Abstract
Background
There is no consensus on whether mitral valve repair or replacement (MVRR) must be performed to treat ischemic mitral regurgitation (MVR) after myocardial infarction. Our objective in this study was to investigate the efficacy of coronary artery bypass grafting (CABG) combined with or without MVRR for the ischemic MVR.
Material/Methods
An article search was performed in OvidSP, PubMed, Cochrane Library, and Embase. In these articles, researchers compared the efficacy of CABG with or without MVRR in treating patients with ischemic MVR after acute coronary syndrome (ACS). We performed a meta-analysis to compare the differences in the short-term and long-term survival rates of patients treated with CABG only and those treated with both CABG and MVRR. Secondary outcomes were compared with the preoperative and postoperative degree of MVR, left ventricular end-systolic volume (LVESV), left ventricular ejection fraction (LVEF), and New York Heart Association (NYHA) class.
Results
Out of the 1183 studies, we selected only 5 articles. A total of 3120 patients were enrolled; the CABG and MVRR group included 575 patients, while the CABG only group included 2545 patients. Long-term survival was higher in the CABG only group (hazard ratio [HR], 1.34; 95% confidence interval [CI] 1.15–1.58, P=0.003). Hospital mortality was similar in both the groups (odds ratio [OR], 2.54; 95% CI, 0.65–9.95; P=0.18). No differences were found in the degree of residual MVR, the mean of LVESV, LVEF, or NYHA class.
Conclusions
In patients with ischemic MVR, the short-term survival rate was similar in both groups. Moreover, there was no significant improvement in the long-term survival rates of patients treated with both CAG and MVRR.
MeSH Keywords: General Surgery, Meta-Analysis, Survival
Background
With the current understanding of the mechanism of coronary artery disease, acute ST segment elevation myocardial infarction, non-ST segment elevation myocardial infarction, and unstable angina can be classified as acute coronary syndrome (ACS). Acute myocardial infarction (AMI) is one of the most common cardiovascular diseases, having the highest morbidity and mortality in the world [1–3]. In the year 2010, more than 6 million patients were hospitalized for treatment of AMI in the United States [4]. Owing to China’s aging population, the incidence rate of this disease has increased drastically. According to official estimates, more than 16 million people annually will succumb to AMI by 2020. Moreover, this number is estimated to rise to 23 million annually by 2030 [5]. Patients suffer AMI due to myocardial necrosis, an ischemic injury that leads to the deformation of mitral annulus. Consequently, the function of papillary muscles becomes impaired. In some cases, the papillary muscles may even rupture. All these adverse events lead to ischemic mitral valve regurgitation (MVR). Ischemic MVR persists in 20% to 30% of patients, even after an AMI [6]. The effects of mild ischemic MVR include left ventricular remodeling and left ventricular enlargement. In severe cases, it can lead to left ventricular dysfunction and left ventricular failure, which is a life-threatening condition. When ischemic MVR is severe, the survival rate is just 1 year in more than 60% cases [7].
Coronary artery bypass grafting (CABG) and mitral valve repair or replacement (MVRR) surgery are carried out on patients who develop significant ischemic MVR after myocardial infarction. These surgical treatments can remove coronary artery blockages and MVR simultaneously, helping restore normal left ventricle geometry. According to current guidelines, surgical intervention is the conventional method for treating ischemic MVR with an ejection fraction (EF) of 30% or greater (class I recommendation); a class II b recommendation is when the EF is 30% or less [8]. However, several studies have reported that the survival rate is not significantly different when patients with ischemic MVR are treated with MVRR and CABG [9–14]. In other words, medical researchers are still dubious that MVRR surgery is suitable for treating patients who develop ischemic MVR after myocardial infarction. In this study, we pooled results of recent studies and performed a meta-analysis to investigate the efficacy and patient prognosis of CABG combined with or without MVRR.
Material and Methods
Data source
A comprehensive search was performed in OvidSP, PubMed, Cochrane Library, and Embase to access medical literature from January 1986 to March 2015. We identified and reviewed all the studies that described the trials comparing the efficacy of only CABG with the combined treatment of CABG and MVRR, which were used to treat patients with moderate to severe ischemic MVR (>2+ grade) after ACS. We reviewed both retrospective observational and prospective randomized studies. In PubMed, search terms were presented in text string format as follows: ischemic mitral valve regurgitation or ischemic mitral valve insufficiency) and (mitral valve repair or mitral valve annuloplasty or mitral valve replacement) and (coronary bypass grafting or surgical revascularization) and (acute myocardial infarction or acute coronary syndrome or ACS).
We performed the meta-analysis to compare the differences in the short-term (mean hospital mortality rate) and long-term survival rates of patients treated with CABG only and those treated with both CABG and MVRR. Secondary outcomes were compared with the preoperative and postoperative degree of MVR, left ventricular end-systolic volume (LVESV), left ventricular ejection fraction (LVEF), and New York Heart Association (NYHA) class.
Exclusion criteria
The criteria for excluding the articles were as follows: (1) no direct comparison between only CABG and the combined technique of CABG and MVRR; (2) etiologies for MVR were myxomatous, rheumatic, infectious, congenital, or degenerative; (3) mitral valve regurgitation was accompanied by mitral valve prolapse, tendon rupture, or papillary muscle rupture; (4) no survival curves or hazard ratios; (5) articles were not written in English.
Data quality evaluation
The quality of each included study was evaluated by 2 researchers who cross-checked the extracted data from the selected case control study or randomized controlled trials. If they had differences of opinion, then they sought the opinion of a third researcher.
Statistical analysis
Baseline characteristics were described using proportions of discrete variables, while medians and standard deviation were calculated for continuous variables. To compare discrete variables of different groups, the Pearson χ2 test was performed. To compare continuous variables of different groups, the Kruskal-Wallis test was conducted. In each individual study, the odds ratio was calculated for short-term survival, while hazard ratios were calculated for determining long-term survival. In each study, the survival curves compared the efficacy of only CABG with the combined technique of CABG and MVRR. These survival curves were checked and evaluated for time intervals of 2, 3, or 6 months depending on their mean follow-up years.
The heterogeneity of patients and treatment procedures were incorporated in the random-effects model, including the study of statistical data and the 95% confidence intervals (CIs). The heterogeneity of patients was examined using Cochran’s Q test and the I2 statistic. The degree of heterogeneity was divided into 3 grades: low (<25%), moderate (25%–75%), and high (>75%) [15]. All the values were considered to be statistically significant when P<0.05. Funnel plots were examined to evaluate potential publication bias. The results of the meta-analysis were represented in forest plots. Publication bias was depicted in funnel plots. Meta-analysis was conducted using Review Manager, version 5.3 (The Cochrane Collaboration, Update Software, Oxford).
Results
In total, we reviewed 1183 studies, including 1159 studies in OvidSP and 24 studies in PubMed; however, we could not find any related studies in Embase or Cochrane Library. We excluded 224 studies because they were repetitive articles. Moreover, we excluded over 499 articles that had no full texts. While perusing through title, abstract, and full text of manuscripts, we found that a cohort of 5 studies met the inclusion criteria [9,11,16–18]. Among them, only 1 study [17] was a prospective randomized trial, while the other studies were retrospective observational trials (Figure 1).
Figure 1.
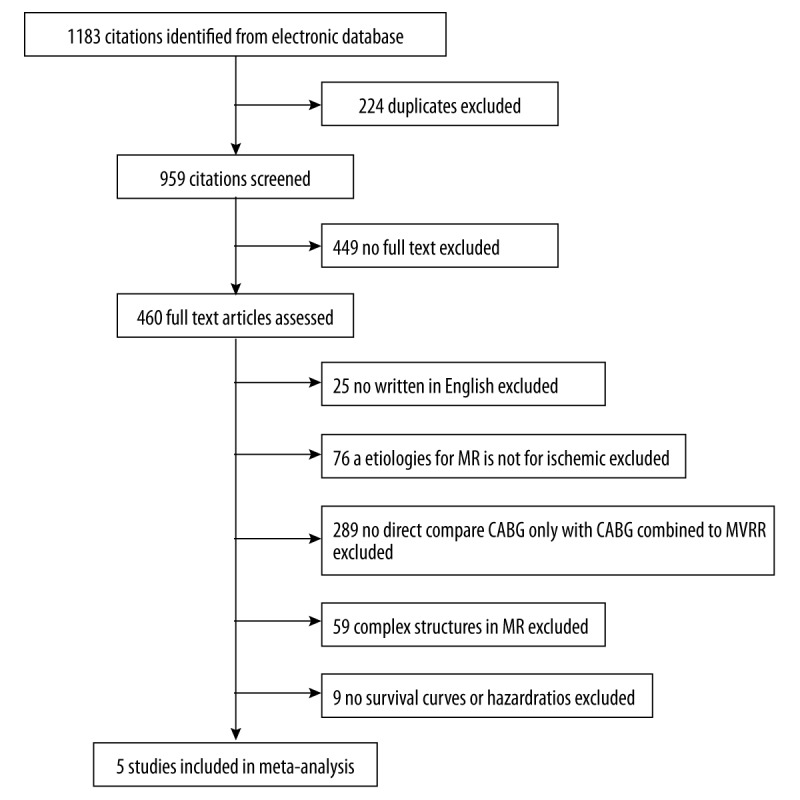
Flowchart depicting study selection for meta-analysis.
Demographic characteristics
A total of 3120 patients were included in all the studies that met our inclusion criteria. Among them, 2545 (82%) patients were treated with only CABG, while 575 (18%) patients underwent both CABG and MVRR. Table 1 presents the basic clinical characteristics of patients included in the analysis. The average age of the study population was 67 years; the patients were in the age group of 61 to 70 years. The mean percentage of male patients was 61%. In the analyzed studies, the proportion of male patients varied from 26% to 81%. An average of 35% patients also had diabetes. The study presented by Kang [4] had no published data on the number of patients with hypertension. However, in other studies more than half of the included patients also suffered from hypertension. The mean number of graft vessels was 3.3. An average of 65% patients had a history of myocardial infarction.
Table 1.
Clinical characteristics of patients included in the analysis.
| Study | Time of study | Mean follow-up | Patients | Mean age (SD) | Male (%) | HBP (%) | Diabetes (%) | Graft vessels (SD, n) | Previous of MI (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | |||
| Castleberry et al. [16] | 1990–2009 | 5.4 years | 1651 | 243 | 66 | 66 | 1074 (65) | 132 (54)* | 1133 (68) | 149 (61) | 590 (35) | 84 (34)* | NS | NS | NS | NS |
| Chan et al. [17] | 2007–2011 | 1 year | 38 | 38 | 70 (8) | 70 (10) | 10 (26) | 9(26) | 23 (59) | 17 (50) | 15 (38) | 12 (35) | 3.1 | 2.7 | 28 (72) | 25 (74) |
| Kang et al. [11] | 1997–2003 | 3 years | 57 | 50 | 63 (9) | 61 (10) | 39 (68) | 37 (74) | NS | NS | 32 (56) | 26 (52) | 3.8 (1.5) | 3.7 (1.5) | NS | NS |
| Trichon et al. [9] | 1986–2001 | 5 years | 687 | 228 | 68 | 68 | 364 (53) | 120 (53)* | 448 (65) | 140 (61) | 230 (34) | 63 (28)* | NS | NS | 474 (69) | 110 (48)* |
| Gangemi et al. [18] | 1993–1998 | 1 years | 121 | 16 | 64 (1) | 65 (2) | 98 (81) | 10 (63) | 78 (64) | 9 (56) | 45 (37) | 6 (38) | 3.3 (0.1) | 2.6 (0.2) | 94 (78) | 6 (38)* |
CABG – indicates coronary artery bypass graftion; MVRR – indicates mitral valve repair of replacement; HBP – indicates hypertension; MI – indicates myocardial infarction; NS – indicates no significant;
indicates P<0.05.
Preoperative and postoperative conditions are presented in Table 2. The degree of MVR was more than 2+ in all the patients that were included preoperatively. After performing revascularization surgery, the degree of residual MVR in patients treated with only CABG was less than 1+, but the degree of MVR was lower in patients treated with both CABG and MVRR (0.9±0.6 vs. 0.6±0.6). Nevertheless, there was no significant difference between the 2 groups [11]. CABG combined with MVRR was better than only CABG in reducing the mean LVESV and NYHA classes [11,17], but only 1 study indicated that there were significant differences between the 2 surgical procedures [17]. In the improvement of the mean LVEF, the results of the 2 surgical procedures were similar [11], except for 1 study showing no improvement in CABG combined with MVRR [18].
Table 2.
Operative results of patients included in the analysis, (A) represents studies conducted on patients preoperatively; (B) represents studies conducted on patients postoperatively.
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Mean MR grade (SD) | Mean LVESV (SD, ml) | Mean LVEF (SD, %) | Mean NYHA class (SD) | ||||
| CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | |
| Castleberry et al. | 2.2 | 3.5 | NS | NS | 48 | 45* | 0.6 | 3.5 |
| Chan et al. | NS | NS | 72 (16) | 78 (27) | 40 (16) | 40 (17) | 2.3 | 2.3 |
| Kang et al. | 2.5 (0.5) | 2.8 (0.4) | 84 (34) | 92 (41) | 36 (9) | 36 (11) | 3.1 (0.9) | 3.1 (0.8) |
| Trichon et al. | 2 | 2.4 | NS | NS | 42 | 45* | NS | NS |
| Gangemi et al. | NS | NS | NS | NS | 26 (0.4) | 23 (1.1)* | NS | NS |
| B | ||||||||
| Sudy | Mean MR grade (SD) | Mean LVESV (SD, ml) | Mean LVEF (SD, %) | Mean NYHA class (SD) | ||||
| CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | CABG | CABG + MVRR | |
| Castleberry et al. | NS | NS | NS | NS | NS | NS | NS | NS |
| Chan et al. | NS | NS | 67 (20) | 56 (15)* | NS | NS | 1.9 | 1.3* |
| Kang et al. | 0.9 (0.6) | 0.6 (0.6) | 58 (25) | 57 (25) | 47 (9) | 47 (9) | 1.5 (0.7) | 1.5 (0.6) |
| Trichon et al. | NS | NS | NS | NS | NS | NS | NS | NS |
| Gangemi et al. | NS | NS | NS | NS | 37 (0.4) | 23 (1.1)* | NS | NS |
CABG – indicates coronary artery bypass graft; MVRR – indicates mitral valve repair or replacement; LVEF – indicates left ventricular ejection fraction; LVESV – indicates left ventricular end systolic volume; MR – indicates mitral valve regurgitation; NS – indicates no significance;
indicates P<0.05.
Short-term survival
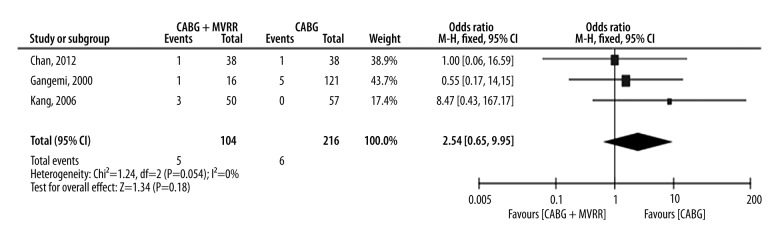
Short-term survival was defined as patients who died within 30 days despite being admitted in a hospital. In 3 studies, a total of 320 patients had hospital mortality [11,17,18]. The total hospital mortality rate in patients treated with both CABG and MVRR techniques was 4.8% (5/104), which was higher than the 2.8% hospital mortality rate of patients treated with only CABG (6/216). However, there was no significant difference in the hospital mortality rates of these patients (P=0.358). Odds ratio (OR) range varied from 1.00 (no favoring) [17] to 8.47 (favoring CABG only) [11]. We also assessed the heterogeneity of patients included in the studies (chi2=1.24, P=0.54, and I2=0%). This indicates that the heterogeneity was null. The pooled OR was 2.54 (95% CI, 0.65–9.95; P=0.18) (Figure 2), indicating that the hospital mortality in patients treated with both CABG and MVRR techniques was similar to that witnessed in patients treated with only CABG. Through the short-term funnel plots, we deduced that there was no publication bias in the included studies (Figure 3).
Figure 2.
Short-term survival forest plots. (CI – confidence interval; DF – degrees of freedom; M-H – Mantel-Haenszel).
Figure 3.
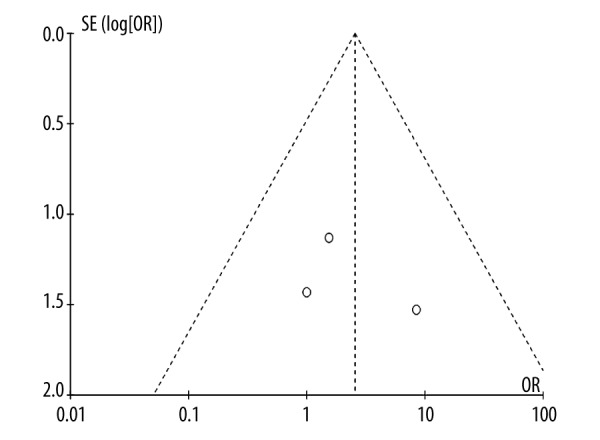
Short-term survival funnel plots.
Long-term survival
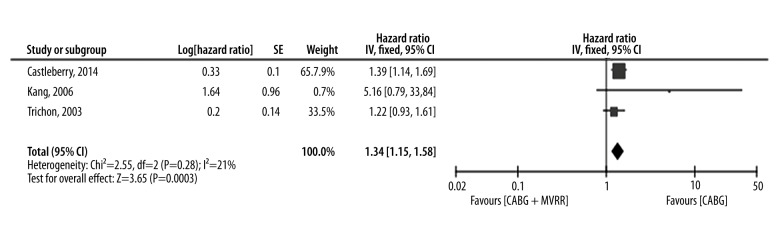
Three studies provided important information of survival curves [9,11,16]. The shortest observation period of these studies was approximately 5 years [9], while the longest observation period was over 10 years [16]. Because these studies were not randomized controlled trials, the chosen survival curves were adjusted before analyzing. The study hazard ratios varied from 1.22 [9] to 5.16 [11] (Figure 4). While assessing potential heterogeneity, (Chi2=2.55 and P=0.28) we found that there was no significant heterogeneity in patients included in various studies. Furthermore, I2=21%, so the variability between these studies was due to low heterogeneity. In summary, the hazard ratio was 1.34 (95% CI 1.15–1.58), P=0.003. The pooled study population showed that compared to the CABG group, long-term survival was more pronounced in the group treated with both CABG and MVRR techniques. Moreover, there was no publication bias in the included articles (Figure 5).
Figure 4.
Long-term survival forest plots. (CI – confidence interval; DF – degrees of freedom; SE – standard error; IV – inverse variance).
Figure 5.
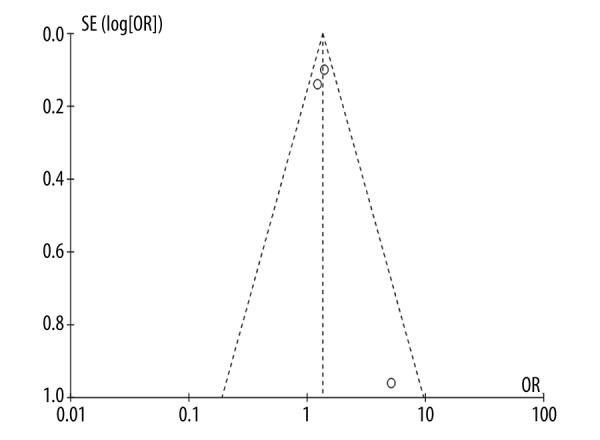
Short-term survival funnel plots.
Discussion
Ischemic MVR is one of the common complications of coronary heart disease. This complication is more pronounced in patients who suffer AMI: the incidence rate of ischemic MVR is up to 13–50% in patients who have suffered AMI [6,19]. In most patients with mild MVR, the symptoms are not very obvious. However, in patients with moderate to severe MVR, the typical symptoms include palpitation, angina, heart failure, or even death [20]. Therefore, many scholars dispute whether patients with ischemic MVR must be treated with concomitant mitral valve surgery.
Ischemic MVR leads to left ventricular volume overload, resulting in left ventricular remodeling that aggravates MVR [21,22]. Therefore, MVRR surgery can reduce left ventricular volume overload by reducing the mitral valve flow, which is also beneficial in left ventricular remodeling. We found that after the surgery, the degree of residual MVR in patients treated with both CABG and MVRR was lower than that in patients treated with only CABG. The combinatorial technique of CABG and MVRR was also better than that of only CABG, regardless of the mean of LVESV, LVEF, or NYHA classes. These results had been confirmed by most studies, and they were also suitable for patients with end-stage cardiomyopathy [23,24]. Except for the Gangemi [18] study, no improvement was reported in the heart function of patients treated with MVRR surgery, perhaps because preoperative left ventricular function had been overestimated in these studies.
Several studies reported that the prognosis of patients improved when they were treated with both CABG and MVRR surgeries. In a case-controlled study, the long-term survival rates in 58 patients with moderate MVR were similar to the 58 case-matched patients without MVR. These 58 patients with moderate MVR were treated with only CABG [25]. Several studies, including a multicenter randomized trial by Deja et al., have reported that MV repair can significantly improve the survival rate of patients as compared to only CABG [26–32]. The risk associated with surgical and postoperative mortality does not increase in patients treated with combinatorial techniques of CABG and MVRR surgeries, although the operation time increases as compared to patients treated with only CABG. However, in guiding clinical management of ischemic MVR, the results of our meta-analyses have some limitations: most studies were carried out on a small number of patients, so the sample size is small in most studies [27,30]. Furthermore, outdated studies do not include current ischemic MVR assessment techniques adequately, increasing perioperative surgical risk [26]. The comparison of different groups could not fully summarize the total range of treatment modalities, including CABG with or without MVRR [26–30].
According to our meta-analysis, compared to patients treated with only CABG, long-term survival rates were lower in patients treated with both CABG and MVRR. Several studies, including a 2009 meta-analysis, have reported that there is no enhancement in the survival rates of patients with ischemic MVR when they are treated with both CABG and MVRR [9–14]. Ischemic MVR occurs because of left ventricular (LV) remodeling and dilatation after myocardial infarction, which tethers and pulls the mitral valve apart; the mitral valve is normal in structure but is incompetent as a result of a dilated and dysfunctional left ventricle [33,34]. Therefore, ischemic MVR can be corrected with only CABG; this surgical intervention can successfully restore coronary flow. Subsequently, there is improvement in coronary wall motion and LV geometry. Thus, we can eliminate the additional operative mortality associated with MVRR [35]. Therefore, the survival rate of patients treated with only CABG might be higher than those treated by both CABG and MVRR techniques.
Limitations
There are several limitations to our study. The search method was very limited and failed to include all the published literature, including published papers of organizations and conferences. We only reviewed manuscripts that were published in English, so papers published in other languages were not included. Therefore, we may have missed out some important findings of research studies published in other languages.
The keyword search included ischemic mitral valve regurgitation, functional mitral valve regurgitation, ischemic mitral valve insufficiency and mitral valve repair, mitral valve annuloplasty, mitral valve replacement or MVS, and coronary bypass grafting or surgical revascularization. Although these keywords were consistent with other studies [36–38], we also included acute myocardial infarction or acute coronary syndrome. Therefore, our selected literature may not be same as the meta-analysis of 2009 [13]. Thus, different studies were included and reviewed in our meta-analysis.
Most of the studies that we reviewed were not randomized controlled trials. Most patients with serious MVR were treated with both CABG and MVRR techniques, so there could be bias in selected population.
Myocardial viability also plays an important role in the surgical management of ischemic MVR. After performing the CABG technique, the dysfunctional yet viable myocardium undergoes significant recovery. This improves the functionality of ischemic MVR. We did not perform routine tests to determine the myocardial viability of patients. In the near future, studies must try to determine the relationship between viability and ischemic MVR.
Conclusions
The patients with ACS complicated with ischemic MVR can achieve reduced mitral regurgitation and improved left ventricular function through either only CABG or both CABG and MVRR surgery. There is still no evidence that the long-term survival rate of CABG combined with MVRR is superior to that of only CABG, while the hospital mortality and secondary outcomes were similar in both. We expect that the new technology of mitral valve surgery can bring about some changes. The optimal surgery plan still needs to be adjusted according to the individual situation of the patient.
Acknowledgments
We thank Prof. Yili Liu for providing constructive criticism that helped us in improving this manuscript.
Footnotes
Source of support: Departmental sources
Conflicts of interest and source of funding
None.
References
- 1.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2.Sodha NR, Clements RT, Feng J, et al. Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. J Thorac Cardiovasc Surg. 2009;138:977–84. doi: 10.1016/j.jtcvs.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–9. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HCUPnet. Healthcare cost and Utilization Project (HCUP) Rochville, MD: Agency for Healthcare Research and Quality; 2010. [Accessed July 15, 2012]. Available from: URL: http://www.hcupnet.ahrq.gov/ [PubMed] [Google Scholar]
- 5.Moran A, Gu D, Zhao D, et al. Future cardiovascular disease in china: markov model and risk factor scenario projections from the coronary heart disease policy model-china. Circ Cardiovasc Qual Outcomes. 2010;3:243–52. doi: 10.1161/CIRCOUTCOMES.109.910711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 7.Hickey MS, Smith LR, Muhlbaier LH, et al. Current prognosis of ischemic mitral regurgitation. Implications for future management. Circulation. 1988;78(3 Pt 2):I51–59. [PubMed] [Google Scholar]
- 8.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Trichon BH, Glower DD, Shaw LK, et al. Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation. 2003;108(Suppl 1):Ii103–10. doi: 10.1161/01.cir.0000087656.10829.df. [DOI] [PubMed] [Google Scholar]
- 10.Diodato MD, Moon MVR, Pasque MK, et al. Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: a propensity analysis. Ann Thorac Surg. 2004;78:794–99. doi: 10.1016/j.athoracsur.2004.03.022. discussion 794–99. [DOI] [PubMed] [Google Scholar]
- 11.Kim YH, Czer LS, Soukiasian HJ, et al. Ischemic mitral regurgitation: revascularization alone versus revascularization and mitral valve repair. Ann Thorac Surg. 2005;79:1895–901. doi: 10.1016/j.athoracsur.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Kim MJ, Kang SJ, et al. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation. 2006;114:I499–503. doi: 10.1161/CIRCULATIONAHA.105.000398. [DOI] [PubMed] [Google Scholar]
- 13.Benedetto U, Melina G, Roscitano A, et al. Does combined mitral valve surgery improve survival when compared to revascularization alone in patients with ischemic mitral regurgitation? A meta-analysis on 2479 patients. J Cardiovasc Med (Hagerstown) 2009;10:109–14. doi: 10.2459/JCM.0b013e32831c84b0. [DOI] [PubMed] [Google Scholar]
- 14.Goland S, Czer LS, Siegel RJ, et al. Coronary revascularization alone or with mitral valve repair: outcomes in patients with moderate ischemic mitral regurgitation. Tex Heart Inst J. 2009;36:416–24. [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castleberry AW, Williams JB, Daneshmand MA, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: a 20-year experience. Circulation. 2014;129:2547–56. doi: 10.1161/CIRCULATIONAHA.113.005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation. 2012;126:2502–10. doi: 10.1161/CIRCULATIONAHA.112.143818. [DOI] [PubMed] [Google Scholar]
- 18.Gangemi JJ, Tribble CG, Ross SD, et al. Does the additive risk of mitral valve repair in patients with ischemic cardiomyopathy prohibit surgical intervention? Ann Surg. 2000;231:710–14. doi: 10.1097/00000658-200005000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellizzon GG, Grines CL, Cox DA, et al. Importance of mitral regurgitation inpatients undergoing percutaneous coronary intervention for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. 2004;43:1368–74. doi: 10.1016/j.jacc.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 20.Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 21.Hung J, Papakostas L, Tahta SA, et al. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004;110:Ii85–90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 22.Guy TSt, Moainie SL, Gorman JH, III, et al. Prevention of ischemic mitral regurgitation does not influence the outcome of remodeling after posterolateral myocardial infarction. J Am Coll Cardiol. 2004;43:377–83. doi: 10.1016/j.jacc.2003.07.045. [DOI] [PubMed] [Google Scholar]
- 23.Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg. 1998;115:381–86. doi: 10.1016/S0022-5223(98)70282-X. discussion 387–88. [DOI] [PubMed] [Google Scholar]
- 24.Kay GL, Kay JH, Zubiate P, et al. Mitral valve repair for mitral regurgitation secondary to coronary artery disease. Circulation. 1986;74:I88–98. [PubMed] [Google Scholar]
- 25.Duarte IG, Shen Y, MacDonald MJ, et al. Treatment of moderate mitral regurgitation and coronary disease by coronary bypass alone: late results. Ann Thorac Surg. 1999;68:426–30. doi: 10.1016/s0003-4975(99)00516-0. [DOI] [PubMed] [Google Scholar]
- 26.Aklog L, Filsoufi F, Flores KQ, et al. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation. 2001;104:I68–75. doi: 10.1161/hc37t1.094706. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima S, Kobayashi J, Bando K, et al. Late outcomes after isolated coronary artery bypass grafting for ischemic mitral regurgitation. Jpn J Thorac Cardiovasc Surg. 2005;53:354–60. doi: 10.1007/s11748-005-0049-z. [DOI] [PubMed] [Google Scholar]
- 28.Milano CA, Daneshmand MA, Rankin JS, et al. Survival prognosis and surgical management of ischemic mitral regurgitation. Ann Thorac Surg. 2008;86:735–44. doi: 10.1016/j.athoracsur.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Grossi EA, Woo YJ, Patel N, et al. Outcomes of coronary artery bypass grafting and reduction annuloplasty for functional ischemic mitral regurgitation: a prospective multicenter study (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve) J Thorac Cardiovasc Surg. 2011;141:91–97. doi: 10.1016/j.jtcvs.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Sun BJ, Kim DH, et al. Percutaneous versus surgical revascularization in patients with ischemic mitral regurgitation. Circulation. 2011;124:S156–62. doi: 10.1161/CIRCULATIONAHA.110.011254. [DOI] [PubMed] [Google Scholar]
- 31.Deja MA, Grayburn PA, Sun B, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–48. doi: 10.1161/CIRCULATIONAHA.111.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bax JJ, Braun J, Somer ST, et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation. 2004;110(11 Suppl 1):II103–8. doi: 10.1161/01.CIR.0000138196.06772.4e. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe N, Ogasawara Y, Yamaura Y, et al. Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial infarction: a real-time 3-dimensional echocardiographic study. Circulation. 2005;112:I458–62. doi: 10.1161/CIRCULATIONAHA.104.524595. [DOI] [PubMed] [Google Scholar]
- 34.Agricola E, Oppizzi M, Maisano F, et al. Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr. 2004;5:326–34. doi: 10.1016/j.euje.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Arcidi JM, Jr, Hebeler RF, Craver JM, et al. Treatment of moderate mitral regurgitation and coronary disease by coronary bypass alone. J Thorac Cardiovasc Surg. 1988;95:951–59. [PubMed] [Google Scholar]
- 36.Bonacchi M, Prifti E, Maiani M, et al. Mitral valve surgery simultaneous to coronary revascularization in patients with end-stage ischemic cardiomyopathy. Heart Vessels. 2006;21:20–27. doi: 10.1007/s00380-005-0853-5. [DOI] [PubMed] [Google Scholar]
- 37.Buja P, Tarantini G, Del Bianco F, et al. Moderate-to-severe ischemic mitral regurgitation and multivessel coronary artery disease: Impact of different treatment on survival and rehospitalization. Int J Cardiol. 2006;111:26–33. doi: 10.1016/j.ijcard.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 38.Mihaljevic T, Lam BK, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–201. doi: 10.1016/j.jacc.2007.02.043. [DOI] [PubMed] [Google Scholar]