Abstract
Objective
To propose an anatomic classification for fetal nuchal lymphatic anomalies that will be clinically useful and to evaluate the classification’s value in predicting chromosomal abnormalities, pregnancy outcomes, other associated fetal anomalies, and spontaneous resolution of these lesions.
Study Design
Retrospective cohort study.
Setting
Tertiary academic hospital and affiliated tertiary children’s hospital.
Subjects and Methods
Mother-baby pairs diagnosed with fetal nuchal lymphatic anomalies in a prenatal ultrasound database. Anomalies were classified as nuchal thickening, dorsal lymphatic malformation, or ventral lymphatic malformation. Pregnancy outcomes, prevalence of chromosomal and anatomic abnormalities, and rates of spontaneous lesion resolution were determined for each group.
Results
The study included 189 patients: 58 with nuchal thickening, 120 with dorsal lymphatic malformation, and 11 with ventral lymphatic malformation. In fetuses for whom chromosomal analysis was available, chromosomal abnormalities were strongly associated with dorsal lymphatic malformations (83%), less associated with nuchal thickening (29%), and not associated with ventral lymphatic malformations. Dorsal lymphatic malformation predicted high rates of elective (43%) and spontaneous (20%) termination of pregnancy and showed the strongest association with cardiac, renal, and skeletal anomalies. Nuchal thickening was more likely to resolve in utero than dorsal lymphatic malformations, while no ventral lymphatic malformation resolved spontaneously.
Conclusions
Fetal nuchal anomalies demonstrate significant and clinically important prognostic differences depending on their anatomic location. The simple classification system proposed here therefore provides useful information to clinicians involved in the pre- and postnatal management of children with these anomalies.
Keywords: prenatal diagnosis, cervical lymphatic malformation, fetal nuchal anomaly, prognosis, outcomes
Cervical lymphatic anomalies, also known as nuchal anomalies, are often diagnosed on prenatal ultrasound.1 Prenatal diagnosis of these anomalies is of interest to the otolaryngologist because modern high-resolution fetal imaging may allow prediction of peripartum airway obstruction, requiring fetal surgery2 or ex utero intrapartum therapy.3 Prenatal diagnosis of head and neck lymphatic anomalies may also affect parental counseling, because these lesions may be associated with significant functional and subjective impairment.4
In addition to concerns for airway obstruction, nuchal anomalies, as observed on prenatal ultrasound, are routinely used as markers for clinically recognized chromosomal abnormalities, an association that has been well supported by previous studies.5-9 This association is important to both clinicians and families because it may drive further genetic workup, decisions about elective termination of pregnancy, or plans for postnatal management.
Diagnosis, counseling, and treatment planning are hindered, however, by the wide variability of terms used to describe these lesions. Current nomenclature includes cystic hygroma, lymphangioma, nuchal thickening, nuchal translucency, and lymphatic malformation,1,8,9 none of which has a standard definition. We believe that this overlapping terminology leads to confusion among clinicians and families with respect to prevalence, prognostic significance, and understanding of the potential functional impact these diagnoses will have during pregnancy and following birth.
The purpose of this study is to categorize prenatally diagnosed nuchal anomalies in a way that will be useful to clinicians treating these children after birth. Accordingly, we aimed to determine the prognostic value of a simple anatomic classification system in predicting the presence of chromosomal abnormalities, pregnancy outcomes, and likelihood of spontaneous resolution of the prenatally diagnosed lymphatic anomaly. We hypothesized that our categories would be associated with different rates of chromosomal abnormalities, elective and spontaneous pregnancy termination, and spontaneous prenatal lesion resolution.
Methods
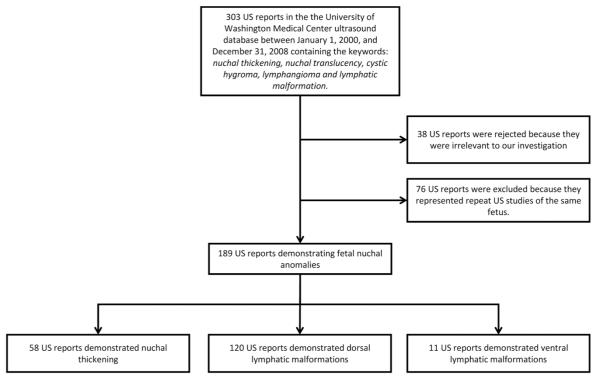
We conducted a retrospective cohort study of mother-fetus pairs at a tertiary academic medical center with a busy obstetric program. The cohort included any mother-baby pair whose prenatal ultrasound studies demonstrated fetal nuchal anomalies. Patients were identified using the University of Washington Medical Center ultrasound database. The database was queried for ultrasound (US) reports recorded between January 1, 2000, and December 31, 2008, containing the keywords nuchal thickening, nuchal translucency, cystic hygroma, lymphangioma, or lymphatic malformation. In cases where multiple studies of the same fetus were available, we included the earliest study and excluded all later studies (Figure 1).
Figure 1.
Cohort assembly demonstrating patient exclusion resulting in final study sample. US, ultrasound.
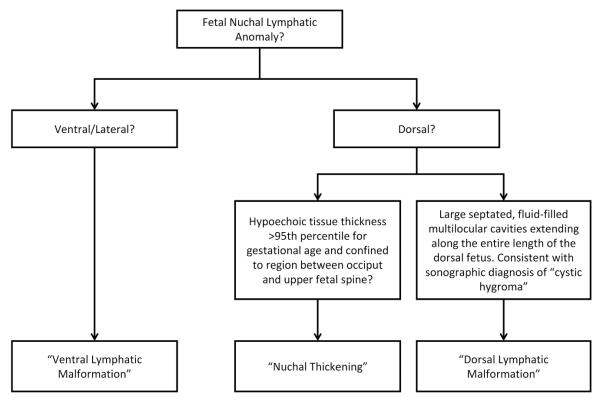
In this study, lymphatic anomalies of the fetal neck were categorized into 3 groups based on their sonographic anatomy: nuchal thickening (NT), dorsal lymphatic malformation (DLM), and ventral lymphatic malformation (VLM) (Figure 2). Examples are presented (Figure 3).
Figure 2.
Flowchart demonstrating nomenclature used to describe fetal nuchal anomalies.
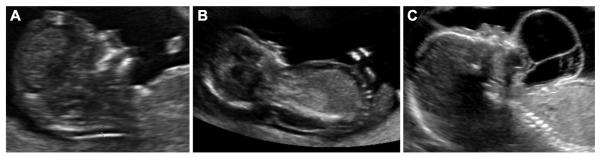
Figure 3.
Ultrasound images demonstrating (A) nuchal thickening, (B) dorsal lymphatic malformation, and (C) ventral lymphatic malformation.
Nuchal translucency was defined as any hypoechoic region between the skin and soft tissues bordered by the fetal occiput and cervical spine. Nuchal translucency was considered NT when it exceeded the 95th percentile for gestational age (Figure 3A). Dorsal lymphatic malformations were defined as septated, fluid-filled multilocular cavities extending along the entire length of the dorsal fetus (Figure 3B). Ventral lymphatic malformations were defined as lymphatic anomalies occurring on the anterior and/or lateral fetal neck (Figure 3C). All US images used in this study were reexamined and classifications confirmed in a blinded fashion by an attending radiologist at the University of Washington. Inconsistencies between the original and the repeat ultrasound interpretation were settled by a blinded third party.
Through chart review, we determined the incidence of spontaneous resolution of lymphatic anomalies; presence of chromosomal anomalies as determined by analysis of chorionic villi, amniotic fluid, or fetal tissue in the case of nonviable birth; and pregnancy outcomes in fetuses included in the study sample. Pregnancy outcomes were classified as spontaneous termination/fetal demise, elective termination, or live birth. Chromosomal abnormalities were classified as trisomy 21, trisomy 13, trisomy 18, Turner syndrome, or other.
Data were analyzed using STATA 11.0 (StataCorp, College Station, Texas) and Microsoft Excel (Microsoft Corporation, Redmond, Washington). This study was approved by the University of Washington Institutional Review Board.
Results
Our database query generated an initial sample of 303 ultrasound studies. Thirty-eight studies were removed from our study database because they were not performed in the prenatal period. Seventy-six studies were excluded because they represented repeat imaging of the same fetus. Following these exclusions, studies demonstrating fetal nuchal anomalies were available for 189 unique patients. Based on our classification criteria, 58 studies (30.7%) demonstrated nuchal thickening, 120 (63.5%) demonstrated dorsal lymphatic malformations, and 11 (5.8%) demonstrated ventral lymphatic malformations.
The study population demographics and characteristics are presented (Table 1). The average maternal age was similar between mothers of fetuses with NT (31.7 years) and DLM (30.1 years). However, mothers of fetuses demonstrating VLM were considerably younger (mean maternal age 24.4 years). Nuchal thickening and DLM were detected on average at just under 18 weeks’ gestational age (using either the last menstrual period or ultrasound findings to define gestational age); VLM tended to be detected significantly later, at 27.9 weeks’ gestational age.
Table 1.
Demographics and Study Sample Characteristics.
| Characteristic | Nuchal Thickening (n = 58) |
Dorsal Lymphatic Malformation (n = 120) |
Ventral Lymphatic Malformation (n = 11) |
|---|---|---|---|
| Sex, No. (%) | |||
| Male | 31 (53.4) | 29 (24.2) | 5 (45.5) |
| Female | 14 (24.1) | 56 (46.6) | 6 (54.5) |
| Undetermined | 13 (22.4) | 35 (29.2) | 0 (0) |
| Maternal age, mean, y | 31.7 | 30.1 | 24.4 |
| US gestational age, mean, wk | 17.7 | 16.8 | 27.9 |
| LMP gestational age, mean, wk | 17.8 | 17.4 | 27.9 |
Abbreviations: LMP, last menstrual period; US, ultrasound.
Chromosomal analysis results were available for 53.4% of fetuses with NT, 54.2% with DLM, and 36.4% of fetuses with VLM (Table 2). These totals include 7 DLM fetuses who underwent postmortem chromosomal analysis of fetal tissue. Among 31 fetuses with NT undergoing chromosomal analysis, 9 (29.0%) had chromosomal anomalies. Among 65 with DLM, 54 (83.1%) had chromosomal anomalies. None of the 4 fetuses with VLM had chromosomal anomalies. Fetuses with DLM were significantly more likely to have chromosomal abnormalities than either fetuses with NT (P < .001) or VLM fetuses (P < .001). Fetuses with NT and those with VLM did not show a significantly different prevalence of chromosomal abnormalities (P = .21). Trisomy 21 was the most common chromosomal abnormality in the NT group (7/31 subjects, 22.6%), whereas Turner syndrome was the most common abnormality in the DLM group (23/65 patients, 35.4%).
Table 2.
Distribution of Results from Genetic Testing in Fetuses with Identified NT, DLM, or VLM.
| Characteristic | NT (n = 31), No. (%) |
P Value, NT vs DLM Comparison |
P Value, NT vs VLM Comparison |
DLM (n = 65), No. (%) |
P Value, DLM vs VLM Comparison |
VLM (n = 4), No. (%) |
|---|---|---|---|---|---|---|
| No chromosomal anomaly | 22 (71.0) | 11 (16.9) | 4 (100) | |||
| Chromosomal anomaly | 9 (29.0) | <.001 | .21 | 54 (83.1) | <.001 | 0 (0.0) |
| Trisomy 21 | 7 (22.6) | 16 (24.6) | NA | |||
| Turner | 0 (0.0) | 23 (35.4) | NA | |||
| Trisomy 13 | 1 (3.2) | 2 (3.1) | NA | |||
| Trisomy 18 | 0 (0.0) | 8 (12.4) | NA | |||
| Other | 1 (3.2) | 5 (7.7) | NA | |||
| Missinga | 27 | 55 | 7 |
Abbreviations: DLM, dorsal lymphatic malformation; NA, not applicable; NT, nuchal thickening; VLM, ventral lymphatic malformation.
Unknown chromosomal anomaly/no chromosomal testing performed.
Fetuses with DLM had the highest rates of adverse pregnancy outcomes, with 52 of 120 (43%) undergoing elective termination of pregnancy and 24 (20%) undergoing spontaneous fetal demise (Table 3). In the NT group, 3 of 58 (5.2%) underwent elective termination of pregnancy, and 2 (3.4%) experienced fetal demise. In the VLM group, 1 of 11 (9.1%) underwent elective termination, with no cases of fetal demise. Dorsal lymphatic malformation predicted a significantly higher risk of adverse pregnancy outcomes than either NT (P < .001) or VLM (P < .001). Nuchal thickening and VLM did not differ in their rates of adverse pregnancy outcomes (P = .96). Fetuses with DLM were likely to have other anomalies in addition to their DLM; 46 of 120 (38.3%) had confirmed cardiac, renal, or skeletal malformations. Additional fetal anomalies were significantly less likely in the NT (5.2%, P < .001) and VLM (0%, P = .01) groups (Table 3).
Table 3.
Pregnancy and Morphologic Outcomes in Fetuses with NT, DLM, or VLM.
| Characteristic | NT (n = 58), No. (%) |
P Value, NT vs DLM Comparison |
P Value, NT vs VLM Comparison |
DLM (n = 120), No. (%) |
P Value, DLM vs VLM Comparison |
VLM (n = 11), No. (%) |
|---|---|---|---|---|---|---|
| Live birth | 53 (91) | 44 (36.7) | 10 (90.9) | |||
| Adverse pregnancy outcome | <.001 | .96 | <.001 | |||
| Elective termination | 3 (5.2) | 52 (43.3) | 1 (9.1) | |||
| Fetal demise | 2 (3.4) | 24 (20.0) | 0 (0) | |||
| Resolution of nuchal anomaly at birth | 47 (81.0) | <.001 | <.001 | 22 (18.3) | .002 | 0 (0) |
| Presence of renal, cardiac, or skeletal anomalies |
3 (5.2) | <.001 | .44 | 46 (38.3) | .01 | 0 (0) |
Abbreviations: DLM, dorsal lymphatic malformation; NT, nuchal thickening; VLM, ventral lymphatic malformation.
Spontaneous resolution of nuchal anomalies was common in the NT group, with 47 (81.0%) demonstrating resolution at the time of birth. We observed an 18.3% (22/120) prevalence of resolution at time of birth in the DLM group. However, many fetuses with DLM never proceeded to parturition due to elective and spontaneous termination; their lesions were therefore considered unresolved. Considering only those fetuses with DLM who actually proceeded to parturition, 50% demonstrated complete resolution. In the VLM group, there was a 0% (0/11) prevalence of resolution of nuchal anomaly at time of birth. Examining only the subset of DLM fetuses with live births, NT was significantly more likely to resolve by birth than DLM (P < .001), which in turn was significantly more likely to resolve by birth than VLM (P = .002).
Discussion
This study describes an anatomic classification for fetal cystic neck abnormalities, providing a consistent nomenclature and clear definitions for these lesions. We encourage its adoption because use of precise and accurate categories has the potential to improve communication and treatment planning between otolaryngologist–head and neck surgeons, radiologists, obstetricians, and other medical specialties involved in the management of these unusual lesions. This is immediately relevant at birth, when VLM may be associated with airway obstruction, and DLM may be associated with renal, cardiac, or skeletal anomalies. Consistent terminology will also facilitate prenatal counseling for families, particularly if it has prognostic value, as described below. In addition, it will promote comparability across future research studies of children with these malformations.
Also of interest to the pediatrician and pediatric head and neck surgeon are the relationships we demonstrate between the anatomic location of fetal nuchal anomalies and important prognostic findings. Importantly, VLM showed several clear differences from NT and DLM. Dorsal lymphatic malformations are associated with chromosomal anomalies and often spontaneously resolve, while NT is even more likely to spontaneously resolve. Ventral lymphatic malformations are not associated with chromosomal anomalies and do not resolve before birth.10-12 Nearly one-third of fetuses with NT showed chromosomal abnormalities, while no chromosomal abnormalities were identified in fetuses with VLM. While this comparison was not statistically significant, the observed difference is intriguing. Other authors have presented similar associations with specific chromosomal abnormalities (trisomy 21, trisomy 18, trisomy 13, and Turner syndrome).13 Although the previous lack of stratification by anatomic location makes direct comparison of our results challenging, the overall prevalence of chromosomal abnormalities in our sample appears similar to previous reports.14
These differences in natural history and chromosomal findings suggest that, although histologically identical,11 VLM may have embryologic origins distinct from posterior lesions. While we make an anatomic distinction between NT and DLM in this study because it is clinically apparent, we do not believe that these represent separate pathologic processes. Instead, we suggest that mild NT is a benign occurrence in many chromosomally normal babies, whereas increased nuchal thickening (greater than 95th percentile) may be an earlier point in a continuum that leads to eventual accumulation of cystic fluid-filled cavities, or DLM. This idea is supported by our finding that NT was more likely to resolve spontaneously than DLM, while DLM was more predictive than NT of chromosomal and anatomic (renal, cardiac, and skeletal) abnormalities and adverse pregnancy outcomes. More severe pathology along this continuum may be associated with serious chromosomal abnormalities.6,7,15
We acknowledge limitations to this preliminary study. First, many fetuses who underwent spontaneous demise did not undergo chromosomal analysis. Our study may therefore underrepresent the true prevalence of chromosomal abnormalities in fetuses with nuchal anomalies, particularly in those with DLM. This would suggest that the differences between DLM and the other groups is underestimated in this study, further supporting the idea of a continuum of disease in these lesions. Our sample of VLM patients was also small relative to the DLM and NT groups, reducing our ability to precisely estimate differences in outcomes between groups. Despite this obstacle, several of our comparisons had sufficient power to detect statistically significant differences between DLM, NT, and VLM. Nevertheless, larger sample sizes would be ideal in future studies to further validate our classification system and to confirm these early findings.
Conclusions
This study proposes a simple, anatomic classification of nuchal lymphatic anomalies identified on prenatal ultrasound, with significant prognostic value for clinicians managing these lesions before and after birth. Dorsal lymphatic malformations and nuchal thickening above the 95th percentile are predictive of chromosomal anomalies and often spontaneously resolve, whereas ventral lymphatic malformations are not associated with chromosomal anomalies and do not spontaneously resolve. Fetuses with dorsal lymphatic malformations demonstrate a higher prevalence of chromosomal anomalies, anatomic malformations, and adverse pregnancy outcomes compared with those with nuchal thickening alone, while nuchal thickening is more likely to resolve spontaneously than dorsal lymphatic malformation. Further study is needed to validate these findings and to clarify the developmental relationships between these 3 groups.
Acknowledgment
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health.
Sponsorships: None.
Funding source: This research was made possible by grant TL1 RR 025016 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Footnotes
Author Contributions
Beck Longstreet, data collection, analysis, interpretation, drafting, final approval; Karthik Balakrishnan, conception, design, analysis, interpretation, drafting, critical revision, final approval; Babette Saltzman, analysis, interpretation, critical revision, final approval; Jonathan A. Perkins, conception, design, analysis, interpretation, critical revision, final approval; Manjiri Dighe, conception, design, data collection, interpretation, critical revision, final approval.
Disclosures
Competing interests: None.
References
- 1.Bloom DC, Perkins JA, Manning SC. Management of lymphatic malformations. Curr Opin Otolaryngol Head Neck Surg. 2004;12:500–504. doi: 10.1097/01.moo.0000143971.19992.2d. [DOI] [PubMed] [Google Scholar]
- 2.Rahbar R, Vogel A, Myers LB, et al. Fetal surgery in otolaryngology: a new era in the diagnosis and management of fetal airway obstruction because of advances in prenatal imaging. Arch Otolaryngol Head Neck Surg. 2005;131:393–398. doi: 10.1001/archotol.131.5.393. [DOI] [PubMed] [Google Scholar]
- 3.Scott AR, Nguyen H, Kelly JC, Sidman JD. Prenatal consultation with the pediatric otolaryngologist. Int J Pediatr Otorhinolaryngol. 2014;78:679–683. doi: 10.1016/j.ijporl.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Balakrishnan K, Edwards TC, Perkins JA. Functional and symptom impacts of pediatric head and neck lymphatic malformations: developing a patient-derived instrument. Otolaryngol Head Neck Surg. 2012;147:925–931. doi: 10.1177/0194599812450838. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi DW, Bianchi DW. Fetology: Diagnosis and Management of the Fetal Patient. 2nd ed McGraw-Hill Medical; New York, NY: 2010. [Google Scholar]
- 6.Breathnach FM, Malone FD, Lambert-Messerlian G, et al. First- and second-trimester screening: detection of aneuploidies other than Down syndrome. Obstet Gynecol. 2007;110:651–657. doi: 10.1097/01.AOG.0000278570.76392.a6. [DOI] [PubMed] [Google Scholar]
- 7.Comstock CH, Malone FD, Ball RH, et al. Is there a nuchal translucency millimeter measurement above which there is no added benefit from first trimester serum screening? Am J Obstet Gynecol. 2006;195:843–847. doi: 10.1016/j.ajog.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P, Burton BK. Congenital Malformations: Evidence-Based Evaluation and Management. McGraw-Hill Medical; New York, NY: 2008. [Google Scholar]
- 9.Malone FD, Canick JA, Ball RH, et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353:2001–2011. doi: 10.1056/NEJMoa043693. [DOI] [PubMed] [Google Scholar]
- 10.Perkins JA, Maniglia C, Magit A, Sidhu M, Manning SC, Chen EY. Clinical and radiographic findings in children with spontaneous lymphatic malformation regression. Otolaryngol Head Neck Surg. 2008;138:772–777. doi: 10.1016/j.otohns.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Perkins JA, Manning SC, Tempero RM, et al. Lymphatic malformations: current cellular and clinical investigations. Otolaryngol Head Neck Surg. 2010;142:789–794. doi: 10.1016/j.otohns.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Perkins JA, Tempero RM, Hannibal MC, Manning SC. Clinical outcomes in lymphocytopenic lymphatic malformation patients. Lymphat Res Biol. 2007;5:169–174. doi: 10.1089/lrb.2007.5304. [DOI] [PubMed] [Google Scholar]
- 13.Chen C-P. Pathophysiology of increased fetal nuchal translucency thickness. Taiwan J Obstet Gynecol. 2010;49:133–138. doi: 10.1016/S1028-4559(10)60029-0. [DOI] [PubMed] [Google Scholar]
- 14.Noia G, Pellegrino M, Masini L, et al. Fetal cystic hygroma: the importance of natural history. Eur J Obstet Gynecol Reprod Biol. 2013;170:407–413. doi: 10.1016/j.ejogrb.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 15.Harsha WJ, Perkins JA, Lewis CW, Manning SC. Pediatric admissions and procedures for lymphatic malformations in the United States: 1997 and 2000. Lymphat Res Biol. 2005;3:58–65. doi: 10.1089/lrb.2005.3.58. [DOI] [PubMed] [Google Scholar]