Abstract
Introduction
Chordoid Gliomas (CG) are rare neoplasms which frequently arise within the third ventricle. Surgery remains the mainstay treatment for CG. The present study comprehensively reviews all reported cases of CG within the literature in order to identify risk factors for surgical complications and tumor recurrence.
Methods
A comprehensive search on MEDLINE (OVID and Pubmed), Scopus, Embase, and Web of Science was conducted following PRISMA guidelines to identify all reported cases of CG.
Results
A total of 81 patients met the study criteria, comprised of 33 males and 48 females. Median age at diagnosis was 48 years with a range from 5 to 72 years, and mean tumor size was 3.1 cm. Biopsy, subtotal resection (STR), and gross total resection (GTR) was achieved in 8, 34, and 33 patients, respectively, with 6 cases not reporting extent of eresection (EOR). Thirteen patients underwent adjuvant radiotherapy. Postoperative complications were noted in 30 cases (37%), with new onset diabetes insipidus being the most common. Postoperative morbidity was not associated with age, tumor size, or extent of resection. A trans-lamina terminalis approach demonstrated a strong trend towards decreased overall rates of postoperative morbidity compared to other approaches (p=0.051). GTR was associated with improved progression-free survival (PFS; p=0.028), while adjuvant radiotherapy, age, tumor size and MIB-I were not predictive of patient outcomes.
Conclusion
GTR should be the primary goal for the management of CG, as it is associated with improved rates of tumor control without an increased rate of postoperative complications. Surgical approach was a stronger predictor of complication rates than extent of resection. Morbidity remains high, and future studies to further elaborate on factors predictive of postoperative complications are critical.
Keywords: chordoid glioma, surgery, radiation therapy, hypothalamic dysfunction, diabetes insipidus
Introduction
Intraventricular neoplasms can be highly challenging to treat due to an elevated morbidity profile associated with surgical approach to resection. Tumors that originate in the anterior portion of the 3rd ventricle include ependymomas, central neurocytomas, craniopharyngiomas, and suprasellar meningiomas. Chordoid gliomas (CG) are rare intracranial tumors characterized by the histological presence of both glial and chordoid elements that also occur primarily in this location. Initially described by Brat et al[1], CG are classified as World Health Organization (WHO) grade II neuroepithelial tumors of uncertain histogenesis.[2] CG are typically found within the suprasellar region and the anterior third ventricle. These tumors present as solid, hyperdense lesions on computed tomography (CT), with uniform contrast enhancement on T1-weighted MRI, and mild hyperintensity on T2-weighted MRI. Cystic components have also been observed on rare occasions.[3] Histologically, CG are comprised of clusters and cords of epithelial cells in a mucinous matrix background with a strong presence of GFAP staining on immunohistochemistry.[4]
Their specific location of origin results in a unique symptom profile at presentation consisting of intracranial hypertension symptoms associated with obstructive hydrocephalus, hypothalamic dysfunction, and/or visual impairment. Their close association, and sometimes tight adherence, to the hypothalamus makes en bloc resection difficult due to the increased risk of post-operative diabetes insipidus (DI) and other neuroendocrine dysfunction. Their growth is indolent and patients may present with persistent symptoms that originated months, or even years, previously. Surgeons have attempted to balance maximal cytoreduction with complication avoidance, and radiotherapy is often used as an adjuvant therapy in patients that undergo subtotal resection. Factors that govern morbidity, mortality, and recurrence in this disease have not been clearly elucidated and there is a lack of large retrospective studies on these tumors due to their rare nature. Various case reports and case series have attempted to illustrate some of these important points. The current systematic review accrued data from 81 patients presented in various case reports and smaller case series in the literature in order to elucidate factors that influence recurrence, morbidity, and mortality, of patients with chordoid gliomas.
Methods
Literature search
Two researchers (LA, WC) each performed independent literature searches on MEDLINE (OVID and Pubmed), Embase, Scopus, and Web of Science with the keywords “chordoid” AND “glioma” to identify all published reports on CGs. Each database was searched on 3/9/2015 and no publication date restriction was placed on the study. The search was further refined by limiting to manuscripts published in English. Each reviewer constructed a data sheet for each searched database, which were utilized to compare and remove duplicate studies. Data sheets were also compared between researchers in order to agree and confirm the eligible studies included. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was applied to this review. The protocol was not registered. (Figure 1) Our inclusion criteria were simplified by including all pathologically confirmed cases of intracranial CGs with disaggregated data available for each patient reported. Cases were excluded for studies that did not provide adequate treatment or clinical parameters for each individual patient.
Figure 1.
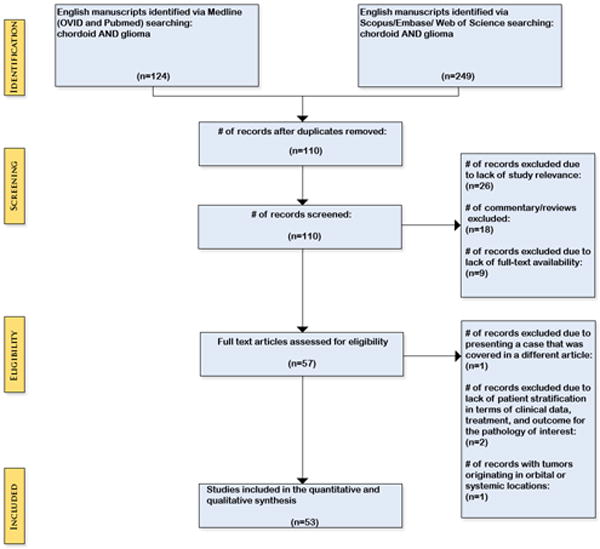
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
Data collection
The following variables were collected from each study: age, gender, tumor location, size, duration of symptoms, surgical approach, extent of resection, Ki-67/MIB-1 proliferation index, post-operative complications, adjuvant radiotherapy, progression-free survival, recurrence status, follow-up time, patient clinical status on last follow-up, and overall survival. All components were not documented in each retrospective study and case report included in the review.
Statistical analysis
SPSS version 20.0 (Armonk, NY, IBM Corp) was utilized for all statistical analysis. Evaluation of rates of recurrence and overall survival were performed through Kaplan Meier analysis and log rank tests. Further subgroup analysis was also performed based on possible prognostic factors through univariate Kaplan Meier analysis. Fisher’s exact test was utilized to evaluate categorical data, and student’s t-test for comparing differences in means between subgroups. Tests were two tailed, and P < 0.05 was considered statistically significant.
Results
A total of 81 patients met study criteria and were included in our analysis. (Table 1 and Table 2) There were 33 males and 48 females. Median age at diagnosis was 48 years (range 5–72 years). Tumor diameter was reported in 42 patients, with a mean diameter of 3.1 cm (range 1.5 to 7.0 cm). Tumor diameter at presentation was neither correlated with MIB-1 labeling (p = 0.52) or time from symptom onset to clinical diagnosis (p =0.35).
Table 1.
Summary of studies and patients included within analysis.
| Author (year) | # of patients | Mean Age | Extent of Resection | Adjuvant Radiotherapy | Recurrences | Deaths |
|---|---|---|---|---|---|---|
| Morais et al. (2015)[56] | 1 | 13 | NA | 0 | 2 | 0 |
| Tanboon et al. (2014)[30] | 1 | 29 | GTR | 0 | 0 | 0 |
| Al-Zubidi et al. (2014)[41] | 1 | 37 | STR | 0 | 0 | 0 |
| Kobayashi et al. (2013)[47] | 3 | 49 | Bx (n=2) STR (n=1) |
3 | 0 | 1 |
| Ni et al. (2013)[17] | 4 | 40 | GTR (n=4) | 0 | 0 | 0 |
| Shiramizu et al. (2013)[54] | 1 | 49 | GTR | 0 | 0 | 0 |
| Scheurkogel et al. (2012)[55] | 1 | 30 | STR | 0 | 0 | 0 |
| Romero-Rojas et al. (2012)[10] | 1 | 39 | GTR | 0 | 0 | 0 |
| Can et al. (2012)[38] | 1 | 37 | GTR | 0 | 0 | 0 |
| Sanches et al. (2012)[49] | 1 | 59 | Bx | 0 | 0 | 0 |
| Xian et al. (2012)[39] | 1 | 53 | GTR | 0 | 0 | 0 |
| Bastin et al. (2012)[24] | 1 | 31 | STR | 0 | 0 | 0 |
| Ghosal et al. (2012)[59] | 1 | 48 | STR | 0 | 0 | 0 |
| Vij et al. (2011)[15] | 1 | 48 | STR | 0 | 0 | 0 |
| Liu et al. (2011)[31] | 1 | 45 | STR | 0 | 0 | 0 |
| Tu et al. (2010)[7] | 1 | 46 | STR | 0 | 0 | 0 |
| Kim et al. (2010)[21] | 1 | 27 | STR | 0 | 0 | 0 |
| DeSouza et al. (2010)[60] | 2 | 42 | GTR (n=1) STR (n=1) |
0 | 0 | 0 |
| Sugita et al. (2010)[37] | 1 | 55 | STR | 0 | 0 | 0 |
| Horbinski et al. (2009)[12] | 5 | 47 | GTR (n=1) NA (n=4) |
0 | 0 | 0 |
| Dziurzynski et al. (2009)[22] | 1 | 41 | Bx | 0 | 0 | 0 |
| Kawasaki et al. (2009)[26] | 2 | 47 | GTR (n=1) STR (n=1) |
0 | 0 | 1 |
| Vanhauwaert et al. (2008)[52] | 1 | 58 | GTR | 0 | 0 | 0 |
| Molnar et al. (2008)[11] | 1 | 34 | STR | 0 | 0 | 1 |
| Carrasco et al. (2008)[4] | 1 | 53 | GTR | 0 | 0 | 0 |
| Jain et al. (2008)[14] | 2 | 31 | GTR (n=2) | 0 | 0 | 1 |
| Gallina et al. (2007)[6] | 1 | 56 | GTR | 0 | 0 | 0 |
| Chung et al. (2007)[13] | 1 | 48 | Bx | 1 | 1 | 0 |
| Goyal et al. (2007)[27] | 1 | 5 | STR | 0 | 0 | 1 |
| Jung et al. (2006)[25] | 1 | 50 | STR | 0 | 1 | 1 |
| Nga et al. (2006)[51] | 1 | 49 | NA | 0 | 1 | 1 |
| Leeds et al. (2006)[48] | 1 | 57 | Bx | 0 | 0 | 0 |
| Baehring et al. (2006)[32] | 1 | 71 | Bx | 0 | 0 | 0 |
| Hsu et al. (2005)[36] | 1 | 50 | GTR | 0 | 0 | 0 |
| Kurian et al. (2005)[18] | 2 | 35 | STR (n=1) Bx (n=1) |
2 | 1 | 1 |
| Buccoliero et al. (2004)[5] | 1 | 56 | GTR | 0 | 0 | 0 |
| Suh et al. (2003)[33] | 1 | 48 | GTR | 0 | 0 | 0 |
| Taraszewska et al. (2003)[20] | 2 | 57 | GTR (n=2) | 0 | 0 | 1 |
| Sato et al. (2003)[40] | 1 | 65 | STR | 0 | 0 | 1 |
| Raizer et al. (2003)[50] | 1 | 57 | GTR | 0 | 0 | 0 |
| Nakajima et al. (2003)[44] | 1 | 49 | STR | 1 | 0 | 0 |
| Grand et al. (2002)[34] | 1 | 41 | STR | 0 | 0 | 0 |
| Pasquier et al. (2002)[35] | 2 | 37 | STR (n=2) | 0 | 0 | 0 |
| Hanbali et al. (2001)[29] | 1 | 57 | STR | 1 | 1 | 1 |
| Castellano-Sanchez et al. (2001)[28] | 1 | 12 | STR | 0 | 0 | 0 |
| Galloway et al. (2001)[8] | 1 | 54 | GTR | 0 | 0 | 0 |
| Cenacchi et al. (2001)[23] | 3 | 39 | GTR (n=3) | 0 | 0 | 1 |
| Kochi et al. (2000)[46] | 1 | 51 | STR | 1 | 0 | 0 |
| Castellano-Sanchez et al. (2000)[42] | 1 | 36 | STR | 0 | 0 | 0 |
| Ricoy et al. (2000)[53] | 1 | 41 | GTR | 0 | 0 | 0 |
| Tonami et al. (2000)[19] | 1 | 42 | STR | 1 | 0 | 0 |
| Reifenberger et al. (1999)[16] | 4 | 52 | GTR (n=3) STR (n=1) |
1 | 0 | 2 |
| Vajtai et al. (1999)[45] | 1 | 60 | STR | 0 | 0 | 1 |
| Brat et al. (1998)[9] | 8 | 47 | GTR (n=2) STR (n=6) |
2 | 3 | 3 |
Abbreviations: GTR=Gross Total Resection/STR=Subtotal Resection/Bx=Biopsy
Table 2.
Summary of patient demographics and tumor characteristics
| Median | Range | |
|---|---|---|
| Age | 48 | 5 to 72 |
| Sex | ||
| Female | 48 | |
| Male | 33 | |
| Tumor diameter (cm) | 3 | 1.5 to 7 |
| MIB-LI | 1.7 | 1 to 20 |
| Treatment | ||
| BX | 8 (9.9%) | |
| STR | 34 (42.0%) | |
| GTR | 33 (40.7%) | |
| Unknown | 6 (9.9%) | |
| Recurrences | 8 | |
| Death | 18 |
Clinical presentation
Due to the characteristic location within the third ventricle, nearly all patients presented symptomatically, with common symptoms of headache, nausea, vomiting, and ataxia, presumably from obstructive hydrocephalus. (Table 3). Only 3 of 81 (3.7%) patients reviewed presented incidentally.[10–12] Among the remaining 78 patients, the most common presenting symptoms, in order, were headache, visual symptoms, mental status changes and memory deficits (likely from forniceal involvement), nausea/vomiting, and lethargy/somnolence. Less frequently reported symptoms included ataxia/gait instability [8, 9, 13–16], seizures [6, 17], syncopal episodes [12, 15], and speech difficulties. [7] History of endocrine dysfunction was noted in a number of patients, including amenorrhea in 4 [8, 18–20], diabetes insipidus in 4 [8, 21–23], and hypothyroidism in 2 patients.[8] Psychotic features were noted in 2 patients.[7, 8] In one series, presenting symptoms for 3 patients was not reported.[24]
Table 3.
Frequency of presenting symptoms
| Symptoms | n | % |
|---|---|---|
| Headache | 33 | 40.7 |
| Visual deficits | 29 | 35.8 |
| Mental status/memory changes | 22 | 27.2 |
| Nausea/Vomiting | 11 | 13.6 |
| Lethargy/somnolence | 9 | 11.1 |
| Ataxia | 7 | 8.6 |
| Amenorrhea | 4 | 4.9 |
| Diabetes Insipidus | 4 | 4.9 |
| Seizures | 2 | 2.5 |
| Syncope | 2 | 2.5 |
| Hypothyroidism | 2 | 2.5 |
| Psychotic features | 2 | 2.5 |
| Speech difficulty | 1 | 1.2 |
| Incidental | 3 | 3.7 |
Mean time from onset of symptoms to surgical management was 22.1 months (Range 0 to 20 years). There were 2 outliers with notably longer duration of symptoms. One patient was a 53 year old woman presenting visual deficits for 20 years, and a 50 kg weight gain over the past 10 years. [7] On evaluation, the only neurological symptom was bitemporal hemianopsia, with a 2.5 cm suprasellar tumor on MRI. [7] The other patient was a 55 year old male presenting with a 10 year history of memory deficits, seizures, and urinary incontinence who was noted to have a large mass occupying the anterior third ventricle. [6]
Imaging
Reporting of CT and MR findings in the literature was inconsistent. On CT, CG typically appear as well circumscribed, hyperdense lesions that homogenously enhance following contrast administration. Of the 41 cases with CT performed, 22 reports commented on signal intensity. Of these, 50% of the lesions appeared hyperdense, 18% appeared hypodense and 23% appeared isodense. The remaining 2 CTs demonstrated mixed density.[6, 25] Atypical CT features reported included calcification, intratumoral hemorrhage, and heterogeneous contrast enhancement. Presence of calcification was reported in 7 cases, of which 2 (28.6%) had calcification.[23, 26] Intratumoral hemorrhage was noted in 3 patients.[5, 15, 27] Heterogenous enhancement was noted in 3 patients [6, 28], and includes the present case.
On MRI, lesions are characteristically either isointense or hypointense, and homogenously contrast-enhancing on T1 weighted imaging. Iso- to hyperintense features were noted in 2 cases.[17, 20] Less frequently, CG demonstrated heterogenous enhancement on T1 weighted MRI, which was noted in 6 out of 56 patients (10.7%). Ring enhancement was noted in 1 patient. [22] On T2 weighted MRI, CG frequently appeared as hyperintense or isointense lesions that were homogenously enhancing. Mixed signal intensity was noted in 3 patients.[17, 29, 30] None of the tumors were hypointense. Edema on MRI was noted in 3 patients. [17, 25, 31] Less frequently, cystic components were noted on imaging in 9 patients. [4, 6, 10, 17, 23, 25, 31–36]
Histology
CG share nearly uniform pathological findings comprised of ovoid or polygonal epithelioid cells with abundant cytoplasm organized in clusters and chords. [5, 7, 18, 37] Cells are typically found within a mucinous vacuolated stroma.[5, 7, 18, 37] Tumor immunopositivity for GFAP, EMA, CD34, cytokeratin, S100, and vimentin is commonly present. [5, 11, 38–40] Some degree of lymphocytic infiltrate appears to be common. Of the 57 cases that elaborated on extent of lymphocyte infiltration, 4 reported sparse infiltrate (7.0%). [13, 19, 26] Of the remaining cases, extent of infiltration was highly variable, ranging from occasional [27, 41] to prominent infiltration.[4, 10, 42, 43] Distribution of tumor infiltrating lymphocytes was also highly variable, with peripheral [21], scattered [30], and focal appearance.[44] Mitoses were consistently low [7, 10, 24, 33, 45–48] or absent [35] when noted. Necrosis was not reported in any study. CG characteristically exhibit low MIB-1 labeling, with all but one case with MIB-1 ≤5%. Mean MIB-1 was 2.5% (SD: 3.0%, range 0.3% to 20%) and was reported in 46 patients. Despite typically low proliferation indexes, Sanches et al. reported a case of a 59 year old female presenting with a 2 cm CG demonstrating GFAP and vimentin positivity, EMA negativity, and Ki67 expression in 20% of cells. [49]
Treatment
The most common surgical approaches described to resect CG include transcallosal in 7 [12, 29, 37, 43, 44, 50], transcortical in 5 [22, 31, 51, 52], and trans-lamina terminalis in 17 [4, 11, 13, 17, 20, 23, 42, 43, 46, 47, 53–55]. Surgical approach was not specified in 52 patients. Extent of surgical resection was reported in 75 cases, of which 10.7%, 45.3%, and 44.0% received biopsy, subtotal resection (STR), or gross total resection (GTR), respectively.
Radiotherapy for CG has largely been reserved as adjuvant therapy for the management of residual tumor following resection. However, the optimal role of radiotherapy remains unclear as reported experience in the literature is limited. Only patients who received either biopsies or STR received adjuvant radiotherapy. Specifically, 50% of biopsies (4/8) and 26.5% of STR (9/34) patients also received radiotherapy following resection. [7, 8, 19, 20, 29, 44, 46, 47] Although a number of radiation modalities have been reported, the most frequently cited was stereotactic radiosurgery, which was utilized in 8 cases.[7, 16, 20, 44, 46, 47] Marginal dose of radiosurgery ranged from 12 to 20 Gy.[16, 47] Kobayashi et al. reported on the long term safety and efficacy of Gamma Knife radiosurgery, delivering a lower marginal dose of 10.5 to 12 Gy following either subtotal resection or biopsy to minimize surgical morbidity.[47] Tumor size stabilized in one case and regressed in another at 70 and 66 months follow up, respectively. One patient died from causes unrelated to disease. However, Chung et al. reported recurrence at 8 months following Gamma Knife radiosurgery with a marginal dose of 20 Gy that eventually required subsequent surgery for recurrent disease.[16] Hanbali et al. reported use of focal fractionated radiotherapy delivering a total dose of 54 Gy in 30 fractions that resulted in recurrence at 7 months. [29] Kurian et al. reported poor outcomes following placement of radioactive iridium-192 seeds in 2 patients. One patient had hypothalamic dysfunction and poor clinical outcome at 15 months, while the other patient died shortly after repeat resection for recurrent disease at 9 months.[19] Further data is necessary to determine the optimal modality and dose of radiotherapy in the treatment of these rare tumors.
Complications
Postoperative complications were noted in 31 cases, and are summarized in Table 4. There were 18 cases of hypothalamic dysfunction, of which 13 were new onset diabetes insipidus and 3 were syndrome of inappropriate antidiuretic hormone (SIADH). Less frequently, panhypopituitarism [15, 43] was noted in 2 and weight gain was noted in another 2 cases.[34, 43] New onset seizures were noted 2 days following STR in a 36 year old male.[42] Short-term memory deficits were present in 7 (8.8%) patients postoperatively. In particular, one of these patients continues to have severe amnesia at last follow up of 40 months. [43] Non-neurologic complications including hematoma formation [6, 31, 47] and bacterial meningitis [4, 50] were also reported. There were 5 reported cases of pulmonary embolism (6.3%). [7, 8, 24, 29, 44]
Table 4.
Postoperative complications stratified by EOR and surgical approach
| Overall | GTR | STR/Bx | Transcortial | Translaminar | Transcallosal | Other | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 81 | 33 | 42 | 4 | 17 | 7 | 53 | |||||||
| Number of complication | 31 | 38.3% | 13 | 39.4% | 18 | 42.9% | 3 | 75.0% | 5 | 29.4% | 5 | 71.4% | 18 | 34.0% |
| Hypothalamic dysfunction | 18 | 22.2% | 8 | 24.2% | 9 | 21.4% | 1 | 25.0% | 2 | 11.8% | 4 | 57.1% | 11 | 20.8% |
| DI | 13 | 16.0% | 8 | 24.2% | 4 | 9.5% | 1 | 25.0% | 2 | 11.8% | 3 | 42.9% | 7 | 13.2% |
| SIADH | 3 | 3.7% | 0 | 0.0% | 3 | 7.1% | 1 | 25.0% | 0 | 0.0% | 2 | 28.6% | 1 | 1.9% |
| Hypothermia | 2 | 2.5% | 2 | 6.1% | 1 | 2.4% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 3.8% |
| Panhypopituitarism | 2 | 2.5% | 2 | 6.1% | 0 | 0.0% | 0 | 0.0% | 1 | 5.9% | 0 | 0.0% | 1 | 1.9% |
| Weight gain | 2 | 2.5% | 1 | 3.0% | 1 | 2.4% | 0 | 0.0% | 1 | 5.9% | 0 | 0.0% | 1 | 1.9% |
| Hypothyroidism | 2 | 2.5% | 1 | 3.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 3.8% |
| Organic psychosis | 2 | 2.5% | 1 | 3.0% | 1 | 2.4% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 3.8% |
| Memory deficits | 7 | 8.6% | 2 | 6.1% | 7 | 16.7% | 0 | 0.0% | 1 | 5.9% | 0 | 0.0% | 6 | 11.3% |
| Seizures | 1 | 1.2% | 0 | 0.0% | 1 | 2.4% | 0 | 0.0% | 1 | 5.9% | 0 | 0.0% | 0 | 0.0% |
| Hematoma | 3 | 3.7% | 1 | 3.0% | 2 | 4.8% | 1 | 25.0% | 1 | 5.9% | 0 | 0.0% | 1 | 1.9% |
| Meningitis | 2 | 2.5% | 2 | 6.1% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.9% |
| Myocardial infarction | 2 | 2.5% | 1 | 3.0% | 1 | 2.4% | 0 | 0.0% | 1 | 5.9% | 1 | 14.3% | 0 | 0.0% |
| Atrial fibrillation | 1 | 1.2% | 1 | 3.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 14.3% | 0 | 0.0% |
| Pulmonary embolism | 5 | 6.2% | 2 | 6.1% | 3 | 7.1% | 0 | 0.0% | 0 | 0.0% | 1 | 14.3% | 4 | 7.5% |
| Stroke | 1 | 1.2% | 0 | 0.0% | 1 | 2.4% | 0 | 0.0% | 0 | 0.0% | 1 | 14.3% | 0 | 0.0% |
| Somnolence/lethargy | 3 | 3.7% | 1 | 3.0% | 2 | 4.8% | 1 | 25.0% | 1 | 5.9% | 0 | 0.0% | 1 | 1.9% |
| Persistent hydrocephalus | 1 | 1.2% | 0 | 0.0% | 1 | 2.4% | 0 | 0.0% | 0 | 0.0% | 1 | 14.3% | 0 | 0.0% |
| Visual deficits | 1 | 1.2% | 0 | 0.0% | 1 | 2.4% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.9% |
Incidence of postoperative morbidity was not associated with age (< 50 vs ≥ 50 years old, p=0.77) or tumor size (<3 vs ≥3cm, p=1.00). The rate of overall complications was 39.4% and 42.9% within the GTR and STR/biopsy groups, respectively, with no statistical difference between groups (p=0.82). While there was a higher incidence of DI following GTR compared to biopsy/STR (24.2% vs 9.5%), these differences were not statistically significant (p=0.12).
Of the cases in which surgical approach was specified, rate of postoperative complication were 75.0%, 71.4%, and 29.4% for transcortical, transcallosal, and trans-lamina terminalis aproaches, respectively. Compared to transcortical and transcollasal approaches, trans-lamina terminalis demonstrated a strong trend towards decreased overall rates of postoperative morbidity (p=0.051), although this difference did not quite meet statistically significance. Of note, data on surgical approach was only available in the minority of cases.
Recurrence and Survival
There were a total of 9 tumor progressions noted with a mean follow up of 14.9 months. The rate of 1, 3, and 5 year progression free survival (PFS) was 88.4%, 82.5%, and 70.7%, respectively, for all patients. For the 9 patients with progression, time to progression ranged from 4 to 96 months postoperatively. Progression was noted in 25% following biopsy and 14.7% of patients following STR. There were no progressions within the group of patients receiving GTR. In one patient with progression, extent of resection for initial resection was not reported. [56] Four patients had repeat resections for progressive disease following initial surgery. [16, 19, 29, 56].Of these, 2 later died from causes unrelated to disease, and 1 remains disease free at 29 months of follow up.[16] One 13 year old female had 2 repeat resections for recurrent disease at 8 and 9 years following initial resection.[56]
The extent of tumor resection was the most robust predictor of improved rates of tumor control (Figure 2). GTR was associated with improved outcomes compared to both STR and biopsy alone (p=0.028) (Table 4). Within the subset of patients with STR, rates of 1, 3, and 5 year PFS was 85.2%, 71.0%, and 35.5%, respectively. Of the 8 patients that received biopsy, rates of 1, 3, and 5 year PFS were all 60.0%. Differences in rates of progression were not statistically significant between STR and biopsy groups (p= 0.86), likely related to small sample size. When controlling for extent of resection, adjuvant radiotherapy did not predict rates of disease progression (p=0.11). Additionally, patient age (p=0.61), tumor size (p=0.48) and MIB-1 labeling (p=0.38) also did not predict progression rates on univariate analysis.
Figure 2.
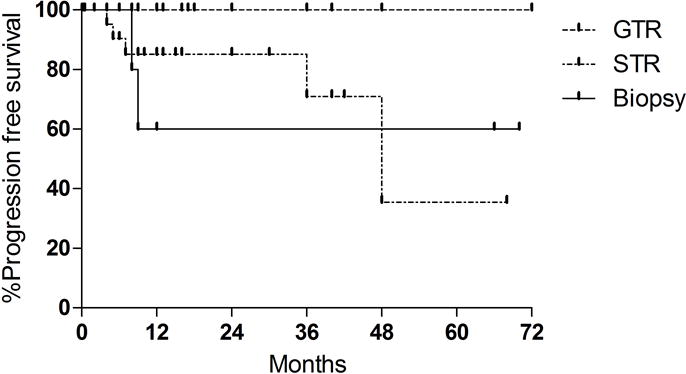
Kaplan Meier analysis of recurrence free survival (PFS) based on extent of resection. STR: subtotal resection. GTR: gross total resection. There were no recurrences within the GTR group. 1-, 3-, and 5 year PFS for STR was 85.2%, 71.0%, and 35.5% respectively. GTR was associated with an improved rate of tumor control than STR and biopsy (p= 0.0276).
Although there were 18 deaths (22.2%) at last follow up, the majority of deaths were not related to disease progression. Nine deaths occurred within 18 days of surgery. Four patients died from pulmonary embolism postoperatively[1, 7, 20, 24], 4 from infectious causes [6, 9, 26, 40], two from myocardial infarction.[5, 21] No deaths from tumor progression were reported in any study. Age, tumor size, MIB, EOR, and RT did not predict survival. For all patients 1 and 5 year overall survival was 78.2% and 63.7%, respectively.
Discussion
Chordoid gliomas are relatively rare tumors considered to be indolent, but still carry an increased risk of recurrence in cases of sub-optimal resection. They are classified as grade II tumors by World Health Organization (WHO) and the current mainstay of treatment consists of surgical intervention with the possibiliy of post-operative radiotherapy in cases of STR. The lesion itself appears pathologically akin to chordomas, possessing clusters and cords, also similar to chordoid meningiomas. The hallmark of this neoplasm is the specific suprasellar and anterior third ventricular region from which it originated. Additionally, these lesions may be adhered to the hypothalamus, which has been thought to carry a greater risk of hypothalamic-associated complications in cases where total resection is attempted. The majority of published studies have been small case series, which makes risk assessment and outcomes of patients with CG unclear and difficult to determine. The present comprehensive literature review represents the largest summarized report of this tumor, reporting 80 distinct cases of CG.
While CG is classified as a WHO grade II neoplasm [57], rates of tumor progression, particularly following STR or biopsy remain very high in a relatively short follow-up period. [47] The 3 and 5 year PFS rate was 71.0% and 35.5% for patients with STR. Therefore, the standard of care for CG remains surgery, and GTR should be the goal when it can be safely performed. There were no reports of recurrence following GTR in any study reported in the literature. However, it is unclear whether GTR is truly curative, as the majority of cases lacked long term follow up. For those patients who did have progression, specifically within the STR group, time to progression ranged from 4 months to 4 years. The limited follow up for select patients undergoing GTR[7, 13, 14, 21, 38, 39, 41, 50, 53] may have precluded detection of later recurrences.
However, treatment should be tailored to individual patients, and GTR may not be safely achievable in select cases of CG. GTR was only achieved in 44% of patients with CG in our analysis. Although extent of resection did not predict postoperative morbidity and mortality in our analysis, complications were frequent following resection. Hypothalamic dysfunction was present in 22.2% of all patients, and most frequently comprised transient DI, which occurred at a rate of 16.0% overall. Other hypothalamic complications include SIADH[22, 29], temperature dysregulation [19], and panhypopituitarism.[15, 43] Surgical approach appears to play a more prognostic role in dictating postoperative morbidity. In our analysis of the studies with adequate reporting of surgical technique, the trans-lamina terminalis approach demonstrated a strong trend toward decreased rates of adverse effects compared to transcortical and transcallosal approaches. The trans-lamina terminalis approach was the most frequently cited surgical approach and demonstrated a complication rate of 29.4% overall, while rates of complications were 75.0% and 71.4% for transcortical and transcollosal approaches. Thus, surgical approach appears to be a stronger predictor of postoperative mobidity than extent of resection. A caveat to this conclusion is the limited number of studies that reported their surgical approach. Of the 81 reported cases within the literature, surgical approach was specified in only 28 patients. Given the limited number of studies that specify surgical approach, statistical analysis of individual complications were insufficiently powered to correlate complication rates to surgical technique. Hypothalamic deficits were reported in only 2 out of 17 trans-lamina terminalis cases, although differences were not statistically significant between surgical approaches. Moreover, surgical approach did not predict rates of memory deficits or seizures.
When a complete resection could not be accomplished, adjuvant radiotherapy or radiosurgery was occasionally administered. The use of adjuvant radiotherapy in literature has been solely reserved for the management of residual disease, either in the setting of biopsy [16, 47] or STR. [19, 20, 46] Kobayashi advocated the role of adjuvant Gamma Knife radiosurgery with lower marginal doses to the hypothalamus and optic nerves in the management of these tumors to minimize adverse neuroendocrine sequelae.[47] Two patients underwent biopsy followed by Gamma Knife, delivering a maximal dose of 21 tyo 22 Gy and a maginal dose of 10.5 to 11 Gy. These two patients were alive without disease progression or radiation toxicity at last follow up. A patient who underwent STR received a marginal dose of 12 Gy, with less than 10 Gy to the optic nerve, and eventually died of causes unrelated to tumor progression. Tumor size was noted to have decreased at 5 months following radiosurgery. In all cases, adjuvant therapy was given within 4 months postoperatively.
Other series have reported the safe management of CG with fractionated radiotherapy [19, 20, 44] [7], and benefits of RT have been demonstrated in the management of other low grade gliomas involving the hypothalamus and optic nerves.[58] The use of adjuvant radiotherapy may allow minimization of surgical complications, and result in improved tumor control or even regression. [47] However, due to the small number of patients in literature, the present study is underpowered to definitively delineate the role of radiotherapy in the management of CG. The use of adjuvant chemotherapy for CG has not been reported in literature.
Although CG appears to be highly recurrent in cases of STR, and a number of deaths were reported, no death was attributed to disease progression. A total of 18 deaths were reported, with 15 (83.3%) deaths occurring within 13 months and 9 (50%) within 18 days of surgery. Most common causes of death were pulmonary embolism [7, 8, 24], myocardial infarction [5, 21], and infection [6, 9, 26]. Pulmonary embolism was reported in 5 patients (6.3%), of which 4 resulted in death. Two patients died within a few days of surgery [7, 8], and the remaining two within 14 days [7] and 15 days [24]of surgery. Proper prophylaxis for thromboembolic events is critical for patients with CG given its high reported incidence.[43] The high rate of postoperative mortality associated with a low-grade tumor that carries a low rate of disease-associated mortality should be carefully considered when planning surgery and peri-operative care. Although better tumor control can be achieved with more aggressive resection, early complications may negate the benefit of a more aggressive approach.
While our analysis comprises the largest literature review of CG, there are several important limitations to consider given our methodology and design. As an analysis of cases presented in literature, our review carries biases inherent to all retrospective studies. Given the rarity of this tumor type, the number of patients receiving adjuvant radiotherapy is limited, and larger studies will be critical in elucidating the role of radiotherapy in the treatment of CG. Additionally, while there was no reported local progression following GTR, length of follow-up may be too limited to capture late recurrences.
Conclusion
CG is a rare subtype of low grade glioma typically located within the third ventricle. Surgical intervention remains critical in the management of these tumors. GTR should be the primary goal, as it is associated with improved rates of tumor control and has not been shown to increase rates of postoperative complications. However, GTR may not always be achievable given the proximity to sensitive neurovascular structures. The application of adjuvant radiotherapy has mainly been utilized in the management of residual disease in cases where GTR may not be achievable. The true benefits of radiotherapy remain unclear. To minimize postoperative complications, a greater emphasis should be placed on surgical approach and perioperative management. Morbidity remains high, and future studies will be critical in optimizing the use radiotherapy and surgical techniques to improve outcomes while minimizing complications.
| Author (year) | # of patients | Average Age | Extent of Resection (initial tumor) | Reported # that underwent radiotherapy† | Reported patient recurrences | Reported deaths |
|
| ||||||
| Al-Zubidi et al. (2014)[41] | 1 | 37 | STR | 0 | 0 | 0 |
| Kobayashi et al. (2013)[47] | 3 | 49 | Bx (n=2) STR (n=1) |
3 | 0 | 1 |
| Ni et al. (2013)[18] | 4 | 40 | GTR (n=4) | 0 | 0 | 0 |
| Shiramizu et al. (2013)[54] | 1 | 49 | GTR | 0 | 0 | 0 |
| Scheurkogel et al. (2012)[55] | 1 | 30 | STR | 0 | 0 | 0 |
| Romero-Rojas et al. (2012)[14] | 1 | 39 | GTR | 0 | 0 | 0 |
| Can et al. (2012)[38] | 1 | 37 | GTR | 0 | 0 | 0 |
| Ghosal et al. (2012)[59] | 1 | 48 | STR | 0 | 0 | 0 |
| Vij et al. (2011)[17] | 1 | 48 | STR | 0 | 0 | 0 |
| Liu et al. (2011)[31] | 1 | 45 | STR | 0 | 0 | 0 |
| Tu et al. (2010)[12] | 1 | 46 | STR | 0 | 0 | 0 |
| Horbinski et al. (2009)[15] | 5 | 47 | GTR (n=1) NA (n=4) |
0 | 0 | 0 |
| Dziurzynski et al. (2009)[23] | 1 | 41 | Bx | 0 | 0 | 0 |
| Kawasaki et al. (2009)[27] | 2 | 47 | GTR (n=1) STR (n=1) |
0 | 0 | 1 |
| Vanhauwaert et al. (2008)[52] | 1 | 58 | GTR | 0 | 0 | 0 |
| Carrasco et al. (2008)[4] | 1 | 53 | GTR | 0 | 0 | 0 |
| Jain et al. (2008)[6] | 2 | 31 | GTR (n=2) | 0 | 0 | 1 |
| Gallina et al. (2007)[11] | 1 | 56 | GTR | 0 | 0 | 0 |
| Goyal et al. (2007)[5] | 1 | 5 | STR | 0 | 0 | 1 |
| Jung et al. (2006)[26] | 1 | 50 | STR | 0 | 1 | 1 |
| Nga et al. (2006)[51] | 1 | 49 | NA | 0 | 1 | 1 |
| Leeds et al. (2006)[48] | 1 | 57 | Bx | 0 | 0 | 0 |
| Baehring et al. (2006)[32] | 1 | 71 | Bx | 0 | 0 | 0 |
| Kurian et al. (2005)[19] | 2 | 35 | STR (n=1) Bx (n=1) |
2 | 1 | 1 |
| Buccoliero et al. (2004)[10] | 1 | 56 | GTR | 0 | 0 | 0 |
| Suh et al. (2003)[33] | 1 | 48 | GTR | 0 | 0 | 0 |
| Taraszewska et al. (2003)[21] | 2 | 57 | GTR (n=2) | 0 | 0 | 1 |
| Sato et al. (2003)[40] | 1 | 65 | STR | 0 | 0 | 1 |
| Raizer et al. (2003)[50] | 1 | 57 | GTR | 0 | 0 | 0 |
| Nakajima et al. (2003)[44] | 1 | 49 | STR | 1 | 0 | 0 |
| Grand et al. (2002)[34] | 1 | 41 | STR | 0 | 0 | 0 |
| Pasquier et al. (2002)[35] | 2 | 37 | STR (n=2) | 0 | 0 | 0 |
| Hanbali et al. (2001)[29] | 1 | 57 | STR | 1 | 1 | 1 |
| Castellano-Sanchez et al. (2001)[28] | 1 | 12 | STR | 0 | 0 | 0 |
| Galloway et al. (2001)[13] | 1 | 54 | GTR | 0 | 0 | 0 |
| Cenacchi et al. (2001)[24] | 3 | 39 | GTR (n=3) | 0 | 0 | 1 |
| Castellano-Sanchez et al. (2000)[42] | 1 | 36 | STR | 0 | 0 | 0 |
| Ricoy et al. (2000)[53] | 1 | 41 | GTR | 0 | 0 | 0 |
| Tonami et al. (2000)[20] | 1 | 42 | STR | 1 | 0 | 0 |
| Reifenberger et al. (1999)[7] | 4 | 52 | GTR (n=3) STR (n=1) |
1 | 0 | 2 |
| Vajtai et al. (1999)[45] | 1 | 60 | STR | 0 | 0 | 1 |
| Brat et al. (1998)[8] | 8 | 47 | GTR (n=2) STR (n=6) |
2 | 3 | 3 |
| Tanboon et al. (2014)[30] | 1 | 29 | GTR | 0 | 0 | 0 |
| Sanches et al. (2012)[49] | 1 | 59 | Bx | 0 | 0 | 0 |
| Xian et al. (2012)[39] | 1 | 53 | GTR | 0 | 0 | 0 |
| Kim et al. (2010)[22] | 1 | 27 | STR | 0 | 0 | 0 |
| DeSouza et al. (2010)[60] | 2 | 42 | GTR (n=1) STR (n=1) |
0 | 0 | 0 |
| Sugita et al. (2010)[37] | 1 | 55 | STR | 0 | 0 | 0 |
| Hsu et al. (2005)[36] | 1 | 50 | GTR | 0 | 0 | 0 |
| Chung et al. (2007)[16] | 1 | 48 | Bx | 1 | 1 | 0 |
| Bastin et al. (2012)[25] | 1 | 31 | STR | 0 | 0 | 0 |
| Molnar et al. (2008)[9] | 1 | 34 | STR | 0 | 0 | 1 |
| Kochi et al. (2000)[46] | 1 | 51 | STR | 1 | 0 | 0 |
| Morais et al. (2015)[56] | 1 | 13 | NA | 0 | 2 | 0 |
| † Radiation soon after initial resection | ||||||
| Abbreviations: GTR=Gross Total Resection/STR=Subtotal Resection/Bx=Biopsy | ||||||
Table 5.
Univariate Kaplan Meier analysis for tumor recurrence. Analysis of radiotherapy compared STR only to STR + RT, and excluded patients with GTR as no patients with GTR received adjuvant therapy.
| Factor | Hazard Ratio | 95% CI | p |
|---|---|---|---|
| Age (>50 vs ≤ 50years) | 0.77 | 0.16–3.71 | 0.618 |
| Tumor diameter (≥3 vs <3cm) | 0.33 | 0.01–7.36 | 0.484 |
| MIB-LI (≥2.5 vs <2.5%) | 0.25 | 0.01–5.75 | 0.383 |
| EOR (GTR vs STR/Bx) | 0.18 | 0.04–0.83 | 0.028 |
| Radiotherapy | 0.16 | 0.03–0.82 | 0.114 |
Acknowledgments
Grant support was derived from the Howard Hughes Medical Institute (LA). Author contributions to the manuscript are as follows: Conception and design (Ampie and Bloch), acquisition of data (Ampie and Choy), statistical analysis (Choy), interpretation of data (Choy and Ampie), drafting article (Choy and Ampie), revising the article (all authors).
Footnotes
Disclosures
There are no conflicts of interest to report.
References
- 1.Brat DJ, Scheithauer BW, Staugaitis SM, Cortez SC, Brecher K, Burger PC. Third ventricular chordoid glioma: A distinct clinicopathologic entity. J Neuropathol Exp Neurol. 1998;57:283–290. doi: 10.1097/00005072-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. Journal of neuropathology and experimental neurology. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 3.Tu A, Yeo T, Steinke D, Resch L, Mehta V. Chordoid glioma: Imaging pearls of a unique third ventricular tumor. Can J Neurol Sci. 2010;37:677–680. [PubMed] [Google Scholar]
- 4.Carrasco R, Pascual JM, Reina T, Nieto S, Linera J, Sola RG. Chordoid glioma of the third ventricle attached to the optic chiasm. Successful removal through a trans-lamina terminalis approach. Clinical neurology and neurosurgery. 2008;110:828–833. doi: 10.1016/j.clineuro.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Goyal R, Vashishta RK, Singhi S, Gill M. Extraventricular unusual glioma in a child with extensive myxoid change resembling chordoid glioma. Journal of clinical pathology. 2007;60:1294–1295. doi: 10.1136/jcp.2005.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain D, Sharma MC, Sarkar C, Suri V, Rishi A, Garg A, Vaishya S. Chordoid glioma: report of two rare examples with unusual features. Acta Neurochir (Wien) 2008;150:295–300. doi: 10.1007/s00701-008-1420-x. discussion 300. [DOI] [PubMed] [Google Scholar]
- 7.Reifenberger G, Weber T, Weber RG, Wolter M, Brandis A, Kuchelmeister K, Pilz P, Reusche E, Lichter P, Wiestler OD. Chordoid glioma of the third ventricle: immunohistochemical and molecular genetic characterization of a novel tumor entity. Brain pathology. 1999;9:617–626. doi: 10.1111/j.1750-3639.1999.tb00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brat DJ, Scheithauer BW, Staugaitis SM, Cortez SC, Brecher K, Burger PC. Third ventricular chordoid glioma: a distinct clinicopathologic entity. Journal of neuropathology and experimental neurology. 1998;57:283–290. doi: 10.1097/00005072-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Molnar PP, Barla SP, Nagy D, Nagy G, Berenyi E, Dobai J. Suprachiasmatic Extraventricular Chordoid Glioma. Neuro-Oncology. 2008;10:1140–1140. [Google Scholar]
- 10.Buccoliero AM, Caldarella A, Gallina P, Di Lorenzo N, Taddei A, Taddei GL. Chordoid glioma: clinicopathologic profile and differential diagnosis of an uncommon tumor. Archives of pathology & laboratory medicine. 2004;128:e141–145. doi: 10.1043/1543-2165(2004)128<e141:CGCPAD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Gallina P, Pansini G, Mouchaty H, Mura R, Buccoliero AM, Di Lorenzo N. An incidentally detected third ventricle chordoid glioma. Neurology India. 2007;55:406–407. doi: 10.4103/0028-3886.33301. [DOI] [PubMed] [Google Scholar]
- 12.Tu A, Yeo T, Steinke D, Resch L, Mehta V. Chordoid glioma: imaging pearls of a unique third ventricular tumor. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2010;37:677–680. [PubMed] [Google Scholar]
- 13.Galloway M, Afshar F, Geddes JF. Chordoid glioma: an uncommon tumour of the third ventricle. British journal of neurosurgery. 2001;15:147–150. doi: 10.1080/02688690120036865. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Rojas AE, Diaz-Perez JA, Ariza-Serrano LM. CD99 is expressed in chordoid glioma and suggests ependymal origin. Virchows Archiv: an international journal of pathology. 2012;460:119–122. doi: 10.1007/s00428-011-1170-2. [DOI] [PubMed] [Google Scholar]
- 15.Horbinski C, Dacic S, McLendon RE, Cieply K, Datto M, Brat DJ, Chu CT. Chordoid glioma: a case report and molecular characterization of five cases. Brain pathology. 2009;19:439–448. doi: 10.1111/j.1750-3639.2008.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung SB, Park SH, Kim JE. Chordoid glioma of the third ventricle with unusual MRI features. J Korean Neurosurg S. 2007;42:224–227. [Google Scholar]
- 17.Vij M, Jaiswal S, Jaiswal AK, Jain M, Behari S. Chordoid glioma of the third ventricle: a case report with review of literature. Neurology India. 2011;59:469–471. doi: 10.4103/0028-3886.82740. [DOI] [PubMed] [Google Scholar]
- 18.Ni HC, Piao YS, Lu DH, Fu YJ, Ma XL, Zhang XJ. Chordoid glioma of the third ventricle: four cases including one case with papillary features. Neuropathology: official journal of the Japanese Society of Neuropathology. 2013;33:134–139. doi: 10.1111/j.1440-1789.2012.01333.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurian KM, Summers DM, Statham PF, Smith C, Bell JE, Ironside JW. Third ventricular chordoid glioma: clinicopathological study of two cases with evidence for a poor clinical outcome despite low grade histological features. Neuropathology and applied neurobiology. 2005;31:354–361. doi: 10.1111/j.1365-2990.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 20.Tonami H, Kamehiro M, Oguchi M, Higashi K, Yamamoto I, Njima T, Okamoto K, Akai T, Iizuka H. Chordoid glioma of the third ventricle: CT and MR findings. Journal of computer assisted tomography. 2000;24:336–338. doi: 10.1097/00004728-200003000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Taraszewska A, Bogucki J, Andrychowski J, Koszewski W, Czernicki Z. Clinicopathological and ultrastructural study in two cases of chordoid glioma. Folia neuropathologica/Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 2003;41:175–182. [PubMed] [Google Scholar]
- 22.Kim JW, Kim JH, Choe G, Kim CY. Chordoid Glioma: A Case Report of Unusual Location and Neuroradiological Characteristics. J Korean Neurosurg S. 2010;48:62–65. doi: 10.3340/jkns.2010.48.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dziurzynski K, Delashaw JB, Gultekin SH, Yedinak CG, Fleseriu M. Diabetes insipidus, panhypopituitarism, and severe mental status deterioration in a patient with chordoid glioma: case report and literature review. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2009;15:240–245. doi: 10.4158/EP.15.3.240. [DOI] [PubMed] [Google Scholar]
- 24.Cenacchi G, Roncaroli F, Cerasoli S, Ficarra G, Merli GA, Giangaspero F. Chordoid glioma of the third ventricle: an ultrastructural study of three cases with a histogenetic hypothesis. The American journal of surgical pathology. 2001;25:401–405. doi: 10.1097/00000478-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Bastin F, Pirotte B, Bouquey D, Roger T. Chordoid Glioma of Third Ventricle: Case Report and Review of Literature. Neuro-Oncology. 2012;14:64–64. [Google Scholar]
- 26.Jung TY, Jung S. Third ventricular chordoid glioma with unusual aggressive behavior. Neurologia medico-chirurgica. 2006;46:605–608. doi: 10.2176/nmc.46.605. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki K, Kohno M, Inenaga C, Sato A, Hondo H, Miwa A, Fujii Y, Takahashi H. Chordoid glioma of the third ventricle: a report of two cases, one with ultrastructural findings. Neuropathology: official journal of the Japanese Society of Neuropathology. 2009;29:85–90. doi: 10.1111/j.1440-1789.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 28.Castellano-Sanchez AA, Schemankewitz E, Mazewski C, Brat DJ. Pediatric chordoid glioma with chondroid metaplasia. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2001;4:564–567. doi: 10.1007/s10024001-0087-1. [DOI] [PubMed] [Google Scholar]
- 29.Hanbali F, Fuller GN, Leeds NE, Sawaya R. Choroid plexus cyst and chordoid glioma. Report of two cases. Neurosurgical focus. 2001;10:E5. doi: 10.3171/foc.2001.10.6.6. [DOI] [PubMed] [Google Scholar]
- 30.Tanboon J, Aurboonyawat T, Chawalparit O. A 29-year-old man with progressive short term memory loss. Brain pathology. 2014;24:103–106. doi: 10.1111/bpa.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu WP, Cheng JX, Yi XC, Zhen HN, Fei Z, Li Q, Zhang X. Chordoid glioma: a case report and literature review. The neurologist. 2011;17:52–56. doi: 10.1097/NRL.0b013e3181e7db67. [DOI] [PubMed] [Google Scholar]
- 32.Baehring JM, Bannykh S. Chordoid glioma of the third ventricle. Journal of neuro-oncology. 2006;76:269. doi: 10.1007/s11060-006-6054-y. [DOI] [PubMed] [Google Scholar]
- 33.Suh YL, Kim NR, Kim JH, Park SH. Suprasellar chordoid glioma combined with Rathke’s cleft cyst. Pathology international. 2003;53:780–785. doi: 10.1046/j.1440-1827.2003.01549.x. [DOI] [PubMed] [Google Scholar]
- 34.Grand S, Pasquier B, Gay E, Kremer S, Remy C, Le Bas JF. Chordoid glioma of the third ventricle: CT and MRI, including perfusion data. Neuroradiology. 2002;44:842–846. doi: 10.1007/s00234-002-0820-0. [DOI] [PubMed] [Google Scholar]
- 35.Pasquier B, Peoc’h M, Morrison AL, Gay E, Pasquier D, Grand S, Sindou M, Kopp N. Chordoid glioma of the third ventricle: a report of two new cases, with further evidence supporting an ependymal differentiation, and review of the literature. The American journal of surgical pathology. 2002;26:1330–1342. doi: 10.1097/00000478-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Hsu Y-C, Kao H-W, Lo C-P, Juan C-J, Chin S-C, Chen C-Y. Chordoid glioma: CT and MR features. Chin J Radiol 2005 [Google Scholar]
- 37.Sugita Y, Ohshima K, Shigemori M, Arakawa M, Kuramoto T, Nakayama K. The tumor of the third ventricle. Neuropathology: official journal of the Japanese Society of Neuropathology. 2010;30:97–100. doi: 10.1111/j.1440-1789.2009.01057.x. [DOI] [PubMed] [Google Scholar]
- 38.Can B. Cytology of chordoid glioma of the third ventricle. Diagnostic cytopathology. 2012;40:185–187. doi: 10.1002/dc.21619. [DOI] [PubMed] [Google Scholar]
- 39.Xian G, Yaodong Z, Meiqing L. Chordoid Glioma of The Third Ventricle: A Case Report and Review of Literature. Journal of Neurological Sciences (Turkish) 2012 [Google Scholar]
- 40.Sato K, Kubota T, Ishida M, Yoshida K, Takeuchi H, Handa Y. Immunohistochemical and ultrastructural study of chordoid glioma of the third ventricle: its tanycytic differentiation. Acta neuropathologica. 2003;106:176–180. doi: 10.1007/s00401-003-0713-2. [DOI] [PubMed] [Google Scholar]
- 41.Al-Zubidi N, McGlynn MM, Chevez-Barrios P, Yalamanchili S, Lee AG. Neuro-ophthalmologic features of chordoid glioma. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2014;34:47–49. doi: 10.1097/WNO.0b013e3182a595b7. [DOI] [PubMed] [Google Scholar]
- 42.Castellano-Sanchez AA, Recine MA, Restrepo R, Howard LH, Robinson MJ. Chordoid glioma: a novel tumor of the third ventricle. Annals of diagnostic pathology. 2000;4:373–378. doi: 10.1053/adpa.2000.19369. [DOI] [PubMed] [Google Scholar]
- 43.Desouza RM, Bodi I, Thomas N, Marsh H, Crocker M. Chordoid glioma: ten years of a low-grade tumor with high morbidity. Skull base: official journal of North American Skull Base Society [et al] 2010;20:125–138. doi: 10.1055/s-0029-1246223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakajima M, Nakasu S, Hatsuda N, Takeichi Y, Watanabe K, Matsuda M. Third ventricular chordoid glioma: case report and review of the literature. Surgical neurology. 2003;59:424–428. doi: 10.1016/s0090-3019(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 45.Vajtai I, Varga Z, Scheithauer BW, Bodosi M. Chordoid glioma of the third ventricle: confirmatory report of a new entity. Human pathology. 1999;30:723–726. doi: 10.1016/s0046-8177(99)90102-8. [DOI] [PubMed] [Google Scholar]
- 46.Kochi N, Nikaido O, Ametani T, Takamori Y, Nakae T. A case of third ventricular chordoid glioma: Immunohistochemical and electron microscopic studies. Brain pathology. 2000;10:584–585. [Google Scholar]
- 47.Kobayashi T, Tsugawa T, Hashizume C, Arita N, Hatano H, Iwami K, Nakazato Y, Mori Y. Therapeutic approach to chordoid glioma of the third ventricle. Neurologia medico-chirurgica. 2013;53:249–255. doi: 10.2176/nmc.53.249. [DOI] [PubMed] [Google Scholar]
- 48.Leeds NE, Lang FF, Ribalta T, Sawaya R, Fuller GN. Origin of chordoid glioma of the third ventricle. Archives of pathology & laboratory medicine. 2006;130:460–464. doi: 10.1043/1543-2165(2006)130[460:OOCGOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Sanches P, Yamashita S, Freitas C, Resende L. Chordoid glioma of the third ventricle: A new case report [Glioma cordoide do terceiro ventrículo: Descrição de um novo caso] Radiologia Brasileira. 2012;45 [Google Scholar]
- 50.Raizer JJ, Shetty T, Gutin PH, Obbens EA, Holodny AI, Antonescu CR, Rosenblum MK. Chordoid glioma: report of a case with unusual histologic features, ultrastructural study and review of the literature. Journal of neuro-oncology. 2003;63:39–47. doi: 10.1023/a:1023752717042. [DOI] [PubMed] [Google Scholar]
- 51.Nga ME, Tan KB, Laporte JP, Takano A. Test and teach. A recurrent third ventricular brain tumour. Diagnosis: Chordoid glioma of the third ventricle. Pathology. 2006;38:254–257. doi: 10.1080/00313020600699151. [DOI] [PubMed] [Google Scholar]
- 52.Vanhauwaert DJ, Clement F, Van Dorpe J, Deruytter MJ. Chordoid glioma of the third ventricle. Acta neurochirurgica. 2008;150:1183–1191. doi: 10.1007/s00701-008-0014-6. [DOI] [PubMed] [Google Scholar]
- 53.Ricoy JR, Lobato RD, Baez B, Cabello A, Martinez MA, Rodriguez G. Suprasellar chordoid glioma. Acta neuropathologica. 2000;99:699–703. doi: 10.1007/s004010051183. [DOI] [PubMed] [Google Scholar]
- 54.Shiramizu H, Hori T, Matsuo S, Niimura K, Yoshimoto H, Ishida A, Asakuno K, Yuzawa M, Moriyama T. Anterior callosal section is useful for the removal of large tumors invading the dorsal part of the anterior third ventricle: operative technique and results. Neurosurgical review. 2013;36:467–475. doi: 10.1007/s10143-013-0455-0. [DOI] [PubMed] [Google Scholar]
- 55.Scheurkogel MM, van Duinen SG, Verstegen MJ, Lycklama a Nijeholt GJ. Chordoid glioma: a rare suprasellar mass. Acta neurologica Belgica. 2012;112:311–314. doi: 10.1007/s13760-012-0084-3. [DOI] [PubMed] [Google Scholar]
- 56.Morais BA, Menendez DF, Medeiros RS, Teixeira MJ, Lepski GA. Chordoid glioma: Case report and review of the literature. International journal of surgery case reports. 2015;7C:168–171. doi: 10.1016/j.ijscr.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cenacchi G, Giangaspero F. Emerging tumor entities and variants of CNS neoplasms. Journal of neuropathology and experimental neurology. 2004;63:185–192. doi: 10.1093/jnen/63.3.185. [DOI] [PubMed] [Google Scholar]
- 58.Kida Y, Kobayashi T, Mori Y. Gamma knife radiosurgery for low-grade astrocytomas: results of long-term follow up. Journal of neurosurgery. 2000;93(Suppl 3):42–46. doi: 10.3171/jns.2000.93.supplement3.0042. [DOI] [PubMed] [Google Scholar]
- 59.Ghosal N, Thakar S, Kumaran SP, Hegde AS. Chordoid glioma in suprasellar location with extension into the third ventricle: smear preparation morphology of a rare tumor. Diagnostic cytopathology. 2012;40:155–158. doi: 10.1002/dc.21606. [DOI] [PubMed] [Google Scholar]
- 60.DeSouza RM, Bodi I, Thomas N, Marsh H, Crocker M. Chordoid Glioma: Ten Years of a Low-Grade Tumor with High Morbidity. Skull Base-Interd Ap. 2010;20:125–138. doi: 10.1055/s-0029-1246223. [DOI] [PMC free article] [PubMed] [Google Scholar]


