Abstract
Despite the importance of herbivory for the structure and functioning of species-rich forests, little is known about how herbivory is affected by tree species richness, and more specifically by random vs. non-random species loss.
We assessed herbivore damage and its effects on tree growth in the early stage of a large-scale forest biodiversity experiment in subtropical China that features random and non-random extinction scenarios of tree mixtures numbering between one and 24 species. In contrast to random species loss, the non-random extinction scenarios were based on the tree species’ local rarity and specific leaf area – traits that may strongly influence the way herbivory is affected by plant species richness.
Herbivory increased with tree species richness across all scenarios and was unaffected by the different species compositions in the random and non-random extinction scenarios. Whereas tree growth rates were positively related to herbivory on plots with smaller trees, growth rates significantly declined with increasing herbivory on plots with larger trees. Our results suggest that the effects of herbivory on growth rates increase from monocultures to the most species-rich plant communities and that negative effects with increasing tree species richness become more pronounced with time as trees grow larger.
Synthesis. Our results indicate that key trophic interactions can be quick to become established in forest plantations (i.e. already 2.5 years after tree planting). Stronger herbivory effects on tree growth with increasing tree species richness suggest a potentially important role of herbivory in regulating ecosystem functions and the structural development of species-rich forests from the very start of secondary forest succession. The lack of significant differences between the extinction scenarios, however, contrasts with findings from natural forests of higher successional age, where rarity had negative effects on herbivory. This indicates that the effects of non-random species loss could change with forest succession.
Keywords: Associational susceptibility, BEF-China, biodiversity and ecosystem functioning, extinction scenarios, functional traits, plant–herbivore interactions, resource concentration, succession, trophic interactions
Introduction
High rates of deforestation and forest degradation world-wide increase rates of species extinctions and seriously threaten essential functions and services provided by forests (Kremen et al. 2000; Bala et al. 2007). The resulting loss of biodiversity is a major driver of ecosystem change (Hooper et al. 2012), but its effects on many of the processes crucial to the functioning of forests, such as herbivory, are not fully understood.
Herbivory is a key process in many forest ecosystems, mediating species coexistence and ecosystem functions, such as productivity and nutrient cycling (Schowalter 2012; Bagchi et al. 2014; Metcalfe et al. 2014). Plant species richness can have significant effects on the levels of herbivore damage (Jactel & Brockerhoff 2007; Cardinale et al. 2012), but the strength and direction of these effects vary among ecosystems (see Dinnage 2013 and Scherer-Lorenzen 2014 for an overview). Differences in the degree of host specialization of the dominant herbivores may be one of the reasons for this observed variability (Jactel & Brockerhoff 2007). While increasing plant species richness might decrease specialist herbivore damage by decreasing resource availability (the resource concentration hypothesis; Root 1973), the performance and consumption rates of many generalist herbivores might be promoted by allowing them to better balance the intake of different nutrients and toxins (the dietary mixing hypothesis; see e.g. Bernays et al. 1994). The actual strength of these effects probably depends on the extent to which plant species richness influences the community-level distribution and diversity of key palatability and defence traits (Loranger et al. 2013; Schuldt et al. 2014a). Herbivore community composition (e.g. Ebeling et al. 2014) and herbivory levels (e.g. Loranger et al. 2013) can be affected significantly by such plant traits, for example leaf chemical compounds, morphological characteristics such as specific leaf area (SLA, increasing values of which indicate decreasing leaf toughness and higher palatability to herbivores; e.g. Salgado-Luarte & Gianoli 2012), but also the local rarity and apparency of plant species (with abundant and apparent species often more visible and readily available to herbivores; e.g. Schuldt et al. 2012; Castagneyrol et al. 2013). However, although species loss in natural communities is often trait-dependent (Srivastava & Vellend 2005; Naeem, Duffy & Zavaleta 2012), it remains poorly studied how such non-random extinctions affect the relationship between plant species richness and processes such as herbivory. This is despite the fact that plant traits playing a crucial role in determining extinction risk might strongly correlate with potential key drivers of herbivore damage (such as rarity and SLA; see e.g. Bruelheide et al. 2014).
This lack of knowledge also hinders a deeper understanding of how feedback effects of herbivory on the plant community are modified by plant species loss. Plant species richness has been shown to influence the strength of herbivory effects on processes such as plant growth (e.g. Mulder et al. 1999; Massad et al. 2011; Riedel et al. 2013). Differences in the response of herbivores to non-random vs. random plant species loss may thus affect the way herbivory influences species coexistence and community-level relationships among plant species richness, productivity and nutrient cycling. However, to our knowledge, no study has quantified the impact of herbivory on plant growth along gradients of plant species richness under contrasting scenarios of species extinction.
Here, we analyse herbivory and its effects on tree growth across a gradient of tree species richness – ranging from monocultures to mixtures of 24 species – in a large-scale forest biodiversity experiment in subtropical China under different scenarios of plant species loss. This biodiversity–ecosystem functioning experiment (henceforth referred to as BEF-China experiment) is currently the world’s largest forest biodiversity experiment and, in contrast to most previous experiments, features gradients of tree species richness based on both random and non-random (trait-based) extinction scenarios (Bruelheide et al. 2014). The non-random scenarios are based on the tree species’ local rarity and SLA, with the most common species and those with the smallest SLA considered most likely to persist in the least diverse species mixtures. Here, we present results from the initial stage of the experiment, 2.5 years after planting. Fast tree growth and conditions that promote strong trophic interactions (Schemske et al. 2009; Rodriguez-Castaneda 2013) might lead to a fast development of relationships between herbivory and plant species richness in our study region.
Assuming that generalist herbivores potentially play a dominant role in such an early-successional ecosystem (Brown 1985; Siemann, Haarstad & Tilman 1999), we hypothesize that (i) community-level herbivore damage increases along the gradient of tree species richness of our experimental sites and therefore that (ii) the resulting higher herbivore pressure will cause a decrease in tree growth with increasing tree species richness. Moreover, we expect that (iii) these effects differ between the random and non-random extinction scenarios. In the rarity-based scenario, high local commonness of species at low levels of species richness may cause a higher degree of herbivory and a stronger herbivory-mediated reduction of tree growth than in the randomly assembled communities. This might lead to an attenuation of positive plant species richness effects on herbivory. In contrast, in the SLA scenario, an increase of species with large SLA with increasing tree species richness might promote generalist herbivores preferring the more palatable and readily digestible leaves of those species. This might result in herbivory levels and thus effects of herbivory on tree growth that are more similar to those in the random scenarios. However, for some herbivores, tree species with a large SLA might be unattractive as they offer lower nutrient content per ingestion effort (Lusk et al. 2010). Patterns might then resemble more strongly those of the rarity-based scenario (see also Schuldt et al. 2012 for similar effects of a related morphological trait, leaf dry matter content).
Effects of tree species richness on community-level herbivory, combined with effects of herbivory on tree growth and potential differences between randomly and non-randomly assembled communities, at such an early stage of our experiment would have important implications – not only for our understanding of how herbivores contribute to the processes that drive the assembly and functioning of establishing tree communities in species-rich forests, but for reforestation and the design of sustainable plantation forests as well (Massad et al. 2011). This is particularly important when considering that early-successional stages in forests constitute an important developmental phase in which the survival rates of tree individuals are often determined.
Materials and methods
Study Sites and Experimental Design
The subtropical BEF-China tree diversity experiment is located close to Xingangshan, Jiangxi Province, in south-east China (29°08′–29°11′ N, 117°90′–117°93′ E). Mean annual temperature is 16.7°C and mean annual precipitation is around 1800 mm (Yang et al. 2013). The experiment consists of two experimental sites (Site A and Site B) of ca 20 ha each, located in sloped terrain between 100 and 300 m.a.s.l. Details of the experimental design are provided in Bruelheide et al. (2014). In short, each site consists of 271 plots of ca 25.8 × 25.8 m (= 1 mu in the traditional Chinese areal unit). Each of the experimental plots consists of 400 trees planted in a grid of 20 × 20 individuals at a horizontal planting distance of 1.29 m. Species were randomly assigned to individual planting positions within the plots, with the total number of individuals per plot divided equally among the species planted in a given plot. Plots were planted in 2009 (Site A) and 2010 (Site B) with either monocultures or mixtures of 2, 4, 8, 16 or 24 tree species. In total, 40 native broad-leaved tree species were planted in the experiment. The species pools of the two sites overlapped by eight species (planted in one of the random extinction scenario replicates of each site). The species composition of the mixtures at both sites followed either a random or one of two non-random (trait-oriented) extinction scenarios. In the random extinction scenario (replicated with three different species pools per site, each composed of 16 species), the tree species of the less diverse mixtures were selected by randomly partitioning the species composition of the 16-species plots into non-overlapping fractions by means of a bootstrapping procedure (see Bruelheide et al. 2014). This ensures that all species are equally represented at all diversity levels. The 24-species plots were included as an additional high species-richness level by combining species from two different 16-species sets out of the three sets per site. Species compositions in the two non-random scenarios were based on local rarity and SLA of the tree species, respectively, with the rarest species or those with the largest SLA being sequentially eliminated with decreasing diversity of the species mixtures (such that only the most common species or those with the smallest SLA remained in the least diverse mixtures; Bruelheide et al. 2014). All plots were weeded twice a year, with all upcoming vegetation between the planted trees being removed.
Herbivory Assessment
Herbivore damage was assessed for the two experimental sites on a total of 296 plots: in the random extinction scenarios 80, 64, 32, 16, 8 and 4 plots of the tree richness levels 1, 2, 4, 8, 16 and 24, respectively, and in each of the two non-random extinction scenarios 24 plots of 2, 4, 8 and 16 species each. Plots with additional manipulation of shrub species richness or of seed family richness were excluded (see Bruelheide et al. 2014). The assessments were conducted at the end of the main growing season in September and October 2011 (Site A) and 2012 (Site B), that is 2.5 years (three growing seasons) after the initial planting of seedlings at each site. In each plot, the central 6 × 6 (= 36) tree individuals were monitored for herbivore damage. While the random planting design might affect the tree species composition of these 36 central tree individuals in some of the more species-rich plots, we were primarily interested in community-level patterns and accounted for this with the mixed model approach described below. On each tree, seven leaves on three randomly selected branches from different parts of the canopy (= 21 leaves per tree) were visually inspected. Herbivory was quantified as the overall leaf damage caused by chewing, mining, galling and (if visible) sucking insects per leaf. We used predefined percentage classes (estimated as 0, < 5, < 25, < 50, < 75, > 75%, with mean values per class used in the statistical analyses), a common approach to visually assess standing levels of leaf damage (see e.g. Scherber et al. 2010; Schuldt et al. 2010, 2012; Ness, Rollinson & Whitney 2011; Salgado-Luarte & Gianoli 2012). Damage levels for individual types of herbivores (e.g. chewers, miners, etc.) are not available, but we recorded which type of herbivore was responsible for most of the damage observed on each tree. To ensure that the analysis was consistent among species, we only used young, fully expanded leaves produced in the current growing season.
Tree growth Measurements
Tree height on all planting positions included in the herbivory assessment (i.e. at 36 positions per plot) was measured as the total length of a tree from the stem base to the apical meristem (in cm), ground diameter as the stem diameter 5 cm above the ground (to the nearest millimetre). Tree height as a size parameter used in the herbivory model, as specified below, was assessed as part of the herbivory measurements on each tree. Tree height and ground diameter as growth parameters were measured in separate campaigns in October 2010 and 2011 (Site A) and in October 2011 and 2012 (Site B), that is both 1 year before and in the same year as the herbivory census. We calculated relative growth rates (RGR) as the relative increase in tree height (RGRheight) and ground diameter (RGRgd) per year as ln(size in year 2/size in year 1) (see e.g. Paine et al. 2012).
Predictors of Herbivore Damage and Growth Rate
In addition to the planted species richness of the plots, we included extinction scenario (three different random scenarios as well as non-random rarity and non-random SLA), experimental site, tree height, elevation and degree of ‘northness’ (cosine-transformed radian values of aspect) of the plots in the analyses. Elevation and northness were chosen to account for differences in the topographic heterogeneity of the experimental sites. Data were obtained from a 5-m digital elevation model (DEM) that was established based on differential GPS measurements when the experiment was started. Due to the large number of plots and tree individuals, the herbivory assessments took place over several weeks and we recorded the day of assessment for each plot and included it as a covariate in the statistical analyses.
Statistical Analysis
We used mixed-effects models with mean leaf damage per tree individual as well as means of RGR per species and plot (with the two response variables RGRheight and RGRgd) as response variables. While these models use data on the tree-individual level (or species level in the growth rate analyses), they model the overall, general response averaged across all tree individuals in a given plot and thus essentially show community-level patterns. Therefore, unless we explicitly mention species-specific patterns (for a general overview of leaf damage across species, calculated as the mean across all individuals of a given species), the data provided in the results section refer to the community level.
In the herbivory model, tree species identity (n = 40), plot identity (n = 296) and species composition of plots (n = 232), as well as the interactions between plot and species composition and between species pool and species composition were used as crossed random effects to account for the hierarchical structure of the data. We also tested for random slope effects of tree species richness depending on species identity. In the herbivory model, we included experimental site as a fixed effect (to account for potential differences between locations and years, treated as a fixed effect as there were only two factor levels; Zuur, Ieno & Smith 2007), as well as day of the assessment, tree height, elevation, the degree of ‘northness’, extinction scenario (three replicates of the random scenario with three different species pools, two non-random scenarios) and tree species richness. To account for potential differences in effects among sites and extinction scenarios, we also included the two-way interactions between site and day, site and scenario, site and tree species richness, scenario and tree species richness, as well as the three-way interaction among site, scenario and tree species richness (i.e. the effects of tree species richness might differ between the random and non-random scenarios, and at the same time depend on the study site due to differences in tree age and time of assessment).
In the tree growth models, we included mean leaf damage and initial tree size (i.e. size data of the first measurement campaign) as additional explanatory variables. We used mean values per species and plot for the response and all predictor variables, as tree positions measured for tree growth could not be reliably matched with the tree positions measured for herbivory in all cases (i.e. although 36 planting positions were checked for both growth and damage, labels identifying the tree positions in the field were not in place in all plots and on all tree individuals at the time of the herbivory assessments, which obviously led to some inconsistencies in the actual tree positions being sampled in the growth and herbivory assessments (but not in the growth assessments between years, which used identical tree positions in each year); note that the tree height data used in the herbivory analysis were measured together with leaf damage in one sampling campaign and can thus be used on a tree-individual level, whereas the growth data were assessed in a separate campaign that led to the above-mentioned difficulties). Besides mean leaf damage and initial tree size, we included experimental site, elevation, the degree of ‘northness’, extinction scenario and tree species richness as fixed effects. As we were primarily interested in the effects of herbivory on tree growth, we included the two- and three-way interactions of leaf damage with extinction scenario, experimental site and tree species richness in the models. We also tested for an interaction between initial tree size and leaf damage, as tree size may influence the degree of herbivore damage. We used the same random effects structure as in the herbivory model, and we tested for a random slope effect of leaf damage depending on species identity. Mean leaf damage as the response variable and tree species richness and initial tree size as predictors were log-transformed to improve modelling assumptions, and all continuous predictors were standardized (mean = 0; SD = 1) before analysis.
We tested for model simplification in two steps. As the experiment was in a very early stage and potential effects of the different extinction scenarios might not yet have had an effect on observed levels of herbivory, we first checked three model variants that reduced the three random extinction scenario replicates and the two non-random extinction scenario levels to (i) one overall random scenario vs. the two non-random scenarios (i.e. three levels), (ii) a contrast between an overall random and an overall non-random scenario (i.e. two levels), or that assumed no differences among scenarios by (iii) completely disposing of the extinction scenario (and its interactions) as a predictor. The three model variants were compared to the initial model (using maximum likelihood for parameter estimation), and the model with the lowest AIC was used for further analysis (Crawley 2007). With this model, we then tested for uninformative predictors and in a stepwise procedure deleted those predictors whose removal resulted in a reduction in the AIC of the model (Burnham & Anderson 2004). The model with the smallest number of predictors and the lowest global AIC was chosen as the most parsimonious, best-fit model and then rerun with restricted maximum likelihood (REML) estimation. Model residuals were checked for normality and homogeneity of variances. All analyses were conducted in R 3.1.0 (http://www.R-project.org) with the packages lme4 (Bates et al. 2014) and lmerTest (Kuznetsova, Brockhoff & Christensen 2014).
Results
The mean damage on the tree-individual level across species and sites was 8.3% ± 0.1 SE (based on the damage class mean values). Manglietia yuyuanensis Y. W. Law and Alniphyllum fortunei (Hemsley) Makino exhibited the lowest mean damage levels (1.6% ± 0.1 SE and 2.8% ± 0.2 SE, respectively); Acer davidii Franchet and Quercus serrata Murray showed the highest damage levels (17.8% ± 3.4 SE and 13.2% ± 0.7 SE, respectively) (Fig.1; see Fig. S1 in Supporting Information for data restricted to monocultures, which shows a very similar ranking of species). Chewing damage was recorded as the main damage type on 75% of all tree individuals, sucking damage on 24% and both mining and galling damage on < 1% of all trees.
Figure 1.
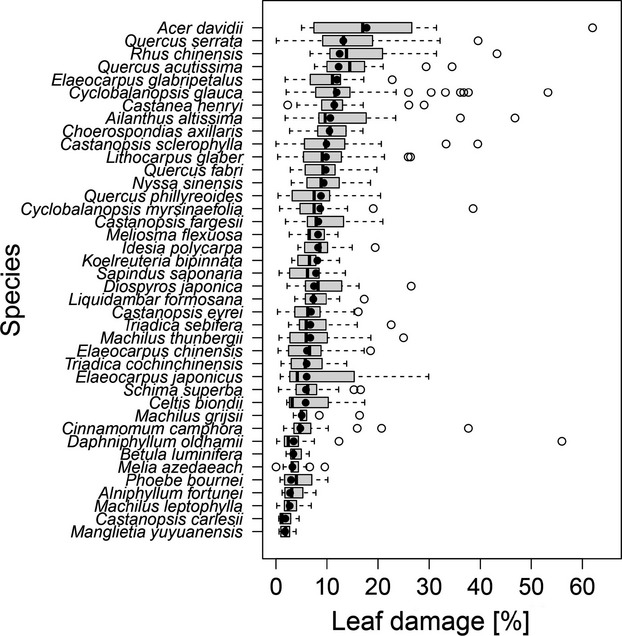
Leaf damage (%) on the 40 tree species planted in the experiment. Species are ordered by mean leaf damage levels (filled circles; black lines show medians) across all plots of the large-scale tree diversity experiment in subtropical China.
Community-level Herbivory
Log-transformed tree species richness showed a significant positive relationship with log-transformed herbivory on the plot level, with a predicted doubling in leaf damage from the monocultures (predicted mean = 5.7%) to the 24 tree species mixtures (predicted mean = 10.6%; Table1, Fig.2; see Fig. S2 for single regressions for the 40 study species, which show that the relationship between herbivory and tree species richness was not due to a sampling effect of including particularly susceptible species in more species-rich plots, but rather to an increase in damage levels with tree species richness in individual species). This relationship and the mean herbivory levels did not differ significantly between sites (Table1) or among the different extinction scenarios, and the simplified model variant that did not differentiate among scenarios (AIC = 17 563) was preferred to the variants that included all scenarios (AIC = 17 578), one combined random vs. the two non-random scenarios (AIC = 17 569), or one combined random and one combined non-random scenario (AIC = 17 563, but BIC = 17 691, compared to BIC =17 664 for the more parsimonious model variant ignoring the scenarios). This result was confirmed by separate tests of potential differences in herbivory between plots of the random and non-random extinction scenarios within the individual tree species richness levels, all of which were non-significant. Including a random slope effect of tree species richness depending on species identity did not improve the fit of the initial model and was dropped from the analysis (AIC = 17 579 vs. AIC = 17 578 of the initial model). In addition to the increase in herbivory with tree species richness, herbivory increased with individual tree height (see also Fig. S3 for species-specific differences in initial tree height) and decreased with the elevation of the plots (Table1). The time at which the plots were checked for herbivory during the assessment campaigns also played a role, but the effect differed between the two experimental sites (Table1).
Table 1.
Minimal mixed-effects models (with standard errors, degrees of freedom, t and P values) for (a) herbivore damage, (b) relative growth rates (RGR) of tree height (mean per species and plot) and (c) RGR of ground diameter (mean per species and plot) across the two sites of the large-scale tree diversity experiment in subtropical China. Estimates were standardized; thus, their magnitude is proportional to the effect size in the final model
| Fixed effects | Std. Est. | Std. Error | d.f. | t | P |
|---|---|---|---|---|---|
| (a) Leaf damage | |||||
| (Intercept) | 1.40 | 0.11 | 50 | 12.3 | < 0.001 |
| Site B | 0.10 | 0.08 | 295 | 1.2 | 0.215 |
| Day | −0.17 | 0.05 | 208 | −3.2 | 0.001 |
| Tree height | 0.17 | 0.02 | 5276 | 8.9 | < 0.001 |
| Elevation | −0.07 | 0.03 | 200 | −2.1 | 0.038 |
| Tree species richness (log) | 0.08 | 0.03 | 224 | 2.8 | 0.006 |
| Site B: day | 0.38 | 0.06 | 218 | 6.7 | < 0.001 |
| (b) RGRheight | |||||
| (Intercept) | 0.61 | 0.02 | 31.0 | 33.2 | < 0.001 |
| Initial gd (log) | −0.12 | 0.01 | 681.6 | −12.7 | < 0.001 |
| Elevation | 0.04 | 0.01 | 270.7 | 4.2 | < 0.001 |
| Mean leaf damage | 0.01 | 0.01 | 828.3 | 1.3 | 0.198 |
| Initial height: leaf damage | −0.02 | 0.01 | 864.3 | −3.3 | < 0.001 |
| (c) RGRgd | |||||
| (Intercept) | 0.611 | 0.021 | 37.2 | 29.4 | < 0.001 |
| Initial gd (log) | −0.102 | 0.010 | 687.4 | −10.2 | < 0.001 |
| Elevation | 0.023 | 0.011 | 319.8 | 2.1 | 0.036 |
| Mean leaf damage | 0.001 | 0.009 | 823.0 | 0.1 | 0.910 |
| Initial gd: leaf damage | −0.030 | 0.008 | 830.5 | −3.9 | < 0.001 |
Figure 2.
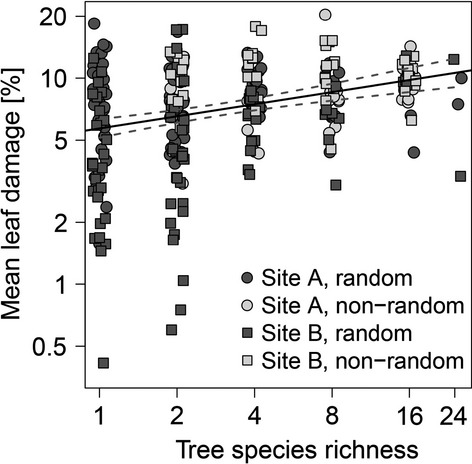
Relationships between mean leaf damage and tree species richness as predicted from the mixed-effects model shown in Table1. Relationship significant at P < 0.05 (see Table1 for details). Note that both axes are on a log scale.
Community-level Tree Growth
The mean RGR in tree height per species and plot over 1 year was 0.61 ± 0.01 SE, the mean RGR in ground diameter 0.63 ± 0.01 SE. Both growth rate metrics were significantly related to the initial tree size and to the interaction between initial size and mean leaf damage on the study plots (Table1). While RGRs per species and plot generally decreased with initial tree size (height and ground diameter, respectively), the strength of this effect increased with increasing leaf damage (Fig.3a,b). This was due to the fact that the relationships between leaf damage and both metrics of RGR changed from positive when trees were initially small to negative when trees were initially larger in size (Fig.3a,b).
Figure 3.
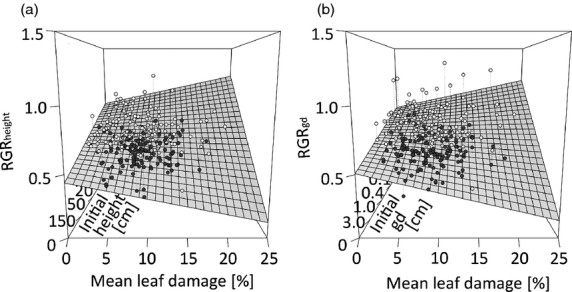
Relationships among mean leaf damage, initial tree size and the relative growth rates (RGR) of (a) tree height and (b) ground diameter as predicted for plot means from the mixed-effects models shown in Table1. Points show plot-level mean values of RGR (open symbols = observed ≥ predicted values, filled symbols = observed ≤ predicted values). Relationships significant at P < 0.05 (see Table1 for details). Initial height data are shown on a log scale.
Based on the predicted values of the RGR models, plot-level RGRs can be expected to increase from 0.78 ± 0.03 (RGRheight) and 0.76 ± 0.04 (RGRgd) in plots without leaf damage to 0.97 ± 0.04 (RGRheight) and 0.97 ± 0.05 (RGRgd) in plots with highest mean leaf damage (20.7%) when trees have an average height of 17 cm and an average ground diameter of 0.2 cm. In contrast, plot-level RGRs are predicted to decrease from 0.35 ± 0.04 and 0.46 ± 0.04 (no damage) to 0.17 ± 0.05 and 0.26 ± 0.04 (20.7% damage) at an average tree height of 364 cm and an average ground diameter of 4.0 cm (Fig.3a,b). For the predicted means of leaf damage identified in the above herbivory analyses for monocultures (5.7%) and 24-species mixtures (10.6%), the RGR models indicate an increase in average RGR from 0.78 ± 0.03 (RGRheight) and 0.76 ± 0.04 (RGRgd) (no leaf damage) to 0.83 ± 0.03 and 0.82 ± 0.03 (average monoculture damage levels) and 0.88 ± 0.03 and 0.87 ± 0.03 (damage levels of the most species-rich mixtures) for the smallest tree size and a decrease in average RGR from 0.35 ± 0.04 and 0.46 ± 0.04 (no damage) to 0.30 ± 0.03 and 0.41 ± 0.03 (monoculture damage) and 0.26 ± 0.03 and 0.36 ± 0.03 (damage in 24-species mix) for the largest tree size.
The models for both relative growth rate metrics did not differentiate among extinction scenarios (AIC = −152.0 for tree height and AIC = −82.7 for ground diameter), which was preferred to the inclusion of all scenarios (AIC = −139.2 for tree height and AIC = −72.0 for ground diameter), one combined random vs. the two non-random scenarios (AIC = −144.3 and −75.0), or the difference between one combined non-random and one combined random extinction scenario (AIC = −148.0 and −79.5). Including a random slope effect of mean leaf damage depending on species identity did not improve the fit of the initial models and was dropped from the analysis (AIC = −135.8 vs. AIC = −139.2 of the initial model for tree height and AIC = −70.0 vs. AIC = −72.0 for ground diameter). Moreover, no interactions with tree species richness were retained in the minimal models, and only elevation had additional significant, positive effects on the relative growth rate metrics (Table1).
Discussion
Our study shows that tree species richness can strongly mediate the degree of herbivore damage in the controlled set-up of a large-scale forest biodiversity experiment, irrespective of whether species compositions in the tree species richness levels were assembled randomly or were informed by rarity or SLA. Importantly, tree growth rates were significantly related to the observed herbivory levels when controlled for initial tree size, with negative effects becoming more pronounced as tree size increased. The results of our study thus have important implications for our understanding of herbivory effects and their relationship with plant species richness in species-rich ecosystems. Moreover, our findings are relevant for the assessment of the conceivable impacts of herbivory on tree recruitment and the competitive performance of saplings, as well as the development success of tree plantations with different tree species richness.
Herbivory and Tree Species Richness
The positive correlation between tree species richness and herbivore damage is in line with other recent studies reporting an increase in herbivory across gradients of plant species richness that included relatively high richness levels of up to 70 plant species per study plot (e.g. Schuldt et al. 2010; Loranger et al. 2014). Early-successional stages such as our experimental sites (Brown 1985; Siemann, Haarstad & Tilman 1999), or forests in general even in species-rich tropical regions (e.g. Novotny & Basset 2005), can be dominated by generalist herbivores that benefit from the diversity of resources in species-rich plant communities (Pfisterer, Diemer & Schmid 2003; Jactel & Brockerhoff 2007). Herbivory in our study plots was largely due to leaf chewers, with a particularly high abundance of grasshoppers and lepidopteran caterpillars (A. Schuldt, unpublished data). Many grasshoppers have a relatively broad host plant spectrum (Bernays & Chapman 2000), and the same probably applies to dominant caterpillars in our study region (see Schuldt et al. 2014a,b). Increases in the abundance (e.g. Ebeling et al. 2014) and increased performance of these herbivores by dietary mixing of different plant species, balancing the intake of different nutrients or toxins, are thus a probable explanation for the higher levels of herbivory in plots of higher tree species richness. Consistent with this interpretation, Lefcheck et al. (2013) showed that, while the overall effects of dietary mixing can be variable, many herbivores may benefit from mixed diets under natural conditions. This may also be one of the reasons for deviating results in other forest systems with potentially more specialized herbivore assemblages (and in most cases with relatively low levels of plant species richness; Jactel & Brockerhoff 2007; Vehviläinen, Koricheva & Ruohomaki 2007; Sobek et al. 2009; Plath et al. 2011; Castagneyrol et al. 2013).
Community and Ecosystem Consequences
The fact that the herbivory levels observed in our study were related to significant changes in the trees’ RGR indicates that even the moderate differences in leaf damage across the tree species richness gradient might have a perceivable impact on the young forest stands. Similar and constant levels of herbivory (i.e. 4 and 8%) have also been shown in another study to strongly affect tree growth (−34 and −45%) and – in the long term – might even influence tree performance to a larger extent than the effects of more severe but only periodical insect outbreaks (Zvereva, Zverev & Kozlov 2012). In this context, it is interesting to note that Yang et al. (2013) found an unexpected increase with elevation in the survival rate (and our results show that growth rates increased as well) of the seedlings planted in our experiment, and our finding that herbivory decreased with elevation (see also Rasmann et al. 2014) could potentially contribute to an explanation for this pattern. Moreover, it is conceivable (and preliminary data indicate such an effect for our experiment; A. Schuldt & L. Hantsch, unpublished data) that the increase in herbivory with increasing tree species richness promotes subsequent attack by fungal plant pathogens, which might potentially amplify negative effects of leaf damage on tree growth (Stout, Thaler & Thomma 2006).
Our results suggest that as trees become larger, negative effects of herbivory may actually become more pronounced, and thus, long-term consequences of herbivory could become more severe with time. The contrasting finding of increasing RGR with herbivory when trees were still comparatively small could be due to a lower apparency of smaller trees. In our study, tree height was negatively correlated with herbivory. This might reduce the probability of generalist herbivores finding suitable combinations of tree species for dietary mixing and could potentially result in a higher incidence of specialist damage. It might also involve (over)compensation for damage in a life stage where maximizing growth can be particularly crucial for survival (Blundell & Peart 2001; Boege, Barton & Dirzo 2011). It should be noted, however, that the potential effects of herbivory on tree growth seem to be a direct consequence of tree species richness-mediated differences in herbivore pressure. This means that there was no indication that equal levels of herbivory had differential effects in plots varying in tree species richness, which would have been seen in significant interactions of herbivory and tree species richness.
In addition to the effects on growth rates, which can result in plot-level effects on plant biomass production and might influence plant community structure in the long term, the observed changes in herbivory levels with increasing tree species richness may well affect further ecosystem functions such as nutrient fluxes. While we can only speculate on such effects in our study, slightly higher levels (12–19%) but similar differences in herbivory among study plots (7%) were found to strongly increase the flux of nutrients to the soil in tropical forests (adding up to more than 50% of ecosystem N inputs and 260% of P inputs; Metcalfe et al. 2014; see also Belovsky & Slade 2000). Such effects may be of particular importance for nutrient-limited ecosystems such as subtropical and tropical forests (Metcalfe et al. 2014).
Interestingly, we observed the effects of tree species richness on herbivory at a very early stage of the experiment. In contrast, several studies in newly established plant communities documented a time-lag in the response of important ecosystem processes to differences in plant species richness or an increase in the strength of this response over time (see Cardinale et al. 2012; Eisenhauer, Reich & Scheu 2012). Some of these effects were attributed to a lag in the establishment of biotic interactions with higher trophic levels (Eisenhauer, Reich & Scheu 2012). For our study system, this indicates that key trophic interactions became established quickly, possibly due to the fact that our study was conducted in a much more biodiverse region – where trophic interactions are often assumed to have a greater impact (Schemske et al. 2009) – than most previous studies. Our results on herbivory and tree growth rates thus suggest that herbivory could play an important role in the regulation of ecosystem functions and the structural development of species-rich forests from the very start of secondary forest succession.
Non-random vs. Random Species Loss
The local abundance of tree species and traits related to the palatability of their leaves, such as SLA, were expected to strongly influence leaf damage levels. In a secondary forest close to our study site, for instance, local commonness and leaf dry matter content (which often scales negatively with SLA; Cornelissen et al. 2003) of the tree species increased herbivory levels (Schuldt et al. 2012), possibly due to increased apparency and the feeding preferences of generalist herbivores. We might thus have expected the greatest herbivore damage at low levels of species richness for the plots of the non-random extinction scenarios. However, there appeared to be no significant differences in herbivory and its relationship with tree species richness among the random and non-random extinction scenarios of our experiment. Neither was there a significant interaction between the effects of extinction scenario and herbivory on tree growth. However, the secondary forests in which the relationships between plant traits and herbivory were observed were much older in terms of successional age and more species-rich than our experimental set-up (Schuldt et al. 2012, 2014b). It is conceivable that herbivores which dominate in the very early stages of our experiment show feeding preferences that do not necessarily represent those of herbivores associated with later successional forest stages. Thus, effects of specific plant traits on herbivory observed at later stages might not be detectable in the initial stages of forest succession and only develop over time with changes in the tree and herbivore assemblages (and potentially also shifts in species-specific trait values, or, alternatively, depend on even higher levels of tree species richness) and their interactions (see also Vehviläinen, Koricheva & Ruohomaki 2007; Loranger et al. 2014). This may also be reflected by the change in the relationship between herbivory and tree growth with increasing tree size observed in our study. Considering the above-mentioned time-lag of biodiversity effects in newly established communities, this could mean that differences between random and non-random species loss on ecosystem processes such as herbivory can become stronger with time as well and, in our case, depend on forest age. However, continuous monitoring over longer time periods in an experimental context, and preferably including even higher levels of tree species richness, is required to evaluate this hypothesis.
Conclusions
Our results have important implications for our understanding of the processes that influence community assembly and interspecific competition of tree species in highly diverse regions, and they may inform reforestation projects that adopt an ecological perspective. Our findings highlight that the effects of herbivory as one of the potential drivers of plant community assembly (HilleRisLambers et al. 2012; Coley & Kursar 2014) can vary with tree species richness and tree size in the early stages of the community assembly process. These patterns, in turn, may potentially have repercussions on tree species richness (see e.g. Schuldt et al. 2014a) and herbivory might thus not only respond to, but actively influence assembly processes of tree communities.
Acknowledgments
We are indebted to Xuefei Yang, Chen Lin, Sabine Both, Keping Ma and all members of the BEF-China consortium that coordinated and helped with the establishment and maintenance of the experiment. We thank Daria Golub, Friederike Rorig and Rhea Struck for their commitment in the herbivory surveys. Comments of two anonymous referees helped to improve the manuscript. We gratefully acknowledge funding by the German Research Foundation (DFG FOR 891/1 and 891/2) and the Sino-German Centre for Research Promotion in Beijing (GZ 524, 592, 698, 699, 785, 970 and 1020).
Data accessibility
Data on plot characteristics are publicly available on the BEF-China data portal at http://china.befdata.biow.uni-leipzig.de/datasets/46 and http://china.befdata.biow.uni-leipzig.de/datasets/71. Data on herbivory and tree growth will be publicly available 12 months after publication on the BEF-China data portal at http://china.befdata.biow.uni-leipzig.de/datasets/478.
Supporting Information
Figure S1. Leaf damage per species in monocultures.
Figure S2. Relationship between herbivory of the single study species and tree species richness.
Figure S3. Initial tree height per species.
References
- Bagchi R, Gallery RE, Gripenberg S, Gurr SJ, Narayan L, Addis CE, Freckleton RP. Lewis OT. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature. 2014;506:85–88. doi: 10.1038/nature12911. [DOI] [PubMed] [Google Scholar]
- Bala G, Caldeira K, Wickett M, Phillips TJ, Lobell DB, Delire C. Mirin A. Combined climate and carbon-cycle effects of large-scale deforestation. Proceedings of the National Academy of Sciences of the USA. 2007;104:6550–6555. doi: 10.1073/pnas.0608998104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DM, Maechler M, Bolker B. Walker S. lme4: Linear Mixed-Effects Models using Eigen and S4. 2014. R package version 1.1-6. available at http://CRAN.R-project.org/package=lme4. Last accessed 30 June 2014. [Google Scholar]
- Belovsky G. Slade J. Insect herbivory accelerates nutrient cycling and increases plant production. Proceedings of the National Academy of Sciences of the USA. 2000;97:14412–14417. doi: 10.1073/pnas.250483797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernays EA. Chapman RF. Plant secondary compounds and grasshoppers: beyond plant defenses. Journal of Chemical Ecology. 2000;26:1773–1794. [Google Scholar]
- Bernays EA, Bright KL, Gonzalez N. Angel J. Dietary mixing in a generalist herbivore: tests of two hypotheses. Ecology. 1994;75:1997–2006. [Google Scholar]
- Blundell AG. Peart DR. Growth strategies of a shade-tolerant tropical tree: the interactive effects of canopy gaps and simulated herbivory. Journal of Ecology. 2001;89:608–615. [Google Scholar]
- Boege K, Barton KE. Dirzo R. Influence of tree ontogeny on plant-herbivore interactions. In: Dawson TE, editor; Meinzer FC, Lachenbruch B, editors. Size-and Age-Related Changes in Tree Structure and Function. Dordrecht: Springer; 2011. pp. 193–214. [Google Scholar]
- Brown VK. Insect herbivores and plant succession. Oikos. 1985;44:17–22. [Google Scholar]
- Bruelheide H, Nadrowski K, Assmann T, Bauhus J, Both S, Buscot F, et al. Designing forest biodiversity experiments: general considerations illustrated by a new large experiment in subtropical China. Methods in Ecology and Evolution. 2014;5:74–89. [Google Scholar]
- Burnham KP. Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer; 2004. [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- Castagneyrol B, Giffard B, Péré C. Jactel H. Plant apparency, an overlooked driver of associational resistance to herbivory. Journal of Ecology. 2013;101:418–429. [Google Scholar]
- Coley PD. Kursar TA. On tropical forests and their pests. Science. 2014;343:35–36. doi: 10.1126/science.1248110. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Crawley MJ. The R Book. Chichester: Wiley; 2007. [Google Scholar]
- Dinnage R. Phylogenetic diversity of plants alters the effect of species richness on invertebrate herbivory. PeerJ. 2013;1:e93. doi: 10.7717/peerj.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling A, Pompe S, Baade J, Eisenhauer N, Hillebrand H, Proulx R, Roscher C, Schmid B, Wirth C. Weisser WW. Plant diversity impacts decomposition and herbivory via changes in aboveground arthropods. PLoS ONE. 2014;9:e106529. doi: 10.1371/journal.pone.0106529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer N, Reich PB. Scheu S. Increasing plant diversity effects on productivity with time due to delayed soil biota effects on plants. Basic and Applied Ecology. 2012;13:571–578. [Google Scholar]
- HilleRisLambers J, Adler P, Harpole W, Levine J. Mayfield M. Rethinking community assembly through the lens of coexistence theory. Annual Review of Ecology, Evolution, and Systematics. 2012;43:227. [Google Scholar]
- Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L. O’Connor MI. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486:105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- Jactel H. Brockerhoff EG. Tree diversity reduces herbivory by forest insects. Ecology Letters. 2007;10:835–848. doi: 10.1111/j.1461-0248.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- Kremen C, Niles JO, Dalton MG, Daily GC, Ehrlich PR, Fay JP, Grewal D. Guillery RP. Economic incentives for rain forest conservation across scales. Science. 2000;288:1828–1832. doi: 10.1126/science.288.5472.1828. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB. Christensen RHB. lmerTest: Tests for Random and Fixed Effects for Linear Mixed Effect Models (lmer Objects of lme4 Package) 2014. R package version 2.0-6. available at http://CRAN.R-project.org/package=lmerTest. Last accessed 30 June 2014. [Google Scholar]
- Lefcheck JS, Whalen MA, Davenport TM, Stone JP. Duffy JE. Physiological effects of diet mixing on consumer fitness: a meta-analysis. Ecology. 2013;94:565–572. doi: 10.1890/12-0192.1. [DOI] [PubMed] [Google Scholar]
- Loranger J, Meyer ST, Shipley B, Kattge J, Loranger H, Roscher C, Wirth C. Weisser WW. Predicting invertebrate herbivory from plant traits: polycultures show strong nonadditive effects. Ecology. 2013;94:1499–1509. doi: 10.1890/12-2063.1. [DOI] [PubMed] [Google Scholar]
- Loranger H, Weisser W, Ebeling A, Eggers T, De Luca E, Loranger J, Roscher C. Meyer S. Invertebrate herbivory increases along an experimental gradient of grassland plant diversity. Oecologia. 2014;174:183–193. doi: 10.1007/s00442-013-2741-5. [DOI] [PubMed] [Google Scholar]
- Lusk CH, Onoda Y, Kooyman R. Gutiérrez-Girón A. Reconciling species-level vs plastic responses of evergreen leaf structure to light gradients: shade leaves punch above their weight. New Phytologist. 2010;186:429–438. doi: 10.1111/j.1469-8137.2010.03202.x. [DOI] [PubMed] [Google Scholar]
- Massad TJ, Chambers JQ, Rolim SG, Jesus RM. Dyer LA. Restoration of pasture to forest in Brazil’s Mata Atlântica: the roles of herbivory, seedling defenses, and plot design in reforestation. Restoration Ecology. 2011;19:257–267. [Google Scholar]
- Metcalfe DB, Asner GP, Martin RE, Silva Espejo JE, Huasco WH, Farfán Amézquita FF, et al. Herbivory makes major contributions to ecosystem carbon and nutrient cycling in tropical forests. Ecology Letters. 2014;17:324–332. doi: 10.1111/ele.12233. [DOI] [PubMed] [Google Scholar]
- Mulder CPH, Koricheva J, Huss-Danell K, Hogberg P. Joshi J. Insects affect relationships between plant species richness and ecosystem processes. Ecology Letters. 1999;2:237–246. [Google Scholar]
- Naeem S, Duffy JE. Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336:1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- Ness JH, Rollinson EJ. Whitney KD. Phylogenetic distance can predict susceptibility to attack by natural enemies. Oikos. 2011;120:1327–1334. [Google Scholar]
- Novotny V. Basset Y. Host specificity of insect herbivores in tropical forests. Proceedings of the Royal Society B-Biological Sciences. 2005;272:1083–1090. doi: 10.1098/rspb.2004.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine C, Marthews TR, Vogt DR, Purves D, Rees M, Hector A. Turnbull LA. How to fit nonlinear plant growth models and calculate growth rates: an update for ecologists. Methods in Ecology and Evolution. 2012;3:245–256. [Google Scholar]
- Pfisterer AB, Diemer M. Schmid B. Dietary shift and lowered biomass gain of a generalist herbivore in species-poor experimental plant communities. Oecologia. 2003;135:234–241. doi: 10.1007/s00442-002-1169-0. [DOI] [PubMed] [Google Scholar]
- Plath M, Mody K, Potvin C. Dorn S. Establishment of native tropical timber trees in monoculture and mixed-species plantations: small-scale effects on tree performance and insect herbivory. Forest Ecology and Management. 2011;261:741–750. [Google Scholar]
- Rasmann S, Pellissier L, Defossez E, Jactel H. Kunstler G. Climate-driven change in plant–insect interactions along elevation gradients. Functional Ecology. 2014;28:46–54. [Google Scholar]
- Riedel J, Dorn S, Plath M, Potvin C. Mody K. Time matters: temporally changing effects of planting schemes and insecticide treatment on native timber tree performance on former pasture. Forest Ecology and Management. 2013;297:49–56. [Google Scholar]
- Rodriguez-Castaneda G. The world and its shades of green: a meta-analysis on trophic cascades across temperature and precipitation gradients. Global Ecology and Biogeography. 2013;22:118–130. [Google Scholar]
- Root RB. Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea. Ecological Monographs. 1973;43:95–124. [Google Scholar]
- Salgado-Luarte C. Gianoli E. Herbivores modify selection of plant functional traits in a temperate rainforest understory. American Naturalist. 2012;180:E42–E53. doi: 10.1086/666612. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Mittelbach GG, Cornell HV, Sobel JM. Roy K. Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology Evolution and Systematics. 2009;40:245–269. [Google Scholar]
- Scherber C, Eisenhauer N, Weisser WW, Schmid B, Voigt W, Fischer M, et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature. 2010;468:553–556. doi: 10.1038/nature09492. [DOI] [PubMed] [Google Scholar]
- Scherer-Lorenzen M. The functional role of biodiversity in the context of global change. In: Simonson W, editor; Burslem D, Coomes D, editors. Forests and Global Change. Cambridge: Cambridge University Press; 2014. pp. 195–238. [Google Scholar]
- Schowalter TD. Insect herbivore effects on forest ecosystem services. Journal of Sustainable Forestry. 2012;31:518–536. [Google Scholar]
- Schuldt A, Baruffol M, Böhnke M, Bruelheide H, Härdtle W, Lang AC, Nadrowski K, von Oheimb G, Voigt W, Zhou H. Assmann T. Tree diversity promotes insect herbivory in subtropical forests of south-east China. Journal of Ecology. 2010;98:917–926. doi: 10.1111/j.1365-2745.2010.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt A, Bruelheide H, Durka W, Eichenberg D, Fischer M, Kröber W, et al. Plant traits affecting herbivory on tree recruits in highly diverse subtropical forests. Ecology Letters. 2012;15:732–739. doi: 10.1111/j.1461-0248.2012.01792.x. [DOI] [PubMed] [Google Scholar]
- Schuldt A, Assmann T, Bruelheide H, Durka W, Eichenberg D, Härdtle W, Kröber W, Michalski SG. Purschke O. Functional and phylogenetic diversity of woody plants drive herbivory in a highly diverse forest. New Phytologist. 2014a;202:864–873. doi: 10.1111/nph.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt A, Baruffol M, Bruelheide H, Chen S, Chi X, Wall M. Assmann T. Woody plant phylogenetic diversity mediates bottom-up control of arthropod biomass in species-rich forests. Oecologia. 2014b;176:171–182. doi: 10.1007/s00442-014-3006-7. [DOI] [PubMed] [Google Scholar]
- Siemann E, Haarstad J. Tilman D. Dynamics of plant and arthropod diversity during old field succession. Ecography. 1999;22:406–414. [Google Scholar]
- Sobek S, Gossner MM, Scherber C, Steffan-Dewenter I. Tscharntke T. Tree diversity drives abundance and spatiotemporal beta-diversity of true bugs (Heteroptera) Ecological Entomology. 2009;34:772–782. [Google Scholar]
- Srivastava DS. Vellend M. Biodiversity-ecosystem function research: is it relevant to conservation? Annual Review of Ecology Evolution and Systematics. 2005;36:267–294. [Google Scholar]
- Stout MJ, Thaler JS. Thomma B. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annual Review of Entomology. 2006;51:663–689. doi: 10.1146/annurev.ento.51.110104.151117. [DOI] [PubMed] [Google Scholar]
- Vehviläinen H, Koricheva J. Ruohomaki K. Tree species diversity influences herbivore abundance and damage: meta-analysis of long-term forest experiments. Oecologia. 2007;152:287–298. doi: 10.1007/s00442-007-0673-7. [DOI] [PubMed] [Google Scholar]
- Yang X, Bauhus J, Both S, Fang T, Härdtle W, Kröber W, et al. Establishment success in a forest biodiversity and ecosystem functioning experiment in subtropical China (BEF-China) European Journal of Forest Research. 2013;132:593–606. [Google Scholar]
- Zuur AF, Ieno EN. Smith GM. Analysing Ecological Data. New York: Springer; 2007. [Google Scholar]
- Zvereva EL, Zverev V. Kozlov MV. Little strokes fell great oaks: minor but chronic herbivory substantially reduces birch growth. Oikos. 2012;121:2036–2043. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Leaf damage per species in monocultures.
Figure S2. Relationship between herbivory of the single study species and tree species richness.
Figure S3. Initial tree height per species.
Data Availability Statement
Data on plot characteristics are publicly available on the BEF-China data portal at http://china.befdata.biow.uni-leipzig.de/datasets/46 and http://china.befdata.biow.uni-leipzig.de/datasets/71. Data on herbivory and tree growth will be publicly available 12 months after publication on the BEF-China data portal at http://china.befdata.biow.uni-leipzig.de/datasets/478.


