Abstract
The Gram-positive bacterial pathogen methicillin-resistant Staphylococcus aureus (MRSA) can cause infections in the bloodstream, endocardial tissue, respiratory tract, culture-confirmed skin, or soft tissue. There are currently no effective vaccines, and none are expected to become available in the near future. An effective vaccine capable of eliciting both systemic and mucosal immune responses is also urgently needed. Here, we reported a novel oil-in-water nanoemulsion adjuvant vaccine containing an MRSA recombination protein antigen, Cremophor EL-35® as a surfactant, and propylene glycol as a co-surfactant. This nanoemulsion vaccine, whose average diameter was 31.34±0.49 nm, demonstrated good protein structure integrity, protein specificity, and good stability at room temperature for 1 year. The intramuscular systemic and nasal mucosal immune responses demonstrated that this nanoemulsion vaccine could improve the specific immune responses of immunoglobulin (Ig)G and related subclasses, such as IgG1, IgG2a, and IgG2b, as well as IgA, in the serum after Balb/c mice intramuscular immunization and C57 mice nasal immunization. Furthermore, this nanoemulsion vaccine also markedly enhanced the interferon-γ and interleukin-17A cytokine cell immune response, improved the survival ratio, and reduced bacterial colonization. Taken together, our results show that this novel nanoemulsion vaccine has great potential and is a robust generator of an effective intramuscular systemic and nasal mucosal immune response without the need for an additional adjuvant. Thus, the present study serves as a sound scientific foundation for future strategies in the development of this novel nanoemulsion adjuvant vaccine to enhance both the intramuscular systemic and nasal mucosal immune responses.
Keywords: nanotechnology, adjuvant, methicillin-resistant Staphylococcus aureus, recombination protein, immune responses
Video abstract
Introduction
The Gram-positive bacterial pathogen methicillin-resistant Staphylococcus aureus (MRSA) can cause infections in a wide range of human tissues such as the bloodstream, lower respiratory tract, endocardial tissue, soft tissue, or culture-confirmed skin.1 It has a very high mortality rate in the USA, the People’s Republic of China, and Japan. The number of deaths caused by a single infection with MRSA has exceeded that of HIV/AIDS in the USA. Therefore, a safe and effective MRSA vaccine is urgently needed.2 We previously reported a recombination antigen protein vaccine containing alpha-toxin (Hla) gene and iron-regulated surface determinant B (IsdB) gene designed by ourselves. The antibody immune response and protective efficacy were clearly improved after intramuscular immunization with this antigen combined with an aluminum adjuvant in a mouse model of MRSA systemic infection compared with the recombination proteins alone.1
Aluminum is a conventional adjuvant which is widely licensed for human use. The primary mechanisms by which antigens adsorb to aluminum are electrostatic interactions, hydrophobic interactions, and ligand exchange.3 However, some disadvantages are associated with this adjuvant, including side effects and safety concerns, as well as the possibility of diminishing or even suppressing cell-mediated immunity and subsequent cytotoxic T-lymphocyte responses, providing poor adjuvant activity for recombinant protein vaccines. Therefore, aluminum adjuvants are only applied to elicit intramuscular systemic immune responses. These weaknesses necessitate the development of a new adjuvant for vaccines.4
Emulsions have also been researched and are widely used as adjuvant. Unlike the local reaction at the injection site caused by water-in-oil emulsions, oil-in-water (O/W) emulsions have advantages such as good injection ability due to low oil content and viscosity. O/W emulsions efficiently exert adjuvant action and can modulate genes including leukocyte migration and antigen presentation by inducing a primary and potent cytokine- and chemokine-rich environment at injection site.5 Several vaccine adjuvant emulsions are approved for human use, such as MF59® (a squalene-based O/W emulsion, Novartis, US Food and Drug Administration [FDA] approved on October 10, 2011), AS04 (contains monophosphoryl lipid A and aluminum hydroxide, GlaxoSmithKline, FDA approved on October 16, 2009), and AS03 (another squalene-based O/W emulsion, GlaxoSmithKline, FDA approved on November 22, 2013).6 However, the above emulsion adjuvant still has some drawbacks, such as poor adjuvant effects because of the thermodynamic instability of systems with average sizes exceeding 160 nm. In addition, these emulsions, such as the MF59 and AS series, are immunogens that generate an insufficiently active cellular immune response.7 Therefore, MF59 and AS series adjuvants were rarely used to elicit a mucosal response.
Nanotechnology has an increasingly important role in the vaccine adjuvant development; specifically, nanoemulsion adjuvants are safe, effective, and ideal vaccine adjuvants. Nanoemulsions with an average diameter of 1–100 nm are composed of the surfactant or co-surfactant, oil, and water, and possess high thermodynamic stability and exist as a single optically isotropic liquid.8 These nanoemulsions may exhibit a strong and broad antimicrobial, antiviral, and antifungal activity and provide good adjuvant activity for vaccines, especially those using intact viruses including respiratory syncytial virus and influenza.9 A recent study indicated that nanoemulsion adjuvants potently and powerfully induce broad mucosal immune responses.10 However, no nanoemulsion adjuvant has been reported to significantly enhance both the intramuscular systemic and nasal mucosal immune responses.
We have successfully developed a novel bovine serum albumin (BSA) nanoemulsion delivery system by the phase inversion method based on the result of a pseudo-ternary phase diagram discussed in our previous publication.11 Also, this novel system has high encapsulation efficiency and drug loading and can clearly improve the stability of BSA while maintaining good structural integrity, structural specificity, and relative bioactivity.11 However, the utility of nanoemulsions as a vaccine adjuvant for the development of protective immunity by enhancing both the systemic and mucosal immune responses remains to be determined.
Therefore, a novel nanoemulsion adjuvant vaccine based on MRSA subunit recombination protein vaccines derived from IsdB and Hla was designed and optimized in this study using our previous methods. The basic characteristics of this nanoemulsion vaccine, such as the morphology, size, and zeta potential, were studied. In addition, the stability of this nanoemulsion vaccine is reported. Importantly, the novel nanoemulsion acts as a vaccine adjuvant that can produce protective immunity by enhancing both the intramuscular systemic and nasal mucosal immune responses in Balb/c mice and C57 mice.
Materials and methods
Bacterial strains and bacterial culture conditions
MRSA252 strains were purchased from the ATCC (Manassas, VA, USA) and used for all experiments. This bacterium was inoculated in tryptic soy broth and cultured at 37°C for 6 hours. Subsequently, the bacteria were washed three times with sterile phosphate-buffered saline (PBS) after centrifuging at 5,000× g for 5 minutes. Finally, the absorbance of the bacteria was measured by a spectrophotometer at 600 nm after diluting to an appropriate concentration with PBS.
Preparation of MRSA antigen protein
The antigen protein was prepared according to our previously published methods.1 Briefly, the IsdB and Hla genes were ligated into the pGEX-6P-2 plasmid, amplified, double digested, and transformed into the Xl/blue strain of Escherichia coli. Protein expression in transformed E. coli BL21 cells was induced with isopropyl-β-D-1-thiogalactopyranoside. The protein was subsequently isolated with Capto™ MMC, and endotoxin was removed by the Triton X-114 phase-separation method.1 The endotoxin concentration was determined by bacterial endotoxin test methods (Chinese Pharmacopoeia Appendix, 2010 version) and Turtle kits (Batch No 201503180; Zhanjiang Bokang Marine Biological Co., Ltd, Zhanjiang, People’s Republic of China). Endotoxin units (EU)/μg protein antigen is ≤0.06, which is below the internationally accepted standard (1.5 EU/μg). The antigens (48 kDa as determined by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis [SDS-PAGE]) were reconstituted to a concentration of 1.5 mg/mL (bicinchoninic acid method), which yielded a 13.843-minute protein peak time and 99.4% purity (high-performance liquid chromatography) in either endotoxin-free sterile water or PBS, and stored at −70°C.
Preparation of the novel nanoemulsion adjuvant vaccine
Influence of surfactant-to-oil ratio on the nanoemulsion vaccine
To optimize the nanoemulsion formula for the antigen protein, we constructed a pseudo-ternary phase diagram based on the results of our previous report.11 The oil phase (isopropyl myristate), the surfactant (Cremophor EL-35®), and the co-surfactant (propylene glycol) were chosen in this study. The nanoemulsion vaccine was prepared in the following order: surfactant, co-surfactant, oil, and protein, that is, SCOH. The protein concentration was 150 μg/mL. Four mixing ratios for the surfactant and the co-surfactant (Smix) at different mass ratios (2:1, 3:1, 4:1, and 5:1) were constructed based on the pseudo-ternary phase diagram according to our previously described method.11 The formulas that resulted in the largest nanoemulsion formation areas were utilized in the subsequent studies.
Size and zeta potential influence of different protein additives
To determine the optimum protein addition to the MRSA vaccine, six different protein concentrations (0, 150, 225, 300, 450, and 600 μg/mL) were examined. The obtained Smix ratios from the above orders were used. The nanoemulsions generated from the six protein concentrations were prepared by our previously described methods. The key factors including drop diameter and zeta potential of different nanoemulsions were determined by a Nano ZS 90 spectroscope (Malvern Instruments, Malvern, UK) at 25°C.
Effect of the addition order on droplet size and zeta potential
We evaluated various addition orders to optimize the MRSA vaccine formulation. We analyzed the following four orders of addition: (1) HSCO – protein followed by surfactant, co-surfactant, and oil; (2) SHCO – surfactant followed by protein, co-surfactant, and oil; (3) SCHO – surfactant followed by co-surfactant, protein, and oil; and (4) SCOH – surfactant followed by co-surfactant, oil, and protein. These nanoemulsion vaccines were prepared by our previously described methods. The droplet size and zeta potential of these vaccines were determined by the methods described above.
Preparation of the novel nanoemulsion adjuvant vaccine
Based on these results, we optimized the protein content in the novel nanoemulsion vaccine and the order of addition. First, the Smix =4:1 (w/w) of EL-35® and propylene glycol sample was prepared. The optimal ratio of oil (oil:mixture of surfactant and co-surfactant =1:9) and antigen protein was added to the ideal concentration. The novel nanoemulsion vaccine was prepared by the same methods described in our previous report. The novel nanoemulsion vaccine was considered complete when it achieved a low viscosity and clear appearance. The same protocol was used to prepare the blank nanoemulsion (BNE), but distilled water replaced the protein as the aqueous phase.
Novel nanoemulsion adjuvant vaccine characterization
Observation of ultrastructure and morphology
The ultrastructure and the morphology of the novel O/W nanoemulsion vaccine particle were observed by transmission electron microscopy (TEM) as previously described.12 Briefly, 10 μL samples (50 times diluted with distilled water) were placed on the 100-mesh carbon copper grid. Ten microliters of phosphotungstic acid (1%, pH 7.4) solution was added after sitting for 5 minutes at room temperature. All samples were examined with a JEM-1230 (JEOL Ltd., Tokyo, Japan) with 120 kV voltages.
Molecular morphology
The molecular morphology of the novel O/W nanoemulsion vaccine particles was observed by atomic force microscopy (AFM) (IPC-208B; Chongqing University, Chongqing, People’s Republic of China) and G3DR software, at a scan range of 10.5×10.5 nm using point-by-point scanning methods. Briefly, ~50 μL samples were dried at room temperature after being placed on glass slides with a gold coating.
Observation of basic and key indexes
Basic and key physicochemical indexes such as droplet size, zeta potential, refractive index, and viscosity were measured by a Nano ZS 90 with the conditions and treatment methods described above.
Stability assessment of the nanoemulsion vaccine adjuvant
High-speed centrifuge stability and thermodynamic stability
High-speed centrifuge test
The stability of the fresh nanoemulsion vaccine was evaluated after centrifugation for 30 minutes at 13,000× g. The appearance of all samples was observed. The nanoemulsion vaccine is unstable if phase or drug separation, creaming, precipitation, turbidity, or demulsification occurs.
Thermodynamic stability test
In this study, we adopted previously described methods.13 Briefly, the appearance of the fresh and centrifuged (13,000× g for 30 minutes) nanoemulsion vaccine was observed after six cycles (one cycle stored at 4°C for 28 hours and 25°C for 48 hours). The nanoemulsion vaccine is considered unstable if it has different appearances.
Integrity and specificity of protein structure evaluation
The structural integrity and specificity of this novel nanoemulsion vaccine were evaluated by SDS-PAGE and Western blotting. Briefly, a mixture of 0.6 mL absolute ethanol and 0.3 mL nanoemulsion vaccine samples was shaken and centrifuged (13,000× g, 30 minutes). The supernatant was transferred into a new tube, and the precipitate was dissolved in 0.3 mL water. All samples of the supernatant and precipitate of both the blank control and nanoemulsion vaccine obtained after absolute ethanol treatment or the standard treatment and the same concentration of the naive antigen were diluted 50 times with distilled water. The same volume of 2× protein load buffer solution was added, and the sample was boiled for 5 minutes before running 25 μL samples on a 12% SDS-PAGE gel. All samples of the primary structural integrity were stained with Coomassie Gel Code blue stain reagent (Thermo Scientific, Waltham, MA, USA) for 2 hours and then washed for 24 hours with distilled water. A prestained protein ladder was used as the standard. The aforementioned samples were electroblotted using a Bio-Trans Blot semi-dry blotter (Multiphor II Electrophoresis Unit; Bio-Rad Laboratories, Inc., Philadelphia, PA, USA) at 25 mV for 15 minutes with polyvinylidene fluoride membranes. The membranes were blocked with 10 mM PBS/0.05% Tween 20 (PBST; pH 7.4) at 4°C and incubated with 1:5,000 dilutions of self-made goat anti-rabbit polyclonal primary antibody (the recombination antigen of HI [the recombination protein of IsdB and Hla] combined with completed Freud’s adjuvant subcutaneous quadriceps injection immune to rabbit with 250 μg/kg body weight dose, at first, seventh, and 14th day, antibody titers 1:1,280,000) for 1 hour at 37°C. Then, horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin (Ig)G secondary antibody (Zhongshan Gold Bridge of Beijing) (1:10,000) was added and detected with diaminobenzidine reagent (containing 0.05% diaminobenzidine, 0.005% hydrogen peroxide).
Stability of the nanoemulsion vaccine at different temperatures and long-term stability
Nanoemulsion samples were stored at 4°C, 25°C, and 40°C for a period of 1, 2, and 3 months, respectively. After stored for 0, 1, 2, 3, 4, 6, 8, 10, and 12 months at room temperature, the stability including appearance and primary structural integrity of the antigen protein of the nanoemulsion vaccine samples were examined using the previously described methods and conditions.
Animals and experimental groups
All animal experiments were approved by the Animal Ethical and Experimental Committee (Third Military Medical University of Chinese PLA, Chongqing, People’s Republic of China), and animals were maintained in a specific pathogen-free (SPF) laboratory (23°C±2°C; 55%±5% relative humidity; 12:12-hour light:dark) with free access to autoclaved food and water. All mice were anesthetized with sodium pentobarbital and sacrificed by CO2 inhalation. All treatments attempted to minimize suffering. Balb/c mice (6 weeks old, SPF grade, female) and C57 mice (6 weeks old, SPF grade, female) were obtained from Beijing HFK Bioscience Co. Ltd. (Beijing, People’s Republic of China). Mice were separated into four groups (Group 1: PBS group; Group 2: BNE control group; Group 3: naive antigen [protein] group; Group 4: nanoemulsion vaccine group).
Immunization and serum sample collection
All mice were immunized by intramuscular injection (Balb/c mice) into the upper quadriceps muscles (100 μL/15 μg antigen vaccine, two legs) or intranasal droplet administration (C57 mice) of 30 μL (15 μL per nostril, 30 μg antigens) on days 0, 7, and 14 with the antigen alone or the nanoemulsion vaccine. Mice in the control group were given PBS or the BNE alone in an identical fashion. Serum was collected from all mice 1 week after three immunizations were completed. All serum samples were stored at −70°C for further analysis.
ELISA for specific antibodies
The serum levels of IgG and IgG subclasses IgG1, IgG2a, IgG2b, and IgA were quantitatively determined with an enzyme-linked immunosorbent assay (ELISA) in accordance with the previously described protocol.4 Briefly, ELISA plates (Costar, 42592; Corning, Corning, NY, USA) were washed five times with PBST and incubated for 60 minutes at 37°C with 1% BSA (m/v; Roche, Basel, Switzerland) after coating each well with 1 μg antigen protein at 4°C for 12 hours. One hundred microliters of diluted serum samples was added to each well followed by incubation at 37°C for 45 minutes. One hundred microliters of HRP-conjugated goat anti-mouse IgG (Zhongshan Gold Bridge of Beijing, 112,971, 1:5,000), IgG1 (Bethyl, A90-105P, 1:5,000), IgG2a (Bethyl, A90-107P, 1:5,000), IgG2b (Bethyl, A90-109P, 1:5,000), or IgA (Bethyl, A90-103P, 1:5,000) secondary antibody was added to each plate. Thereafter, 100 μL of 3,3′,5,5′-tetramethylbenzidine substrate was added, and plates were first incubated at 37°C for 45 minutes and then incubated at room temperature for 5 minutes. The optical density (OD, 450 nm) was read with an enzyme-linked immunosorbent reader (BioRad 6.0; Bio-Rad Laboratories, Inc.,) after adding 100 μL of 2 M H2SO4 to stop the reaction. For the IgG test, the diluted sera were serially diluted twofold from 1:500 to 1:512,000. All serum samples tested for IgG1, IgG2a, IgG2b, and IgA were diluted at a ratio of 1:500. Only the antibody titer of IgG is given as a log2 value; all other antibody titers are expressed as OD values.
IFN-γ and IL-17A cytokines in serum assay
All immunized mice were evaluated at 1 week after the three immunizations according to the procedures described previously with slight modifications.14,15 The serum interferon (IFN)-γ and interleukin (IL)-17A cytokine levels were also quantified by ELISA with ELISA MAX™ STANDARD SET kit for mouse IFN-γ (No 430803; Biolegend, San Diego, CA, USA) and mouse IL-17A (No 432503; Biolegend).
Protective effect of the nanoemulsion vaccine in vivo
All mice were anesthetized with sodium pentobarbital prior to being intravenously injected with 1×109 CFU (lethal dose of 90%, 100 μL per Balb/c mice) or intranasal administration with 2.5×108 CFU (infection dose, 30 μL [15 μL per nostril]/C57 mice) of MRSA252. Survival rates of all Balb/c mice were observed and recorded for 10 days after MRSA252 infection. When C57 mice were infected with MRSA after 1 and 3 days, the bacterial burden of the lung tissue was measured by plating on the tryptic soy agar (BD Diagnostic Systems, Sparks, MD, USA) after tenfold serial dilutions and PBS homogenization.
Statistical analysis
Values from three replications were utilized to calculate means and standard deviations. For all comparisons, significant differences between the groups were expressed as follows: *P<0.05 and **P<0.01. All data were submitted to multiple comparisons by t-test and one-way analysis of variance and analyzed by SPSS 19.0 statistical software for Windows.
Results
Preparation of the novel nanoemulsion adjuvant vaccine
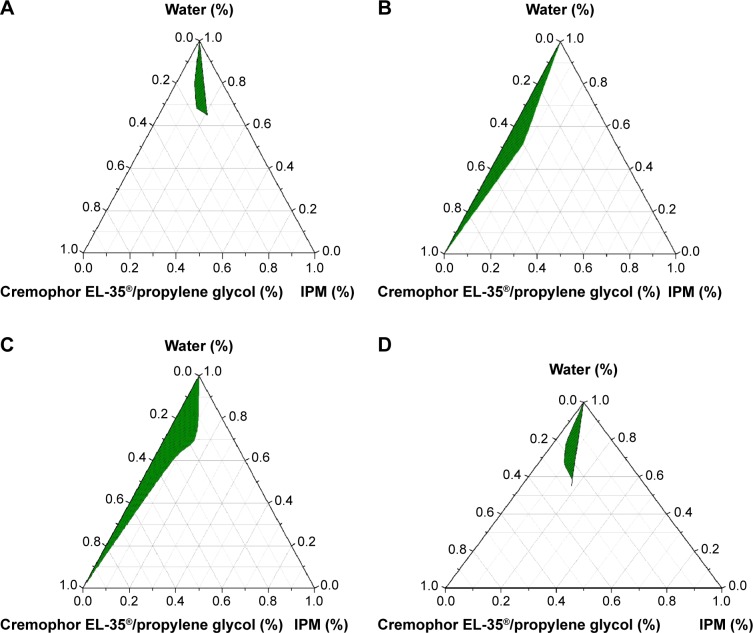
Influence of surfactant-to-oil ratio of the MRSA vaccine Pseudo-ternary phase diagrams of the four Smix ratios are shown in Figure 1. From Figure 1, we can see that the green nanoemulsion areas of Smix =4:1 (Figure 1C) are larger than that of Smix =3:1 (Figure 1B), Smix =2:1 (Figure 1A), and Smix =5:1 (Figure 1D). The nanoemulsion area positively correlated with the Smix ratio (Figure 1A and C). Based on these pseudo-ternary phase diagrams, a 4:1 Smix ratio was chosen and applied for the nanoemulsion vaccine formulation.
Figure 1.
Pseudo-ternary phase diagrams of the novel nanoemulsion adjuvant vaccine.
Notes: (A) Smix =2:1. (B) Smix =3:1. (C) Smix =4:1. (D) Smix =5:1. The green areas represent the region of the nanoemulsion formation.
Abbreviations: IPM, isopropyl myristate; Smix, ratio of surfactant and co-surfactant.
Effect of protein content on the nanoemulsion size and zeta potential
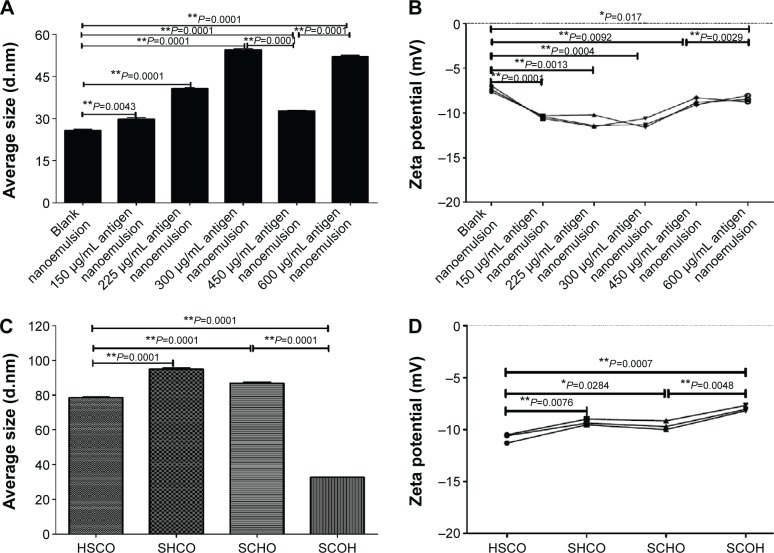
Depending on the preparation method, the protein content could affect the mean particle diameter and zeta potential (Figure 2A and B). Figure 2A shows that the average size positively correlated with the protein content (from 0 to 600 μg/mL), except at a protein antigen concentration of 450 μg/mL, which appeared to minimally affect the particle size of the resultant nanoemulsion (Figure 2A). Importantly, the other key factor, the zeta potential of the nanoemulsion, followed a pattern similar to that of the average size, as shown in Figure 2B. Overall, a significant reduction in average size and zeta potential was only evident at a protein content of 450 μg/mL, as shown by Figure 2A and B. At the same time, the average size and zeta potential both vary significantly in comparison to the nanoemulsion samples with other 150, 225, 300, and 600 μg/mL protein contents. Additionally, these results demonstrate that producing a nanoemulsion with high antigen contents does not confer an added stability benefit, which has positive effects on the industrial manufacturing of nanoemulsion products. Therefore, we identified the optimal addition protein antigen content of 450 μg/mL.
Figure 2.
The average size and zeta potential change in response to different amounts of the adding protein.
Notes: (A) Average size change in response to different addition amounts. (B) Zeta potential change in response to different addition amounts. (C) Average size change in response to different addition orders. (D) Zeta potential change in response to different addition orders. Four addition orders: HSCO represents protein, surfactant, co-surfactant, and oil; SHCO represents surfactant, protein, co-surfactant, and oil; SCHO represents surfactant, co-surfactant, protein, and oil; SCOH represents surfactant, co-surfactant, oil, and protein. *P<0.05 is considered as a difference; **P<0.01 is considered as a significant difference.
Effect of addition order on nanoemulsion size and zeta potential
It is well known that addition order is important to the preparation of the novel nanoemulsion. The addition order exerted an appreciable effect on the average particle diameter and zeta potential (Figure 2C and D). The SCOH addition order minimized the droplet diameter of the nanoemulsion (31 nm). The SHCO order maximized the droplet diameter of the nanoemulsion (~100 nm), as shown in Figure 2C. The other addition orders resulted in mean particle diameters that were approximately SCOH orders of larger than HSCO order, that is, ~80–100 nm (Figure 2C). The difference in particle size between the SCOH order and all other orders, that is, HSCO, SHCO, and SCHO, was statistically significant. Furthermore, the change in the zeta potential of the nanoemulsion vaccine did not significantly differ by average particle size, as shown in Figure 2D. The only significant change was observed for the nanoemulsion vaccine prepared using the SCOH order, as shown in Figure 2C and D. These results are important because they imply that nanoemulsion with small particle sizes (~30 nm) can be formed. Additionally, these findings demonstrate that different production orders do not confer an added stability benefit, which has a positive impact on the industrial manufacturing of nanoemulsion samples. Therefore, all further experiments were carried out using the SCOH addition order because only this production was capable of producing very fine droplets, resulting in a transparent nanoemulsion vaccine.
Characterization of the nanoemulsion adjuvant vaccine
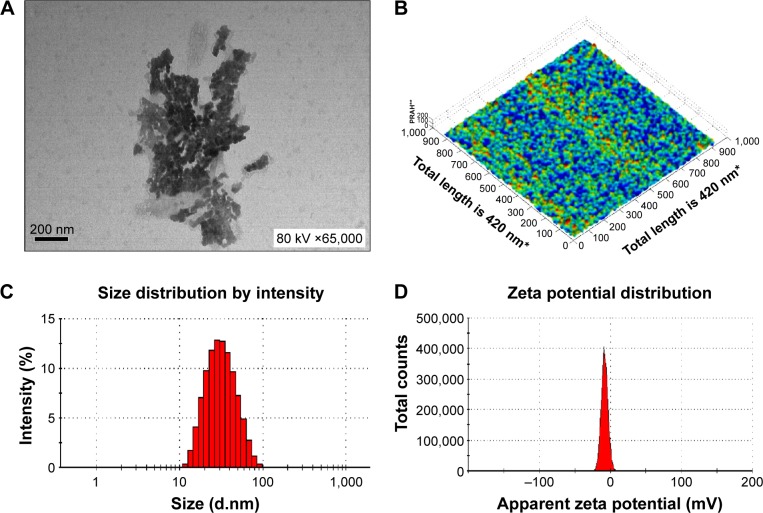
The nanoemulsion vaccine preparation was complete when the liquid became clear. The morphology of the nanoemulsion vaccine was observed by TEM (Figure 3A). Figure 3A shows that the droplet particle sizes were mostly in the 1–100 nm range. Importantly, these nanoemulsion vaccine particles appeared black and dark by TEM (Figure 3A). The molecular morphology of the nanoemulsion vaccine was observed by AFM (Figure 3B). We further found that the nanoemulsion vaccine has a solid spherical morphology with a mean diameter of ~30 nm, as shown in Figure 3B. Moreover, this nanoemulsion surface appears rugged, with sags and crests. The nanoemulsion droplet has good distribution without any aggregation as shown in Figure 3A and B. Figure 3C shows that the nanoemulsion vaccine has a small average size (31.34±0.49 nm) and good particle distribution (polydispersity index [PdI] value is 0.270±0.011, PdI <0.3). It is well known that PdI is a key factor affecting the spread of the particle size distribution; smaller values reflect a narrower size range.11 These data show that the stability of the nanoemulsion vaccine was narrowly distributed and demonstrated good stability. The average zeta potential of this nanoemulsion was −7.33 mV; the zeta potential has a narrow distribution, as shown in Figure 3D. Additional basic indexes such as viscosity (0.8872 cP), dispersant index (1.330), and refractive index (1.59 nD20) were obtained by Nano ZS 90. These results demonstrate that the nanoemulsion vaccine has good stability and displays the basic characteristics of nanoemulsions.
Figure 3.
Physical characteristics of the novel nanoemulsion vaccine.
Notes: (A) Transmission electron micrograph. (B) Atomic force microscopy micrograph. (C) Size diameter and distribution. (D) Zeta potential and distribution. *The X and Y axes both have a total length of 420 nm and are broken up into 1,000 parts; every 100 parts is a unit and represents 42 nm. **The Z axis is measured as percentages (‘100’ represents 100% of the average height measured, and is defined as a standard; ‘200’ represents two times the average height).
Abbreviation: PRAH, percentage of relative average height.
Stability assessment of the nanoemulsion adjuvant vaccine
High-speed centrifugation and thermodynamic stability
The novel nanoemulsion vaccine was stable after centrifugation (13,000× g for 30 minutes). The nanoemulsion vaccine exhibited no turbidity, phase or drug separation, creaming, precipitation, and/or demulsification or any other form of unstable appearance. This result demonstrated that the novel nanoemulsion vaccine is very stable. Thermostability differentiates a nanoemulsion that is kinetically stable but eventually phase separates. Thus, the thermodynamic stability of the formulations was tested after centrifugation with both heating–cooling and freeze–thaw cycle treatment. We have not identified any conditions under which the nanoemulsion vaccine exhibited an unstable appearance. The results of both the centrifugation and thermostability assays confirmed that the nanoemulsion vaccine has good stability.
Evaluation of the integrity and specificity of protein structure
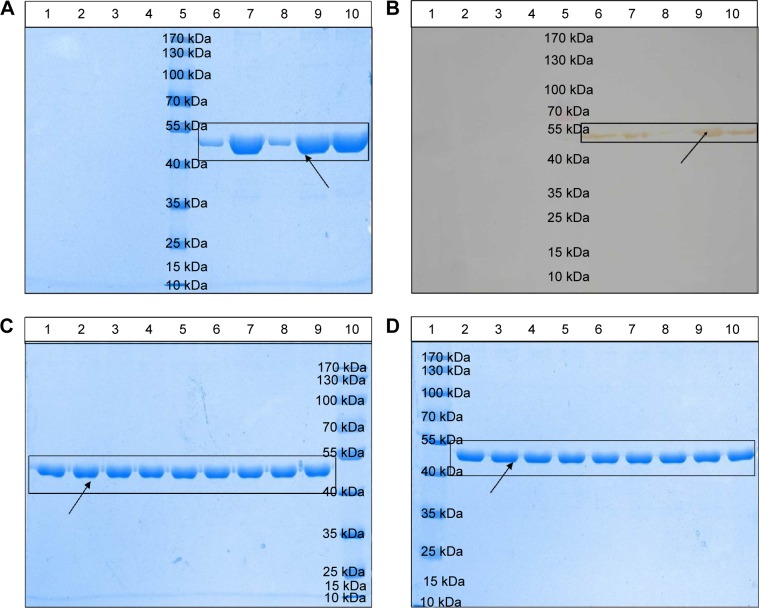
The results of the evaluation of the integrity and specificity of protein structure are shown in Figure 4A and B. The strips of lane 6 (the supernatant of nanoemulsion vaccine), lane 7 (the precipitate of nanoemulsion vaccine), and lane 9 (the precipitate of nanoemulsion vaccine after treatment) have one clear and bright band located at 48 kDa, similar to lane 10 (the aqueous antigen solution control). The strip of lane 9 (the precipitate of nanoemulsion vaccine after treatment) is obviously brighter than that of lane 8 (the supernatant of nanoemulsion vaccine after treatment). This result indicated that most of the antigen emulsion was in the precipitate. The majority of the antigen in the nanoemulsion and emulsions were in the precipitate because lane 7 and lane 9 have obvious bands. The BNE has no obvious bands in lanes 1 and 2 (the supernatant and precipitate of the BNE) and in lanes 3 and 4 (the supernatant and precipitate of BNE after treatment). These data demonstrate that SDS-PAGE and Western blot can be used to evaluate the structural integrity and structural specificity of the protein. No obvious degradation was detected in the nanoemulsion vaccine or in the supernatant and precipitate of the BNE after treatment. Therefore, these results demonstrate good immunogenicity of this vaccine due to good structural integrity and specificity.
Figure 4.
Physical stability of the novel nanoemulsion vaccine.
Notes: (A) Primary structural integrity of the antigen protein. (B) Structural specificity of the antigen protein. (C) Primary structural integrity after storage at different temperatures. (D) Stability after long-term storage. Blank nanoemulsion supernatant: lane 1; blank nanoemulsion precipitate: lane 2; blank nanoemulsion treatment supernatant: lane 3; blank nanoemulsion treatment precipitate: lane 4; prestained marker: lane 5; novel nanoemulsion vaccine supernatant: lane 6; novel nanoemulsion vaccine precipitate: lane 7; novel nanoemulsion vaccine treatment supernatant: lane 8; novel nanoemulsion vaccine treatment precipitate: lane 9; native protein agent: lane 10, in (A) and (B). Lanes 1, 4, and 7 represent the novel nanoemulsion vaccine stored at 4°C for 1, 2, and 3 months individual; lanes 2, 5, and 8 represent novel nanoemulsion vaccine stored at 25°C for 1, 2, and 3 months individual; lanes 3, 6, and 9 represent novel nanoemulsion vaccine stored at 40°C for 1, 2, and 3 months individual, and lane 10 represents prestained protein marker, in (C). Lane 1: prestained marker; lanes 2–10 represent novel nanoemulsion vaccine stored at room temperature for 0, 1, 2, 3, 4, 6, 8, 10, and 12 months in (D). The visibly clear protein lanes were marked by black squares. Black arrows indicate antigen protein.
Temperature and long-term storage stability of the nanoemulsion vaccine
The thermal stability of optimized fresh nanoemulsion vaccine formulation was evaluated at two temperatures for six cycles. It is also very important to study the nanoemulsion vaccine stability when stored at 4°C, 25°C, and 40°C for 1, 2, and 3 months. No nanoemulsion sample happened previously describe instability phenomenon before or after centrifugation at 13,000× g for 30 minutes. The primary structural integrity results of antigen protein in samples kept at 4°C, 25°C, and 40°C for 1, 2, and 3 months are shown in Figure 4C. There was no difference in the protein staining of these vaccines, and no degradation products were found at any time points, as shown in Figure 4C. The protein primary structure of antigen protein stored at room temperature for 1 year was assessed in vitro at 0, 1, 2, 3, 4, 6, 8, 10, and 12 months. The primary structural integrity of the antigen protein stored for 1 year at room temperature is shown in Figure 4D. The primary protein structure tests showed that the protein band did not significantly differ between fresh samples and samples stored at room temperature for 1 year. These results show that the nanoemulsion vaccine is very stable.
Intramuscular systemic immune response assessed in vivo
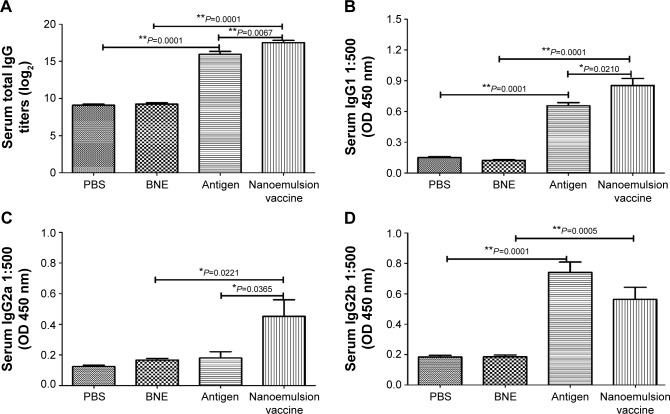
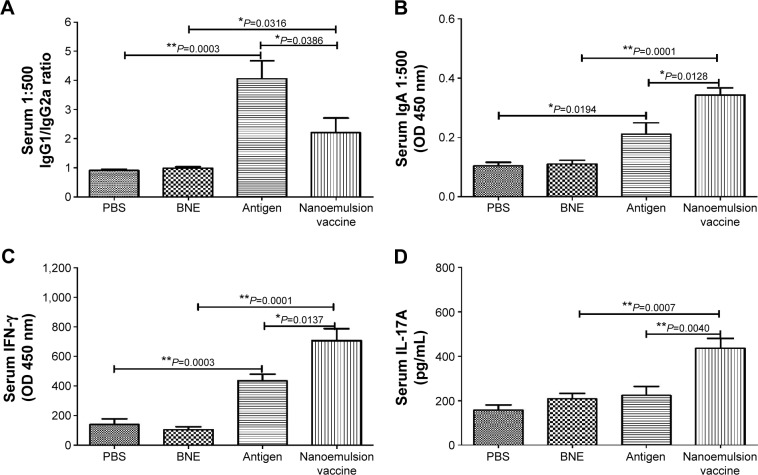
The antigen-specific antibody responses of the novel nanoemulsion vaccine were determined by ELISA methods. The geometric mean titers (GMTs) of IgG against PBS, BNE, naive antigen, and the nanoemulsion vaccine were 500, 552.044, 64,000, and 156,033.7, respectively. The IgG titer log2 value of the nanoemulsion vaccine was the highest among the four groups, as shown in Figure 5A, and it is significantly extremely higher than that of the naive antigen and BNE groups (P=0.0067, P<0.01 and P=0.0001, P<0.01). The IgG titer log2 value of the naive antigen was significantly higher than the PBS control group (P=0.0001, P<0.01). The serum IgG1 OD of the nanoemulsion adjuvant vaccine at a 1:500 serum dilution in PBS at 450 nm was the highest among the four groups as shown in Figure 5B. Furthermore, this IgG1 value was significantly higher than that of naive antigen group and BNE group (P=0.0210, P<0.05 and P=0.0001, P<0.01). The value of the naive antigen was higher than that of the PBS control (P=0.0001, P<0.01). The serum IgG2a OD of the nanoemulsion vaccine at a 1:500 serum dilution in PBS at 450 nm was the highest among the four groups, as shown in Figure 5C. Moreover, this IgG2a value was significantly higher than that of the naive antigen control and BNE group (P=0.0365, P<0.05 and P=0.0211, P<0.05). The serum IgG2b OD of the naive antigen at a 1:500 dilution in PBS at 450 nm was the highest among the four groups as shown in Figure 5D. Moreover, the nanoemulsion vaccine value was lower than that of the naive antigen group, but there was no difference (P>0.05). Additionally, the nanoemulsion vaccine value was higher than that of BNE (P=0.0005, P<0.01). The serum IgG1/IgG2a ratio of the naive antigen at a 1:500 serum dilution in PBS was the highest among the four groups, as shown in Figure 6A. Furthermore, the nanoemulsion vaccine value was significantly lower than that of the naive antigen group (P=0.0366, P<0.05). The naive antigen value was higher than that of the PBS control group (P=0.0003, P<0.01). Furthermore, the value of the novel nanoemulsion vaccine was significantly higher than BNE group (P=0.0316, P<0.05). The serum IgA OD of the novel nanoemulsion vaccine at a 1:500 serum dilution in PBS at 450 nm was highest among the four groups as shown in Figure 6B. Furthermore, this value was significantly higher than that of the naive antigen and BNE groups (P=0.0128, P<0.05 and P=0.0001, P<0.01).
Figure 5.
Specific IgG, IgG1, IgG2a, and IgG2b antibodies in the serum after intramuscular injection of the nanoemulsion vaccine.
Notes: (A) The IgG titer log2 value of the nanoemulsion. (B) The serum IgG1 optical density of the nanoemulsion vaccine at 450 nm. (C) The serum IgG2a optical density of the nanoemulsion vaccine at 450 nm. (D) The serum IgG2b optical density of the nanoemulsion vaccine at 450 nm. **P<0.01 is considered as a significant difference; *P<0.05 is considered as a difference.
Abbreviations: Ig, immunoglobulin; OD, optical density; PBS, phosphate-buffered saline; BNE, blank nanoemulsion.
Figure 6.
IgG1/IgG2a, IgA, IFN-γ, and IL-17A levels in response to an intramuscular injection of the nanoemulsion vaccine.
Notes: (A) IgG1/IgG2a ratio of the nanoemulsion vaccine. (B) Serum IgA optical density at 450 nm of the nanoemulsion vaccine. (C) Serum IFN-γ level in response to the nanoemulsion vaccine. (D) Serum IL-17A level in response to the nanoemulsion vaccine. **P<0.01 is considered as a significant difference; *P<0.05 is considered as a difference.
Abbreviations: Ig, immunoglobulin; IFN, interferon; IL, interleukin; PBS, phosphate-buffered saline; BNE, blank nanoemulsion; OD, optical density.
To assess the ability to induce cell-mediated immune responses, important factors including IFN-γ and IL-17A levels in serum were measured by ELISA. The results confirmed that the novel nanoemulsion vaccine stimulated an increase in the T-helper (Th) 1 cytokine IFN-γ level compared to the naive antigen and BNE (P=0.0137, P<0.05 and P=0.0001, P<0.01), as shown in Figure 6C. In addition, the naive antigen was higher than that of the PBS control (P=0.0003, P<0.01). The nanoemulsion vaccine also stimulated an increase in the Th17 cytokine IL-17A level compared to the naive antigen and BNE (P=0.0040, P<0.01 and P=0.0007, P<0.01) as shown in Figure 6D. These data suggest that the nanoemulsion vaccine can act through a predominant Th17 response.
Nasal mucosal immune response assessed in vivo
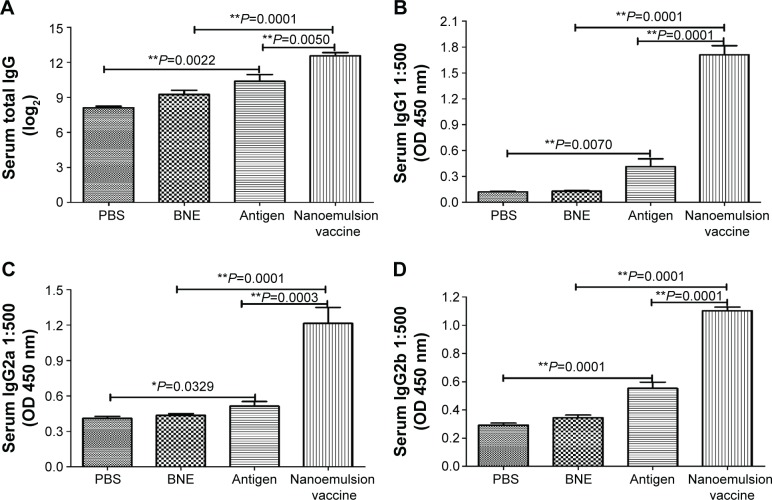
Analysis of the serum IgG antibody titers after 1 week indicated a significantly higher antibody level in the nanoemulsion vaccine-immunized C57 mice compared to the naive antigen and BNE groups (P=0.0050, P<0.01 and P=0.0001, P<0.05), as shown in Figure 7A. The naive antigen stimulated an IgG increase compared to immunization with PBS (P=0.0022, P<0.01). The GMTs of IgG in the PBS, BNE, naive antigen, and nanoemulsion vaccine groups were 125, 609.506, 1,259.921, and 3,482.202, respectively. We also observed that the serum IgG1 levels in response to the novel nanoemulsion vaccination were higher than those generated by the naive antigen following nasal vaccination with the nanoemulsion, as shown in Figure 7B. The serum IgG1 OD of the nanoemulsion vaccine at a 1:500 serum dilution in PBS at 450 nm was the highest among the four groups. Furthermore, this value was significantly higher than that of the naive antigen and BNE groups (P=0.0001, P<0.01 and P=0.0001, P<0.01).
Figure 7.
Specific IgG, IgG1, IgG2a, and IgG2b in the serum antibodies after the nasal mucosal administration of the nanoemulsion vaccine.
Notes: (A) The IgG titer log2 value of the nanoemulsion. (B) The serum IgG1 optical density of the nanoemulsion vaccine at 450 nm. (C) The serum IgG2a optical density of the nanoemulsion vaccine at 450 nm. (D) The serum IgG2b optical density of the nanoemulsion vaccine at 450 nm. **P<0.01 is considered as a significant difference; *P<0.05 is considered as a difference.
Abbreviations: Ig, immunoglobulin; PBS, phosphate-buffered saline; BNE, blank nanoemulsion; OD, optical density.
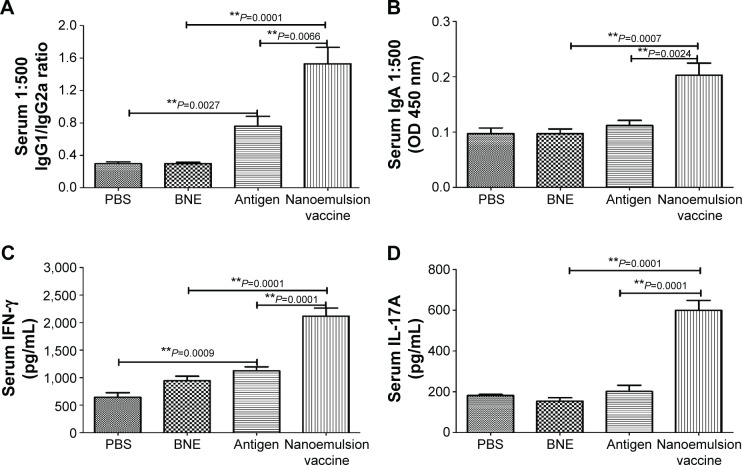
The serum IgG2a OD of the nanoemulsion vaccine at a 1:500 serum dilution in PBS at 450 nm was the highest among the four groups, as shown in Figure 7C. Furthermore, this value was significantly higher than both the naive antigen and BNE control (P=0.0003, P<0.01 and P=0.0001, P<0.01). The OD of serum IgG2b of the novel nanoemulsion vaccine at a 1:500 serum dilution in PBS at 450 nm was the highest among the four groups, as shown in Figure 7D. Furthermore, the OD of the nanoemulsion vaccine was significantly higher than both the naive antigen and BNE control (P=0.0071, P<0.01 and P=0.0001, P<0.01). The serum IgG1/IgG2a ratio of the nanoemulsion vaccine at a 1:500 serum dilution in PBS was the highest among the four groups, as shown in Figure 8A. Moreover, the ratio of the novel nanoemulsion vaccine was higher than that of the naive antigen and BNE groups (P=0.0066, P<0.01 and P=0.0001, P<0.01).
Figure 8.
IgG1/IgG2a, IgA, IFN-γ, and IL-17A level in the serum after a nasal mucosal immune response.
Notes: (A) IgG1/IgG2a ratio of the nanoemulsion vaccine. (B) Serum IgA optical density of the nanoemulsion vaccine at 450 nm. (C) Serum IFN-γ production in response to the nanoemulsion vaccine. (D) Serum IL-17A production in response to the nanoemulsion vaccine. **P<0.01 is considered as a significant difference; *P<0.05 is considered as a difference.
Abbreviations: Ig, immunoglobulin; IFN, interferon; IL, interleukin; PBS, phosphate-buffered saline; BNE, blank nanoemulsion; OD, optical density.
The serum IgA OD of the novel nanoemulsion vaccine at a 1:500 serum dilution in PBS at 450 nm was highest among the four groups, as shown in Figure 8B. This value was significantly higher than that of the naive antigen and BNE control (P=0.0024, P<0.01 and P=0.0007, P<0.01). Interestingly, compared with the naive antigen, the nanoemulsion vaccine not only induced higher titers of IgG1 but also significantly improved the levels of IgG2a, IgG2b, and IgA.
The nasal mucosa produces significant levels of IL-17 and IFN-γ after exposure to the novel nanoemulsion vaccine, but the exact role of IL-17 in the acute phase response of the nasal mucosa is poorly understood. Compared to the naive antigen and BNE, the novel nanoemulsion vaccine stimulated a higher increase of the Th1 cytokine IFN-γ (P=0.0001, P<0.01 and P=0.0001, P<0.01), as shown in Figure 8C. Figure 8D shows the results for this vaccine, indicating a predominant Th17 response. The level of the Th17 cytokine induced by this novel nanoemulsion vaccine was higher than that of the naive antigen and BNE (P=0.0001, P<0.01 and P=0.0001, P<0.01).
Protective immune effect of the nanoemulsion vaccine in vivo
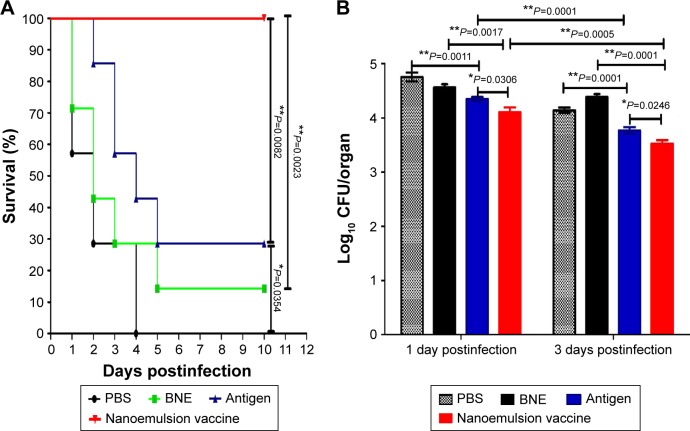
The survival ratio for systemic infection in Balb/c mice All Balb/c mice were infected with the MRSA252 strain 10 days after the third immunization with the novel nanoemulsion vaccine, the naive protein antigen, and BNE or PBS. All mice that received the novel nanoemulsion vaccine adjuvant survived without clinical signs of infection for 10 days. The survival ratio of the nanoemulsion vaccine adjuvant (100%) was significantly higher than that of the naive antigen and BNE groups (28.6%, P=0.0082, P<0.01 and 14.28%, P=0.0023, P<0.01), as shown in Figure 9A. The survival rate of mice given the naive antigen (28.6%, P=0.0082, P<0.01) was higher than that of PBS group, but there is statistical difference (0%, P=0.0354, P<0.05) (Figure 9A).
Figure 9.
Survival ratio and bacterial challenge results.
Notes: (A) The survival ratio of Balb/c mice in response to systemic MRSA infection. (B) Lung bacterial burden of C57 mice infected with MRSA pneumonia. **P<0.01 is considered as a significant difference. *P<0.05 is considered as a difference.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; PBS, phosphate-buffered saline; BNE, blank nanoemulsion.
Lung bacterial burden from pneumonia infection in C57 mice
The protective effect of the novel nanoemulsion vaccines after intranasal vaccination was determined. The microbial burden of lung tissue was measured by bacterial counting methods after 1 and 3 days of MRSA252 infection. The lung bacterial burden is shown in Figure 9B. The lung bacteria number of the novel nanoemulsion vaccine was significantly extremely lower than that of the naive antigen control (P=0.0306, P<0.05 and P=0.0246, P<0.05) and BNE control (P=0.0017, P<0.01 and P=0.0001, P<0.01) at 1 and 3 days, as shown in Figure 9B. The lung bacteria number of the naive antigen was significantly lower than PBS control (P=0.0011, P<0.01 and P=0.0017, P<0.01) on the first and third day. Similarly, the lung bacteria number of the nanoemulsion vaccine was significantly lower than the naive antigen control (P=0.0001, P<0.01 and P=0.0005, P<0.01) on the first and third day. These results suggest that the novel vaccine can reduce the lung bacterial burden and partially protect against MRSA infection after nasal immunization.
Discussion
MRSA causes sepsis, endocardial infection, pneumonia, bacteremia, and meningitis and can induce significant morbidity and mortality.16 The data show that half of patients with pneumonia and sepsis were infected with MRSA.17 Pneumonia and sepsis are life-threatening diseases with high mortality rates (60% and 63.6%, respectively). Therefore, we selected MRSA as a model of both sepsis and pneumonia because of systemic and lung infection. It is well known that IsdB plays a major role in heme iron acquisition as a cell wall-anchored, iron-regulated surface protein.18 It is also confirmed that IsdB can be applied in MRSA vaccination.19 Alpha-toxin protein, one of the most potent known bacterial toxins, can form pores in eukaryotic cells and interfere with bacterial adhesion to epithelial cells.20 A novel recombinant two antigens (IsdB and Hla) of MRSA combined with alum adjuvant could induce and improve the humoral immunity response and decrease the mortality ratio of MRSA infection in our previously study.1 However, the humoral, mucosal, and cell-mediated immune responses of an effective vaccine against MRSA still need to be evaluated.
Despite being used for over 80 years, alum has some drawbacks, including side effects and safety concerns, such as contributing to or even suppressing cell-mediated immunity and subsequent cytotoxic T-lymphocyte responses.21–23 Use of alum with a mild Th2 type adjuvant can only effectively enhance IgG1 antibody responses.4 Alum minimally stimulates cell-mediated immune responses because it may block the activation or differentiation of the T-cell. Subunit vaccines based on protein antigens are usually better tolerated and regarded as safer alternatives to traditional vaccines; they are usually poorly immunogenic when used alone and therefore require an exogenous adjuvant to augment the resultant immune responses. Therefore, a novel vaccine adjuvant to enhance both the systemic immune response and mucosal immune response is urgently needed.
Few adjuvants other than alum are currently approved by the FDA and commercially useful in humans in the USA.24 Vaccine adjuvants are substances that enhance the antigen-specific immune response.25 Broadly speaking, vaccine adjuvants can be separated into two classes, immunostimulatory adjuvant and particulate delivery systems, based on their principal mechanisms of action. In contrast to the former, which is thought to activate the innate immune system, the latter is generally specific in nature and mainly functions as a depot to ensure the immuno-availability of the antigen.5 Some studies suggested that antigen protein combined with MF59 adjuvant in an O/W emulsion can improve the immune response to the antigen vaccine. However, MF59 failed to provide a depot for the antigen, and the mechanism of the adjuvant remains unknown.26 Importantly, previous reports have found post-immunization reactions associated with the above emulsion adjuvant and that average sizes exceeding 160 nm exert a poor adjuvant effect. A recent study showed that as vaccines take on less immunogenic “minimalist” compositions, formulations that boost antigen effectiveness are increasingly needed. For example, a small nanoparticle of 25–40 nm can more easily penetrate tissue barriers and traffic rapidly to the draining lymph nodes than a larger nanoparticle of >100 nm.27 Therefore, smaller nanoparticles can be transported easily to the lymph nodes because they are taken up and trafficked by dendritic cells and are retained for a longer time at the site of the injection.28 At the same time, nanoemulsion has little or no toxicity and no serious adverse effects in multiple species including >200 individuals to date.28 A novel nanoemulsion vaccine with an average size of 31.43 nm (size range of 25–40 nm) based on recombinant antigens with IsdB and alpha-toxin without any vaccine adjuvant, such as Al or MF59, was designed and evaluated in this study.
The average size and zeta potential are well known as the key factors for nanoemulsion formation and stability. Thus, we selected these two factors to assess the influences of protein content and the addition order. We also selected four key indexes, TEM, AFM, average size, and zeta potential, to characterize the novel nanoemulsion and chose three standard analysis methods, centrifugation, SDS-PAGE, and Western blotting, to assess the stability and bioactivity of this novel nanoemulsion vaccine adjuvant. High-speed centrifugation is a simple and standard method to examine the stability of a nanoemulsion. SDS-PAGE and Western blotting are the easiest, most highly sensitive, and most widely used methods to evaluate the structural integrity and specificity of protein vaccines. The results showed that nanoemulsion vaccine has good stability when stored at room temperature for 1 year.
At present, alum has no enough adjuvant activity for protein vaccines, especially subunit antigens. MF59 or AS series emulsions can induce chemokine secretion, lead to immune cell recruitment at the site of injection, and cause the differentiation of the dendritic cell phenotype toward monocytes. However, at present, MF59 or AS series are rarely utilized to elicit a mucosal response.29,30 Therefore, we attempted to design and prepare a novel nanoemulsion adjuvant vaccine with good intramuscular and nasal mucosal immune responses in this study.
We observed that the IgG levels of serum following the intramuscular or nasal mucosal immune response were higher than those generated in response to the naive antigen vaccine. Moreover, the nasal and intramuscular administration routes of the novel nanoemulsion vaccine generated higher levels of MRSA-specific serum IgG subclasses and IgA than an equivalent dose of naive protein antigen. The novel nanoemulsion vaccine specifically combined with recombination antigen protein flexibly shifted the Th1/Th2 balance of the vaccine immune response. A balanced Th1/Th2 response is necessary and desirable. The nanoemulsion vaccine can improve the IgG1 level in the serum, indicating that this vaccine may induce a dominant Th2 humoral immune response after intramuscular immunization. The nanoemulsion vaccine can also improve the IgG2a level in the serum, indicating that it may also induce a dominant Th1 cellular immune response after nasal immunization. It is a known fact that IgG2a can identify a Th1-polarized immune response, and the IgG1/IgG2a ratio can identify a Th2-biased or Th1-biased immune response. The novel nanoemulsion vaccine administered using the above immunization methods can stimulate significantly higher levels of both IgG1 and IgG2a antibodies than the naive antigen and BNE group. In addition, this novel nanoemulsion vaccine clearly affected various IgG antibody isotypes, as shown by the appearance of the IgG1 and IgG2 antibody subclass, while this vaccine may produce an overwhelming level of IgG1 antibodies in the serum. The appearance of IgG2b in the total IgG response provided the confirmation of a Th1 bias in the resultant cellular immunity response after both immunization methods.
In addition, IFN-γ cytokines of Th1 cells can affect intracellular pathogens, especially MRSA infections. In this study, the IFN-γ level generated by this novel nanoemulsion vaccine was higher than the naive antigen and other controls. Therefore, this novel nanoemulsion vaccine can induce Th1 cell immune responses. An ideal vaccine adjuvant should also promote an appropriate and adequate immune response such as Th17 cells. IL-17A, a pro-inflammatory cytokine of Th17 cells production, is well described in host defense against MRSA infection. It can protect mice against MRSA challenge through CD4+ and CD8+ T-cells production. In this study, we also found that the IL-17A level of this novel nanoemulsion vaccine was higher than that of the naive antigen. These data show that the nanoemulsion vaccine can improve the protection efficacy because the Th1/Th17-mediated response plays an important role in host defense against MRSA.
The development of a safe and effective adjuvant has proved to be a major hurdle in the application of vaccines. Several hundred different adjuvants have been studied in the last few decades. However, only few adjuvants have been approved for human use due to lack of efficacy, instability, manufacturing difficulties, unacceptable local or systemic toxicity, high cost, etc. In this work, we reported the strong and novel adjuvant effect of a nanoemulsion vaccine in response to both intramuscular systemic and nasal mucosal immunization. Some research suggests that nanoemulsions with nonspecific pro-inflammatory components can display an acceptable safety profile in all types of animal models and humans.31 Moreover, additional aspects, such as the stability of the novel nanoemulsion vaccine, were also studied. Our results represent a sound scientific foundation for future strategies in the development of this novel adjuvant vaccine that can enhance both intramuscular systemic and nasal mucosal immune responses.
Acknowledgments
This research was supported by the National Key Technology R&D Project Program of China (number 2014BAI15B00/2014BAI15B01), Natural Science Foundation Project Program of Chongqing (CSTC, number 20142014jcyjA10107), National Natural Science Foundation Program of China (number 31370932), and National Natural Science Foundation Program of China (number 31400792).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zuo QF, Yang LY, Feng Q, et al. Evaluation of the protective immunity of a novel subunit fusion vaccine in a murine model of systemic MRSA infection. PLoS One. 2013;8(12):e81212. doi: 10.1371/journal.pone.0081212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Z, Li B, Sun HQ, et al. Fine-mapping of immunodominant linear B-cell epitopes of the Staphylococcus aureus SEB antigen using short overlapping peptides. PLoS One. 2014;9(3):e90445. doi: 10.1371/journal.pone.0090445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morefield G, Touhey G, Lu F, Dunham A, HogenEsch H. Development of a recombinant fusion protein vaccine formulation to protect against Streptococcus pyogenes. Vaccine. 2014;32(30):3810–3815. doi: 10.1016/j.vaccine.2014.04.092. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Wang L, Liu Y, et al. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials. 2014;35(23):6086–6097. doi: 10.1016/j.biomaterials.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Chen WL, Liu SJ, Leng CH, Chen HW, Chong P, Huang MH. Disintegration and cancer immunotherapy efficacy of a squalane-in-water delivery system emulsified by bioresorbable poly(ethylene glycol)-block-polylactide. Biomaterials. 2014;35(5):1686–1695. doi: 10.1016/j.biomaterials.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Deng J, Cai W, Jin F. A novel oil-in-water emulsion as a potential adjuvant for influenza vaccine: development, characterization, stability and in vivo evaluation. Int J Pharm. 2014;468(1–2):187–195. doi: 10.1016/j.ijpharm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Liu Y, Chen Z, et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12(4):2003–2012. doi: 10.1021/nl300027p. [DOI] [PubMed] [Google Scholar]
- 8.Shi R, Hong L, Wu D, et al. Enhanced immune response to gastric cancer specific antigen Peptide by coencapsulation with CpG oligodeoxynucleotides in nanoemulsion. Cancer Biol Ther. 2005;4(2):218–224. doi: 10.4161/cbt.4.2.1472. [DOI] [PubMed] [Google Scholar]
- 9.Lindell DM, Morris SB, White MP, et al. A novel inactivated intranasal respiratory syncytial virus vaccine promotes viral clearance without Th2 associated vaccine-enhanced disease. PLoS One. 2011;6(7):e21823. doi: 10.1371/journal.pone.0021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makidon PE, Belyakov IM, Blanco LP, et al. Nanoemulsion mucosal adjuvant uniquely activates cytokine production by nasal ciliated epithelium and induces dendritic cell trafficking. Eur J Immunol. 2012;42(8):2073–2086. doi: 10.1002/eji.201142346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun H, Liu K, Liu W, et al. Development and characterization of a novel nanoemulsion drug-delivery system for potential application in oral delivery of protein drugs. Int J Nanomed. 2012;7:5529–5543. doi: 10.2147/IJN.S36071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai W, Deng W, Yang H, Chen X, Jin F. A propofol microemulsion with low free propofol in the aqueous phase: formulation, physicochemical characterization, stability and pharmacokinetics. Int J Pharm. 2012;436(1–2):536–544. doi: 10.1016/j.ijpharm.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Myc A, Kukowska-Latallo JF, Bielinska AU, et al. Development of immune response that protects mice from viral pneumonitis after a single intranasal immunization with influenza A virus and nanoemulsion. Vaccine. 2003;21(25–26):3801–3814. doi: 10.1016/s0264-410x(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 14.Park S, Lee JB, Kang S. Topical application of Chrysanthemum indicum L. attenuates the development of atopic dermatitis-like skin lesions by suppressing serum IgE levels, IFN-gamma, and IL-4 in Nc/Nga mice. Evid Based Complement Alternat Med. 2012;2012:821967. doi: 10.1155/2012/821967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jafarzadeh A, Nemati M, Rezayati MT, Ebrahimi M, Hassan ZM. Cimetidine enhances delayed-type hypersensitivity responses and serum interleukin (IL)-2, -10, -12, and IL-17 levels after burn injury in an animal model. J Immunotoxicol. 2013;10(2):201–209. doi: 10.3109/1547691X.2012.708365. [DOI] [PubMed] [Google Scholar]
- 16.Khodaverdian V, Pesho M, Truitt B, et al. Discovery of antivirulence agents against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57(8):3645–3652. doi: 10.1128/AAC.00269-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua L, Hilliard JJ, Shi Y, et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother. 2014;58(2):1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazmanian SK, Ton-That H, Su K, Schneewind O. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc Natl Acad Sci U S A. 2002;99(4):2293–2298. doi: 10.1073/pnas.032523999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebert T, Smith S, Pancari G, et al. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) with functional activity in vitro and in vivo. Hum Antibodies. 2010;19(4):113–128. doi: 10.3233/HAB-2010-0235. [DOI] [PubMed] [Google Scholar]
- 20.Liang X, Ji Y. Alpha-toxin interferes with integrin-mediated adhesion and internalization of Staphylococcus aureus by epithelial cells. Cell Microbiol. 2006;8(10):1656–1668. doi: 10.1111/j.1462-5822.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 21.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9(4):287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25(11):2085–2094. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15(2):51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang J, Fisher EM, Hensley SE, Lustigman S, Murasko DM, Shen H. Antigen sparing and enhanced protection using a novel rOv-ASP-1 adjuvant in aqueous formulation with influenza vaccines. Vaccine. 2014;32(23):2696–2702. doi: 10.1016/j.vaccine.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60(8):915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalvodova L. Squalene-based oil-in-water emulsion adjuvants perturb metabolism of neutral lipids and enhance lipid droplet formation. Biochem Biophys Res Commun. 2010;393(3):350–355. doi: 10.1016/j.bbrc.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 27.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 28.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180(8):5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 30.Mohan T, Verma P, Rao DN. Novel adjuvants & delivery vehicles for vaccines development: a road ahead. Indian J Med Res. 2013;138(5):779–795. [PMC free article] [PubMed] [Google Scholar]
- 31.Das SC, Hatta M, Wilker PR, et al. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine. 2012;30(48):6871–6877. doi: 10.1016/j.vaccine.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]