Abstract
Nicotinamide adenine dinucleotide (NAD+) is an essential coenzyme/cosubstrate for many biological processes in cellular metabolism. The rate-limiting step in the major pathway of mammalian NAD+ biosynthesis is mediated by nicotinamide phosphoribosyltransferase (Nampt). Previously, we showed that mice lacking Nampt in forebrain excitatory neurons (CamKIIαNampt−/− mice) exhibited hyperactivity, impaired learning and memory, and reduced anxiety-like behaviors. However, it remained unclear if these functional effects were accompanied by synaptic changes. Here, we show that CamKIIαNampt−/− mice have impaired induction of long-term depression (LTD) in the Schaffer collateral pathway, but normal induction of long-term potentiation (LTP), at postnatal day 30. Pharmacological assessments demonstrated that CamKIIαNampt−/− mice also display dysfunction of synaptic GluN2B (NR2B)-containing N-methyl-D-aspartate receptors (NMDARs) prior to changes in NMDAR subunit expression. These results support a novel, important role for Nampt-mediated NAD+ biosynthesis in LTD and in the function of GluN2B–containing NMDARs.
Keywords: NAD+, Nampt, Long-term depression, NR2B, GluN2B, N-methyl-D-aspartate receptor
1. Introduction
Nicotinamide adenine dinucleotide (NAD+) is an essential coenzyme/cosubstrate in multiple metabolic reactions, including glycolysis and oxidative phosphorylation [30, 49, 61, 78]. While there are several pathways of NAD+ biosynthesis, most mammalian cells generate NAD+ from nicotinamide [30, 78]. The rate-limiting step of this pathway is performed by nicotinamide phosphoribosyltransferase (Nampt) [65]. Recently, we found that mice lacking Nampt in forebrain excitatory neurons of the hippocampal CA1 subregion and cortical layers II/III (CamKIIαNampt−/− mice) exhibited hyperactivity, impaired learning and memory, and reduced anxiety-like behaviors at 2–3 months of age [79]. And yet, at postnatal day (P) 60, CamKIIαNampt−/− mice had intact tetanic long-term potentiation (LTP), a form of synaptic plasticity considered to be an electrophysiological correlate of learning and memory [20]. Both spatial memory deficits and decreased expression of the immediate early genes Egr1 and Arc, which were exhibited by CamKIIαNampt−/− mice, have been strongly linked to LTP [15, 40, 46, 83]. Thus, it was surprising that the spatial memory performance and loss of immediate early gene expression in CamKIIαNampt−/− mice were not intrinsically linked with LTP. Our findings raised an important question: does loss of Nampt in forebrain excitatory neurons have any synaptic manifestations?
Little is known regarding the importance of NAD+ for synaptic transmission. Besides LTP, another form of synaptic plasticity is long-term depression (LTD), or a persistent decrease in synaptic strength [20, 43, 94]. Consolidation of hippocampal-dependent memory has been linked to N-methyl-D-aspartate receptor (NMDAR) dependent LTD in CA1 [7, 25, 91]. NMDAR activation modulates induction of LTP and LTD [94] and occurs during conditions of energy deprivation, such as hypoxia and hypoglycemia [13, 14, 26, 31, 81]. NMDARs are tetrameric receptors consisting of two obligatory NR1 (GluN1) subunits and two regulatory subunits, usually a combination of GluN2A (NR2A) and GluN2B (NR2B) [94]. Previous work found that mice lacking GluN2B function behave similarly to CamKIIαNampt−/− mice [79], with hyperactivity [1, 29, 85], memory impairments [7, 25], and reduced levels of anxiety-like behaviors [2, 7, 85].
Here, we show that CamKIIαNampt−/− mice exhibit a specific defect in induction of LTD in the Schaffer collateral pathway. Moreover, CamKIIαNampt−/− mice display insensitivity to pharmacological inhibition of synaptic GluN2B–containing NMDARs. These functional changes occur prior to decreased expression of hippocampal NMDARs and in the absence of changes in basal transmission or presynaptic mechanisms. Together, the constellation of phenotypes that we report in CamKIIαNampt−/− mice informs our understanding of the effects of NAD+ depletion, a form of energy deprivation, on synaptic plasticity and function of NMDARs.
2. Materials and Methods
2.1 Animals
Mice were bred and maintained as described [79]. Briefly, Namptflox/flox mice [71] were crossed to CamKIIαCre mice [The Jackson Laboratory, Stock #005359, T29-1 [84]] to generate CamKIIαNampt−/− mice. In all experiments, control mice were age-matched littermates. Since no sex differences were observed, both male and female mice were used. All animal procedures were approved by the Washington University Animal Studies Committee, Division of Comparative Medicine, Washington University School of Medicine, St. Louis, MO, and were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996. All efforts were made to minimize the number of animals used and their suffering.
2.2 Reagents
Chemicals were purchased from Sigma or Tocris (St. Louis, MO). Drugs were freshly dissolved in artificial cerebrospinal fluid (ACSF) at the time of experiment and administered by bath perfusion as noted in the text. The concentrations and durations of drug administration were based on prior studies indicating that the agents are effective at altering synaptic transmission or synaptic plasticity as administered.
2.3 Western Blotting
Protein was isolated and analyzed as previously described [79]. Briefly, protein extracts (15–50 µg) were prepared from acutely isolated mouse hippocampi flash frozen in liquid nitrogen, and stored at −80C until use. Membranes were incubated with primary antibodies in Tris-buffered saline containing 0.1% Tween 20 (TBST) overnight at 4°C. Primary antibodies used: Gapdh (1:1000; Millipore, CB1001, 6C5), Nampt (1:3000; Alexis Biochemicals, ALX-804-717-C100, mouse), GluN2B (1:1000; NeuroMab, N59/36, mouse).
2.4 Quantitative real-time RT-PCR
RNA was isolated and analyzed as previously described [79]. Briefly, mouse hippocampi were flash frozen in liquid nitrogen and stored at −80 C. Total RNA was isolated from the hippocampus using the RNeasy kit (Qiagen) and reverse-transcribed into cDNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Quantitative real-time RT-PCR was conducted with the TaqMan Fast Universal PCR Master mix and appropriate TaqMan primers in the GeneAmp 7500 fast sequence detection system (Applied Biosystems). Relative expression levels were calculated for each gene by normalizing to levels of Gapdh and then to a control.
2.5 Immunohistochemistry
Mice were anesthetized by intraperitoneal injection of ketamine and xylazine, and perfused transcardially through the left ventricle with cold phosphate buffer (0.1 M, pH 7.4) followed by a phosphate-buffered solution of 4% paraformaldehyde (PFA). Brains were postfixed with 4% PFA overnight, equilibrated in 15% sucrose overnight, equilibrated in 30% sucrose overnight, frozen, and stored at −80°C until sectioning. 30 µm coronal sections in a 1 in 8 series were made by cryostat and stored at −30°C in cryoprotectant until use. Every eighth section was processed. Tissue sections were incubated in 50% formamide in 2x saline/ sodium citrate (SSC) at 65°C for 2 h and incubated with 3% H2O2 for 15 min to remove endogenous peroxidase activity. Tissue sections were incubated in blocking/ permeabilization solution containing 10% normal goat serum, 1% bovine serum albumin (BSA), and 0.3% Triton-X in PBS for 45 to 60 min prior to 24 or 48 h of incubation with primary antibodies in 5% normal goat serum and 0.1% Triton-X in PBS at 4°C at the following concentrations: Iba1 (1:500; Wako, #019–19741, rabbit), Gfap (1:1000; Millipore, MAB360, mouse), Nampt (1:1000; Alexis Biochemicals ALX-804-717-C100, mouse), GluN2B (1:10; NeuroMab, N59/36, mouse). Antibody specificity was determined by lack of staining after omission of primary or secondary antibodies. Alexa647 (1:200), Alexa488 (1:200), or Cy3 (1:400) conjugated-secondary antibodies (Jackson ImmunoResearch) diluted in 2% normal goat serum, 1% BSA, and 0.1% Triton-X in PBS were added for 2 h at room temperature. Detection of Nampt and GluN2B was performed using the TSA-Plus kit (PerkinElmer, Boston, MA). Nuclei were stained with 4,6-diamidino-2-phenylindole (Sigma) for 10 min at room temperature. High-magnification (20x, 0.8DICII or 40x oil 1.3DICII) microscopic imaging was performed using a Zeiss Axioimager.Z1 or an Olympus NanoZoomer 2.0-HT. Images were taken in z-stacks of 1 µm steps through the range of tissue section immunoreactivity. ImageJ was used to 3D render z-stacks.
2.6 Hippocampal slice physiology
Hippocampal slices were prepared from postnatal day 30 (P30) mice using standard methods [79]. Mice were anesthetized with isoflurane and decapitated. Right hippocampi were rapidly dissected and placed in ACSF containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 22 NaHCO3, 10 glucose, gassed with 95% O2–5% CO2 at 4–6°C, and sectioned transversely into 400 µm slices using a rotary slicer. Slices were incubated in gassed ACSF for at least 2 h at 30°C. Experiments were performed in a submersion-recording chamber at 30°C with continuous perfusion of ACSF (2 ml/min). Extracellular recordings were obtained from the CA1 apical dendritic region (stratum radiatum) for analysis of field excitatory postsynaptic potentials (fEPSPs) using 2 M NaCl glass electrodes (5–10 MΩ). Responses were elicited with 0.1 ms constant current pulses through a bipolar electrode in the Schaffer collateral pathway. fEPSPs were measured by the maximal slope of their rising phase. A baseline (control) input-output curve was obtained to determine stimulus intensities for subsequent analyses. Input-output curves were generated using stimuli of 6 different intensities to allow determination of half maximal responses. The smallest stimulus was set to evoke a response less than half maximal while the largest stimulus was designed to evoke a fully saturated response. During an experiment, evoked fEPSPs were monitored by applying single stimuli to the Schaffer collateral pathway every 60 s at intensity sufficient to elicit half maximal responses. After establishing a stable baseline, LTD was induced by applying 1 Hz low frequency stimulation (LFS) to the Schaffer collateral pathway for 15 min (900 pulses), as previously described [38]. LTP was induced by a single 100 Hz × 1 s high frequency stimulus (HFS) using a stimulus of the same intensity, as previously described [8, 82]. Input-output curves were repeated 60 min after delivery of either LFS or HFS to determine the magnitude of LTD and LTP based on changes in half maximal responses. Isolated NMDAR synaptic responses were studied in an extracellular solution containing 2 mM calcium and 0.1 mM magnesium. 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 30 µM, Sigma) was added to this solution to inhibit AMPA receptor-mediated fEPSPs, and responses were evoked once per min [32, 36]. fEPSPs were analyzed by measuring their rising slopes.
2.7 Statistical Analyses
Numerical data are presented as mean ± standard error of the mean (S.E.M.). Statistical significance was determined by unpaired Student’s t-tests, Mann-Whitney U-tests in cases of irregular distribution, or Analysis of variance (ANOVA) tests in cases of more than two groups. Statistical analyses and plots of electrophysiology data were performed using Rstudio, SigmaPlot 5.01, SigmaPlot 9.0, and SigmaStat 3.1 (Systat Software Inc., Richmond, CA). P-values of less than 0.050 were considered significant.
3. Results
3.1 CamKIIαNampt−/− mice show impaired induction of LTD but not LTP
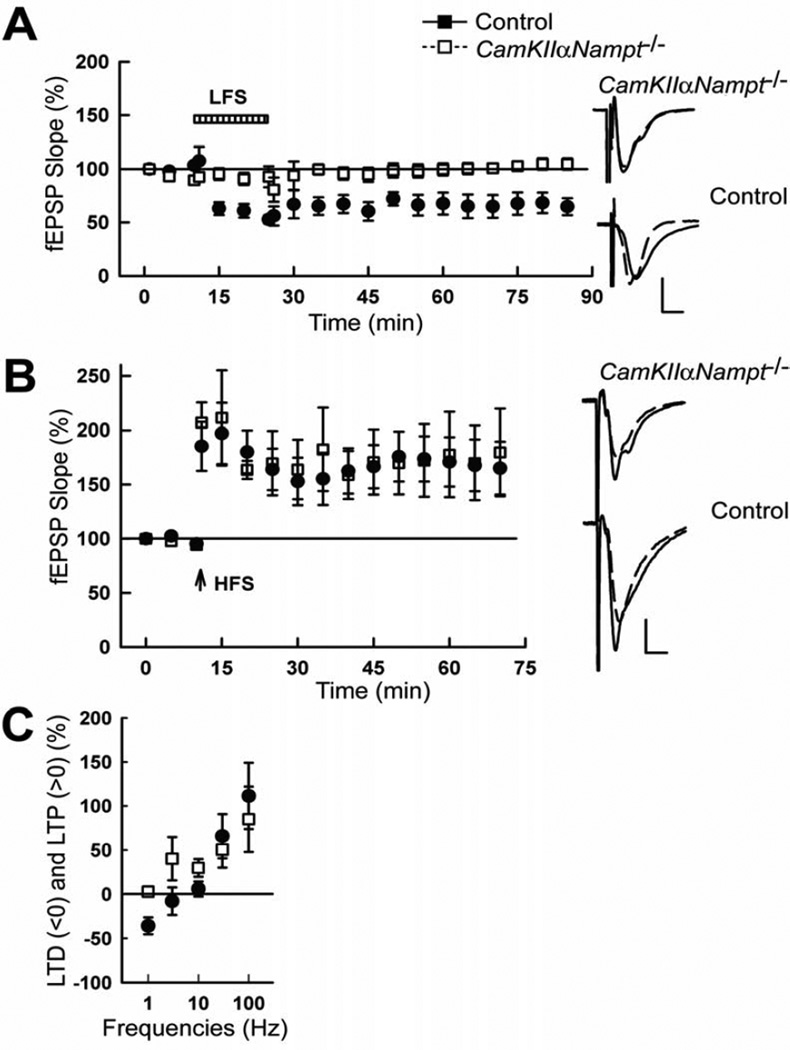
As previously shown [17, 38], low-frequency stimulation (LFS) of the Schaffer collateral pathway in P30 control mice resulted in induction of LTD (Fig. 1A, 64.2 ± 9.5% of baseline 60 min after LFS, n=5). However, the same treatment could not induce LTD in CamKIIαNampt−/− mice (103.0 ± 4.1%, n=5, p=0.006, Student’s t-test). On the other hand, P30 control and CamKIIαNampt−/− mice exhibited normal induction of LTP in response to high-frequency stimulation (HFS, Fig. 1B), as we had previously shown for P60 control and CamKIIαNampt−/− mice [79].
Figure 1.
Postnatal day 30 CamKIIαNampt−/− mice showed impaired induction of long-term depression (LTD) but not long-term potentiation (LTP). A, LTD was not induced by low frequency stimulation (LFS) in CamKIIαNampt−/− mice (white squares), but was induced in control mice (black circles; n=5 each). B, LTP was induced by high frequency stimulation (HFS, arrow) in all CamKIIαNampt−/− and control mice tested (n=5 each). A,B, Traces to the right depict representative field excitatory postsynaptic potentials (fEPSPs) before (dotted lines) and 60 min after HFS or LFS (solid lines). Calibration: 1 mV, 5 ms. C, A frequency response curve for LTD and LTP evoked by 900 pulses delivered at 1, 3, 10, 30, and 100 Hz (n=5). Data are presented as mean ± S.E.M.
A deficit in LTD induced by 1 Hz in the absence of a deficit in LTP induced by 100 Hz may be caused by several mechanisms. For example, synaptic strength could be depressed in CamKIIαNampt−/− mice. Alternatively, the optimal frequency for LTD induction may be altered in CamKIIαNampt−/− mice. To distinguish between these possibilities, we obtained full frequency/response curves in control and CamKIIαNampt−/− mice using 900 pulses administered at various frequencies (Fig. 1C; 1, 3, 10, 30, and 100 Hz; n=5). As expected, there was a significant effect of stimulation strength [Hz, F(4,45)=7.85, p=0.00009, ANOVA] on the percentages of evoked LTP and LTD. However, besides the loss of LTD at 1 Hz in CamKIIαNampt−/− mice, there was no significant difference between genotypes [F(1,48)=0.61, p=0.438, ANOVA]. Thus, the major defect in synaptic plasticity in CamKIIαNampt−/− mice involves a loss in the ability of low frequency Schaffer collateral pathway stimulation to induce LTD.
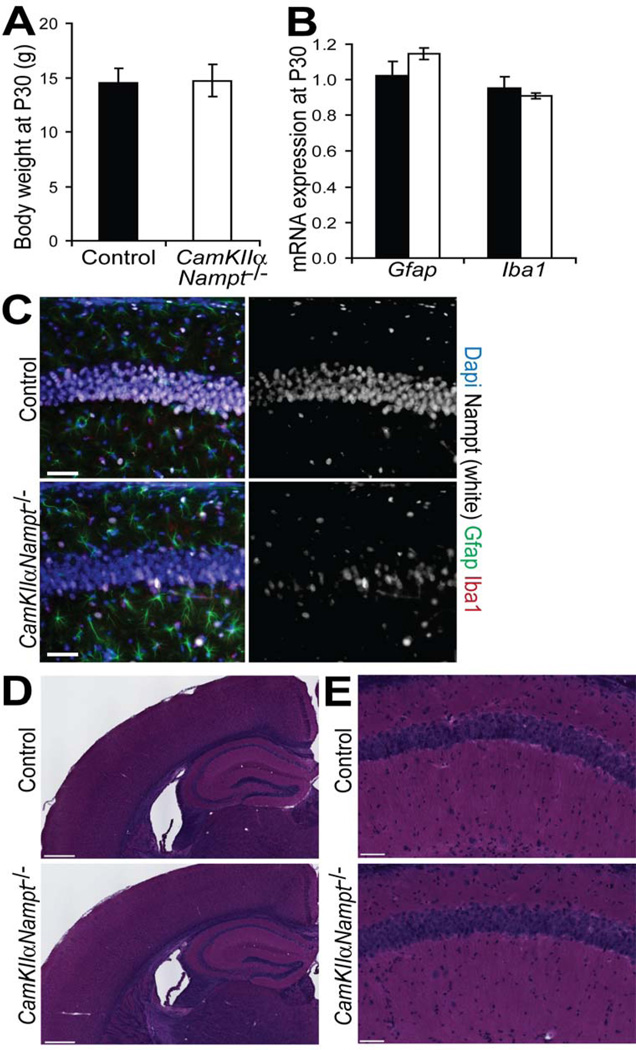
Importantly, at P30, CamKIIαNampt−/− mice lacked the weight loss (Fig. 2A, n=4–5, p=0.92, Student’s t -test), astrogliosis (Fig. 2B,C, Gfap, n=4–5, p=0.23, Student’s t-test), or microgliosis (Fig. 2B,C, Iba1, n=4–5, p=0.60, Student’s t-test) that they displayed at 2–3 months of age [79]. Hematoxylin and eosin (H&E) staining (Fig. 2D,E) and immunofluorescence for Dapi (Fig. 2C) confirmed the lack of the overt cell loss in the pyramidal cell layer that we had observed at 2–3 months of age [79].
Figure 2.
Postnatal day 30 CamKIIαNampt−/− mice were overtly normal. A, The body weights of CamKIIαNampt−/− (n=4) and control (n=5) mice were similar. B, Quantitative PCR analysis of RNA isolated from the hippocampi of CamKIIαNampt−/− and control mice (n = 3–5) showed no astrogliosis or microgliosis. C, Representative images of immunofluorescence for Dapi (blue), Nampt (white), Gfap (green), Iba1 (red) in coronal sections (n = 4–6). Scale bars represent 50 µm. D,E, Hematoxylin and eosin (H&E) staining of coronal brain sections. D, Scale bars represent 500 µm. E, Magnification of the CA1 hippocampal subregion. Scale bars represent 50 µm. Data are presented as mean ± S.E.M.
3.2 CamKIIαNampt−/− mice show impaired GluN2B–containing NMDAR-mediated fEPSPs
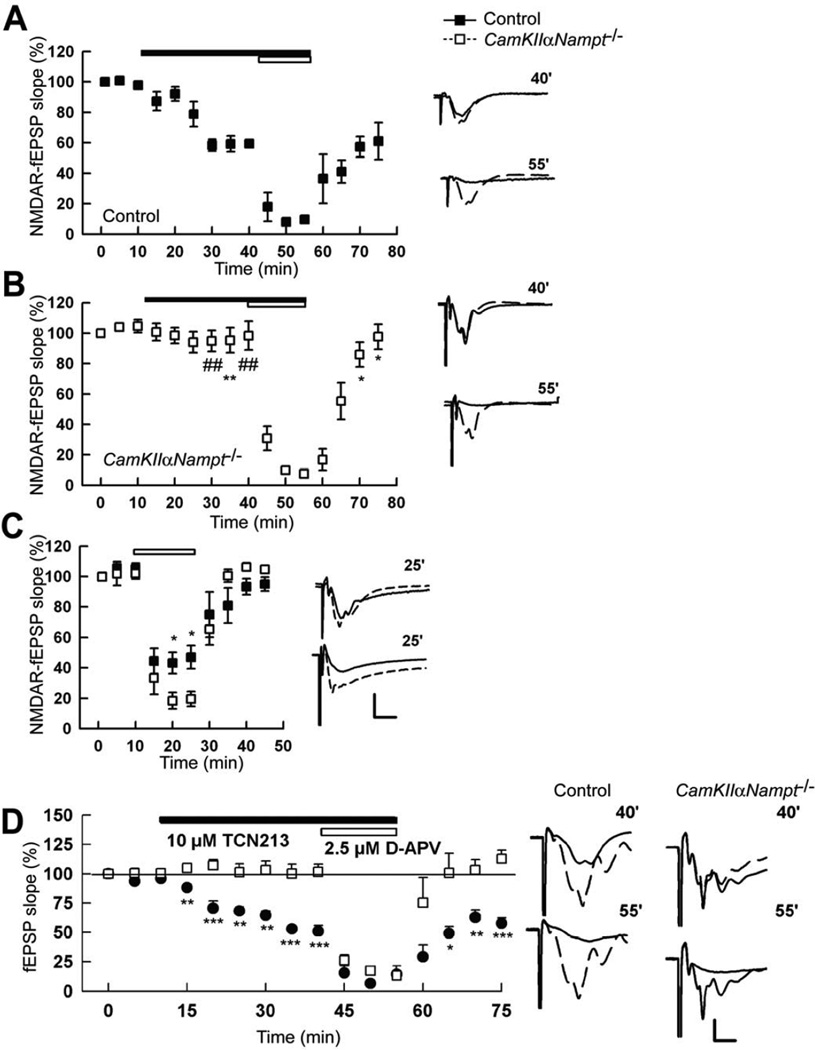
LTD of the Schaffer collateral-CA1 synapse induced by 1 Hz LFS depends on NMDARs [43]. Moreover, energy deprivation often damages the central nervous system by altering NMDAR activity [23, 33, 37, 62, 68]. Thus, we tested if the NAD+ deficiency present in CamKIIαNampt−/− mice altered NMDAR function and expression. Because GluN2B–containing NMDARs are known to contribute to LTD in CA1 [7, 32, 48, 94], we hypothesized that GluN2B–containing NMDAR activity was altered in CamKIIαNampt−/− mice. To test this, we treated hippocampal slices with ifenprodil, a selective antagonist of GluN2B–containing NMDARs [90], alone and in combination with low concentrations of D-2-amino-5-phosphonovalerate (D-APV), a broad-spectrum NMDAR antagonist. Although there are no reliable, selective antagonists against ifenprodil-insensitive NMDAR subtypes [4, 32, 89], low concentrations of D-APV (5 µM or less) have a limited preference for, and thus behave as an antagonist of, ifenprodil-insensitive NMDARs [9, 32, 36]. In all control mice tested, ifenprodil (10 µM) partially depressed NMDAR-mediated fEPSPs (Fig. 3A, 54.6 ± 7.6% compared to baseline, n=5). Subsequent co-administration of D-APV (2.5 µM) almost completely suppressed the remaining NMDAR-mediated fEPSPs (Fig. 3A, 4.7 ± 3.1%), as previously reported [32]. These observations indicate that GluN2B contributes to approximately 45% of synaptic NMDAR activity in P30 control slices, whereas the remainder is mediated by ifenprodil-insensitive NMDARs. In contrast, the depression induced by ifenprodil in CamKIIαNampt−/− mice was minimal (Fig. 3B, 95.4 ± 10.7% of baseline, n=5, p=0.006, Student’s t-test). And yet, coadministration of D-APV (2.5 µM) successfully suppressed NMDAR-mediated fEPSPs (10.8 ± 2.4% of baseline), with the residual fEPSPs likely due to the fact that a minority of CA1 neurons remained positive for Nampt in P30 CamKIIαNampt−/− mice (Fig. 2C). Thus, it appears that fEPSPs mediated by NMDARs are present in CamKIIαNampt−/− mice, but these NMDARs do not display functional GluN2B subunits in response to synaptic stimulation. In support of this notion, in control mice, 2.5 µM APV treatment alone partially depressed NMDAR-mediated fEPSPs (Fig. 3C, 48.8 ± 3.7%, n=5). Since low concentrations of D-APV behave as an antagonist of ifenprodil-insensitive NMDARs [9, 32, 36], the partial depression of NMDAR-mediated fEPSPs induced by 2.5 ^M APV treatment is consistent with an intact GluN2B–containing NMDAR component. On the other hand, in CamKIIαNampt−/− mice, D-APV treatment alone suppressed NMDAR-mediated fEPSPs to a much greater extent than we observed in control mice (16.6 ± 4.2%, p<0.001, Student’s t-test), suggesting a reduced functional GluN2B component. To verify these findings, we repeated this experiment with TCN 213 in place of ifenprodil. TCN 213 [N-(cyclohexylmethyl)-2-[{5 [(phenylmethyl)amino]-1,3,4-thiadiazol-2-yl}thio]acetamide] was initially thought to be a selective antagonist of GluN2A–containing NMDARs [5]. However, we recently showed that inhibition of NMDAR EPSPs by ifenprodil and TCN 213 largely overlapped in the CA1 region of hippocampal slices from 30-day-old rats [39]. We speculated that ifenprodil and TCN 213 may act on the same subset of GluN2A/GluN2B triheteromers [39]. Like ifenprodil, TCN 213 (10 µM) partially depressed NMDAR-mediated fEPSPs in all control mice tested (Fig. 3D, 50.9 ± 6.5% compared to baseline, n=5). Subsequent co-administration of D-APV (2.5 µM) suppressed the remaining NMDAR-mediated fEPSPs (Fig. 3D, 14.4 ± 7.3%). These observations suggest that GluN2B contributes to approximately 48% of synaptic NMDAR activity in P30 control slices, which is similar to the 45% we noted with ifenprodil (Fig. 3A). On the other hand, TCN 213, like ifenprodil, did not depress NMDAR fEPSPs in slices from CamKIIαNampt−/− mice (Fig. 3D, 101.6 ± 6.5% of baseline, n=5, p=0.0002, Student’s t-test). And yet, co-administration of D-APV (2.5 µM) successfully suppressed NMDAR-mediated fEPSPs (13.3 ± 2.0% of baseline), further supporting our notion that CamKIIαNampt−/− mice have altered NMDAR function.
Figure 3.
Postnatal day 30 CamKIIαNampt−/− mice showed impaired GluN2B–containing N-methyl-D-aspartate receptor (NMDAR)-mediated field excitatory postsynaptic potentials (fEPSPs). A, In control mice, ifenprodil (10 µM, filled bar) partially depressed NMDAR-mediated fEPSPs. Co-administration of D-APV (2.5 µM, open bar) thereafter almost completely suppressed the remaining NMDAR-mediated fEPSPs (n=5). B, In CamKIIαNampt−/− mice, ifenprodil only minimally depressed NMDAR-mediated fEPSPs. Co-administration of D-APV thereafter successfully suppressed NMDAR-mediated fEPSPs (n=5). C, D-APV (2.5 µM, open bar) alone suppressed NMDAR-mediated fEPSPs to a greater extent in CamKIIαNampt−/− mice than in control mice. D, In control mice, TCN 213 (10 µM, filled bar) partially depressed NMDAR-mediated fEPSPs. Co-administration of D-APV (2.5 µM, open bar) almost completely suppressed the remaining NMDAR-mediated fEPSPs (n=5). In CamKIIαNampt−/− mice, TCN 213 did not depress NMDAR-mediated fEPSPs. Co-administration of D-APV successfully suppressed NMDAR-mediated fEPSPs in both genotypes (n=5). A-D, Traces to the right depict representative NMDAR-mediated fEPSPs at the times denoted. A-C, Calibration: 1 mV, 5 ms. D, Calibration: 1 mV, 10 ms. Data are presented as mean ± S.E.M. *, # represents significance generated from Student t-tests and Mann-Whitney U-tests, respectively, in comparisons between control and CamKIIαNampt−/− mice. *,#P < 0.05. **,##P < 0.01. ***,###P < 0.001.
3.3 GluN2B expression is unaltered in P30 CamKIIαNampt−/− mice
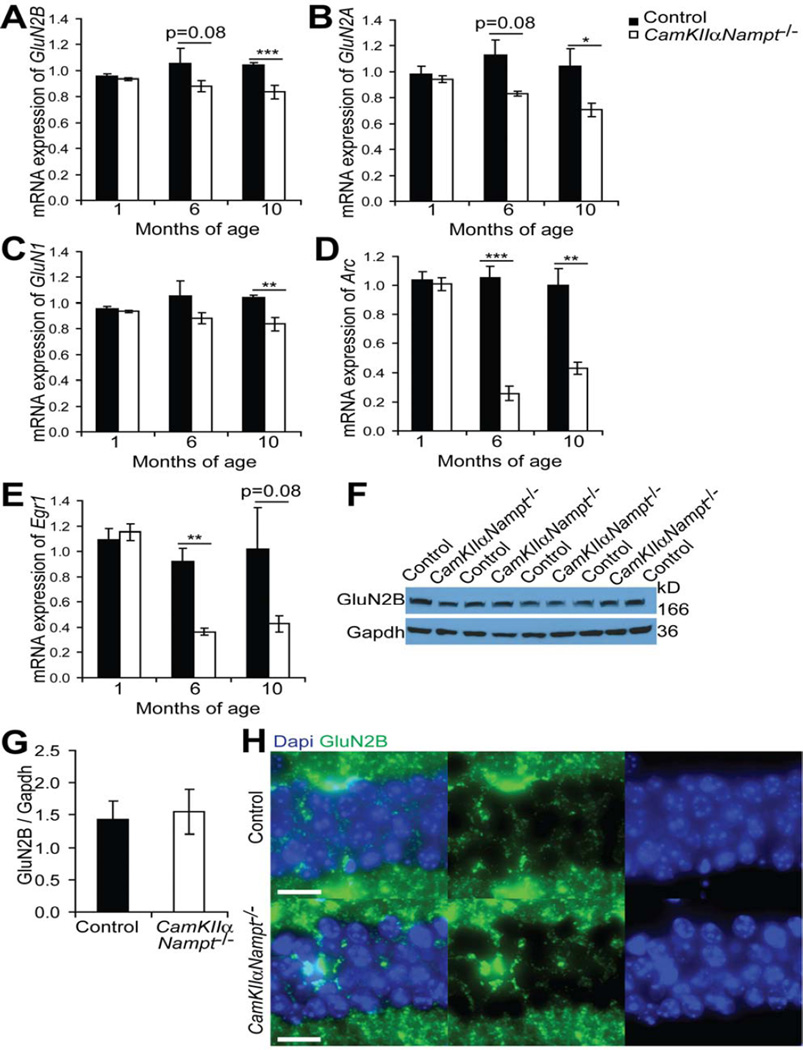
Given the lack of responsiveness of CamKIIαNampt−/− mice to ifenprodil, we examined the mRNA expression of GluN2B. Quantitative RT-PCR analysis of hippocampal RNA samples showed no difference in GluN2B mRNA expression between P30 control and CamKIIαNampt−/− mice (Fig. 3A). While GluN2B mRNA expression was not altered at P30, it decreased with age, showing a 20% reduction by 6 months of age (n=3–4, p=0.076, Student’s t-test) and a 27% reduction by 10 months of age (Fig. 4A, n=4–5, p=0.0004, Student’s t -test). To determine if this change in GluN2B mRNA expression was specific, we also assessed age-related changes in other NMDAR subunits, GluN2A and GluN1, as well as two immediate early genes shown to have altered expression by 2–3 months of age [79], Arc and Egr1. Like GluN2B, GluN2A expression was not altered at P30, but decreased with age (Fig. 4B), showing 26% and 32% reductions by 6 (n=3–4, p=0.084, Student’s t-test) and 10 months of age (n=4–5, p=0.039, Student’s t-test), respectively. GluN1 expression also significantly decreased with age (Fig. 4C), but at a lesser extent, showing 16% and 20% reductions by 6 (n=3–4, p=0.288, Student’s t-test) and 10 months of age (n=4–5, p=0.009, Student’s t-test), respectively. Expression of the immediate early genes Arc and Egr1 were even more affected (Fig. 4D,E), showing an approximately 60% decrease at 6 (n=3–4, Arc: p=0.0006, Egr1: p=0.008, Student’s t-test) and 10 months of age (n=4–5, Arc: p=0.0013, Egr1: p=0.083, Student’s t-test). These data suggest that the change in GluN2B function at P30 is unlikely to result from transcriptional modifications and that the change in GluN2B mRNA expression with age is representative of broader hippocampal defects.
Figure 4.
CamKIIαNampt−/− mice showed an age-related decline in N-methyl-D-aspartate receptor (NMDAR) subunits and immediate early genes. A-E, Quantitative PCR analysis in RNA isolated from the hippocampi of CamKIIαNampt−/− and control mice at the ages indicated (n = 3–5). F, Representative immunoblot of hippocampal extracts of postnatal day 30 CamKIIαNampt−/− and control mice for GluN2B normalized by Gapdh. G, Quantification of F. H, Representative images of immunofluorescence in postnatal day 30 CamKIIαNampt−/− and control mice for Dapi (blue) and GluN2B (green) in CA1 (n = 4–6). Scale bars represent 20 µM Data are presented as mean ± S.E.M. *P < 0.05. **P < 0.01. ***P < 0.001.
To determine whether GluN2B protein levels were altered in CamKIIαNampt−/− mice, we immunoblotted hippocampal extracts for GluN2B. However, control and CamKIIαNampt−/− mice showed similar protein levels of GluN2B (Fig. 4F,G). To examine whether the distribution of GluN2B was changed in CamKIIαNampt−/− mice, we performed immunohistochemistry for GluN2B on P30 brain sections. Control and CamKIIαNampt−/− mice appeared to show similar patterns of GluN2B protein distribution on hippocampal sections (Fig. 4H). Thus, loss of Nampt affects the function of GluN2B–containing NMDARs through a mechanism independent of global GluN2B expression profiles.
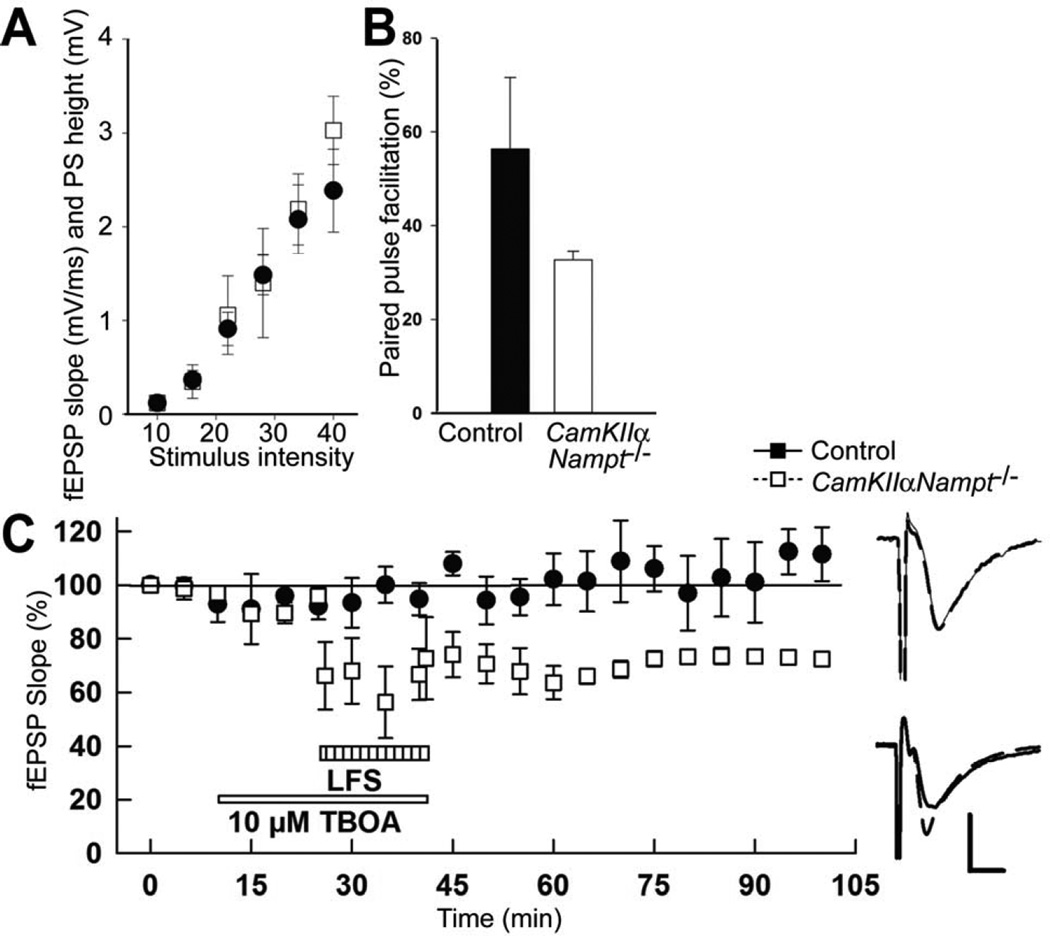
To assess if changes in GluN2B sensitivity involved alterations in presynaptic mechanisms, we assessed the paired pulse ratio by delivering two identical stimuli at an interval of 21 msec. We then used a baseline stimulus-response curve to calculate changes in the second fEPSP slope at a stimulus intensity that evoked a half maximal response for the first response (Fig. 5A). In this paradigm, the second fEPSP evoked by the second stimulus with the same intensity are usually bigger (>100%) than the half maximal fEPSP evoked by the initial stimulus. Such an occurrence is termed paired pulse facilitation. Similar paired pulse facilitation was seen in slices from control and CamKIIαNampt−/− mice (Fig. 5B, p=0.151, Mann-Whitney Rank Sum Test). Thus, presynaptic mechanisms do not appear to be significantly affected in CamKIIαNampt−/− mice and cannot explain their insensitivity to LTD or GluN2B antagonists.
Figure 5.
Postnatal day 30 CamKIIαNampt−/− mice showed normal baseline synaptic responses as well as presynaptic mechanisms and their impaired induction of LTD was rescued by inhibition of glutamate uptake. A, Base line response was evaluated by obtaining baseline stimulus response curves with the same set of 6 stimuli (10, 16, 22, 28, 34, and 40 V; n=5). B, Paired pulse facilitation was evaluated by delivering two identical stimuli at an interval of 21 msec and calculated changes in the second EPSP slopes a stimulus intensity that evoked a half maximal response for the first response based on a baseline stimulus-response curve. Compared to the half maximal fEPSP evoked by the initial stimulus the second fEPSP evoked by the second stimulus with the same intensity are usually bigger (>100%). Similar paired pulse facilitation was seen in slices from control and CamKIIαNampt−/− mice (n=5). C, The presence of the glutamate uptake inhibitor DL-threo-β-benzyloxyaspartate (DL-TBOA) at 10 µM for 30 min allowed 1 Hz LFS to induce LTD in slices from CamKIIαNampt−/− mice (65.9±7.5%, white squares, n=5). The failure of LFS to induce LTD in the absence of DL-TBOA is shown in black circles. Calibration: 1 mV, 5 ms.
Having found that synaptic responses from CamKIIαNampt−/− mice lacked sensitivity to GluN2B antagonists despite normal expression of GluN2B and normal presynaptic function, we next investigated if GluN2B was properly targeted to synapses. To address this possibility, we administered DL-threo-β-benzyloxyaspartate (DL-TBOA), a potent, selective nontransportable inhibitor of excitatory amino acid transporters [16, 75], at 10 µM for 30 min. DL-TBOA blocks recycling of presynaptically-released glutamate and causes accumulation of glutamate in the synaptic cleft, thus enhancing ‘spillover’ and increasing the likelihood of extrasynaptic GluN2B activation [52, 92]. DL-TBOA did not depress basal response in CamKIIαNampt−/− mice (Fig. 5C, 111.8±6.5, n=5). However, in the presence of DL-TBOA, 1 Hz LFS induced LTD in CamKIIαNampt−/− mice (Fig. 5C, 65.9±7.5%, n=5). These data suggest that blocking glutamate uptake results in the induction of LTD by allowing glutamate released by LFS to activate receptors not normally activated during LFS.
4. Discussion
Here we report a novel assessment of Nampt, the rate-limiting enzyme in the major pathway of mammalian NAD+ biosynthesis [65], in LTD and NMDAR-mediated synaptic transmission. We show that CamKIIαNampt−/− mice have impaired induction of LTD and function of GluN2B–containing NMDARs which manifest prior to changes in NMDAR subunit expression.
Why do CamKIIαNampt−/− mice exhibit intact induction of LTP but abolished induction of LTD? NMDARs can drive both LTP and LTD, but potentially in a subunit dependent manner [94]. The idea that subunit identity differentially affects synaptic plasticity is reasonable as GluN2A–containing NMDARs have faster rise and decay times than GluN2B–containing NMDARs [11, 18]. Several studies have shown that LTP requires GluN2A–containing NMDARs, but not GluN2B–containing NMDARs [48, 73, 77, 85]. However, we and others have found that NMDAR-mediated LTP is not subunit specific [4, 32, 89]. In contrast, many studies have shown that LTD involves GluN2B–containing NMDARs [7, 32, 36, 48, 94]. While not all studies support this functional dichotomy [3, 27, 45], our results are consistent with a link between LTD and GluN2B–mediated synaptic transmission. We show that CamKIIαNampt−/− mice have markedly dampened responsiveness to GluN2B antagonism at synapses, suggesting significantly reduced function of synaptic GluN2B–containing NMDARs. Moreover, a specific impairment of GluN2B synaptic transmission is consistent with the behavioral phenotypes of CamKIIαNampt−/− mice, including hyperactivity, impaired spatial memory and fear conditioning, and diminished anxiety-like behaviors [79]. Similar phenotypes are also seen in mice lacking functional GluN2B [1, 2, 7, 29, 85].
A selective impairment in LTD accompanied by intact LTP has been observed before [19, 32, 35, 43, 54]. However, the finding that CamKIIαNampt−/− mice have impaired induction of LTD but intact LTP was surprising since we previously showed that CamKIIαNampt−/− mice exhibit impaired spatial memory [79]. Traditionally, LTP has been considered a major contributor to hippocampal spatial memory [40, 43, 83]. In contrast, LTD has been given an auxiliary role in signal-to-noise regulation or in forgetting [43]. However, LTD may also contribute to hippocampal-dependent memory as mice with impaired LTD displayed impaired spatial memory [7, 19, 25, 43, 54, 55]. LTD may also be relevant to novelty detection, acquisition [42, 50], and habituation [19]. Thus, impaired LTD is consistent with the hyperactivity and lack of habituation displayed by CamKIIαNampt−/− mice [79].
Importantly, these phenotypes are not typical effects of energy deprivation. While P30 CamKIIαNampt−/− mice exhibit relatively normal CA1 population spikes and fEPSPs, other conditions involving energy deprivation, such as hypoglycemia [72, 81], anoxia [31, 33], or ischemia [60, 87] are associated with reduced or abolished population spikes and fEPSPs. Similarly, CamKIIαNampt−/− mice exhibit normal LTP, whereas hypoglycemia [37], anoxia [33], ischemia [13, 87], or pharmacological glycolytic blockade [34] can abolish LTP. LTD has been less studied in the context of energy deprivation. However, hypoxia and/or hypoglycemia can cause non-tetanic LTD [76] and depress synaptic transmission in CA1 neurons [23]. Similarly, reduced Na,K-ATPase activity, which occurs upon energy deprivation [53], induces, rather than abolishes, LTD [64]. Energy deprivation also occurs with NMDAR over-activation [13, 14, 26, 31, 33, 81]. In fact, GluN2B–containing NMDARs appear to play a key role in this over-activation as antagonism of GluN2B improved fEPSP recovery following hypoxia [68] and blocked non-tetanic LTP post-ischemia [62]. Metabolic inhibition also increased the expression of GluN2B in CA1 [10]. Thus, our analysis of CamKIIαNampt−/− mice suggests that NAD+ depletion generates specific effects that differ from other forms of energy deprivation and/or that energy deprivation is not the primary cause of CamKIIαNampt−/− phenotypes.
What could be the potential cause of CamKIIαNampt−/− phenotypes? One possibility would be the accumulation of Nampt’s substrate, nicotinamide. However, nicotinamide appears to have unrelated effects from those we find upon deletion of Nampt. In particular, bath applied nicotinamide blocks LTP [44, 74] and presynaptic injection of nicotinamide reduces basal transmission without affecting LTP [59]. Moreover, in preliminary studies, we found that application of nicotinamide (200 µM, 15 min) prior to LFS did not inhibit LTD (58.0 ± 8.1%, n=3). Thus, accumulation of nicotinamide is unlikely to play a causal role.
Another possibility is the functional defect in key mediators produced from NAD+ or requiring NAD+. One potential mediator is cyclic ADP (cADP)-ribose. cADP-ribose is a second messenger hydrolyzed from NAD+ by cADPR-ribose synthases (CD38 and CD157) [78], and cADPR-mediated signaling pathways play a key role in LTD induction [66, 67]. Induction of LTD begins with calcium entry via NMDARs and this calcium influx activates CD38, which produces cADP-ribose from NAD+. cADP-ribose binds ryanodine receptors, thus modulating calcium release from intracellular stores that is necessary for induction of LTD [66, 67, 69]. In addition to cADP-ribose synthases, NAD+ is a substrate for poly(ADP-ribosyl) polymerases (ie. Parp1) and sirtuins (ie. Sirt1) [30, 78]. However, to our knowledge, neither factor has been assessed in LTD. Therefore, further investigation will be necessary.
The age-dependent decline in the expression of the immediate early gene Arc could be a possible cause, as genetic reduction of Arc reduced LTD [63]. While Egr1 mRNA expression also declined with age, Egr1 appears to be more important for LTP than LTD [12, 88]. Both Egr1 [41, 58] and Arc [70] are upregulated after ischemia and/or hypoxia, suggesting that their downregulation in CamKIIαNampt−/− mice is a specific change.
Currently, the possibilities outlined above do not provide a direct connection to the impaired GluN2B–containing NMDAR function exhibited by CamKIIαNampt−/− mice. Although we show that NMDAR subunit mRNA expression significantly decreases with age in CamKIIαNampt−/− mice, GluN2B mRNA and protein levels were not altered at P30. Moreover, the immunostaining profile of GluN2B did not reveal an overt difference. Thus, the reduced activity of GluN2B in P30 CamKIIαNampt−/− mice likely resulted from something besides GluN2B expression levels. Nevertheless, it is important to acknowledge that immunohistochemical analysis was performed on slices from mice anesthetized with ketamine. Some have found that ketamine down-regulated GluN2B in rat forebrain culture [21] and upon subcutaneous injection in neonatal mice [47]. However, these effects were shown with ketamine exposure for 12 hours [21] and upon reaching adulthood [47], respectively. We sacrificed our mice within 15 minutes of intraperitoneal ketamine administration, which is scant time for ketamine to reach the hippocampus and alter GluN2B expression. Thus, we perceive it to be unlikely that use of ketamine affected our results.
One potential explanation is that the trafficking of GluN2B is altered in CamKIIαNampt−/− mice. It is still possible that GluN2B subunits were present, but intracellular or extrasynaptic [93]. This notion is supported by our finding that in the presence of the glutamate uptake inhibitor, DL-TBOA, 1 Hz stimulation induced LTD in CamKIIαNampt−/− mice. Indeed, other studies have found that spillover of glutamate released by LFS results in the induction of GluN2B–dependent LTD [51]. While it is currently difficult to distinguish between synaptic and extrasynaptic NMDARs, others have defined extrasynaptic receptors as those that are not activated by 1 Hz LFS unless a glutamate uptake blocker is applied [51]. This definition implies that the GluN2B–containing NMDARs in CamKIIαNampt−/− mice are extrasynaptic. As such, future work with surface biotinylation, measurements of intra- and extra-synaptic receptors, and electron microscopy are prime areas for future pursuit. Another potential explanation is that GluN2B is correctly localized, but inactive. NMDAR function is dependent upon intracellular oxidation-reduction state [6], which could change upon loss of NAD+ biosynthesis. Thus, maybe oxidation-reduction state can inactivate GluN2B subunits. It should be noted that GluN2B–containing NMDARs are vulnerable to a variety of insults [22, 35]. Thus, an alterative explanation is that rather than a specific effect on GluN2B, GluN2B function is a particularly sensitive synaptic component.
In any genetic model, effects could result from compensatory changes during development. However, with the CamKIIαCre driver, recombination begins in CA1 at P14–18 and plateaus at P28 [56, 84, 86]. Thus, the CamKIIαCre driver avoids any compensatory changes during embryonic and early postnatal development and is therefore a preferred Cre driver [24]. Other models for inducible genetic deletion also have caveats. For example, the tetracycline regulatory system requires long-term exposure to doxycycline or tetracycline just as the Cre lines CrePR, CreERT, CreERT2, CreER™ lines require tamoxifen or RU486. The inducible models not only have lower efficiency in the brain, but can have other serious drug-induced side effects [24, 28, 57, 80]. Nevertheless, recapitulation of these effects in an adult knockdown model is a prime area for future work and a study that we are currently undertaking.
In conclusion, in a model that minimizes compensatory changes during development by postnatal activation of Cre recombinase [56, 84, 86], loss of Nampt selectively impairs LTD and the function of GluN2B–containing NMDARs. These findings support an intricate link between neuronal metabolic status and synaptic plasticity, but are not typical effects of energy deprivation. Thus, loss of neuronal NAD+ and/or the manner in which the brain compensates for loss of neuronal NAD+ appears to generate significant and novel effects. Further analysis of CamKIIαNampt−/− mice should provide meaningful insight into the involvement of GluN2B in synaptic transmission.
Highlights.
CamKIIαNampt−/− mice lack the NAD+ biosynthetic enzyme Nampt in forebrain neurons.
CamKIIαNampt−/− mice have impaired induction of long-term depression (LTD).
CamKIIαNampt−/− mice have normal induction of long-term potentiation (LTP).
CamKIIαNampt−/− mice have dysfunctional GluN2B–containing NMDARs.
Nampt plays a critical, novel role in LTD and GluN2B–containing NMDAR function.
Acknowledgements
We thank Kazuko A. O’Dell for help with the electrophysiology. This work was supported in part by the National Institute on Aging (AG024150, AG037457), the Ellison Medical Foundation to S.I., and the National Institute of Mental Health (MH077791) to C.F.Z., the National Institutes of Health Training Grant T32 GM007067 to L.R.S., and the Alafi Neuroimaging Laboratory, the Hope Center for Neurological Disorders, and NIH Neuroscience Blueprint Center Core Grant P30 NS05105 to Washington University.
Abbreviations
- CA1
Cornu ammonis region 1
- fEPSP
Field excitatory postsynaptic potential
- HFS
High frequency stimulation
- LTD
Long-term depression
- LTP
Long-term potentiation
- LFS
Low frequency stimulation
- NAD+
Nicotinamide adenine dinucleotide
- Nampt
Nicotinamide phosphoribosyltransferase
- NMDAR
N-methyl-D-aspartate receptor
- P
Postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
S.I. is a co-founder of Metro Midwest Biotech. C.Z. serves on the scientific advisory board of Sage Therapeutics. L.R.S. and Y.I. declare no competing financial interests.
Author contributions: L.R.S., C.F.Z., S.I., and Y.I. designed research, analyzed data, and wrote the paper. L.R.S. and Y.I. performed research.
Contributor Information
Liana Roberts Stein, Email: LianaLRoberts@gmail.com.
Charles F. Zorumski, Email: zorumskc@psychiatry.wustl.edu.
Shin-ichiro Imai, Email: imaishin@wustl.edu.
Yukitoshi Izumi, Email: izumiy@psychiatry.wustl.edu.
References
- 1.Badanich KA, Doremus-Fitzwater TL, Mulholland PJ, Randall PK, Delpire E, Becker HC. NR2B–deficient mice are more sensitive to the locomotor stimulant and depressant effects of ethanol. Genes, brain, and behavior. 2011;10:805–816. doi: 10.1111/j.1601-183X.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. European journal of pharmacology. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B–containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, Cardullo F. Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B–containing receptors. The Journal of pharmacology and experimental therapeutics. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- 6.Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B–containing NMDA receptors in CA1 hippocampus cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukalo O, Dityatev A. Analysis of neural cell functions in gene knockout mice: electrophysiological investigation of synaptic plasticity in acute hippocampal slices. Methods in enzymology. 2006;417:52–66. doi: 10.1016/S0076-6879(06)17005-6. [DOI] [PubMed] [Google Scholar]
- 9.Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camacho A, Montiel T, Massieu L. Sustained metabolic inhibition induces an increase in the content and phosphorylation of the NR2B subunit of N-methyl-D-aspartate receptors and a decrease in glutamate transport in the rat hippocampus in vivo. Neuroscience. 2007;145:873–886. doi: 10.1016/j.neuroscience.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 11.Chen N, Luo T, Raymond LA. Subtype-dependence of NMDA receptor channel open probability. J Neurosci. 1999;19:6844–6854. doi: 10.1523/JNEUROSCI.19-16-06844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheval H, Chagneau C, Levasseur G, Veyrac A, Faucon-Biguet N, Laroche S, Davis S. Distinctive features of Egr transcription factor regulation and DNA binding activity in CA1 of the hippocampus in synaptic plasticity and consolidation and reconsolidation of fear memory. Hippocampus. 2012;22:631–642. doi: 10.1002/hipo.20926. [DOI] [PubMed] [Google Scholar]
- 13.Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Progress in neurobiology. 1998;54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- 14.Crepel V, Epsztein J, Ben-Ari Y. Ischemia induces short- and long-term remodeling of synaptic activity in the hippocampus. Journal of cellular and molecular medicine. 2003;7:401–407. doi: 10.1111/j.1582-4934.2003.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behavioural brain research. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 16.Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. The Journal of physiology. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etkin A, Alarcon JM, Weisberg SP, Touzani K, Huang YY, Nordheim A, Kandel ER. A role in learning for SRF: deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50:127–143. doi: 10.1016/j.neuron.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Progress in neurobiology. 2012;96:283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L, Tang R, Bao N, Wang J, Ma H. Ketamine and propofol in combination induce neuroapoptosis and down-regulate the expression of N-methyl-D-aspartate glutamate receptor NR2B subunit in rat forebrain culture. Die Pharmazie. 2011;66:771–776. [PubMed] [Google Scholar]
- 22.Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, Wozniak DF, Milbrandt J. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience. 2007;148:633–643. doi: 10.1016/j.neuroscience.2007.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao TM, Pulsinelli WA, Xu ZC. Prolonged enhancement and depression of synaptic transmission in CA1 pyramidal neurons induced by transient forebrain ischemia in vivo. Neuroscience. 1998;87:371–383. doi: 10.1016/s0306-4522(98)00150-x. [DOI] [PubMed] [Google Scholar]
- 24.Gaveriaux-Ruff C, Kieffer BL. Conditional gene targeting in the mouse nervous system: Insights into brain function and diseases. Pharmacology & therapeutics. 2007;113:619–634. doi: 10.1016/j.pharmthera.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Ge Y, Dong Z, Bagot RC, Howland JG, Phillips AG, Wong TP, Wang YT. Hippocampal long-term depression is required for the consolidation of spatial memory. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16697–16702. doi: 10.1073/pnas.1008200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godukhin O, Savin A, Kalemenev S, Levin S. Neuronal hyperexcitability induced by repeated brief episodes of hypoxia in rat hippocampal slices: involvement of ionotropic glutamate receptors and L-type Ca(2+) channels. Neuropharmacology. 2002;42:459–466. doi: 10.1016/s0028-3908(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 27.Hendricson AW, Miao CL, Lippmann MJ, Morrisett RA. Ifenprodil and ethanol enhance NMDA receptor-dependent long-term depression. The Journal of pharmacology and experimental therapeutics. 2002;301:938–944. doi: 10.1124/jpet.301.3.938. [DOI] [PubMed] [Google Scholar]
- 28.Higashi AY, Ikawa T, Muramatsu M, Economides AN, Niwa A, Okuda T, Murphy AJ, Rojas J, Heike T, Nakahata T, Kawamoto H, Kita T, Yanagita M. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. Journal of immunology. 2009;182:5633–5640. doi: 10.4049/jimmunol.0802413. [DOI] [PubMed] [Google Scholar]
- 29.Higgins GA, Ballard TM, Huwyler J, Kemp JA, Gill R. Evaluation of the NR2B–selective NMDA receptor antagonist Ro 63-1908 on rodent behaviour: evidence for an involvement of NR2B NMDA receptors in response inhibition. Neuropharmacology. 2003;44:324–341. doi: 10.1016/s0028-3908(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 30.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocrine reviews. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu KS, Huang CC. Characterization of the anoxia-induced long-term synaptic potentiation in area CA1 of the rat hippocampus. British journal of pharmacology. 1997;122:671–681. doi: 10.1038/sj.bjp.0701409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izumi Y, Katsuki H, Benz AM, Zorumski CF. Oxygen deprivation produces delayed inhibition of long-term potentiation by activation of NMDA receptors and nitric oxide synthase. J Cereb Blood Flow Metab. 1998;18:97–108. doi: 10.1097/00004647-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Izumi Y, Katsuki H, Zorumski CF. Monocarboxylates (pyruvate and lactate) as alternative energy substrates for the induction of long-term potentiation in rat hippocampal slices. Neuroscience letters. 1997;232:17–20. doi: 10.1016/s0304-3940(97)00567-3. [DOI] [PubMed] [Google Scholar]
- 35.Izumi Y, Kitabayashi R, Funatsu M, Izumi M, Yuede C, Hartman RE, Wozniak DF, Zorumski CF. A single day of ethanol exposure during development has persistent effects on bi-directional plasticity, N-methyl-D-aspartate receptor function and ethanol sensitivity. Neuroscience. 2005;136:269–279. doi: 10.1016/j.neuroscience.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience. 2005;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Izumi Y, Zorumski CF. Involvement of nitric oxide in low glucose-mediated inhibition of hippocampal long-term potentiation. Synapse (New York, N.Y) 1997;25:258–262. doi: 10.1002/(SICI)1098-2396(199703)25:3<258::AID-SYN4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 38.Izumi Y, Zorumski CF. NMDA receptors mGluR5, and endocannabinoids are involved in a cascade leading to hippocampal long-term depression. Neuropsychopharmacology. 2012;37:609–617. doi: 10.1038/npp.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izumi Y, Zorumski CF. Sensitivity of N-methyl-D-aspartate receptor-mediated excitatory postsynaptic potentials and synaptic plasticity to TCN 201 and TCN 213 in rat hippocampal slices. The Journal of pharmacology and experimental therapeutics. 2015;352:267–273. doi: 10.1124/jpet.114.220582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffery KJ, Morris RG. Cumulative long-term potentiation in the rat dentate gyrus correlates with but does not modify, performance in the water maze. Hippocampus. 1993;3:133–140. doi: 10.1002/hipo.450030205. [DOI] [PubMed] [Google Scholar]
- 41.Jin K, Mao XO, Eshoo MW, del Rio G, Rao R, Chen D, Simon RP, Greenberg DA. cDNA microarray analysis of changes in gene expression induced by neuronal hypoxia in vitro. Neurochemical research. 2002;27:1105–1112. doi: 10.1023/a:1020913123054. [DOI] [PubMed] [Google Scholar]
- 42.Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends in neurosciences. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Kleppisch T, Pfeifer A, Klatt P, Ruth P, Montkowski A, Fassler R, Hofmann F. Long-term potentiation in the hippocampal CA1 region of mice lacking cGMP-dependent kinases is normal and susceptible to inhibition of nitric oxide synthase. J Neurosci. 1999;19:48–55. doi: 10.1523/JNEUROSCI.19-01-00048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohr G, Jensen V, Koester HJ, Mihaljevic AL, Utvik JK, Kvello A, Ottersen OP, Seeburg PH, Sprengel R, Hvalby O. Intracellular domains of NMDA receptor subtypes are determinants for long-term potentiation induction. J Neurosci. 2003;23:10791–10799. doi: 10.1523/JNEUROSCI.23-34-10791.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learning & memory (Cold Spring Harbor, N.Y) 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- 47.Lecointre M, Vezier C, Benard M, Ramdani Y, Dupre N, Brasse-Lagnel C, Henry VJ, Roy V, Marret S, Gonzalez BJ, Jegou S, Leroux-Nicollet I. Age-dependent alterations of the NMDA receptor developmental profile and adult behavior in postnatally ketamine-treated mice. Developmental neurobiology. 2015;75:315–333. doi: 10.1002/dneu.22232. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science (New York, N.Y) 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 49.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annual review of cell and developmental biology. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 50.Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B–containing NMDA receptors in cortical long-term potentiation and long-term depression. J. Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B–containing NMDA receptors in cortical long-term potentiation and long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 55.Min SS, Quan HY, Ma J, Lee KH, Back SK, Na HS, Han SH, Yee JY, Kim C, Han JS, Seol GH. Impairment of long-term depression induced by chronic brain inflammation in rats. Biochemical and biophysical research communications. 2009;383:93–97. doi: 10.1016/j.bbrc.2009.03.133. [DOI] [PubMed] [Google Scholar]
- 56.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biological psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 57.Morimoto M, Kopan R. rtTA toxicity limits the usefulness of the SP-C-rtTA transgenic mouse. Developmental biology. 2009;325:171–178. doi: 10.1016/j.ydbio.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagata T, Takahashi Y, Sugahara M, Murata A, Nishida Y, Ishikawa K, Asai S. Profiling of genes associated with transcriptional responses in mouse hippocampus after transient forebrain ischemia using high-density oligonucleotide DNA array. Brain research. Molecular brain research. 2004;121:1–11. doi: 10.1016/j.molbrainres.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 59.Pavlidis P, Montgomery J, Madison DV. Presynaptic protein kinase activity supports long-term potentiation at synapses between individual hippocampal neurons. J Neurosci. 2000;20:4497–4505. doi: 10.1523/JNEUROSCI.20-12-04497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedersen JZ, Bernardi G, Centonze D, Pisani A, Rossi L, Rotilio G, Calabresi P. Hypoglycemia hypoxia, and ischemia in a corticostriatal slice preparation: electrophysiologic changes and ascorbyl radical formation. J Cereb Blood Flow Metab. 1998;18:868–875. doi: 10.1097/00004647-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Pellerin L. Brain energetics (thought needs food) Current opinion in clinical nutrition and metabolic care. 2008;11:701–705. doi: 10.1097/MCO.0b013e328312c368. [DOI] [PubMed] [Google Scholar]
- 62.Picconi B, Tortiglione A, Barone I, Centonze D, Gardoni F, Gubellini P, Bonsi P, Pisani A, Bernardi G, Di Luca M, Calabresi P. NR2B subunit exerts a critical role in postischemic synaptic plasticity. Stroke; a journal of cerebral circulation. 2006;37:1895–1901. doi: 10.1161/01.STR.0000226981.57777.b0. [DOI] [PubMed] [Google Scholar]
- 63.Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 64.Reich CG, Mason SE, Alger BE. Novel form of LTD induced by transient, partial inhibition of the Na,K-pump in rat hippocampal CA1 cells. Journal of neurophysiology. 2004;91:239–247. doi: 10.1152/jn.00722.2003. [DOI] [PubMed] [Google Scholar]
- 65.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. The Journal of biological chemistry. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 66.Reyes-Harde M, Empson R, Potter BV, Galione A, Stanton PK. Evidence of a role for cyclic ADP-ribose in long-term synaptic depression in hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4061–4066. doi: 10.1073/pnas.96.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reyes-Harde M, Potter BV, Galione A, Stanton PK. Induction of hippocampal LTD requires nitric-oxide-stimulated PKG activity and Ca2+ release from cyclic ADP-ribose-sensitive stores. Journal of neurophysiology. 1999;82:1569–1576. doi: 10.1152/jn.1999.82.3.1569. [DOI] [PubMed] [Google Scholar]
- 68.Reyes M, Reyes A, Opitz T, Kapin MA, Stanton PK. Eliprodil a non-competitive NR2B–selective NMDAantagonist, protects pyramidal neurons in hippocampal slices from hypoxic/ischemic damage. Brain research. 1998;782:212–218. doi: 10.1016/s0006-8993(97)01280-8. [DOI] [PubMed] [Google Scholar]
- 69.Reyes M, Stanton PK. Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J Neurosci. 1996;16:5951–5960. doi: 10.1523/JNEUROSCI.16-19-05951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rickhag M, Teilum M, Wieloch T. Rapid long-term induction of effector immediate early genes (BDNF, Neuritin and Arc) in peri-infarct cortex and dentate gyrus after ischemic injury in rat brain. Brain research. 2007;1151:203–210. doi: 10.1016/j.brainres.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Rongvaux A, Galli M, Denanglaire S, Van Gool F, Dreze PL, Szpirer C, Bureau F, Andris F, Leo O. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008;181:4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 72.Sadgrove MP, Beaver CJ, Turner DA. Effects of relative hypoglycemia on LTP and NADH imaging in rat hippocampal slices. Brain research. 2007;1165:30–39. doi: 10.1016/j.brainres.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 74.Schuman EM, Meffert MK, Schulman H, Madison DV. An ADP-ribosyltransferase as a potential target for nitric oxide action in hippocampal long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11958–11962. doi: 10.1073/pnas.91.25.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Molecular pharmacology. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 76.Singh V, Carman M, Roeper J, Bonci A. Brief ischemia causes long-term depression in midbrain dopamine neurons. The European journal of neuroscience. 2007;26:1489–1499. doi: 10.1111/j.1460-9568.2007.05781.x. [DOI] [PubMed] [Google Scholar]
- 77.Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- 78.Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends in endocrinology and metabolism: TEM. 2012;23:420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stein LR, Wozniak DF, Dearborn JT, Kubota S, Apte RS, Izumi Y, Zorumski CF, Imai S. Expression of nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. J Neurosci. 2014;34:5800–5815. doi: 10.1523/JNEUROSCI.4730-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takebayashi H, Usui N, Ono K, Ikenaka K. Tamoxifen modulates apoptosis in multiple modes of action in CreER mice. Genesis. 2008;46:775–781. doi: 10.1002/dvg.20461. [DOI] [PubMed] [Google Scholar]
- 81.Tekkok S, Krnjevic K. Long-term potentiation in hippocampal slices induced by temporary suppression of glycolysis. Journal of neurophysiology. 1995;74:2763–2766. doi: 10.1152/jn.1995.74.6.2763. [DOI] [PubMed] [Google Scholar]
- 82.Tokuda K, O’Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci. 2002;22:9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 85.von Engelhardt J, Doganci B, Jensen V, Hvalby O, Gongrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JN, Seeburg PH, Bannerman DM, Monyer H. Contribution of hippocampal and extra-hippocampal NR2B–containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60:846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 86.Wang L, Budolfson K, Wang F. Pik3c3 deletion in pyramidal neurons results in loss of synapses, extensive gliosis and progressive neurodegeneration. Neuroscience. 2011;172:427–442. doi: 10.1016/j.neuroscience.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang S, Kee N, Preston E, Wojtowicz JM. Electrophysiological correlates of neural plasticity compensating for ischemia-induced damage in the hippocampus. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2005;165:250–260. doi: 10.1007/s00221-005-2296-8. [DOI] [PubMed] [Google Scholar]
- 88.Wei F, Xu ZC, Qu Z, Milbrandt J, Zhuo M. Role of EGR1 in hippocampal synaptic enhancement induced by tetanic stimulation and amputation. The Journal of cell biology. 2000;149:1325–1334. doi: 10.1083/jcb.149.7.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A–containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Molecular pharmacology. 1993;44:851–859. [PubMed] [Google Scholar]
- 91.Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, Brebner K, Liu L, Weinberg J, Christie BR, Phillips AG, Wang YT. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4288–4293. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neuroscience and biobehavioral reviews. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]