Abstract
Development of Alzheimer’s disease (AD) has been linked to the de-regulation of estrogen and gonadotropins such as luteinizing hormone (LH). In this study, we found increases in AD pathology in the hippocampi of aged female 3xTg AD mice after ovariectomy that were unable to be reduced by estrogen therapy or down-regulation of serum LH levels. Despite the lack of effect of these treatments on AD pathology, down-regulation of serum LH but not estrogen improved factors associated with neuronal plasticity such as spatial memory, inhibition of glycogen synthase kinase-3 beta, expression of beta-catenin, and brain-derived neurotrophic factor transcription. Contrasting previous studies in younger mice, estrogen replacement was not able to rescue behavioral deficits, reduced glycogen synthase kinase-3 beta inhibition and increased hippocampal phosphorylation of tau. Of critical importance, serum LH was negatively correlated with brain LH in regions associated with spatial memory, and increases in brain LH correlated with cognitive improvement. This paralleled changes in human female AD brains which showed a significant reduction in brain LH mRNA compared to healthy age- and PMI-matched controls. Taken together, these findings should promote further research into the LH-dependent mechanisms associated with AD cognitive deficits as well as the effects of estrogen within the aged brain.
Keywords: 3xTg AD, Alzheimer’s disease, estrogen, GSK3β, luteinizing hormone, ovariectomy
Current research supports the protective effects of estrogen on neuronal processes associated with cognition (Garcia-Segura et al. 2001; Roepke et al. 2011), particularly within the hippocampus (Sudo et al. 1997; Yang et al. 2010). However, these benefits seem predominantly confined to a critical period of effectiveness both in rodents (Bimonte and Denenberg 1999; Rissanen et al. 1999; Daniel et al. 2006; Bohacek and Daniel 2010) and humans (Rapp et al. 2003; Daviglus et al. 2010; Chlebowski et al. 2010, 2013).
An aspect that may partially account for this paradox of hormone effects is the fact that estrogen becomes progressively less effective at providing negative feedback onto gonadotropin synthesis/release in women (Rossmanith et al. 1994) and in rodents (King et al. 1987; Lloyd et al. 1994). Epidemiologically, higher Alzheimer’s disease (AD) risk in women (Payami et al. 1996; Gao et al. 1998) is paralleled by higher levels of gonadotropins in women compared with men (Chakravarti et al. 1976; Neaves et al. 1984). Furthermore, human studies also show that serum luteinizing hormone (LH) levels are higher in AD patients compared to controls (Short et al. 2001) and in non-AD patients with cognitive deficits (Rodrigues et al. 2008).
Rodent studies also support a role for gonadotropins such as LH in cognition and AD. For example, blocking serum LH synthesis improves cognition in intact AD-Tg2576 female mice (Casadesus et al. 2006) and memory loss can be prevented in a neurotoxin-induced AD rat model (Ziegler and Thornton 2010). Cognitive benefits through the reduction in gonadectomy-induced rises in serum LH are also observed in male rats (McConnell et al. 2012) and female mice (Bryan et al. 2010). Furthermore, peripheral administration of human chorionic gonadotropin (hCG), which shares the same receptor as LH, leads to cognitive deficits in rats (Berry et al. 2008). Collectively, these findings support a role of LH in cognitive processes and AD pathogenesis, however, the mechanisms of action remain unclear.
To this end, while lowering peripheral LH provides cognitive benefits, it is yet to be defined how choriogonadotropin/luteinizing hormone receptor (CG/LHR) signaling affects cognition given the extensive expression of the receptor in the hippocampus (Lei et al. 1993; Lukacs et al. 1995), midbrain, and cortex (Apaja et al. 2004). In addition, it remains to be clarified if the cognitive improvements associated with peripheral gonadotropin down-regulation are a direct result of reductions in neuronal CG/LHR signaling cascades that are intimately involved in learning and memory (Salvador et al. 2002) and neuroplasticity (Roy et al. 2009). Therefore, the aim of this study was to determine the influence of peripheral gonadotropin down-regulation on learning and memory and AD pathology processes relative to estrogen depletion/replacement within advanced stages of AD and to extricate the involvement of the CG/LHR in these aspects.
Methods
Animals and housing
Twenty-three female 18-month-old triple-transgenic mice (Oddo et al. 2003a,b) were purchased from Jackson Laboratory (Bar Harbor, ME, USA), 8 wild-type animals were used to determine the magnitude of effect of transgenes and our treatments. Animals were group-housed (3/cage), provided ad libitum access to food and water, and maintained on a 12 h light/dark cycle. All protocols were approved by The Institutional Animal Care and Use Committee of Case Western Reserve University.
Ovariectomy and treatment
All subjects underwent either an ovariectomy (OVX) or sham-surgery (SHAM; n = 6) as described in Bryan et al. (2010). Treatment groups were chosen randomly and received physiological saline (OVX+SAL; n = 6) (0.9%), leuprolide acetate (OVX+LA; n = 6) (3.6 μg/day) or 17β-estradiol (OVX+E2; n = 5) (1.1 ng/day) in Alzet pumps (Pump 1004, Durect Corporation, Cupertino, CA, USA) implanted once a month for 3 months. It has been previously shown that a slow-release formulation of LA significantly reduces LH levels and improves cognitive function in Tg2576 mice (Casadesus et al. 2006) as well as female wild-type mice (Bryan et al. 2010), and the estrogen dosage was selected from previous literature that showed cognitive improvement in OVX rats (Takuma et al. 2007). All animals were weighed prior to initial capsule implant and before each capsule replacement for the 3-month-treatment duration as an analysis of health.
Behavioral testing
The Morris Water Maze was used to measure hippocampal-dependent spatial learning and memory following previous protocol (Fugger et al. 1998). After 3 months of treatment, all animals were trained within a black circular tub (diameter = 120 cm) that was filled daily with water at 23°C and colored white with non-toxic paint. Within the experimental room, distal visual cues were available on each wall as reference points. In each trial, latency to the platform (0.5 cm submerged; diameter = 10.5 cm) was recorded with a time limit of 60 s. The platform remained in the NW quadrant for each mouse, and the starting location was moved between four equidistant points around the maze to control for location bias. After four consecutive trials, the mouse was placed in a clean, warm, bedded cage and rested for 30 min until the second round of four training trials. Each mouse had eight trials per day for four training days followed by a probe trial on the fourth day. For the 60 s probe trial, the platform was removed and the mouse entered the maze opposite the platform quadrant and percent time spent in the platform quadrant was recorded. Behavior was analyzed with a digital video recorder and computer using Ethovision tracking software (Noldus Information Technology, Wageninegen, Netherlands).
Tissue and blood processing
Blood was drawn from all mice by terminal cardiac puncture under anesthesia (2.5% Avertin; intraperitoneal injection). The samples were measured by sandwich-ELISA RIA analysis to determine serum levels of LH (Ligand Assay and Analysis Core; University of Virginia) and E2 (University of Colorado State). Following blood collection, each subject was promptly killed by cervical dislocation and the uterine and fallopian tubes were removed, weighed, and inspected for successful ovariectomy. The brain was harvested and divided by a mid-sagittal section. The hippocampus and cortex was sectioned and frozen from one hemisphere and the other hemisphere was post-fixed in 4% paraformaldehyde and embedded in paraffin for immunohistochemistry.
Cell culture
Primary cortical neuron cultures were grown from Sprague–Dawley rat brains at embryonic day 18 as previously described (Lee et al. 2009). Briefly, cultures were run twice in duplicate in six well-plates coated with Poly-D-Lysine/Laminin (BD Biosciences, San Jose, CA, USA) in neurobasal medium supplemented with 2% B27/0.5 mM glutamine (Invitrogen, Carlsbad, CA, USA). Cultures were maintained at 37°C in a humidified, 5% CO2 atmosphere and treated in parallel with hCG (Sigma, St. Louis, MO, USA) for 2 or 6 h with concentrations of 10, 30, 100, 200, and 400 ng. After treatment, cells were lysed (Lee et al. 2009) for immunoblotting.
Western blotting
Frozen samples of hippocampus were homogenized and total protein concentration was quantified (Pierce Biotechnology Inc., Rockford, IL, USA) from tissue and cell culture extract. 20 μg of protein allotted from each supernatant was separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA), blocked for 1 h at 21°C with 10% non-fat dry milk in tris-buffered saline and tween-20 (TBS-T), and incubated in primary antibodies diluted in TBS-T overnight at 4°C: actin (1 : 100 000; Millipore), beta-catenin (1 : 1000; BD Biosciences), pGSK3βser9 (1 : 1000; Cell Signaling Technology, Beverly, MA, USA), glycogen synthase kinase-3 beta (GSK3β) (1 : 1000; Millipore), glyceraldehyde 3-phosphate dehydrogenase (1 : 100 000; Sigma), CG/LHR (1 : 1000; Santa Cruz, Dallas, TX, USA). Primary antibody binding was detected with horseradish peroxidase-linked secondary antibodies diluted in TBS-T for 1 h at 21°C (1 : 5000; Cell Signaling Technology). Western blot images were captured using FlourChem M imaging system (ProteinSimple, Santa Clara, CA, USA). Quantitative values of optical density were acquired using ImageJ freeware (NIH). Protein load for each sample was normalized to actin or glyceraldehyde 3-phosphate dehydrogenase.
Immunohistochemistry and immunofluorescence
The paraffin-fixed hemispheres were sliced at 10 microns on a microtome and mounted on Superfrost Plus treated slides (Fisher Scientific, Pittsburgh, PA, USA). High-temperature antigen retrieval was performed in 10 mM citrate buffer (pH 6.0) for three 5 min increments at 50% power in a microwave, avoiding excessive boiling. For amyloid-beta (Aβ) staining, the tissue was pre-treated for 15 min in 70% formic acid. Sections were blocked with 3.0% hydrogen peroxide in phosphate-buffered saline (PBS) for 1 h at 21°C then in 3.0% normal goat serum (NGS; Vector Laboratories, Burlingame, CA, USA) in PBS for 1 h at 21°C. Primary antibodies were diluted in 3.0% NGS and the slides were incubated overnight at 4°C: Aβ (4G8; 1 : 1000; Covance, Princeton, NJ, USA), Tau tangles (AT8; 1 : 1000; Thermo Fisher Scientific, Wayne, MI, USA). Slides were washed with PBS and incubated in biotinylated goat anti-mouse secondary antibody (Vector Laboratories) for 1 h at 21°C (1 : 400 in 1.5% NGS). After secondary incubation, ABC reagent (Vector Laboratories) was applied for 1 h at 21°C. Slides were then rinsed with water and developed in ImmPACT DAB (Vector Laboratories) until optimal staining was reached (1–1.5 min). All slides were treated identically and processed in parallel. After development, the tissue was dehydrated in ethanol and xylene and mounted using Permount.
Fluorescent staining involved high-temperature antigen retrieval in a pressure cooker with decloaker solution (Biocare Medical, CA, USA). After blocking in 3.0% NGS, primary antibody BetaLH-IC-3 (Courtesy of: A.F. Parlow; National Hormone & Pituitary Program) was applied in 3.0% NGS at a 1 : 200 dilution and was incubated overnight at 4°C. Secondary antibody goat anti-rabbit DyLight 488 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was applied for 1 h at 21°C in 1.5% NGS after washing in PBS. Sections were then stained in 0.3% Sudan black to reduce background fluorescence. Slides were mounted using Vectashield Hard Set (Vector Laboratories).
All images were captured using Leica DM4000B microscope (Leica Microsystems) and quantification was performed with MetaMorph (Molecular Devices, Palo Alto, CA, USA). Within MetaMorph, 10 distinct equal sized regions of the CA1 region of the hippocampus were superimposed on three non-serial brain sections for each mouse. The region of interest pattern was maintained across all quantifications, and the threshold for staining was manually set and correlated between two investigators. The percent area stained for all 10 regions was then averaged.
Real time RT-PCR
Human hippocampus and cortex were matched from the same case and were obtained through the Case Western Reserve University Pathology department (mean age: 82 years, mean PMI: 5 h). Total RNA was extracted from human and 3xTg-AD mice samples using Trizol solution and RNeasy Plus Mini Kits (Qiagen, Valencia, CA, USA). The extracted RNA was quantified using Qubit 2.0 Broad Range Assay (Life Technologies, Grand Island, NY, USA) and cDNA was generated using High Capacity cDNA Reverse Transcription Kits (Life Technologies). Real-time PCR reactions utilized TaqMan master mix and probes (Life Technologies). In human tissue, LH (Hs00751207_s1) and follicle-stimulating hormone (FSH) (Hs00174919_m1) transcription was normalized to transcription of 18S (Hs99999901_s1). Cortical mouse tissue was handled following identical protocol and brain-derived neurotrophic factor (BDNF) (Mm04230607_s1) transcription was normalized to 18S (Mm03928990_g1). The results were analyzed using relative quantification to 18S without amplification efficiency correction.
Statistical analysis
All data were analyzed with the assistance of SPSS statistical software (SPSS, Inc., Chicago, IL, USA). Morris water maze training performance was compared using repeated measures ANOVA. Probe trial performance, pathology and in vivo signaling analyses used one-way ANOVA with LSD post hoc comparisons. Independent t tests were used to compare average expression of LHβ in healthy and AD human brain tissue. Cell culture data were analyzed with a one-way ANOVA and Dunnett post hoc tests. Pearson correlations were performed on the 3xTg AD mouse data to examine the relationships between cognition, serum and brain LH levels, pathology and associated signaling markers. Statistical outliers were identified using Grubb’s test at the 0.05 alpha level.
One OVX+SAL animal was removed from all analysis because of low serum LH levels and high uterine weight, indicating an incomplete ovariectomy.
Results
Morris Water Maze
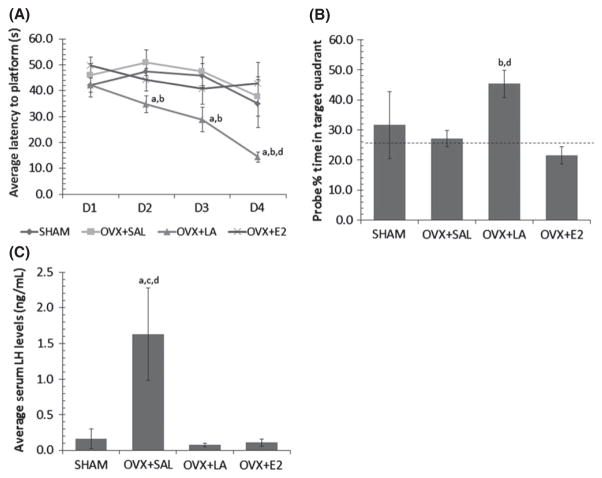
One-way repeated measures ANOVA showed a significant difference between treatment groups in Morris Water Maze (MWM) training(F(3,18) = 3.53, p = 0.036; Fig. 1a). OVX+LAtreatment significantly improved performance in the maze across days in comparison to SHAM (p = 0.036), OVX+SAL (p = 0.012), and OVX+E2 (p = 0.018). However, there was no significant day*group interaction (p = 0.08) suggesting treatment did not affect speed of learning across groups. There were no statistical differences between SHAM, OVX+SAL, and OVX+E2 groups between day one and day four, suggesting a floor effect owing to the advanced age of the SHAM operated group. Across MWM training we found no differences in swimming speed between groups (p = 0.6) indicating that shorter escape latencies were not because of locomotor differences.
Fig. 1.
(A) LA treatment in ovariectomized 21-month-old 3xTg Alzheimer’s disease (AD) female mice improves learning in the Morris Water Maze. Mean and SE plot escape latency of experimental groups across four training days (eight trials per day). Each day of training was analyzed separately with a one-way ANOVA to show significant differences within each day. (B) Ovariectomized mice treated with LA show increased memory retention in the Morris Water Maze probe trial. Bars with SE plot the mean percent time spent in the target quadrant. Mice were released opposite the targetquadrant and their activity was recorded for 60 s. Performance at the level of random chance (25%) is indicated by the dotted line. (C) Serum levels of luteinizing hormone (LH) in 3xTg AD female mice at sacrifice. Blood samples were taken through terminal cardiac puncture and measured by RIA analysis (University of Virginia). Significant differences were found between transgenic groups as measured by a one-way ANOVA. Significant difference (p ≤ 0.05) from SHAM (a), from OVX+SAL (b), from OVX+LA (c), from OVX+E2 (d).
The ability to retain and utilize spatial information was tested using the probe trial. In this regard, there were significant differences between treatment groups (F(3,17) = 3.11, p = 0.05; Fig. 1b) and LA-treated animals showed improved retention of the task compared to OVX+E2 (p = 0.013) and OVX+SAL (p = 0.048) treatments. There were no statistical differences in retention between the SHAM, OVX+SAL, and OVX+E2 treatments as they all performed near the level of chance (25%).
Efficacy of treatments
Radioimmunoassay (performed by: Ligand Assay and Analysis Core; University of Virginia) was used to verify that circulating LH and estrogen were within physiological levels and that LA and E2 treatments effectively regulated-targeted gonadotropins. The overall ANOVA was significant (F(3,18) = 37.42, p ≤ 0.001; Fig. 1c) and as shown previously (Bryan et al. 2010), ovariectomy significantly elevated levels of serum LH in comparison to SHAM, OVX+LA, and OVX+E2 (p ≤ 0.001). Furthermore, E2 levels in OVX+E2 animals were within physiological levels (12–85 pg/mL). These data support that the observed effects were not because of confounds associated with abnormal hormone levels.
Treatment effects on the pathological markers of AD – amyloid and tau pathology
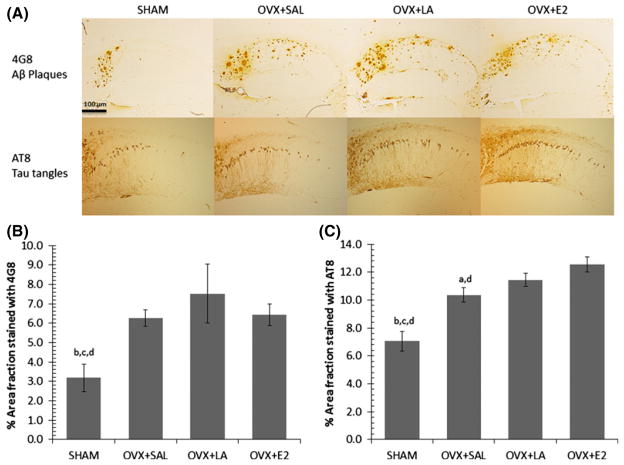
A one-way ANOVA of Aβ plaque load (4G8) in 3xTg AD hippocampus indicated significant differences between treatments (F(3,19) = 8.50, p = 0.001; Fig. 2a and b). There was an ovariectomy-associated increase in Aβ plaques (p = 0.003), but we found no significant differences owing to treatment. Furthermore, we found no significant differences in oligomeric Aβ levels across all treatments.
Fig. 2.
(A) Aβ plaque (4G8) and tau tangle (AT8) immunoreactivity in the hippocampi of 21 month female 3xTg Alzheimer’s disease (AD) mice. Images were captured at 10× magnification; scale bar distance shows 100 μm. (B) Ovariectomy is associated with increased hippocampal Aβ plaques that are not reduced by LA treatment or E2 replacement in 21-month 3xTg AD mice. Bars with SE show the area fraction of 4G8 staining for Aβ plaques in equivalent regions of the hippocampus. (C) Ovariectomy is associated with increased phosphorylated tau in the hippocampi of 21 month 3xTg AD mice and tau load is further elevated by E2 replacement. Bars with SE show the area fraction of AT8 staining for phosphorylated tau in equivalent regions of the hippocampus. Significant difference (p ≤ 0.05) from SHAM (a), from OVX+SAL (b), from OVX+LA (c), from OVX+E2 (d).
A one-way ANOVA of hippocampal PHF-tau (AT8) showed significant differences between groups (F(3,19) = 6.23, p ≤ 0.001; Fig. 2a and c). An ovariectomy-associated increase (p = 0.039) was unaltered by LA treatment, but was further aggravated by estrogen replacement in comparison to the SHAM (p ≤ 0.001) and OVX+SAL control (p = 0.005).
Modulation of AD and synaptic plasticity signaling cascades
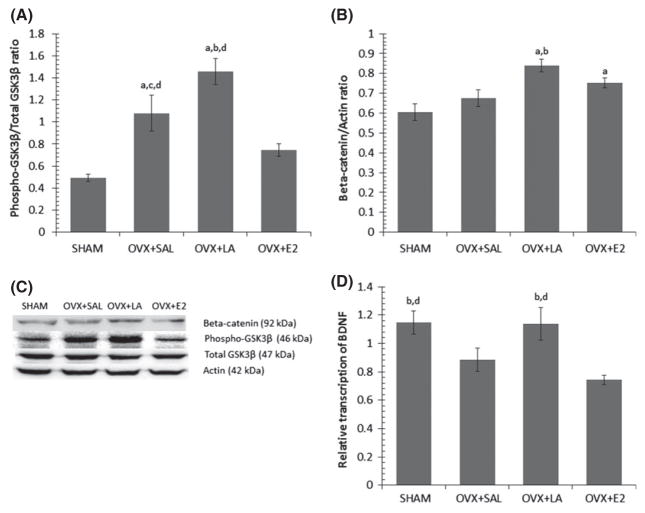
GSK3β can be inhibited by phosphorylation at ser9 (Hur and Zhou 2010) and this aspect is associated with activation of neuroprotective signaling cascades (Chen et al. 2006; Hur and Zhou 2010). A one-way ANOVA showed a statistical difference between groups (F(3,17) = 15.56, p ≤ 0.001; Fig. 3a and c). Ovariectomy increased GSK3β inhibition (p = 0.002), that was further increased by LA treatment in comparison to the OVX+SAL control (p = 0.023). Interestingly, estrogen treatment in the transgenic mice significantly reduced levels of GSK3β inhibition compared to OVX+SAL (p = 0.048) and OVX+LA (p ≤ 0.001).
Fig. 3.
(A) LA treatment increases phosphorylation of GSK3βser9, and E2 treatment decreases GSK inhibition in comparison to OVX controls. Bars with SE show the ratio of (pGSK3βser9/actin): (totalGSK3β/actin) expressed in 3xTg Alzheimer’s disease (AD) mouse hippocampus. (B) LA treatment increases expression of beta-catenin. Bars with SE show ratio of beta-catenin/actin expressed in 3xTg hippocampus. (C) Representative western blots of 3xTg AD hippocampus. (D) OVX-associated decrease in cortical brain-derived neurotrophic factor (BDNF) transcription is rescued by LA treatment. Bars with SE show the relative transcription of BDNF to 18S in the cortex of 3xTg mice. Significant difference (p ≤ 0.05) from SHAM (a), from OVX+SAL (b), from OVX+LA (c), from OVX+E2 (d).
Beta-catenin is stabilized through the inhibition of GSK3β and results in memory improvements in mice (Maguschak and Ressler 2008; Toledo and Inestrosa 2010). To this end, we found significant differences in beta-catenin expression across treatments (F(3,18) = 3.97, p = 0.025; Fig. 3b and c). LA treatment significantly increased beta-catenin in comparison to SHAM (p = 0.004) and OVX+SAL control (p = 0.036), and estrogen replacement increased beta-catenin in comparison to SHAM (p = 0.05). Interestingly, while OVX resulted in increases in GSK3b inhibition, these increases did not translate into stabilization of beta-catenin.
These findings resulted in additional inquiry into markers of synaptic plasticity including BDNF, an up-stream modulator of GSK3β and beta-catenin (Chen et al. 2013) known to be altered in AD (Phillips et al. 1991). Real-time PCR analysis of cortical BDNF transcription showed a significant difference between treatment groups (F(3,19) = 5.12, p = 0.01; Fig. 3d). OVX significantly decreased BDNF transcription compared to SHAM-operated animals (p = 0.049) and this loss was normalized by LA treatment (p = 0.05), but not estrogen replacement.
Gonadotropin immunofluorescence and transcription
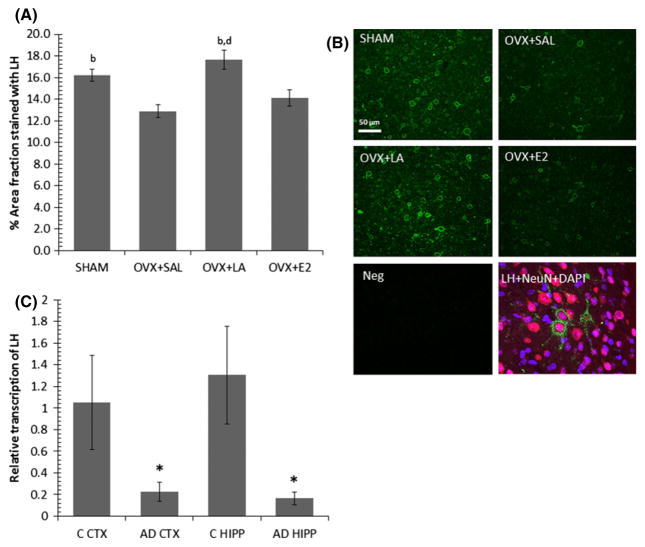
LH immunofluorescent staining of mid-sagittal sections of 3xTg AD brains was observed regionally, and was found to be particularly strong in the superior colliculus with significant differences between treatment groups (F(3,13) = 6.67, p = 0.006; Fig. 4a and b). There was a substantial loss of LH in the superior colliculus because of ovariectomy (p = 0.035). LA treatment rescued this loss to the level of SHAM, exceeding OVX+SAL control (p = 0.002) and OVX+E2 (p = 0.005).
Fig. 4.
(A) LA treatment rescues OVX-associated reductions in luteinizing hormone (LH) levels in the superior colliculi of 3xTg Alzheimer’s disease (AD) mice. Bars with SE show randomly sampled regions of immunofluorescent LH staining in the superior colliculi of female 21 month old 3xTg AD mice. Significant difference (p ≤ 0.05) from SHAM (a), from OVX+SAL (b), from OVX+LA (c), from OVX+E2 (d). (B) Representative samples of LH immunofluorescent staining in the superior colliculi of 21 month female 3xTg AD mice, specificity control (Neg), and colocalization of LH with a neuronal marker (NeuN). Images for LH immunostaining were captured at 20× magnification; scale bar distance shows 50 μm. Triple labeling with LH, NeuN and DAPI was taken at 40×. (C) Human female AD brains show significantly less LH transcription in the hippocampus and cortex. Bars with SE show a loss in the relative transcription of LH to 18S in the cortex (CTX) and hippocampus (HIPP) of AD-diagnosed female patients (AD) compared to healthy age-matched female controls (C) *significant difference from controls, p ≤ 0.05.
Based on the rodent findings, we examined whether LH transcription was present in human brain, and if it was different between AD diagnosed patients and non-demented age-matched and PMI-matched controls. Independent t tests revealed a significant loss of LH transcription in AD compared to controls for both the cortex (t(12) = 2.16, p = 0.05; Fig. 5c) and hippocampus (t(11) = 2.29, p = 0.043; Fig. 5c). In addition, we also measured brain mRNA FSH levels, another gonadotropin altered by our treatment, and found those to be transcribed at extremely low levels compared to LH and unchanged between these two groups, lending further credence to the importance of LH and not FSH in cognitive processes.
Fig. 5.
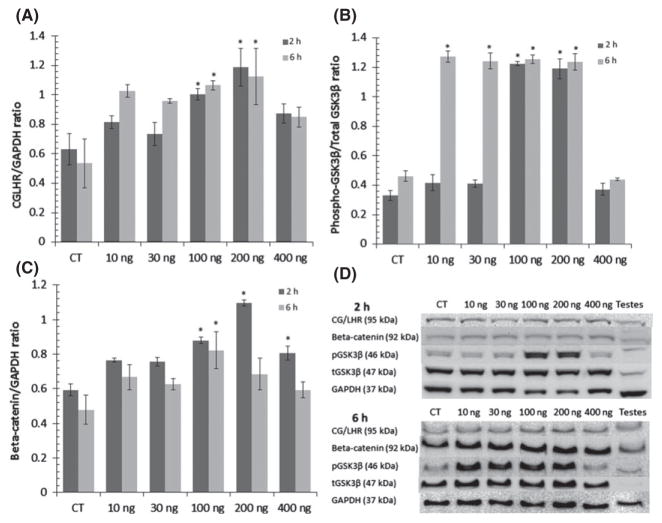
(A) Choriogonadotropin/luteinizing hormone receptor (CG/LHR) is expressed in rat cortical primary neurons and its expression is increased by human chorionic gonadotropin (hCG) treatment over 2 and 6 h, but is desensitized at high doses. Bars with SE show the ratio of (CG/LHR): [glyceraldehyde 3-phosphate dehydrogenase (GAPDH] expression in rat cortical culture treated with hCG. (B) GSK3β is inhibited by hCG treatment on rat cortical culture over 2 and 6 h. Bars with SE show the ratio of (pGSK3βser9/actin): (totalGSK3β/actin) in rat cortical culture treated with hCG. (C) Beta-catenin expression in rat cortical culture is increased over 2 and 6 h treatments with hCG. Bars with SE show the ratio of beta-catenin/GAPDH expression in rat cortical culture treated with hCG *significant difference from controls, p ≤ 0.05. (D) Representative western blots of rat cortical culture.
Correlations
We examined the presence of relationships between our treatments and effects using Pearson correlations. Day 4 latency in the MWM was tightly correlated with the MWM probe trial performance indicating accuracy between these behavioral measures (r(21) = −0.625, p = 0.002). Importantly, MWM probe performance positively correlated GSK3β inhibition (r(16) = 0.511, p = 0.043) and beta-catenin expression (r(16) = 0.623, p = 0.01). BDNF transcription correlated negatively with MWM day 4 latency (r(22) = −0.419, p = 0.05) and had a positive correlation toward MWM probe performance (r(21) = 0.572, p = 0.007). To our surprise, we found that superior colliculus LH levels positively correlated with MWM performance (r(17) = 0.574, p = 0.016) and GSK3β inhibition (r(14) = 0.524, p = 0.05), indicating a possible role of the hormone in neuronal signaling pathways that regulate this enzyme. Superior colliculus LH showed strong positive correlation trends with beta-catenin and BDNF, whereas GSK3β inhibition trended toward a positive correlation with beta-catenin expression; however, these relationships were not statistically significant (p = 0.09–0.12). Importantly, our data also showed a strong negative relationship trend between serum LH and superior colliculus LH (p = 0.07) suggesting an inverse relationship between brain and pituitary LH synthesis.
GSK3β and beta-catenin modulation by hCG in primary culture
To determine whether CG/LHR activation led to changes in GSKβ inhibition and beta-catenin stabilization in the CNS, we treated rat cortical primary neurons with hCG which shares the CG/LHR with LH but has a longer half-life. First, CG/LHR expression was confirmed in vitro and the overall ANOVA analysis showed significant dose and time-dependent receptor expression changes after the 2 h treatment (F(5,18) = 5.76, p = 0.002) but not statistically significant after the 6 h treatment (p = 0.06; Fig. 5a and d). Dunnett post hoc comparisons indicated that 100 and 200 ng treatments increased CG/LHR expression from control for the both the 2 h (p = 0.02, p = 0.001) and 6 h treatments (p = 0.044, p = 0.025) but returned to normal at high doses of hCG.
Statistical differences in GSK3β inhibition were observed after both 2 h (F(5,18) = 98.50, p ≤ 0.001) and 6 h treatments (F(5,18) = 105.70, p ≤ 0.001; Fig. 5b and d). Post hoc analysis indicated GSK3β was significantly inhibited by 100 ng and 200 ng of hCG after 2 h (p ≤ 0.001), and by 10, 30, 100 and 200 ng doses after 6 h of treatment (p ≤ 0.001).
An overall ANOVA indicated beta-catenin expression was altered by hCG treatment after the 2 h (F(5,18) = 10.69, p ≤ 0.001) but not after the 6 h of treatment (p = 0.1; Fig. 5c and d). Post hoc analysis indicated beta-catenin expression was significantly increased by 100 ng and 200 ng treatments after 2 h (p = 0.004, p ≤ 0.001), and only the 100 ng dose differed from control after the 6 h treatment (p = 0.021).
Discussion
Our data demonstrate three main and novel findings: (i) down-regulation of serum gonadotropins, not estrogen replacement, is able to improve hippocampal spatial memory in 3xTg AD female mice in advanced stages of disease, (ii) improvements in cognitive function by down-regulation of serum LH are independent of amyloid and tau pathology, but appear to be associated with GSK3β and alterations in synaptic plasticity, and (iii) changes in locally produced brain LH may drive the effects on cognition and signaling-associated markers that are observed after down-regulation of serum LH.
Previous findings show that estrogen-associated cognitive improvements observed in this 3xTg AD mouse strain (Carroll et al. 2007, 2010) appear to be eliminated by 12 months of age (Singh et al. 2012). In accordance with these results, our data show that estrogen did not provide cognitive benefit in 3xTg AD female mice at 21 months despite the presence of physiological levels of estrogen. This lends support to a critical period hypothesis in which brain health is necessary to benefit from estrogenic supplementation (Sohrabji 2005). As such, while estrogen treatment prior to neurological damage is neuroprotective, pathology-associated or independent injuries (Brinton 2008; Yao and Brinton 2012) that are present in advanced AD stages may lead to an opposite outcome of estrogen replacement (Brinton 2005).
In addition to the healthy cell bias hypothesis, it is clear from our data that lowering serum gonadotropins is beneficial to cognition. These support previous findings of gonadotropin inhibition-associated improvements in behavior in amyloid precursor protein 2576 Tg mice (Casadesus et al. 2007) as well as in wild-type rodents after ovariectomy (Bryan et al. 2010; Ziegler and Thornton 2010). However, this study fails to support previous evidence showing reductions in Aβ after LA treatment (Bowen et al. 2004; Casadesus et al. 2006). This discrepancy may be methodologically based as animals in previous studies were not ovariectomized, or because of a transgene effect as 3xTg AD mice have dual amyloid and tau mutations (Oddo et al. 2003b). Future studies should concentrate on understanding the relationship between ovariectomy and pathology as well as carefully dissecting the contribution of combined tau and amyloid precursor protein mutations on hormone processes associated with cognition.
Previous research from our laboratory has shown that down-regulation of serum gonadotropins can regulate cascades important for memory formation such as calcium/calmodulin-dependent protein kinase II and cAMP-response-element-binding protein phosphorylation (Bryan et al. 2010). These signaling cascades have been shown to be associated with the regulation of BDNF (Chen et al. 2012), that is known to be critically involved in neuroplasticity (Bartoletti et al. 2002; Bittner et al. 2010; Blurton-Jones et al. 2009; Nagahara et al. 2009), protective against Aβ toxicity (Li et al. 2012), and reduced in AD (Phillips et al. 1991). Here we show that OVX-associated BDNF transcription reductions are normalized when LH, but not E2, is brought to pre-OVX levels, suggesting that menopausal alterations in LH levels may modulate transcription of this protein. Importantly, the inability of E2 to normalize OVX-associated BDNF transcription reductions, despite its ability to down-regulate serum LH via pituitary negative feedback, implicates gonadotropin-independent mechanisms associated with the lack of effects of E2 replacement within an advanced AD context (Brinton 2005). Nevertheless, the fact that our SHAM operated animals showed cognitive impairment despite BDNF transcription levels being similar to the LA-treated group suggests that other aspects beyond BDNF transcription are at play in the observed cognitive improvement. This is supported by the fact that while SHAM operated animals did not show improvements, BDNF transcription levels were positively correlated with cognitive function. Therefore, aspects such as translational efficiency of the transcript, the maturation of BDNF or changes in levels or truncation of tropomyosin related kinase B may influence the outcome with regard to cognition and need to be further studied.
The Wnt pathway is also intimately associated with neuroplasticity (Chen et al. 2006, 2013) as well as AD (Balaraman et al. 2006; Hooper et al. 2008; Martinez and Perez 2008). To this end, our data show that down-regulation of gonadotropins has a powerful effect on GSK3β inhibition that goes beyond SHAM operated animals. Importantly, changes in GSK3β are correlated with cognitive improvements in our animals suggesting a relationship between this pathway and cognition. Interestingly, our results show that GSK3β inhibition was also increased by OVX but these animals showed no cognitive improvement. Based on this, it is clear that depletion of estrogen increases GSK3β inhibition. Nevertheless, in comparison to the OVX control, only the LA-treated group showed enhancements in beta-catenin expression, a protein regulated by GSKβ inhibition (Boonen et al. 2009) and known to be reduced in AD (He and Shen 2009). Furthermore, beta-catenin expression was strongly correlated with cognitive function, suggesting that other signaling mechanisms inhibit this pathway independently of GSK3β regulation (Topol et al. 2003).
Taken together, these findings indicate that hormonal changes have a profound impact on Wnt pathway proteins such as GSK3β and beta-catenin, and that changes in these signaling proteins are associated with cognitive changes after menopause. Interestingly, while we found GSK3β inhibition to be powerfully up-regulated by OVX and down-regulation of LH, we did not detect statistically significant changes in tau phosphorylation, an aspect dependent on GSK3β inhibition, but rather we saw large increases in tau pathology in the OVX groups. Our data therefore suggests that tau phosphorylation increases after ovariectomy are GSK3β inhibition-independent in this AD model. Consequently, while down-regulation of LH may have been effective at further inhibiting GSK3β and regulating aspects that are beneficial for cognitive function, this treatment as well as estrogen replacement were unable to regulate mechanisms associated with tau phosphorylation and thus reduce pathology.
In connection with our signaling findings, it is well known that the activation of the LH receptor in the gonads drives the inhibition of GSK3β and results in the increased stability of beta-catenin (Roy et al. 2009). As such, given the apparent conflict observed between what is known with regard to CG/LHR activation and our in vivo data demonstrating activation of receptor-dependent cascades despite reduced levels of peripheral LH, we determined whether endogenous brain LH existed in human and rodent brain tissue and whether a relationship existed between brain LH, serum LH, and cognition in the female 3xTg AD mice.
To this end, we found that transcription of LH was significantly lower in the cortex and hippocampus tissue of human female AD brains in comparison to healthy controls. Similarly, we found that inhibition of serum LH in rodents led to increases in brain LH in cognition-associated areas including the cingulate, thalamus, hippocampus, hypothalamus, and superior colliculi: all regions of the brain that have been previously shown to express the CG/LHR (Lei et al. 1993; Hämäläinen et al. 1999; Apaja et al. 2004). As a result of the sagittal preparation of our paraffin-embedded tissue, and the precise anatomical localization of LH in the brain, we were only able to systematically quantify LH expression in the superior colliculus, which is relevant given its critical involvement in spatial ability in primates (Klier et al. 2003; Takaura et al. 2011), and its importance for rodent MWM performance (Jeljeli et al. 1999). Importantly, we found that LH increases in the superior colliculus were positively correlated with cognitive performance and negatively correlated with serum LH.
Lastly, we sought to confirm whether CG/LHR activation in neurons mimics the signaling events which it mediates in gonadal cell lines (Roy et al. 2009), and thus could explain our in vivo findings. Our data show that in vitro hCG treatment, which shares the same receptor with LH, led to marked increases in GSK3β inhibition and beta-catenin expression as well as the up-regulation of the CG/LHR in a dose- and time-dependent manner. Conversely, high doses of hCG led to inhibition of signal and receptor expression, an aspect that is known to occur in gonadal cells via down-regulation of receptor transcription and increased internalization of the receptor (Bockaert et al. 1976; Hunzicker-Dunn et al. 1979; Ascoli et al. 2002). Taken together our findings suggest that the CG/LHR receptor is functional in neurons and drives cascades similar to those observed in vivo, when ligand levels brain LH are high.
Taken together, while the mechanisms underlying the inverse relationship between peripheral and central LH remain unclear, our data demonstrates that the cognitive improvements observed when peripheral/serum LH is inhibited could be potentially mediated via the up-regulation of central LH synthesis and the consequent increased activation of the CG/LHR. Future studies should seek to further understand the mechanisms underlying CG/LHR activation in the CNS.
Acknowledgments
This work is dedicated to Mark A Smith Ph.D. who passed away during the conclusion of these studies. Because of his critical contribution to the conception of this work, he is listed as an author of this publication. Funds for this study were derived from NIA - R01 AG032325-01.
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations used
- AD
Alzheimer’s disease
- CG
choriogonadotropin
- hCG
human chorionic gonadotropin
- LH
luteinizing hormone
- LHR
luteinizing hormone receptor
- MWM
Morris Water Maze
- NGS
normal goat serum
Footnotes
conflict of interest disclosure
The authors have no conflict of interest to declare.
References
- Apaja PM, Harju KT, Aatsinki JT, Petäjä-Repo UE, Rajaniemi HJ. Identification and structural characterization of the neuronal luteinizing hormone receptor associated with sensory systems. J Biol Chem. 2004;279:1899–1906. doi: 10.1074/jbc.M311395200. [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3beta and Alzheimer’s disease: pathophysiological and therapeutic significance. Cell Mol Life Sci. 2006;63:1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti A, Cancedda L, Reid SW, Tessarollo L, Porciatti V, Pizzorusso T, Maffei L. Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation. J Neurosci. 2002;22:10072–10077. doi: 10.1523/JNEUROSCI.22-23-10072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bittner T, Fuhrmann M, Burgold S, Ochs SM, Hoffmann N, Mitteregger G, Kretzschmar H, LaFerla FM, Herms J. Multiple events lead to dendritic spine loss in triple transgenic Alzheimer’s disease mice. PLoS ONE. 2010;5:e15477. doi: 10.1371/journal.pone.0015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Hunzicker-Dunn M, Birnbaumer L. Hormone-stimulated desensitization of hormone-dependent adenylyl cyclase. Dual action of luteninizing hormone on pig graafian follicle membranes. J Biol Chem. 1976;251:2653–2663. [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology. 2010;35:694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Boonen RA, Van Tijn P, Zivkovic D. Wnt signaling in Alzheimer’s disease: up or down, that is the question. Ageing Res Rev. 2009;8:71–82. doi: 10.1016/j.arr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-β precursor protein and amyloid-β deposition. J Biol Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan KJ, Mudd JC, Richardson SL, Chang J, Lee H, Zhu X, Smith MA, Casadesus G. Down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits. J Neurochem. 2010;112:870–881. doi: 10.1111/j.1471-4159.2009.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Villamagna A, Pike CJ. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3xTransgenic-Alzheimer’s disease mice. Endocrinology. 2010;151:2713–2722. doi: 10.1210/en.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-β deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao C, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269:107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Collins WP, Forecast JD, Newton JR, Oram DH, Studd JW. Hormonal profiles after the menopause. Br Med J. 1976;2:784–787. doi: 10.1136/bmj.2.6039.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci. 2012;15:1707–1714. doi: 10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW, Luo ZJ. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J Neurosci Res. 2013;91:30–41. doi: 10.1002/jnr.23138. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105:526–535. doi: 10.1093/jnci/djt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010;27:1–30. Available online at http://www.ncbi.nlm.nih.gov/pubmed/20445638. [PubMed] [Google Scholar]
- Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ER[alpha] on spatial learning. Horm Behav. 1998;34:163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Hämäläinen T, Poutanen M, Huhtaniemi I. Age- and sex-specific promoter function of a 2-Kilobase 5′-flanking sequence of the murine luteinizing hormone receptor gene in transgenic mice. Endocrinology. 1999;140:5322–5329. doi: 10.1210/endo.140.11.7115. [DOI] [PubMed] [Google Scholar]
- He P, Shen Y. Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer’s disease. J Neurosci. 2009;29:6545–6557. doi: 10.1523/JNEUROSCI.0421-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Jungmann R, Derda D, Birnbaumer L. LH-induced desensitization of the adenylyl cyclase system in ovarian follicles. Adv Exp Med Biol. 1979;112:27–44. doi: 10.1007/978-1-4684-3474-3_3. [DOI] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeljeli M, Strazielle C, Caston J, Lalonde R. Effects of electrolytic lesions of the lateral pallidum on motor coordination, spatial learning, and regional brain variations of cytochrome oxidase activity in rats. Behav Brain Res. 1999;102:61–71. doi: 10.1016/s0166-4328(98)00162-4. [DOI] [PubMed] [Google Scholar]
- King JC, Anthony EL, Damassa DA, Elkind-Hirsch KE. Morphological evidence that luteinizing hormone-releasing hormone neurons participate in the suppression by estradiol of pituitary luteinizing hormone secretion in ovariectomized rats. Neuroendocrinology. 1987;45:1–13. doi: 10.1159/000124698. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. Three-dimensional eye-head coordination is implemented downstream from the superior colliculus. J Neurophysiol. 2003;89:2839–2853. doi: 10.1152/jn.00763.2002. [DOI] [PubMed] [Google Scholar]
- Lee H, Zhu X, Casadesus G, Pallàs M, Camins A, O’Neill MJ, Nakanishi S, Perry G, Smith MA. The effect of mGluR2 activation on signal transduction pathways and neuronal cell survival. Brain Res. 2009;1249:244–250. doi: 10.1016/j.brainres.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Rao C, Kornyei J, Licht P, Hiatt E. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132:2262–2270. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- Li QS, Yang W, Pan YF, Min J, Zhang Z, Gao HZ, Qi JS. Brain-derived neurotrophic factor prevents against amyloid beta protein-induced impairment of hippocampal in vivo long-term potentiation in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2012;28:425–429. [PubMed] [Google Scholar]
- Lloyd JM, Hoffman GE, Wise PM. Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology. 1994;134:1800–1805. doi: 10.1210/endo.134.4.8137745. [DOI] [PubMed] [Google Scholar]
- Lukacs H, Hiatt ES, Lei ZM, Rao CV. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav. 1995;29:42–58. doi: 10.1006/hbeh.1995.1004. [DOI] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. β-Catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Perez DI. GSK-3 inhibitors: a ray of hope for the treatment of Alzheimer’s disease? J Alzheimer’s Dis. 2008;15:181–191. doi: 10.3233/jad-2008-15204. [DOI] [PubMed] [Google Scholar]
- McConnell SEA, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm Behav. 2012;61:479–486. doi: 10.1016/j.yhbeh.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaves WB, Johnson L, Porter JC, Jr, Parker CrR, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59:756–763. doi: 10.1210/jcem-59-4-756. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003a;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular A[beta] and synaptic dysfunction. Neuron. 2003b;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Payami H, Montee K, Grimslid H, Shattuc S, Kaye J. Increased risk of familial late-onset Alzheimer’s disease in women. Neurology. 1996;46:126–129. doi: 10.1212/wnl.46.1.126. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rissanen A, Puoliväli J, van Groen T, Riekkinen P. In mice tonic estrogen replacement therapy improves non-spatial and spatial memory in a water maze task. NeuroReport. 1999;10:1369–1372. doi: 10.1097/00001756-199904260-00039. [DOI] [PubMed] [Google Scholar]
- Rodrigues MA, Verdile G, Foster JK, et al. Gonadotropins and cognition in older women. J Alzheimer’s Dis. 2008;13:267–274. doi: 10.3233/jad-2008-13304. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci. 2011;16:1560–1573. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmanith WG, Reichelt C, Scherbaum WA. Neuroendocrinology of aging in humans: attenuated sensitivity to sex steroid feedback in elderly postmenopausal women. Neuroendocrinology. 1994;59:355–362. doi: 10.1159/000126678. [DOI] [PubMed] [Google Scholar]
- Roy L, McDonald CA, Jiang C, Maroni D, Zeleznik AJ, Wyatt TA, Hou X, Davis JS. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3β/β-catenin signaling in corpus luteum progesterone synthesis. Endocrinology. 2009;150:5036–5045. doi: 10.1210/en.2009-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology. 2002;143:2986–2994. doi: 10.1210/endo.143.8.8976. [DOI] [PubMed] [Google Scholar]
- Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- Singh C, Liu L, Wang JM, Irwin RW, Yao J, Chen S, Henry S, Thompson RF, Brinton RD. Allopregnanolone restores hippocampal-dependent learning and memory and neural progenitor survival in aging 3xTgAD and nonTg mice. Neurobiol Aging. 2012;33:1493–1506. doi: 10.1016/j.neurobiolaging.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F. Estrogen: a neuroprotective or proinflammatory hormone? Emerging evidence from reproductive aging models. Ann N Y Acad Sci. 2005;1052:75–90. doi: 10.1196/annals.1347.006. [DOI] [PubMed] [Google Scholar]
- Sudo S, Wen T, Desaki J, Matsuda S, Tanaka J, Arai T, Maeda N, Sakanaka M. Beta-Estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil. Neurosci Res. 1997;29:345–354. doi: 10.1016/s0168-0102(97)00106-5. [DOI] [PubMed] [Google Scholar]
- Takaura K, Yoshida M, Isa T. Neural substrate of spatial memory in the superior colliculus after damage to the primary visual cortex. J Neurosci. 2011;31:4233–4241. doi: 10.1523/JNEUROSCI.5143-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Matsuo A, Himeno Y, et al. 17beta-estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neuroscience. 2007;146:60–68. doi: 10.1016/j.neuroscience.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol Psychiatry. 2010;15:272–285. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS ONE. 2010;5:e9851. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Brinton RD. Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer’s disease. Adv Pharmacol. 2012;64:327–371. doi: 10.1016/B978-0-12-394816-8.00010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SG, Thornton JE. Low luteinizing hormone enhances spatial memory and has protective effects on memory loss in rats. Horm Behav. 2010;58:705–713. doi: 10.1016/j.yhbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]