Abstract
Production of gametocytes in vitro is essential for studies of Plasmodium falciparum sexual stages. Here, we describe procedures for the high-yield production and fractionation of P. falciparum gametocytes stages I to V.
Keywords: Plasmodium falciparum, Gametocyte, Purification, Percoll-sorbitol gradient, Multilayer Percoll gradient
1. Introduction
During the erythrocytic life cycle, P. falciparum can follow one of two developmental fates: cyclic asexual propagation or terminal sexual differentiation into gametocytes. Previous studies have shown that all merozoites originating from a single schizont will be either asexual or sexual (1). Once a sexually committed merozoite invades an erythrocyte, it will begin a complex differentiation and maturation process. It starts as a stage I gametocyte that is morphologically almost identical to an asexual trophozoite and ends with the formation of a large crescent-shaped stage V gametocyte. The mature stage V gametocyte is the only form of the parasite that is able to survive in the mosquito vector. Thus, understanding gametocyto-genesis may lead to improved strategies for malaria control. Nevertheless, producing large numbers of pure gametocytes in vitro is difficult.
There are three main challenges for gametocyte production in vitro. First, only a very small proportion of the parasites (~5%) will commit and differentiate into gametocytes (2). Additionally, different strains of P. falciparum vary in their ability to produce gametocytes (3). Moreover, during continuous culture parasites may lose the ability to produce gametocytes altogether (3). Second, gametocytes take ~10–15 days to mature and require fresh medium changes every day (4), which can make experiments laborious and costly. Finally, while it is generally agreed that “stress” can induce or increase numbers of gametocytes (5–8), it is not entirely clear what defines “stress.” There have been reports that gametocyte numbers can increase with the addition of certain drugs (9), by increase of cAMP signaling (10) or by addition of spent or conditioned medium to the culture (11). However, duplication of these experimental conditions has resulted in variable success (4). In addition to difficulties in producing large numbers of gametocytes, it can be challenging to produce pure preparations of specific stages.
Recently, new protocols have been developed that increase gametocyte numbers and improve the yield of specific stages (4, 12, 13). From these and other published procedures, we have developed protocols for the reliable production of large numbers of gametocytes of multiple P. falciparum strains and for the purification of each gametocyte stage.
2. Materials
2.1. Equipment
37°C incubator.
Slide warmer that can be set to 37°C.
Malaria gas (5% O2, 5% CO2, 90% N2) or candle jar (14).
25-cm2 or 75-cm2 culture flasks with plug caps (Corning) (see Note 1) or 6-well culture plates (if using a candle jar) (Corning).
MACS (Miltenyi Biotec) and LS columns (Miltenyi Biotec) (15).
15-ml and 50-ml conical tubes (BD Biosciences).
Glass Pasteur pipettes (Fisher), autoclaved.
Refrigerated tabletop centrifuge.
2.2. Culture Components
Incomplete culture medium: 10.4 g RPMI 1640 (with L-Gln, no NaHCO3) (Invitrogen), 24 ml 7.5% NaHCO3 (Sigma), 25 ml 1 M HEPES (Sigma), 10 ml 20% glucose (Sigma), 0.5 ml 25 mg/ml hypoxanthine (Sigma), 50 μg/ml gentamicin (Sigma), 900 ml ddH2O. Adjust pH to 7.4, adjust water to 1 L, filter-sterilize, aliquot and store at 4°C. Store some aliquots at 37°C.
Complete culture medium: take above incomplete medium and add heat-inactivated (55°C for at least 45 min after thawing from −80°C) human serum (Interstate Blood Bank) to a final concentration of 10% (see Note 2).
Fresh (less than 1-week old) human red blood cells (RBC), washed and resuspended at 50% hematocrit in complete culture medium (see Notes 3 and 4).
5 M GlcNAc (Sigma) dissolved in RMPI 1640, store at 4°C for short periods of time (<1 month) and at −20°C for longer periods of time.
2.3. Purification Components
5% d-sorbitol: mix 50 g d-sorbitol (Sigma) with 1 L ddH2O, filter-sterilize, and store at 37°C.
10× RPMI-HEPES, pH 6.8: mix 10.4 g RPMI (with l-Gln, no NaHCO3) with 80 ml ddH2O, add 5.84 g HEPES, adjust to 100 ml ddH2O, filter-sterilize, and store at 4°C.
1× RPMI-HEPES: 10 ml 10× RPMI-HEPES mixed with 90 ml ddH2O, filter-sterilize and store at 4°C.
90% Percoll-6% sorbitol gradient solution: mix 20 ml 10× RPMI-HEPES with 180 ml of Percoll (Sigma), add 12 g d-Sorbitol, filter-sterilize, and store at 4°C.
70% Percoll-sorbitol gradient solution: mix 37.5 ml 90% Percoll-6% sorbitol gradient solution with 10.5 ml 1× RPMI-HEPES, filter-sterilize, and store at 4°C.
40% Percoll-sorbitol gradient solution: mix 21 ml of 90% Percoll-6% sorbitol gradient solution with 27 ml of 1× RPMI-HEPES.
Multilayer Percoll Gradients: 80% Percoll (80 ml Percoll, 20 ml incomplete medium, filter-sterilize and store at 4°C); 65% Percoll (65 ml Percoll, 35 ml incomplete medium, filter-sterilize and store at 4°C); 50% Percoll (50 ml Percoll, 50 ml incomplete medium, filter-sterilize and store at 4°C); 35% Percoll (35 ml Percoll, 65 ml incomplete medium, filter-sterilize and store at 4°C).
2.4. Giemsa Stain Components
Microscope slides (Fisher).
Giemsa stain (Sigma).
1× Phosphate saline buffer (Invitrogen).
Microscope with 100× objective.
2.5. Parasite Line
NF54, 3D7A or other P. falciparum line that is able to produce gametocytes in adequately high numbers (see Note 5).
3. Methods
It is very important to keep the parasites at 37°C, especially as the gametocytes mature. Make sure to place the culture flasks on a slide warmer in the culture hood when working. Always warm medium to 37°C before adding it to the cultures. The two “crash” methods (Subheadings 3.1 and 3.2) are best for obtaining high-yield of mid- to late-stage gametocytes and also checking whether a particular clone or strain produces gametocytes. Subheading 3.3 is best for production of synchronous gametocytes but is material- and time-consuming. Subheadings 3.4 and 3.5 are methods by which each specific gametocyte stage may be purified. Subheadings 3.3–3.5 are appropriate for obtaining pure gametocyte preparations to be used for protein isolation, nucleic acid isolation, or immunofluorescence.
3.1. Production of High Numbers of Asynchronous Gametocytes (the “Crash” Method)
Maintain a healthy culture (<6% parasitemia) at 4% hematocrit.
When the culture reaches 3–5% rings, synchronize with 5% d-sorbitol (day 0) (see Note 6) (16).
The next day (day 1), change medium by removing spent medium with a sterile Pasteur pipette. Be sure to only take off medium and not RBC. Add fresh 37°C medium and resuspend the culture. Make a Giemsa-stained smear to check for rings.
When rings appear (usually on day 2), dilute culture to 0.1% rings.
Change medium everyday but do not add more RBC.
You will be able to observe a crash of the asexual stages after ~7 days (day 9) and high numbers of gametocytes, particularly stages III and IV. Stage V gametocytes should be evident on days 10–12.
3.2. Alternative “Crash” Method
Maintain a healthy culture (<6% parasitemia) at 4% hematocrit.
When the culture reaches 3–5% rings, synchronize with 5% d-sorbitol.
Continue to change medium everyday and check parasitemia. Do not add new RBC.
When the culture reaches ~6–10% parasitemia, add GlcNAc to a final concentration of 50 mM.
Change medium and add GlcNAc for the next 72–96 h, making smears.
You should see the asexual stages decreasing and gametocytes peaking about 2–4 days after you stopped adding GlcNAc.
3.3. Production of Synchronous Gametocytes (4, 12)
Maintain a healthy culture (<6% parasitemia) at 4% hematocrit.
When the culture reaches 3–5% rings, synchronize with 5% d-sorbitol.
The next day, make a smear and check for schizonts.
When most parasites are schizonts, collect schizonts by Percoll-sorbitol gradient, as follows.
In a 15-ml conical tube, lay 3 ml 70% Percoll-sorbitol gradient solution and 3 ml 40% Percoll-sorbitol gradient solution (kept sterile and stored at 4°C until use).
Remove the culture from the 37°C incubator. Resuspend the culture and transfer to a 50-ml tube, then centrifuge at RT for 3 min at 2,600 rpm (1,270 × g).
Remove old medium and resuspend to 50% hematocrit.
Remove the Percoll-sorbitol gradient from 4°C and carefully lay the resuspended culture on top of the gradient, then centrifuge at 3,500 rpm (2,300 × g) for 20 min at 20°C.
Collect parasites from the 40/70% interface (see Note 7), add 10× volume of incomplete medium, wash twice, and put the parasites back in culture at 4% hematocrit.
Carefully shake the culture every half hour or place the culture on a rotating shaker in the incubator. Be sure to shake slowly (see Note 8).
3 h after the Percoll-sorbitol gradient, make a smear. If the majority of the parasites are rings, synchronize again with d-sorbitol (this will ensure a very pure population of newly invaded rings).
Put the parasites back to culture, but increase the hematocrit to 10–20% with the addition of fresh RBC.
Change the medium and make smears everyday watching for “stressed” parasites. “Stressed” parasites are characterized by high parasitemia, small tight schizonts, triangular rings, and oddly shaped trophozoites (see Fig. 1). The spent medium will be yellowish-brown instead of pink and the parasitemia may be as high as 20%. This can take up to 1 week (Fig. 1 here).
Once the stressed parasites appear, Percoll gradient-purify the schizonts and follow with sorbitol synchronization 3 h post-gradient purification as before (day-1).
Medium doubling: on day 0, 26 h after sorbitol synchronization, make a smear and check if parasites are trophozoites. Do not remove the old medium. Keep old medium and add an equal volume of fresh complete medium (this will decrease the hematocrit and further stress the parasites while providing necessary nutrients for gametocyte survival) (12).
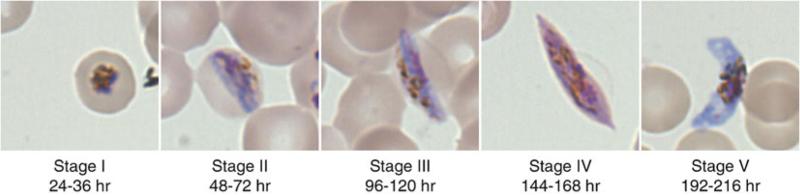
For the following days, change medium as normal, the gametocytes should appear as follows (see Fig. 2): days 1–1.5 (24–48 h post medium doubling), stage I gametocytes; days 2–3 (48–72 h post medium doubling), stage II gametocytes; days 4–5 (96–120 h post medium doubling), stage III gametocytes; days 6–7 (144–168 h post medium doubling), stage IV gametocytes; days 8–9 (192–216 h post medium doubling), stage V gametocytes.
Fig. 1.
“Stressed” versus healthy asexual stage parasites. (a) Stressed ring, trophozoite, and schizont from a culture at ~15% parasitemia. The stressed rings appear triangular and elongated, while the stressed trophozoites and schizonts appear dark and clumped. (b) Healthy ring, trophozoite, and schizont from a culture at ~5% parasitemia. Parasites retain a round shape and the cytoplasm is uniformly distributed.
Fig. 2.
Gametocyte stages. The morphological changes of the gametocyte from stage I through stage V are illustrated. Hours listed are hours post invasion of the sexually committed merozoite as outlined in Subheading 3.3.
3.4. Purification of Stage I and II Gametocytes (13)
Follow Subheading 3.3 to give highly synchronous gametocytes.
On days 1–3, stage I and II gametocytes can be purified.
Percoll-sorbitol gradient-purify parasites as in Subheading 3.3. This time, however, collect the bottom layer. This layer contains noninvaded RBC, rings, and early stage gametocytes. Wash with 37°C incomplete medium and resuspend in 5 ml incomplete medium at 37°C.
Separate the gametocytes from the noninvaded RBC and rings via MACS magnet as follows (see Note 9).
Place the LS column on the MACS magnet.
Equilibrate with 5 ml incomplete medium at 37°C.
Run the 5 ml of rings, noninvaded RBC, and early stage gametocytes through the column at RT (before starting, keep the LS columns at 37°C and make sure all incomplete medium is heated at 37°C).
Wash column three times with 5 ml incomplete medium at 37°C.
Carefully remove the LS column from magnet, place in a 15-ml conical tube, and elute bound parasites with 3 ml incomplete medium at 37°C.
Wash two times and resuspend in desired volume of incomplete medium. Use for SDS-PAGE gels or immunofl uorescence.
3.5. Purification of Stage III, IV, V Gametocytes
Follow Subheading 3.3 to give highly synchronous gametocytes.
On days 4–5, collect stage III gametocytes; on days 6–7, collect stage IV gametocytes; on days 8–9, collect stage V gametocytes.
Pour the Multilayer Percoll gradient in a 15-ml conical tube: 2 ml 80% Percoll, 2 ml 65% Percoll, 2 ml 50% Percoll, 2 ml 35% Percoll, keep sterile at 4°C (17).
Collect the culture in a 50-ml tube, centrifuge for 3 min at 2,600 rpm (1,270 × g), remove the spent medium, and resuspend the pellet in 2 ml of incomplete medium.
Remove the Multilayer Percoll gradient from 4°C and slowly add the parasites (2 ml) to the top of the gradient.
Centrifuge the gradient for 10 min at 2,800 rpm (1,473 × g).
Collect the stage III, IV, or V gametocytes from the 35/50% interface (see Note 10).
Wash by resuspending the collected gametocytes in 5 ml incomplete medium, centrifuge at 2,600 rpm (1,270 × g) for 3 min, remove medium. Resuspend in desired volume of incomplete medium. Use for SDS-PAGE gels or immunofluorescence.
Acknowledgments
We thank Kim Williamson for helpful advice and discussions about production of healthy gametocytes. We also thank Jun Cao for help with Percoll-sorbitol purification protocols and advice on synchronization.
Footnotes
The size of flask, 25 cm2 or 75 cm2, will depend on the purpose of the gametocyte preparation; 5–10-ml cultures in a 25-cm2 flask are appropriate for immunofluorescence, Giemsa smears, RNA or DNA preparations; 25–50-ml cultures in a 75-cm2 flask are appropriate for protein preparations.
Traditionally, gametocyte cultures have only been maintained with human serum; however, it has recently been reported that 1% AlbuMax II (Invitrogen) can also support the growth of gametocytes (12).
To avoid clumping of the RBC in culture due to RBC surface antigens, make sure that the RBC and serum are compatible (i.e., use matching blood type and gender for both the RBC and serum). We use male O-positive for both.
We have found that storing RBC in complete medium (with human serum) greatly enhances their viability and keeps them from lysing during storage. RBC stored this way can be used to for gametocyte production for up to 10 days.
You may wish to clone your parasite line in order to isolate a clone that produces high levels of gametocytes.
Sorbitol is taken up specifically by the trophozoites and schizonts resulting in rupture. Rings do not express the sorbitol receptor and thus are sorbitol-insensitive (18).
Schizonts, trophozoites, and mature gametocytes can be collected from the 40/70 interface of the Percoll-sorbitol gradient. Rings, noninvaded RBC, and early stage gametocytes can be collected from the very bottom of the Percoll-sorbitol gradient.
Shaking of the culture will facilitate successful invasion by the merozoites and will also decrease the numbers of RBC with multiple invasion (19).
When using a MACS magnet, gametocytes that have hemozoin will stick to the magnet, while the rings and uninfected RBC will flow-through (15).
If large numbers of trophozoites and schizonts are present when purifying late stage gametocytes, the cultures can be treated with 5% D-sorbitol. This will kill the trophozoites and schizonts but not the gametocytes.
References
- 1.Bruce MC, et al. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100:191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 2.Eksi S, et al. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:90–99. doi: 10.1016/j.molbiopara.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Brockelman CR. Conditions favoring gametocytogenesis in the continuous culture of Plasmodium falciparum. J Euk Microbiol. 1982;29:454–458. doi: 10.1111/j.1550-7408.1982.tb05432.x. [DOI] [PubMed] [Google Scholar]
- 4.Fivelman QL, et al. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol. 2007;154:119–123. doi: 10.1016/j.molbiopara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Talman AM, et al. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker DA. Malaria gametocytogenesis. Mol Biochem Parasitol. 2010;172:57–65. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyer M, Day KP. Commitment to gametocytogenesis in Plasmodium falciparum. Parasitol Today. 2000;16:102–107. doi: 10.1016/s0169-4758(99)01608-7. [DOI] [PubMed] [Google Scholar]
- 8.Alano P. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol Microbiol. 2007;66:291–302. doi: 10.1111/j.1365-2958.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- 9.Peatey CL, et al. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J Infect Dis. 2009;200:1518–1521. doi: 10.1086/644645. [DOI] [PubMed] [Google Scholar]
- 10.Dixon MW, et al. A green fluorescent protein-based assay for determining gametocyte production in Plasmodium falciparum. Mol Biochem Parasitol. 2009;163:123–126. doi: 10.1016/j.molbiopara.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Williams JL. Stimulation of Plasmodium falciparum gametocytogenesis by conditioned medium from parasite cultures. Am J Trop Med Hyg. 1999;60:7–13. doi: 10.4269/ajtmh.1999.60.7. [DOI] [PubMed] [Google Scholar]
- 12.Buchholz K, et al. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J Infect Dis. 2011;203:1445–1453. doi: 10.1093/infdis/jir037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvestrini F, et al. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2010;9:1437–1448. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen JB, Trager W. Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle jar method. J Parasitol. 1977;63:883–886. [PubMed] [Google Scholar]
- 15.Ribaut C, et al. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar J. 2008;7:45. doi: 10.1186/1475-2875-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 17.Kariuki MM, et al. Plasmodium falciparum : purification of the various gametocyte developmental stages from in vitro-cultivated parasites. Am J Trop Med Hyg. 1998;59:505–508. doi: 10.4269/ajtmh.1998.59.505. [DOI] [PubMed] [Google Scholar]
- 18.Nguitragool W, et al. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell. 2011;145:665–677. doi: 10.1016/j.cell.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen RJ, Kirk K. Plasmodium falciparum culture: the benefits of shaking. Mol Biochem Parasitol. 2010;169:63–65. doi: 10.1016/j.molbiopara.2009.09.005. [DOI] [PubMed] [Google Scholar]