindled cells and infiltrate with diffuse inflammation and positive immunoreactivity of ALK-1, the tumor was diagnosed as IMT of the urinary bladder.
ALK gene translocation in non-small-cell lung cancer was initially reported in 2007 by Soda et al.4 IMTs were also noted to have ALK gene rearrangements, and cytoplasmic reactivity for ALK protein is detectable in 50%-60% of IMTs, which correlates well with the presence of a rearrangement of the ALK gene.1 ALK-1 protein expression and/or ALK gene rearrangements are useful in the differential diagnosis of IMTs versus sarcomatoid carcinomas because these genetic rearrangements have not been documented in the sarcomatoid carcinomas.
Fluorescence in situ hybridization is useful in detecting ALK gene translocation and was used to detect the ALK gene translocation in this case (Fig. 3). In the field of lung cancer, ALK gene translocation is reported to respond well to an ALK inhibitor, and molecularly targeted therapy with crizotinib has already been applied in the clinical setting. In view of the benign course of IMT, complete resection of the tumor is considered to be the standard therapy. However, the course in some patients behaves in a malignant manner.5 Molecular targeted therapy is expected to have a role in the treatment of such patients in the future.
Figure 3.
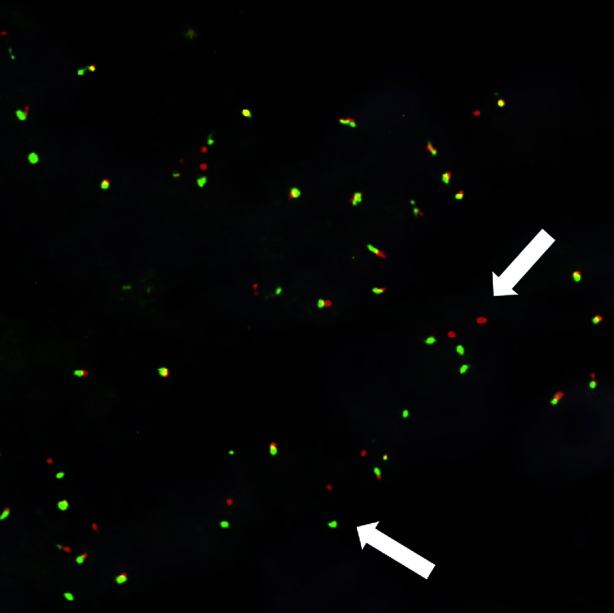
Fluorescence in situ hybridization analysis. White arrowheads indicate signals positive for ALK gene rearrangements.
Conflict of interest
None declared.
References
- 1.Fletcher C.D.M., Bridge J.A., Hogendoorn P., Mertens F. Vol. 5. IARC Press; Lyon, France: 2013. pp. 83–84. (WHO Classification of Tumours). [Google Scholar]
- 2.Teoh J.Y., Chan N.H., Cheung H.Y. Inflammatory myofibroblastic tumors of the urinary bladder: a systematic review. Urology. 2014;84:503–508. doi: 10.1016/j.urology.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Thway K., Wang J., Mubako T., Fisher C. Histopathological diagnostic discrepancies in soft tissue tumours referred to a specialist centre: reassessment in the era of ancillary molecular diagnosis. Sarcoma. 2014;2014:686902. doi: 10.1155/2014/686902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soda M., Choi Y.L., Enomoto M. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 5.Kim H.W., Choi Y.H., Kang S.M. Malignant inflammatory myofibroblastic tumor of the bladder with rapid progression. Korean J Urol. 2012;53:657–661. doi: 10.4111/kju.2012.53.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]


