Significance
Ever since the rapid extranuclear signaling effects of 17β-estradiol (E2) were first identified in the brain decades ago, it has remained an enigma as to how these nonclassical effects are achieved. Using a forebrain-specific knockout animal model, the current study demonstrates that a recently cloned estrogen receptor coregulator protein, Proline-, glutamic acid-, and leucine-rich protein 1 (PELP1), is critical for mediating E2 regulation of rapid extranuclear signaling, as well as E2-induced neuroprotection and cognitive function in the hippocampus after ischemic injury. Our studies also identified PELP1 as a novel interacting protein and a substrate of glycogen synthase kinase-3β (GSK3β). Finally, PELP1 was also shown to mediate E2 genomic effects to regulate genes involved in inflammation, metabolism, and survival after ischemic injury.
Keywords: 17-β estradiol, PELP1, extranuclear, neuroprotection, cognition
Abstract
17-β estradiol (E2) has been implicated as neuroprotective in a variety of neurodegenerative disorders. However, the underlying mechanism remains unknown. Here, we provide genetic evidence, using forebrain-specific knockout (FBKO) mice, that proline-, glutamic acid-, and leucine-rich protein 1 (PELP1), an estrogen receptor coregulator protein, is essential for the extranuclear signaling and neuroprotective actions of E2 in the hippocampal CA1 region after global cerebral ischemia (GCI). E2-mediated extranuclear signaling (including activation of extracellular signal-regulated kinase and Akt) and antiapoptotic effects [such as attenuation of JNK signaling and increase in phosphorylation of glycogen synthase kinase-3β (GSK3β)] after GCI were compromised in PELP1 FBKO mice. Mechanistic studies revealed that PELP1 interacts with GSK3β, E2 modulates interaction of PELP1 with GSK3β, and PELP1 is a novel substrate for GSK3β. RNA-seq analysis of control and PELP1 FBKO mice after ischemia demonstrated alterations in several genes related to inflammation, metabolism, and survival in PELP1 FBKO mice, as well as a significant reduction in the activation of the Wnt/β-catenin signaling pathway. In addition, PELP1 FBKO studies revealed that PELP1 is required for E2-mediated neuroprotection and for E2-mediated preservation of cognitive function after GCI. Collectively, our data provide the first direct in vivo evidence, to our knowledge, of an essential role for PELP1 in E2-mediated rapid extranuclear signaling, neuroprotection, and cognitive function in the brain.
The steroid hormone, 17β-estradiol (E2), exerts multiple actions in the brain, including regulation of synaptic plasticity, neurogenesis, reproductive behavior, and cognition. E2 has also been implicated to serve as an important neuroprotective factor in a variety of neurodegenerative disorders, including stroke and Alzheimer’s disease (1–3). The main source of E2 synthesis in females is the ovary. Circulating E2 levels are known to fluctuate during development and puberty, as well as during the menstrual cycle. After menopause, however, circulating E2 levels decline precipitously (4). Relative to men, women are “protected” against stroke until the years of menopause. However, after menopause, women exhibit a significantly higher disability and fatality rate compared with men (5–7). Intriguingly, the higher risk and poorer outcome of stroke in postmenopausal women parallels the falling E2 levels that occur after menopause (7, 8). Furthermore, exogenous administration of E2 significantly reduces the infarct volume in cortex and hippocampus after focal and global cerebral ischemia (GCI) in various animal models, and female animals exhibit reduced neural damage compared with young adult males after brain injury (1, 9–11). Taken as a whole, these studies suggest E2 functions as an important neuroprotective factor. However, the molecular mechanisms by which E2 exerts its neuroprotective effects remain unclear.
E2 signaling is thought to be primarily mediated by the classical estrogen receptors, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). Both subtypes are expressed in the brain and have been shown to mediate various neural actions of E2, including neuroprotection (4, 12–14). For example, ER genomic actions have been shown to contribute to increased expression of the antiapoptotic gene, bcl-2, and the antiapoptotic prosurvival factor, survivin, which can contribute to E2 neuroprotective effects (11, 15, 16). Recent evidence suggests that E2 also exerts “rapid signaling” in the brain via interaction with extranuclear ER, which can lead to regulation of kinase activation and ion channels (17, 18). ER “extranuclear” signaling has been suggested to contribute to E2 neuroprotective effects by facilitating the rapid activation of extracellular signal-regulated kinases (ERKs) and prosurvival serine threonine kinase Akt (4, 19–22). Furthermore, E2 also interacts with nonclassical ERs such as G protein-coupled estrogen receptor, which can also contribute to activation of ERK and PI3K (4, 23). Transcriptional activity of E2-bound ERs is regulated by several coregulatory proteins that associate with the ER in response to E2 binding (24). Association of the coregulatory proteins with ER leads to formation of an “ER signalosome” that facilitates ER genomic and extranuclear actions in a tissue-dependent manner. However, the composition of the ER-signalosomepi that mediates the beneficial effects of E2 in the brain is not completely clear.
Work by our group led to the cloning of proline-, glutamic acid-, and leucine-rich protein 1 (PELP1) and its characterization as a coregulator of ER (25, 26). PELP1 is a multidomain scaffold protein that serves as a platform for various protein–protein interactions (27). For example, PELP1 can interact with ERs, using the nuclear receptor interaction motif LXXLL; Src kinase, using SH2 domains; and PI3K, using PXXP motifs (28). The PELP1 C-terminal region is unique and contains a 70-aa glutamic acid-rich domain that facilitates PELP1 interactions with histones (29). Recent studies in cancer cells revealed that PELP1 also interacts with and modulates the activity of several chromatin-modifying enzymes such as histone deacetylase 2 (HDAC2), lysine (K)-specific demethylase 1A (KDM1A), and coactivator-associated arginine methyltransferase 1 (CARM1) (27, 30), and thus may contribute to E2-mediated genomic actions by facilitating the recruitment of chromatin modifiers to ER target genes. PELP1 is widely expressed in neuronal tissues, and its expression is modulated by E2 (31). However, the role of PELP1 in mediating the signaling, neuroprotective, and cognitive effects of E2 in the brain remains unknown. In addition, the factors that interact with and modulate PELP1 in the brain remain unidentified.
To address these deficits, in the present study, we developed the first PELP1 knockout model, to our knowledge, that conditionally depletes PELP1 in the forebrain. Our studies discovered that E2-mediated neuroprotection and rapid extranuclear signaling are lost in PELP1 knockout mice compared with floxed control mice. PELP1 antisense oligonucleotide knockdown studies in the rat further confirmed the role of PELP1 in mediating E2 extranuclear signaling and neuroprotection. RNA-seq studies identified several genes uniquely regulated by PELP1 under conditions of ischemia, and they represent pathways related to neuronal survival, apoptosis, and inflammation. PELP1 forebrain-specific knockout (FBKO) mice exhibited defects in spatial learning and memory. Mechanistic studies demonstrated that the PELP1 signalosome contains ERα, Src, and P85, and discovered that although PELP1 is a novel substrate of glycogen synthase kinase-3β (GSK3β) kinase, phosphorylation of PELP1 by GSK3β regulates PELP1’s stability. Collectively, these findings demonstrate a key role for PELP1 in mediating E2 rapid signaling, neuroprotection, and cognitive function in the brain.
Results
Characterization of PELP1 FBKO Mice.
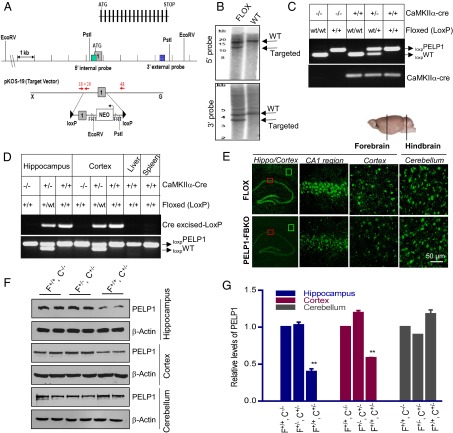
To examine the functional role of PELP1 in the regulation of E2 signaling, we generated conditional PELP1 FBKO mice using a site-specific Cre/loxP recombination system. A transgenic mouse that expresses a floxed allele of PELP1 was custom-generated and preliminarily characterized by Lexicon Pharmaceuticals Incorporated. In this model, exon 1 of PELP1 was flanked by loxP sites; the strategy is depicted in Fig. 1A. The targeted PELP1 allele was confirmed with Southern blot analysis of EcoRV- and Pst1-digested genomic DNA, using 5′ internal and 3′ external probes (Fig. 1B). PELP1 loxp/loxp mice were bred with Cre mice that expressed Cre under the control of the CaMKIIα promoter, which is specifically expressed in excitatory neurons of the forebrain. The CaMKIIα-Cre transgene is not expressed until after birth, thereby allowing normal embryonic development of the transgenic mice. Mice were genotyped by using PCR on genomic DNA of tail biopsies to confirm the genotype of floxed and Cre alleles (Fig. 1C). PELP1 FBKO mice are viable, with no obvious gross immunological, reproductive, or neurological abnormality or phenotype. To confirm knockout effectiveness of PELP1 in the CaMKIIα-Cre mice, we used genomic PCR of forebrain samples (Fig. 1D), as well as immunohistochemistry (Fig. 1E) and Western blot analysis (Fig. 1 F and G) for PELP1. As shown in Fig. 1D, deletion of the PELP1 exon 1 resulted in generation of a unique PCR band because of the expression of CaMKIIα-Cre in the forebrain compared with FLOX control mice. Immunohistochemical analysis of various regions of the forebrain further confirmed the efficient knockdown of PELP1 in the hippocampus and cortex, with no effect noted in the hindbrain (Fig. 1E). In addition, Western blot analysis showed the efficient knockdown of PELP1 in the forebrain (hippocampus and cerebral cortex) of PELP1 FBKO mice compared with FLOX control and PELP1 FBKO heterozygote mice (61% knockdown in hippocampus, 42% knockdown in cortex with no change in cerebellum) (Fig. 1 F and G). The residual amount of PELP1 in the hippocampus and cortex may be attributable to the expression of PELP1 in glia, interneurons, and a small percentage of excitatory neurons that lack Cre expression. PELP1 knockdown was specific to the forebrain, as PELP1 levels did not change in the cerebellum (hindbrain) in PELP1 FBKO versus FLOX control mice.
Fig. 1.
Generation and characterization of PELP1 FBKO mice. (A) Schematic representation of endogenous (WT) and recombined locus mPELP1. (B) Confirmation of targeted clones by Southern blotting of genomic DNA. (C) Tail DNA PCR confirmation of bitransgenic PELP1 FBKO mice expressing CaMKIIα-Cre and loxpPELP1. (D) Confirmation of excision of floxed region in hippocampus and cortex of FLOX control and PELP1 FBKO mice using genomic PCR. (E) Immunohistochemical analysis of coronal sections of hippocampus, cortex, and hindbrain of control and PELP1 FBKO mice. (F) Lysates of hippocampus, cortex and cerebellum collected from control (PELP1 loxp/loxp), heterozygous (PELP1 loxp/−, CaMKIIαCre+/−), and homozygous PELP1 FBKO (PELP1 loxp/loxp, CaMKIIα-Cre+/−) mice were subjected to Western blotting with PELP1 antibody. β-actin was used as a loading control. (G) The data were quantified using Image J software and normalized to β-actin. C, Cre; F, FLOX. Bar graphs represent mean ± SEM. **P < 0.01.
E2-Induced Rapid Extranuclear Signaling Is Attenuated in PELP1 FBKO Mice.
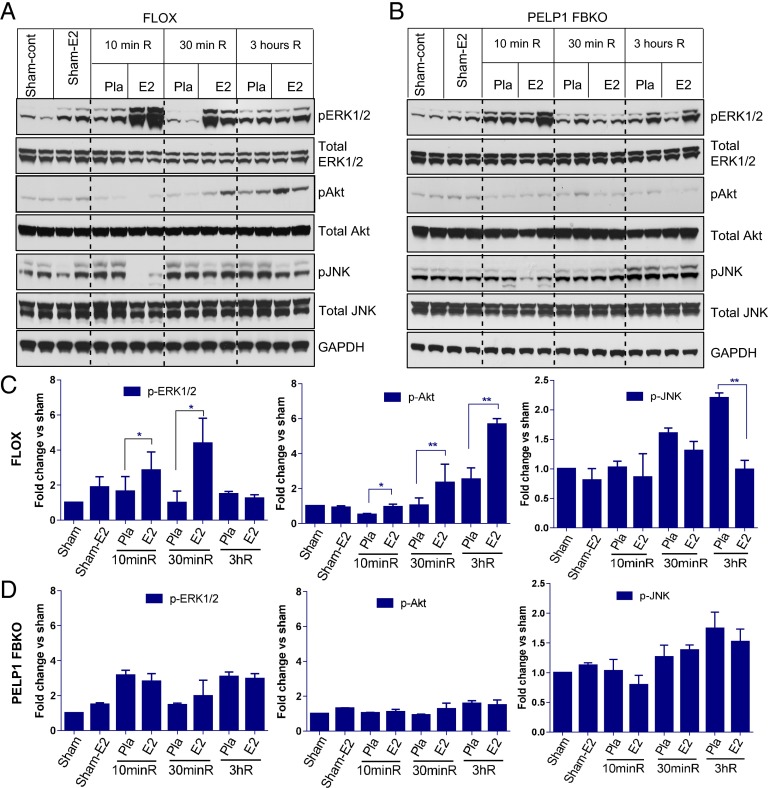
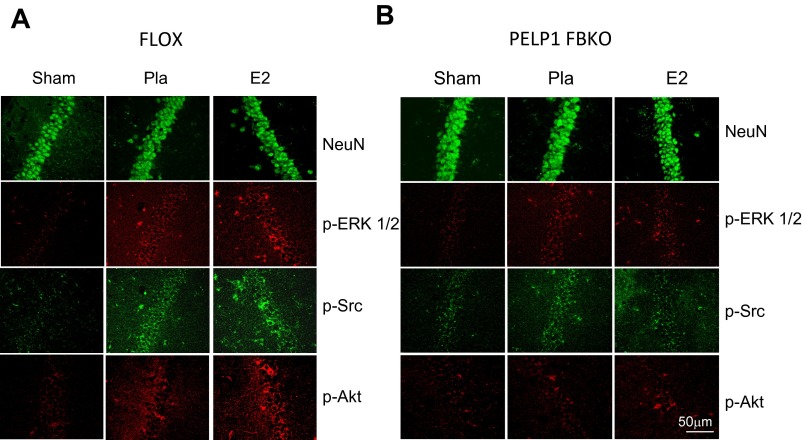
We previously showed that E2, through its cognate receptors ERα and GPR30, activates rapid extranuclear signaling to offer immediate protection to neurons after an insult (19, 23). To evaluate the role of PELP1 in the E2-mediated extranuclear signaling in vivo, ovariectomized placebo- and E2-implanted FLOX control and PELP1 FBKO mice were subjected to GCI, and the hippocampi were isolated after 10 min, 30 min, and 3 h reperfusion. As shown in Fig. 2 A and C, Western blot analysis demonstrated that in FLOX control mice, E2 significantly increased the phosphorylation of survival signaling kinases including pERK1/2 and pAkt. Phosphorylation of ERK1/2 was increased at 10 min and 30 min after reperfusion, with an increase in pAkt observed at 10 min, 30 min, and 3 h after reperfusion. However, E2 was unable to induce these survival signals in PELP1 FBKO mice (Fig. 2 B and D). We previously showed that E2 robustly attenuates phosphorylation/activation of the proapoptotic factor JNK after GCI insult (19, 23). We thus examined the role of PELP1 in this effect, using the PELP1 FBKO mice. As shown in Fig. 2 A and C, although E2 significantly decreased the phosphorylation of JNK in FLOX control mice at 3 h reperfusion, this effect was lost in PELP1 FBKO mice (Fig. 2 B and D), suggesting PELP1 is critical for E2 regulation of JNK activation. Immunofluorescence analysis also demonstrated that E2 significantly increased the phosphorylation of p-Src, p-ERK1/2, and p-Akt in hippocampal CA1 regions of FLOX control mice at 30 min reperfusion. However, in PELP1 FBKO mice, this E2-mediated increase was attenuated (Fig. S1 A and B). These results suggest that PELP1 is essential for the activation of rapid E2-mediated extranuclear signaling under conditions of cerebral ischemia. In addition, use of an alternative knockdown approach (central administration of PELP1 antisense oligonucleotides in the rat) further confirmed the critical role of PELP1 in mediating E2 regulation of rapid signaling (Akt and JNK activation) after GCI (Fig. S2).
Fig. 2.
E2 activation of extranuclear survival signaling and inhibition of proapoptotic JNK signaling is significantly attenuated in PELP1 FBKO mice. (A–D) Hippocampal lysates obtained from sham, sham E2, and at 10 min, 30 min, and 3 h after GCI/reperfusion from placebo and E2-treated ovariectomized female FLOX control mice (A and C) and PELP1 FBKO mice (B and D) were subjected to Western blotting. Blots were probed with phosphor- and total ERK1/2, Akt, and JNK antibodies. GAPDH was used as internal control. Pla = placebo; R = reperfusion. Bar graphs represent mean ± SEM. *P < 0.05; **P < 0.01 versus sham. n = 4–5 mice per group.
Fig. S1.
Immunohistochemistry results confirm that PELP1 FBKO mice exhibit reduced activation of extranuclear survival signaling with E2. Immunofluorescence staining of NeuN, p-Src, p-ERK1/2, and p-Akt in hippocampus CA1 at 30 min after GCI/reperfusion in FLOX control (A) and PELP1 FBKO mice (B). Pla = placebo. Results are representative of staining observed in five (n = 5) individual animals per group.
Fig. S2.
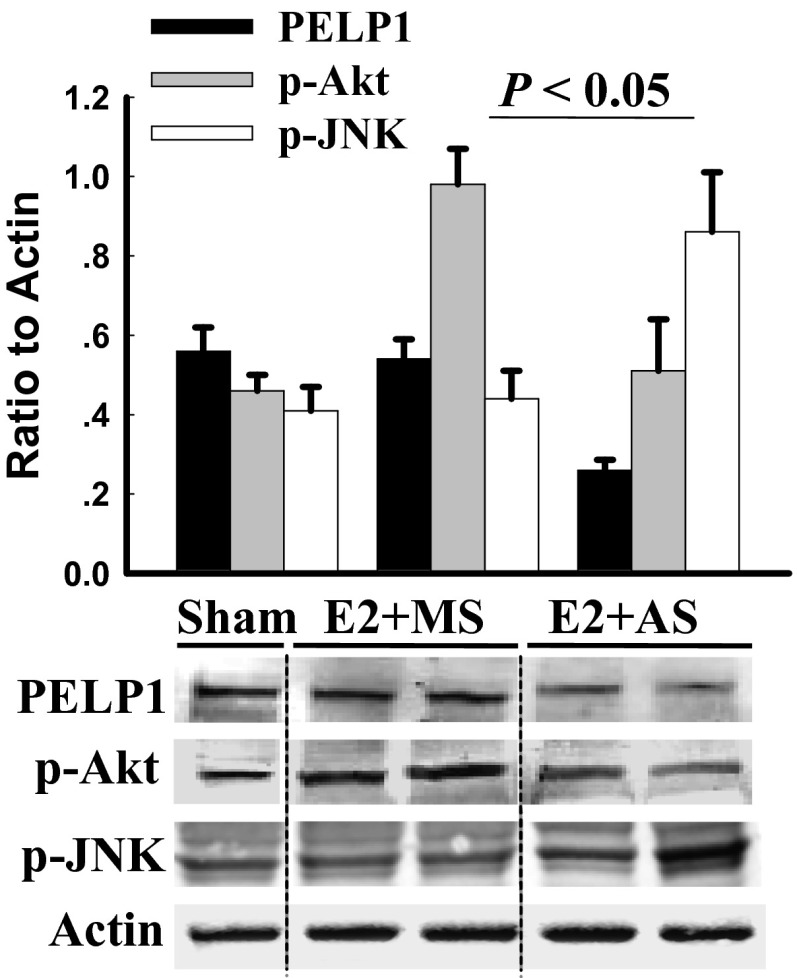
Antisense oligonucleotide knockdown of PELP1 reverses E2-mediated effects on phosphorylation of Akt and JNK at 3h after GCI in the rat. MS or PELP1 AS oligonucleotides (10 μL, 10 nmol) were administered into the lateral cerebroventricle of adult ovariectomized rats every 24 h beginning 3 d before GCI and 1 h before GCI. E2 was administered by minipumps implanted subcutaneously at time of ovariectomy and left in place until the end of the experiment. The minipumps produce low diestrus levels (15–20 pg/mL) of E2 in the bloodstream. Western blot analysis results are shown. Note that black dotted lines between bands indicate representative bands that were noncontiguous. The results demonstrate a robust decrease of PELP1 levels in the hippocampal CA1 region of AS-treated rats versus MS-treated rats. Note also that PELP1 AS treatment reverses the E2-mediated effects upon Akt and JNK activation/phosphorylation after GCI (compare E2+AS to E2+MS). P < 0.05 between MS and AS treated groups. n = 5 per group.
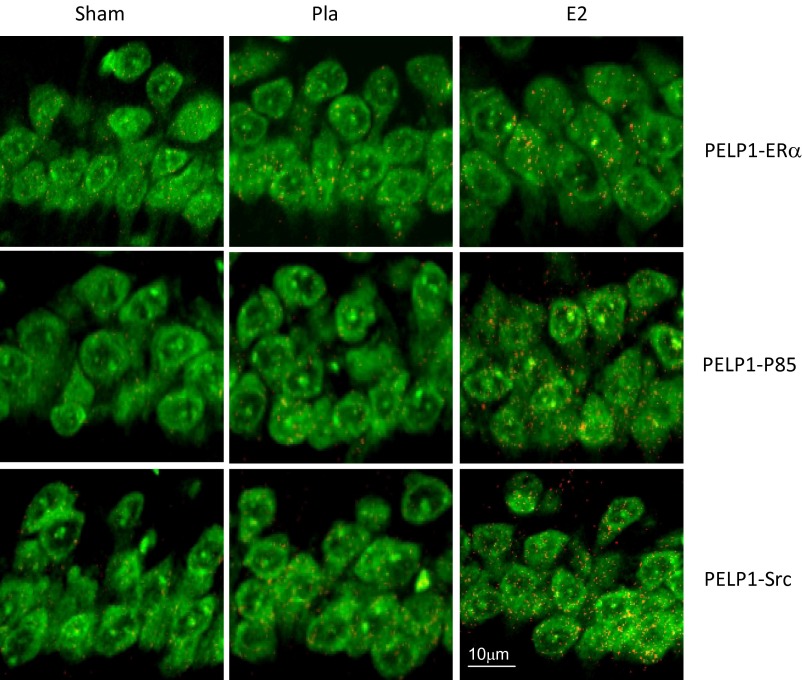
PELP1 is a scaffolding protein that contains several protein–protein interaction motifs that interact with SH2 and SH3 domains present in Src and PI3 kinases. To determine whether PELP1 forms a scaffold complex with Src, PI3 kinase, and ERα in the hippocampus, and whether E2 enhances this effect, we examined the in vivo association of these proteins at 30 min after GCI in wild-type mice treated with placebo or E2, using the Duolink II in situ proximity ligation assay detection kit. The results revealed that there is low PELP1 interaction with ERα, Src, and PI3K in the placebo group after GCI, whereas, in contrast, the E2-treated group showed robust PELP1 interaction with ERα, Src, and PI3K 30 min after ischemia (Fig. S3). Taken together, these results suggest PELP1 is needed for the optimal activation of E2-mediated extranuclear signaling under conditions of ischemic damage.
Fig. S3.
Duo-Link II in situ proximity ligation assay reveals that PELP1 interacts with ERα, PI3K regulatory subunit p85, and Src in vivo in the hippocampal CA1 region at 30 min after GCI, and that estradiol (E2) increases the interaction of PELP1 with these factors. Orange dots indicate protein interaction between PELP1 and ERα, P85, or Src. Tissue sections derived from sham, placebo, and E2 treated mice were blocked in 5% (vol/vol) goat serum for 1 h and incubated overnight with appropriate primary antibodies at 4 °C. Slides were then incubated with Duolink PLA Rabbit MINUS and PLA Mouse PLUS proximity probes for 1 h at 37 °C. Ligation and amplification was carried out using the Duolink detection reagent kit according to manufacturer’s protocol. Images were captured using confocal microscope. (Scale bar, 10 μm.)
E2-Mediated GSK3β Inhibition Is Attenuated in PELP1 FBKO Mice.
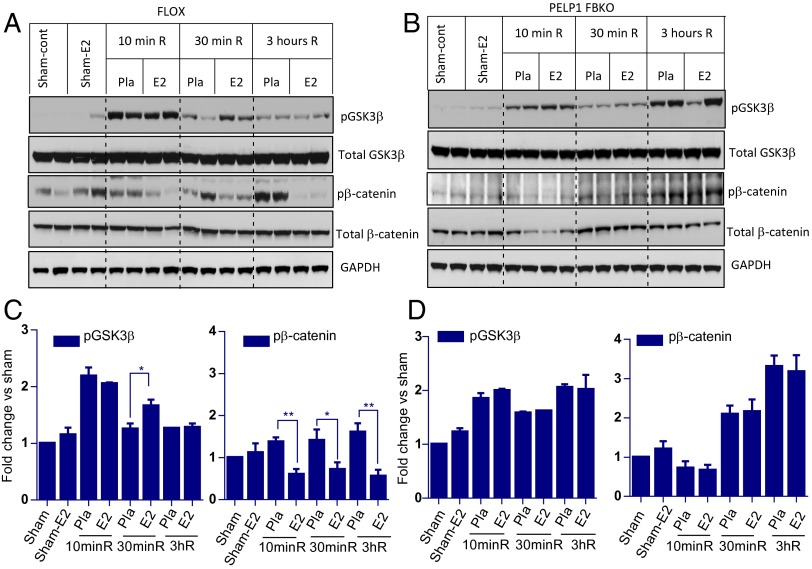
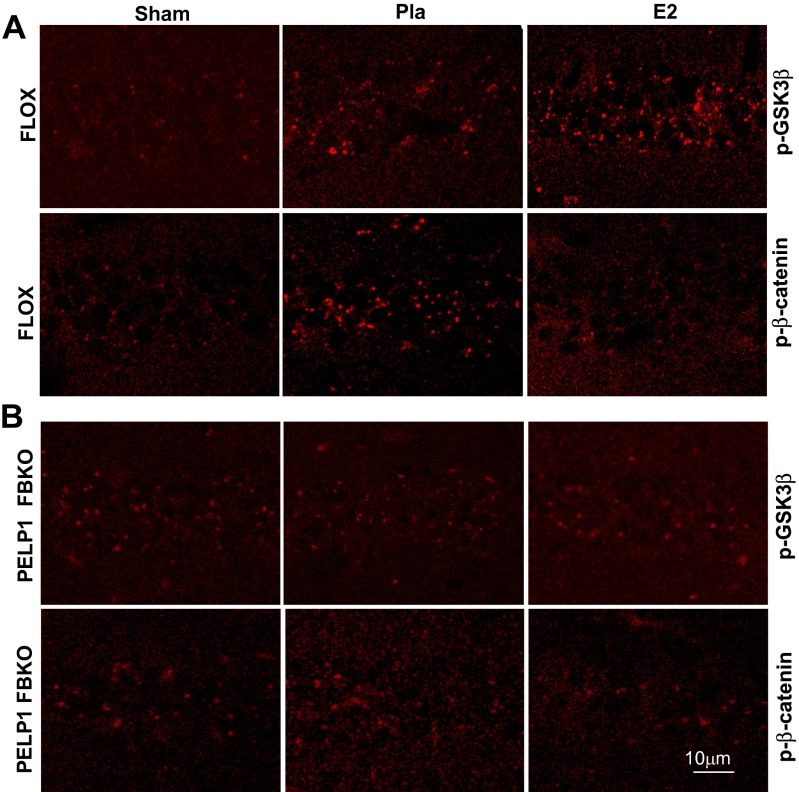
Previous work suggests GSK3β is one of the key mediators of cell death in several neurodegenerative models, including cerebral ischemia and Alzheimer’s disease, and that inhibition of GSK3β improves functional recovery after cerebral ischemia (17, 32–34). GSK3β phosphorylates many substrate proteins to regulate their stability (35, 36). In turn, GSK3β activity is inhibited by several kinases, including the Akt, Wnt, and MAPK pathways (37). It is known that the prosurvival kinase, Akt, can phosphorylate GSK3β at its N terminus, leading to its inactivation. Because we showed that PELP1 status modulates Akt phosphorylation, we examined whether PELP1 might play a role in regulating GSK3β phosphorylation, using FLOX control and PELP1 FBKO hippocampi, after GCI. As shown in Fig. 3 A and C, GSK3β phosphorylation is initially elevated at 10 min after GCI in the hippocampus of FLOX control mice, followed by a robust fall in its phosphorylation at 30 min and 3 h, indicating a transient ischemia-induced inhibition of GSK3β after GCI. Interestingly, E2 treatment did not alter GSK3β phosphorylation at 10 min after GCI, but significantly increased its phosphorylation at 30 min after GCI compared with the placebo control, suggesting E2 maintains a longer period of GSK3β inhibition. However, this regulatory effect of E2 on GSK3β phosphorylation is lost in PELP1 FBKO mice (Fig. 3 B and D). GSK3β is one of the key components in the Wnt signaling pathway. It negatively regulates prosurvival signaling by phosphorylating β-catenin, which leads to its degradation. We previously showed that E2 significantly up-regulates the β-catenin pathway after GCI (11). As shown in Fig. 3 A and C, Western blot analysis revealed that E2 substantially reduced the phosphorylation of β-catenin after GCI compared with placebo in FLOX control mice. However, the ability of E2 to attenuate β-catenin phosphorylation was lost in PELP1 FBKO mice (Fig. 3 B and D). Interestingly, after GCI, phosphorylation of β-catenin was increased more in PELP1 FBKO mice (two- to threefold increase vs. sham) compared with FLOX control mice (1.5–1.7-fold increase vs. sham) (Fig. 3 C and D). Immunohistochemical analysis further demonstrated that E2 increases phosphorylation of GSK3β and decreases phosphorylation of β-catenin in the hippocampal CA1 regions of FLOX control mice at 30 min after reperfusion (Fig. S4A). However, no substantial changes in the phosphorylation of GSK3β and β-catenin were observed between E2 and placebo groups in PELP1 FBKO mice (Fig. S4B). These results suggest that PELP1 is essential for E2-mediated GSK3β inactivation and further activation of β-catenin signaling under conditions of cerebral ischemia.
Fig. 3.
E2-mediated enhancement of p-GSK3β and reduction of p-β-catenin is significantly attenuated in PELP1 FBKO mice. (A–D) Hippocampal lysates obtained from sham, sham E2, and at 10 min, 30 min, and 3 h after GCI/reperfusion from placebo and E2 treated ovariectomized female FLOX control mice (A and C) and PELP1 FBKO mice (B and D) were subjected to Western blotting to detect changes in the levels of p-GSK3β and p-β-catenin. GAPDH was used as an internal control. Representative blots show the attenuation of E2-mediated effects in PELP1 FBKO mice. Pla = placebo; R = reperfusion (C and D). Bar graphs represent mean ± SEM. *P < 0.05; **P < 0.01 versus sham. n = 4–5 mice per group.
Fig. S4.
Immunohistochemistry results confirm that PELP1 FBKO mice show a reduction in E2-mediated inactivation of p-GSK3β and activation of β-catenin. Immunofluorescence staining of p-GSK3β and p-β-catenin in hippocampus CA1 at 30 min after GCI/reperfusion in FLOX (A) and PELP1 FBKO (B) mice. Pla = placebo; R = reperfusion. Results are representative of staining observed in five individual animals per group.
PELP1 Is a Novel Substrate of GSK3β.
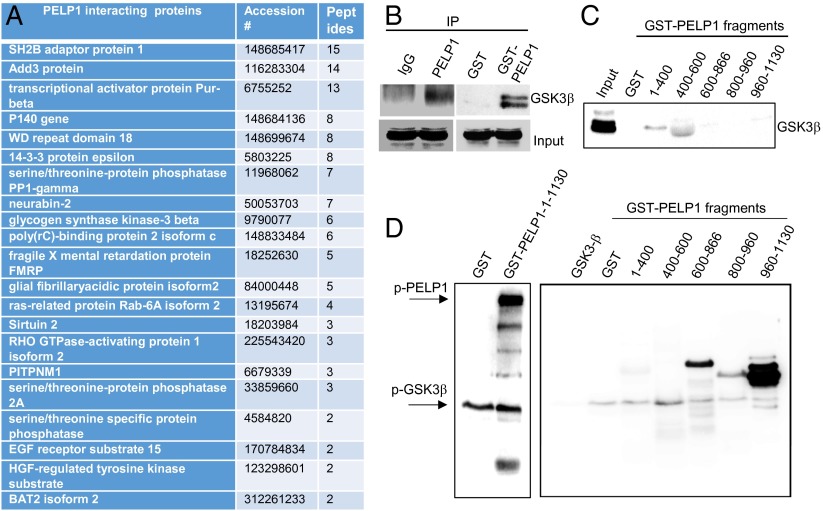
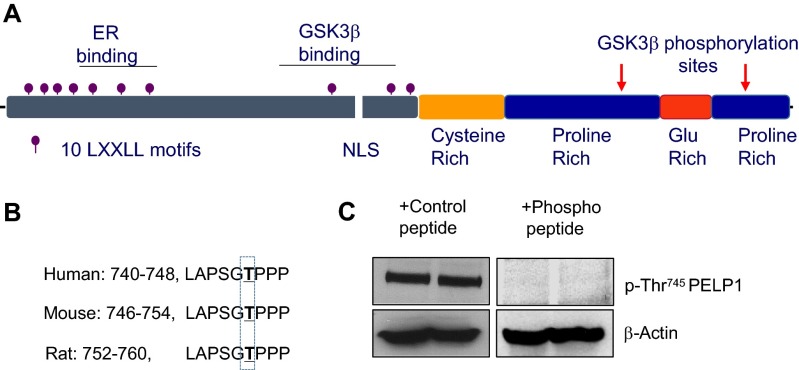
Emerging evidence suggests that PELP1 functions as multifunctional scaffolding protein that interacts with many transcriptional factors, including ERα and chromatin-modifying factors. However, the components of the PELP1 interactome under conditions of cerebral ischemia remain unknown. To identify PELP1-interacting proteins, we performed mass spectrometry analysis of PELP1 immunoprecipitates obtained from hippocampal lysates that were subjected to GCI (24 h reperfusion). We found several cytosolic PELP1 binding proteins that play an important role in neuronal survival and cell death as novel PELP1 interacting proteins (Fig. 4A). Interestingly, GSK3β is one of the proteins that interacted with PELP1 during cerebral ischemia. Because of its established role in cerebral ischemia, we focused on the PELP1-GSK3β interaction in subsequent studies. We confirmed the binding of PELP1 to GSK3β by performing an immunoprecipitation assay using a PELP1 antibody and by performing a GST pull-down assay using GST-PELP1. Results from both assays confirmed that GSK3β specifically interacts with PELP1 (Fig. 4B). To map the region of PELP1 binding, we performed GST pull-down assays by using various PELP1-GST deletion fragments (Fig. S5). The results showed that GSK3β binds to the 400–600-aa region of PELP1 (Fig. 4C). Minor interaction of GSK3β with the 1–400-aa region of PELP1 was also observed. Close examination of the amino acid sequence of PELP1 revealed several GSK3β phosphorylation consensus S/TXXXS/T motifs. To examine whether PELP1 is a novel substrate of GSK3β, we performed an in vitro kinase assay using both GST-PELP1 and various GST-deletion fragments of PELP1. Our results demonstrated that PELP1 indeed is phosphorylated by GSK3β (Fig. 4D). Mapping studies revealed that GSK3β phosphorylates PELP1 at two different residues (Thr745 and Ser1059; Fig. 4D). The proposed locations for GSK3β binding and phosphorylation sites in the PELP1 protein are illustrated in Fig. S6A. Collectively, these studies identified PELP1 as a novel interacting protein and a substrate of GSK3β.
Fig. 4.
PELP1 is a novel substrate of GSK3β. Hippocampal lysates collected 24 h after GCI reperfusion were subjected to immunoprecipitation with PELP1 or IgG antibody. Immunoprecipitates were pulled down by adding dynabeads protein A. Immunoprecipitates were subjected to SDS/PAGE, followed by mass spectrometry analysis. (A) List of unique PELP1-interacting proteins. (B) Forebrain lysates were subjected to immunoprecipitation or GST pull-down assay, and PELP1 interaction with GSK3β was confirmed by Western blotting. (C) Forebrain lysates were used in pull-down assays using GST or various deletion fragments of PELP1, and interaction of PELP1 deletions with GSK3β was analyzed by Western blotting. (D) GST-tagged PELP1 deletions and full-length GST-PELP1 were incubated with purified GSK3β enzyme in kinase buffer with [γ32-P] ATP and 100 μM cold ATP for 30 min at 30 °C, and the phosphorylation of PELP1 fragments was analyzed by PhosphorImager.
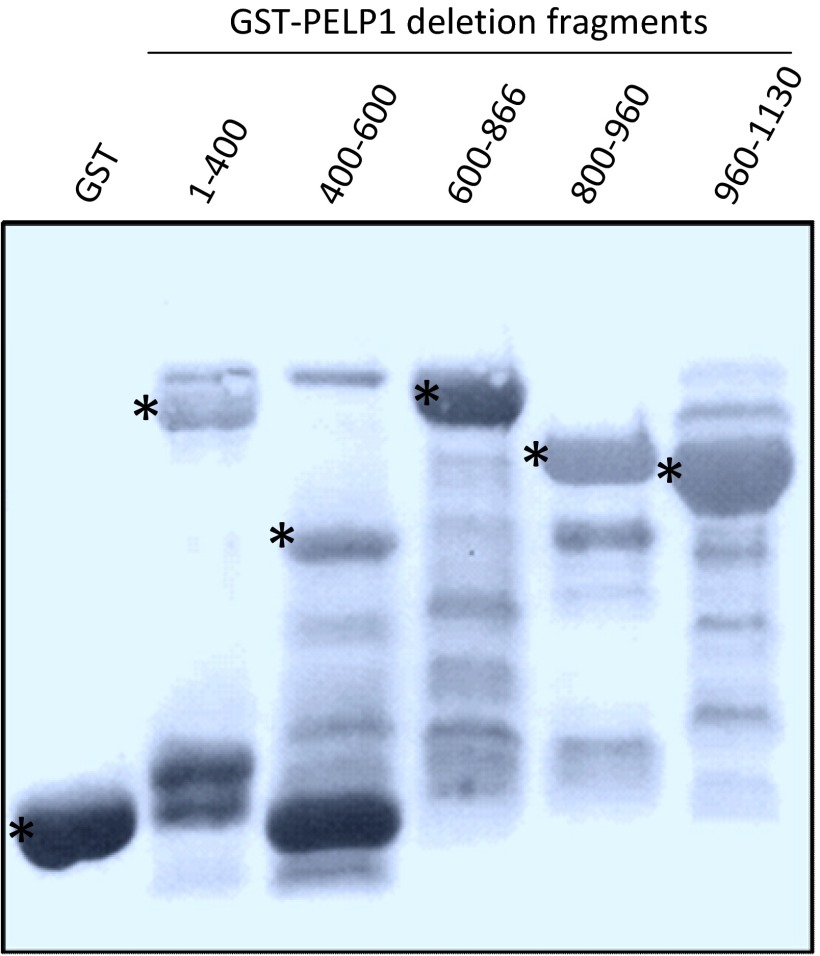
Fig. S5.
SDS/PAGE analysis of expression of various GST-PELP1 deletion fragments used in the study. Bands were visualized by Ponceau S staining. Asterisk points to the GST-PELP1 deletion protein band.
Fig. S6.
Characterization of p-Thr745 PELP1 antibody. (A) Location of GSK3β binding and phosphorylation sites in PELP1. (B) Conservation of peptide sequence that contains the GSK3β phosphorylation site used for antibody preparation. (C) Western blots of hippocampus lysates (from two different mice) were probed with the Thr745 phospho-PELP1 antibody in the presence of control or phosphopeptide.
GSK3β-Mediated Phosphorylation of PELP1 Leads to Its Degradation.
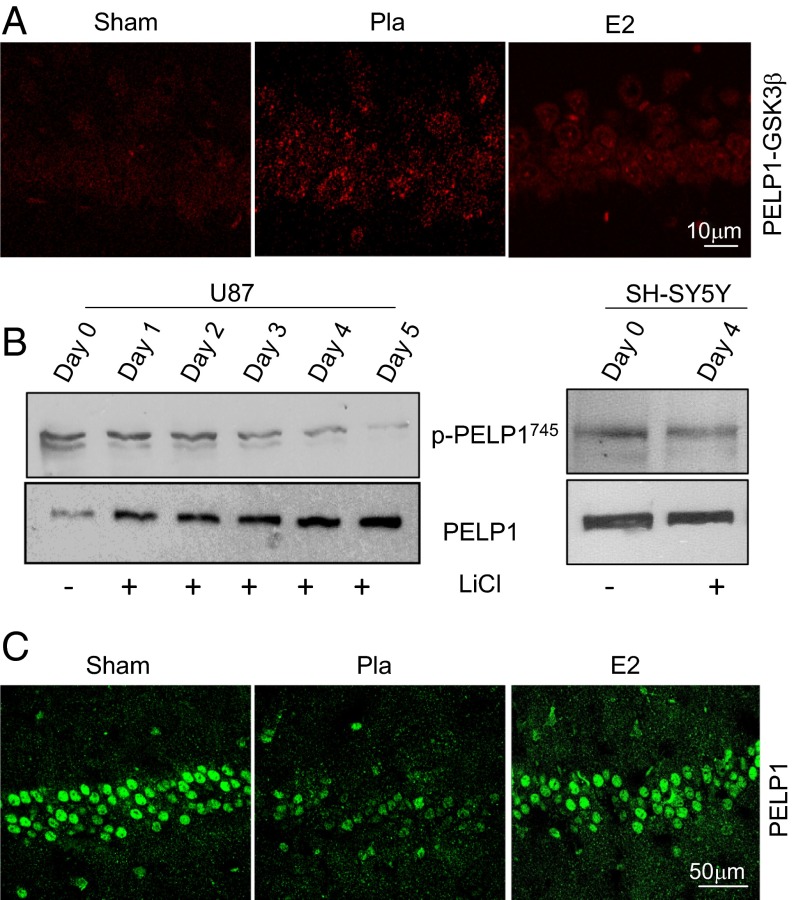
To further demonstrate the effect of E2 on the interaction of GSK3β and PELP1, we examined their interaction under the conditions of GCI (placebo), as well as after E2 treatment, using an in vivo DuoLink proximity ligation assay. The results from this assay demonstrated that GCI significantly increases the interaction of PELP1 with GSK3β in the hippocampal CA1 region compared with sham, whereas E2 treatment significantly decreased their interaction (Fig. 5A). Earlier published studies demonstrated that GSK3β phosphorylates and regulates the stability of many of its substrates. Because GSK3β activity is altered after ischemia, and because PELP1 is phosphorylated by GSK3β in vitro, we tested whether GSK3β phosphorylation of PELP1 also regulates its stability. Although the mapping studies using human PELP1 identified two GSK3β sites, only Thr745 is conserved in mouse. Therefore, to demonstrate that GSK3β phosphorylates PELP1 in vivo, we generated a novel, affinity-purified phospho-Thr745 PELP1 antibody. Antibody specificity was confirmed by competing with blocking peptide that is specific to the Thr745 site (Fig. S6 B and C). To better understand the functional relevance of phosphorylation of PELP1 by GSK3β, we inhibited the GSK3β activity, using lithium chloride in two different cell models (glioblastoma, U87, and neuroblastoma, SH-SY5Y) and analyzed the levels of phospho-Thr745 PELP1. Western blot analysis of total lysates indicated that lithium chloride treatment significantly reduced the phosphorylation of PELP1, and total PELP1 levels were simultaneously stabilized (Fig. 5B). To examine whether such regulation occurs in vivo, we studied the total PELP1 levels at 6 d reperfusion after GCI in the hippocampus by immunofluorescence analysis. The results showed that PELP1 levels were significantly reduced in the placebo group; however, E2 significantly restored the PELP1 levels in hippocampus (Fig. 5C). Collectively, these results suggest that PELP1 is a novel substrate of GSK3β and that phosphorylation of PELP1 by GSK3β at Thr745 leads to its degradation, and that E2 has a protective effect of restoring the PELP1 levels by blocking GSK3β activation.
Fig. 5.
GSK3β phosphorylation of PELP1 results in reduced stability of PELP1. (A) Tissue sections derived from sham, placebo, and E2-treated mice at 24 h after GCI were blocked in 5% (vol/vol) goat serum for 1 h and incubated overnight with appropriate primary antibodies at 4 °C. Slides were then incubated with Duolink PLA Rabbit MINUS and PLA Mouse PLUS proximity probes for 1 h at 37 °C. Ligation and amplification were carried out using the Duolink detection reagent kit according to manufacturer’s protocol. Images were captured using a confocal microscope. (B) Glioblastoma (U87) and neuroblastoma (SH-SY-5Y) cells were treated with GSK3β inhibitor lithium chloride, and lysates were collected at the indicated periods. Lysates were subjected to Western blot analysis with p745PELP1 and total PELP1 antibodies. (C) Coronal brain sections collected from sham, placebo, and E2-treated FLOX mice 6 d after GCI reperfusion were subjected to immunofluorescence for PELP1 antibody.
PELP1 Is Needed for Optimal Activation of E2-Regulated Genes After GCI.
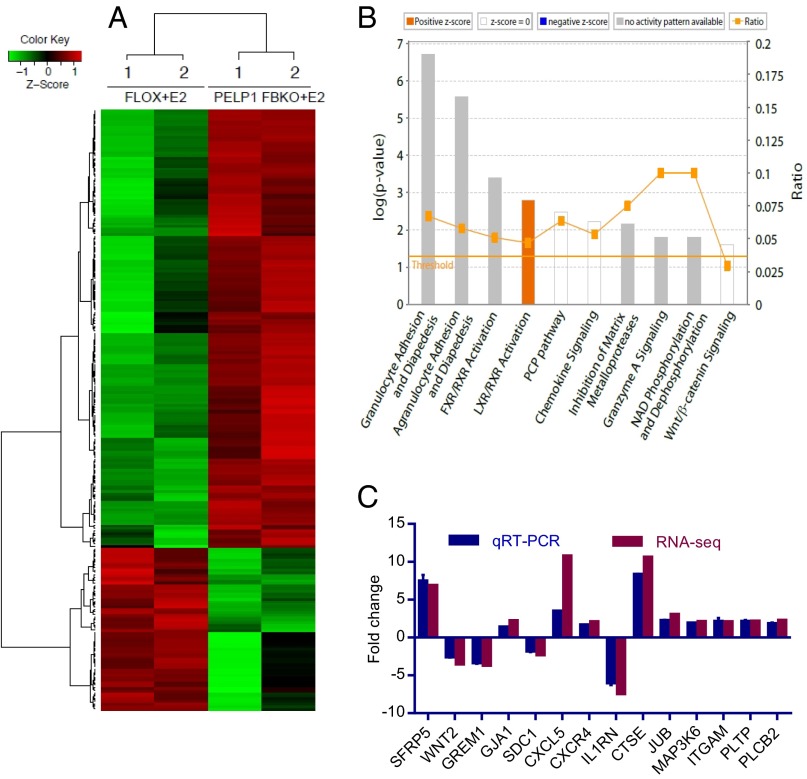
E2-mediated neuroprotective effects involve both nuclear and extranuclear functions of ER (4). PELP1 functions as coregulator of ER and can modulate both extranuclear and nuclear functions of ER in cancer cells (25, 28). To examine the role of PELP1 in E2-ER-mediated transcription in the hippocampus under conditions of GCI, we performed global transcriptome analysis. E2-treated FLOX control, and PELP1 FBKO mice were subjected to GCI, followed by 24 h reperfusion, and the isolated RNA was used for RNA-seq analysis. The genes that showed at least twofold changes (P < 0.01) were chosen for analysis. Overall, 229 genes were differentially expressed in PELP1 FBKO mice compared with FLOX control mice, and among those, 167 genes were up-regulated and 62 genes were down-regulated. A representative heat map is shown in Fig. 6A. The complete list of differentially expressed genes is available at the Gene Expression Omnibus database under accession number GSE72136. To further explore the biological significance of differentially expressed genes in PELP1 FBKO mice, we performed pathway analysis using ingenuity pathway analysis software. The top five networks that were significantly altered in PELP1 FBKO mice include tissue development and function, cellular assembly and organization, neurological disease, and inflammatory disease (Figs. S7A and S8). Some of the top ingenuity pathway analysis canonical pathways that were significantly altered in PELP1 FBKO mice include neuroinflammation/neuronal death (including chemokine signaling, inhibition of matrix metalloproteases, granzyme A signaling) and Wnt pathway. Other pathways regulated by PELP1 were related to activation of FXR/RXR, LXR/RXR, and ketogenesis (Fig. 6B). Further analysis of molecular and cellular functions of PELP1-regulated genes revealed that they are involved in cellular movement, cell-to-cell signaling interaction, cell death, survival, and DNA repair (Fig. S7B). We used quantitative RT-PCR (qRT-PCR) to validate the expression of some of the top differentially expressed genes involved in inflammation, cell death, survival, cellular function, and signaling, as well as some of the important Wnt pathway target genes in FLOX and PELP1 FBKO mice (Fig. 6C). The results suggested that the β-catenin pathway is one of the major pathways activated by E2 after GCI, which is attenuated in PELP1 FBKO mice.
Fig. 6.
PELP1 FBKO mice have alterations in E2-induced transcriptome. Total RNA was isolated from ovariectomized FLOX control mice and PELP1 FBKO mice (implanted with E2 mini pumps) that were subjected to GCI and killed at 24 h reperfusion. Hippocampi samples were subjected to RNA-seq analysis, as detailed in Methods. Differential expression analysis was performed by DEseq, and significant genes with at least twofold change were chosen for analysis. (A) Representative heat map of differentially expressed genes from two groups. (B) Differentially expressed genes with greater than twofold change in expression were further analyzed by ingenuity pathway analysis. The top canonical pathways are shown. (C) Selected differentially expressed genes were further validated by quantitative RT-PCR; RNA-seq expression values are plotted side by side for comparison.
Fig. S7.
PELP1 FBKO mice have alterations in activation of various networks. Differentially expressed genes identified in RNA-seq analysis with greater than twofold change in expression were further analyzed by ingenuity pathway analysis. The top five associated network functions (A) and top five biological functions (B) are shown.
Fig. S8.
Gene interaction networks of differentially expressed genes between FLOX-E2 and PELP1-FBKO-E2 groups. Ingenuity pathway analysis of interaction of differentially expressed genes identified several networks. (A–E) Top five networks are shown. (A) Respiratory system development and function, connective tissue development and function, embryonic development. (B) Cellular assembly and organization, neurological disease, inflammatory disease. (C) Lipid metabolism, molecular transport, small molecule biochemistry. (D) Dermatological diseases and conditions, inflammatory disease, neurological disease. (E) Cell signaling, molecular transport, nucleic acid metabolism. A line represents the biological relationship between molecules, with solid lines representing direct interactions and the dashed lines representing indirect interactions. Genes that were up-regulated or down-regulated in PELP1-FBKO-E2 group were shown in red and green, respectively.
E2-Mediated Neuroprotection Is Compromised in PELP1 FBKO Mice.
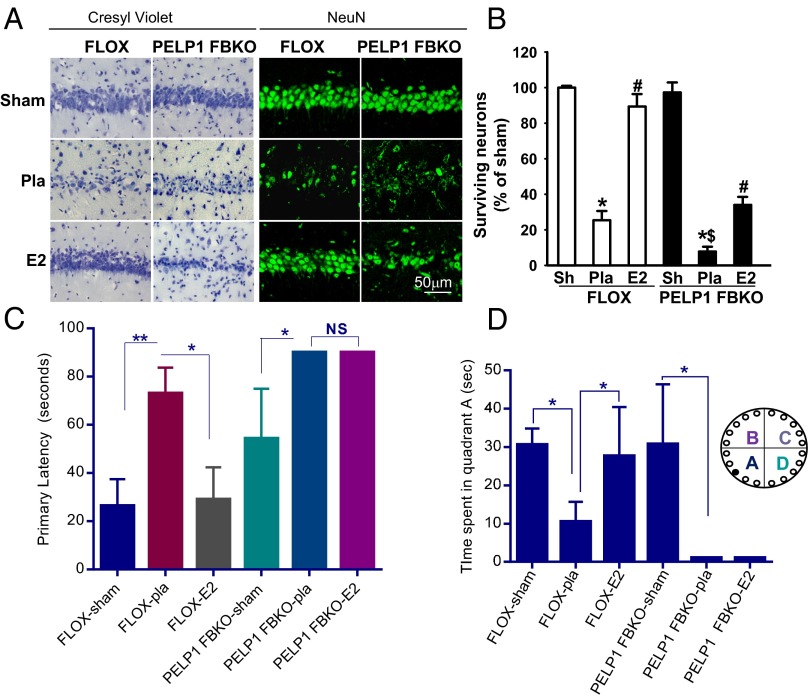
We, and others, have shown previously that E2 exerts neuroprotective functions after global cerebral ischemia (38, 39). We therefore examined the effect of PELP1 knockdown on E2-mediated neuroprotection in the hippocampal CA1 region after GCI. Tissue sections collected at 6 d after GCI were subjected to cresyl violet and NeuN staining. Fig. 7A shows the representative images of the cresyl violet staining and NeuN staining in the hippocampal CA1 region at 6 d reperfusion after GCI in placebo- and E2-treated ovariectomized FLOX control and PELP1 FBKO mice. Sham animals are included as nonischemic controls. The results showed that in FLOX control animals, GCI (placebo) resulted in a significant reduction in the number of surviving neurons, as evidenced by the nonviable cells with condensed, pyknotic, and shrunken nuclei in the CA1 region compared with the sham control group. However, E2 treatment exerted significant neuroprotection in the CA1 region after GCI, as evidenced by dense rounded neuron cells similar to CA1 pyramidal cells. These results were further confirmed by NeuN positive staining (Fig. 7 A and B). Interestingly, PELP1 FBKO mice had increased sensitivity to GCI damage, as evidenced by a decreased number of surviving neurons in PELP1 FBKO (Pla) mice compared with FLOX (Pla) control mice (Fig. 7 A and B). Furthermore, in PELP1 FBKO mice, E2-mediated neuroprotection is markedly attenuated, as evidenced by a decreased number of surviving neurons and NeuN-positive cells in the hippocampal CA1 region (Fig. 7 A and B). In addition, use of an alternative knockdown approach (central administration of PELP1 antisense oligonucleotides in the ovariectomized rat) further confirmed the critical role of PELP1 in mediating E2 neuroprotection after GCI (Fig. S9). Taken together, these results demonstrate that PELP1 is critical for the ability of E2 to induce neuroprotection after GCI.
Fig. 7.
PELP1 FBKO mice display diminished E2-induced neuroprotection: Coronal brain sections collected 6 d after GCI-reperfusion from sham, placebo, and E2-treated female ovariectomized FLOX control and PELP1 FBKO mice were subjected to cresyl violet and NeuN immunostaining, as described in Methods. (A) Representative Image of hippocampal CA1 region showing significant neuroprotection with E2 treatment, as evident from the significant increase in the number of surviving neurons compared with placebo in FLOX control mice. (B) Surviving neuronal counts of NeuN-positive neurons are shown. *P < 0.05 versus sham; #P < 0.05 versus placebo; $P < 0.05 versus FLOX placebo. (C) Spatial learning and memory were assessed in sham, placebo, and E2-treated female ovariectomized FLOX and PELP1 FBKO mice that were subjected to GCI, using the Barnes maze test, as described in Methods. (D) Time spent in each quadrant was quantified for all treatment groups. Data analysis indicated that PELP1 FBKO mice exhibited impaired E2-mediated spatial learning and memory. Data represent mean ± SE, n = 4–7 mice per group. *P < 0.05 and **P < 0.01.
Fig. S9.
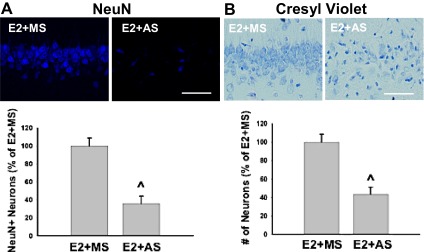
Antisense oligonucleotide knockdown of PELP1 in the ovariectomized rat reverses E2-mediated neuroprotection after global cerebral ischemia. (A) Representative photomicrographs depict hippocampal CA1 neurons immunopositive for the neuronal nuclear marker NeuN 7 d after global cerebral ischemia. The experimental design is described in Fig. S2. PELP1 knockdown efficiency is also shown in Fig. S2. Note that E2-treated PELP1 AS knockdown rats had significantly fewer surviving NeuN-positive neurons compared with E2-treated MS animals after GCI, indicating a loss of E2 neuroprotection after PELP1 knockdown. Quantitative summary of data (means ± SE; n = 5–7 per group) is expressed as a percentage of NeuN-positive neurons per 250-μm medial CA1 of E2+MS and E2+AS animals. ^ = P < 0.05 compared with E2+MS. Magnification 40×. (Scale bar, 50 μm.) (B) Representative cresyl violet staining in the hippocampal CA1 region 7 d after global cerebral ischemia. Quantitative summary of data (means ± SE; n = 5–7 per group) is expressed as a percentage of cresyl violet-positive neurons per 250-μm medial CA1 of E2+MS and E2+AS animals. ^ = P < 0.05 compared with E2+MS. Magnification 40×. (Scale bar, 50 μm.)
E2-Mediated Cognitive Function Is Lost in PELP1 FBKO Mice.
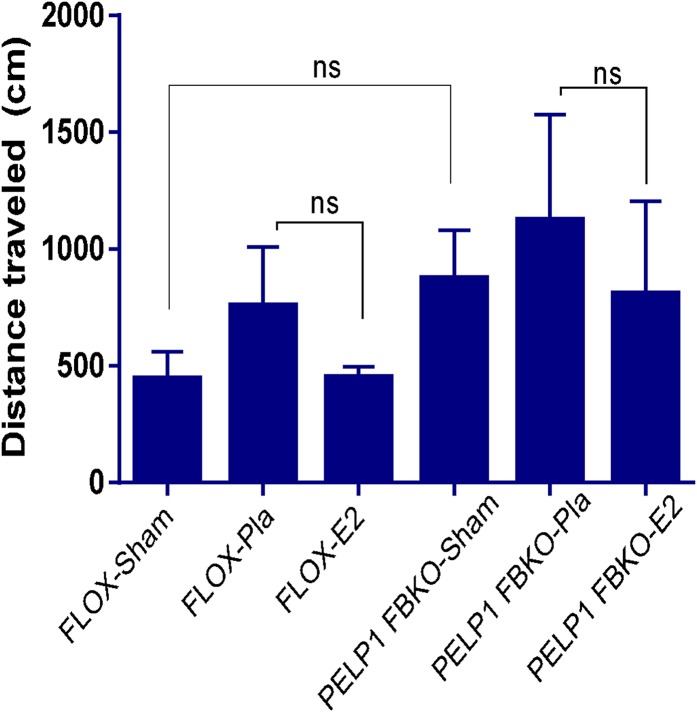
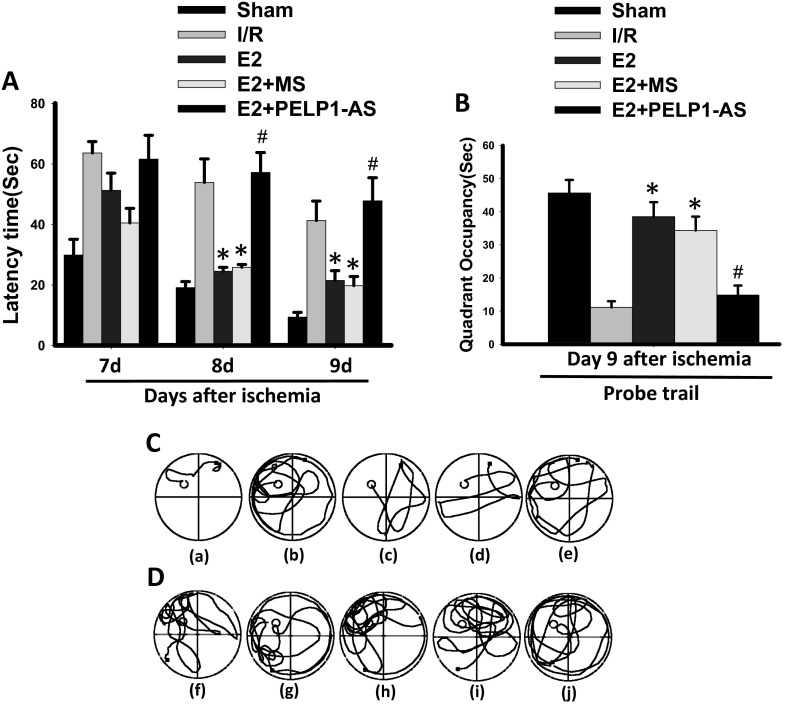
To investigate the importance of PELP1 in E2-mediated cognitive functional recovery after GCI, spatial learning and memory were tested in the Barnes maze, as previously described (40). Both FLOX control and PELP1 FBKO mice were subjected to sham, placebo + GCI, or E2 + GCI treatment, and cognitive testing was initiated 24 after sham/GCI and continued for 4 d, with a probe trail on day 5 after reperfusion. As shown in Fig. 7C, primary latency to locate the correct escape hole during the probe trial was significantly increased by GCI in both FLOX control and PELP1 FBKO mice. E2 treatment rescued the GCI-induced deficit only in FLOX control mice, whereas no difference was observed between placebo and E2 treatment in PELP1 FBKO mice. Further, as shown in Fig. 7D, FLOX control mice subjected to GCI spent less time in the quadrant of the maze where the escape hole was located compared with sham. E2 treatment significantly rescued this deficit during the probe trial, whereas E2-mediated effect was abolished in PELP1 FBKO mice. However, in the open field test, we did not find any significant differences in distance traveled between PELP1 FBKO and FLOX control mice after GCI, suggesting motor deficits were not responsible for the differences in performance on the Barnes maze (Fig. S10). Finally, central administration of PELP1 antisense oligonucleotides in the ovariectomized rat further confirmed the PELP1 FBKO results, as PELP1 antisense (AS) knockdown animals showed a loss of E2-induced cognitive enhancement after GCI as measured, using the Morris water maze (Fig. S11 A–D). In contrast, missense (MS) oligonucleotides did not affect the ability of E2 to enhance cognitive function after GCI. Collectively, these results suggest a critical role for PELP1 in mediating the cognitive-enhancing effects of E2 after cerebral ischemia.
Fig. S10.
Hyperactivity and anxiety-like behavior was assessed in sham, placebo, and E2-treated female ovariectomized FLOX and PELP1 FBKO mice that were subjected to GCI, using the open field test. Two days after reperfusion, mice were placed in an open field for 5 min, and overall activity was monitored using a video tracker. The total distance traveled in the open field chamber was calculated. Data analysis indicates there was no significant difference between placebo and E2-treated groups of FLOX and PELP1 KO mice. ns = nonsignificant.
Fig. S11.
Antisense oligonucleotide knockdown of PELP1 in the ovariectomized rat reverses E2 enhancement of cognitive function after GCI. (A) Latency trail and (B) probe trail in the Morris water maze. Representative tracks for latency trail (C) and probe trail (D), [a and f: sham; b and g: ischemia/reperfusion (I/R); c and h: E2; d and i: E2+MS; e and j: E2+PELP1-AS]. Note that E2-treated animals had significantly reduced latency times to find the hidden platform in the water maze (A and C) and spent more time in the quadrant where the platform was located compared with I/R animals (B and D). MS oligos had no effect on E2 enhancement of cognitive function. PELP1 AS oligo knockdown markedly attenuated E2’s effect to decrease latency to find the platform (A and C) and increase time spent in the quadrant where the platform was located (B and D). The experimental design is as described in Fig. S2. PELP1 AS knockdown efficiency is also shown in Fig. S2. *P < 0.05 versus I/R group, #P < 0.05 versus E2+MS or E2+AS, n = 5–6.
Discussion
PELP1 was initially identified by our group as a coactivator of ERα in cancer cells, and it was suggested that PELP1 functions as a scaffolding protein to facilitate both genomic and extranuclear functions of ER. Further work revealed that PELP1 is expressed in many tissues, with the highest expression noted in the brain (25, 26). Despite it being highly expressed in the brain, virtually nothing is known regarding the role and importance of PELP1 in this key tissue. The current study provides the first in vivo molecular evidence, to our knowledge, that PELP1 is essential for optimal neuroprotective functions of E2. Our study implicates an important role of PELP1 in E2-mediated neuroprotective actions, including its role in rapid extranuclear signaling; anti-inflammatory effects; induction of survival signaling, including Wnt/β-catenin pathway; facilitation of E2 mediated neuroprotection; and improvement in cognitive function. Collectively, these findings suggest PELP1 is essential for optimal E2-mediated functions in the brain under conditions of ischemic injury.
E2 has been implicated to regulate multiple functions in the brain, including synaptic plasticity, adult neurogenesis, reproductive behavior, and cognition. Work from many laboratories, including our own, has provided abundant evidence that E2 serves as a neuroprotective factor in neurodegenerative disorders, including cerebral ischemia (4). Recent studies demonstrated that E2 mediates rapid extranuclear signaling by activating the neuroprotective ERK and PI3K signaling pathways, as well as the inhibition of proapoptotic JNK signaling (17, 22, 23); however, the mechanism or mechanisms by which E2 facilitates these activities are poorly understood. We previously showed that PELP1 is a multidomain scaffold protein that interacts with ERs, using the nuclear receptor interaction motif LXXLL; Src kinase, using SH2 motifs; and PI3K, using PXXP motifs. In the current study, proximity ligation assays confirmed complex formation of PELP1 with ERα, Src, and PI3K in the hippocampus after GCI, which was markedly enhanced by E2 treatment. The importance of this interaction was confirmed using a conditional PELP1 FBKO mouse model, which showed that PELP1 plays a key role in E2’s ability to induce robust activation of p-ERK and p-Akt signaling pathways, and to inhibit activation of the JNK signaling pathway in the hippocampus after GCI. It is important to note that antisense oligonucleotide knockdown of PELP1 in the rat further confirmed the PELP1 FBKO results by also demonstrating a critical role for PELP1 in E2-mediated kinase pathway activation, neuroprotection, and cognitive recovery after GCI.
GSK3β has been implicated in the pathology of many neurodegenerative disorders (41), and inhibition of GSK3β has been shown to be neuroprotective (11, 42). We previously showed that GSK3β inhibition leads to a reduction in Tau phosphorylation and regulates the stability of β-catenin by phosphorylation (11). In the current study, after GCI, we observed that E2 increased phosphorylation of GSK3β on residue Ser9, which is known to lead to its inactivation (43). However, E2’s ability to modulate phosphorylation of GSK3β is lost in PELP1 FBKO mice. GSK3β modulates the Wnt/β-catenin pathway via regulating the stability of β-catenin by phosphorylating at Ser33/37and Thr41 after priming by CK1α at Ser45. It has been shown that E2-mediated β-catenin stability occurs through an ERα–PI3K–Akt–GSK3β signaling cascade (44, 45). In this study, we found that E2 reduces the phosphorylation of β-catenin after GCI reperfusion in E2-treated FLOX control mice; however, in PELP1 FBKO mice, E2-mediated attenuation of β-catenin phosphorylation was abolished. These findings suggest that PELP1 scaffolding function is critical for formation of an ERα-PI3K-Akt-GSK3β-β-catenin multimolecular complex that leads to the activation of further downstream signaling (44, 46). PELP1 FBKO mice also had a higher induction of β-catenin phosphorylation in the hippocampus after GCI compared with FLOX control mice. This finding may explain, in part, our observation of a greater sensitivity of PELP1 FBKO mice to ischemic damage after GCI.
Mass spectrometry data revealed that PELP1 interacts with several proteins under conditions of GCI/reperfusion. These studies identified for the first time, to our knowledge, that GSK3β is one of the novel PELP1-interacting proteins. As a kinase, GSK3β phosphorylates several proteins in vivo, including β-catenin, and regulates a wide range of cellular processes such as cell growth, differentiation, and survival. GSK3β phosphorylates on the consensus sequence Ser/ThrXXXSer/Thr-P, where X is any residue (35, 36). PELP1 contains several GSK3β phosphorylation consensus sites, and our deletion studies identified that PELP1 is phosphorylated by GSK3β at hThr745 (m751) and hSer1059. Because Thr745 is conserved among many species, including mice, we generated a phospho-specific antibody for hThr745 (mThr751). Using this unique reagent (p745), we confirmed that E2 significantly reduced the phosphorylation of PELP1 at p745 by modulating GSK3β activation. Accordingly, proximity ligation assays demonstrated that the in vivo interaction of PELP1 and GSK3β was reduced after E2 treatment. Furthermore, our results also demonstrated that inhibition of GSK3β results in decreased phosphorylation of PELP1 at p745 and stabilization of PELP1 in vitro. We also observed reduction in PELP1 levels in the hippocampus after 6 d of GCI/reperfusion, which was reversed by E2 treatment. Finally, global profiling of phosphoproteins in the developing brain also identified phosphorylation of PELP1 at Thr745 in brain tissue, further confirming the physiological relevance of p745 of PELP1 in the brain (47). Collectively, these results establish PELP1 as a novel substrate of GSK3β and suggest the possibility of an autocrine loop involving E2-ERα-GSK3β signaling in modulating the levels of PELP1 at the site of neuronal damage.
Work in cancer cells has shown that PELP1 is an important coactivator of ERα that facilitates ERα target gene expression by modulating the activities of several chromatin modifiers, including histone acetyl transferase (creb-binding protein/p300) (26, 29), histone deacetylases (HDAC2) (48), and histone demethylase (KDM1A) (30). PELP1 is widely expressed in neuronal tissues, but little is known about PELP1-regulated genes and pathways in the brain. Our studies using global RNA-sequencing revealed several pathways that are directly or indirectly regulated by PELP1 in the presence of E2. The top PELP1-regulated pathways include several immunological, inflammatory, metabolic pathways and survival pathways. Interestingly, canonical and noncanonical Wnt pathways were also differentially expressed in FLOX control and PELP1 FBKO mice and are known to play vital roles in E2-mediated neuronal survival (11, 49). In an earlier RNA-seq study using in vitro breast epithelial model cells, we found that PELP1 has the potential to regulate multiple signaling pathways, including oxidative stress response, DNA repair, protein ubiquitination, and p53 (50). Our current RNA-seq results using PELP1 FBKO mouse tissues also confirm PELP1’s ability to modulate multiple pathways in the brain after ischemic injury, thus extending the list of PELP1-regulated genes to include immunological, inflammatory, and metabolic pathways.
Postischemic inflammation in the brain plays a key role in tissue injury and repair (51, 52). Several studies showed that apart from its neuroprotective functions, E2 also exhibits immunomodulatory effects (53, 54). The results from RNA-seq analysis conducted in this study showed that inflammatory pathways are among the top enriched pathways that are differentially expressed between E2-treated FLOX control and PELP1 FBKO mice after GCI. Robust activation of chemokines and cytokines contributes either to cerebral damage or neuroprotection, depending on their concentration and time of release (55–58). Previous studies showed that E2 could inhibit production of the neutrophil chemoattractants CXCL1, CXCL2, and CXCL3, and secretion of proinflammatory cytokines, such as IL-1β, IL-6, and TNFα (53, 54). Our RNA-seq analysis demonstrated that several chemokines, including CXCL5 and CXCR4, were up-regulated, whereas ccl9, ccl2, ccl7, CXCL10, and MMP12 were down-regulated in PELP1 KO mice. In addition, we also found that interleukin-1 receptor antagonist (IL1RN) was down-regulated in PELP1 FBKO mice. However, additional detailed studies are needed to better understand the role of PELP1 in modulating genes involved in inflammation in the ischemic brain. In conclusion, the current study provides the first direct in vivo evidence, to our knowledge, of an essential role for PELP1 in E2-mediated rapid extranuclear signaling, genomic signaling, neuroprotection, and cognitive function in the brain. As such, it significantly advances our understanding of how E2 exerts its important actions in the brain and sets the stage for future studies to further unlock the mysteries of this key hormone.
Materials and Methods
Details regarding animals, PELP1 FBKO mouse generation, antibodies and reagents, two-vessel occlusion global cerebral ischemia animal model, E2 replacement, histology, immunohistochemistry, mass spectrometry, immuno-precipitation, confocal analysis, Western blot analysis, RNA Seq and qRT-PCR, Barnes maze, and statistical analysis are contained in SI Materials and Methods. All procedures used were approved by the University of Texas Health Science Center at San Antonio (UTHSCSA) Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health guidelines for animal research.
Materials and Methods
Generation of Forebrain-Specific PELP1 Knockout Mice.
To test the role of PELP1 in the regulation of E2 signaling, we have created forebrain-neuron-specific PELP1 knockout mice. Transgenic mice expressing a floxed allele of PELP1 in C57BL/6 background were generated by Taconic/Lexicon, using a homologous recombination system. In this model, PELP1 was targeted by inserting two loxP sites flanking exon 1. Removal of exon 1 causes a shift in the ORF, leading to a stop termination codon within exon 2 sequences. Target vector (pKOS-19) was electroporated into ES cells, and targeted ES cells (two different clones) were then injected into C57BL/6 blastocysts, using standard protocols. Targeted clones were confirmed by Southern blotting of genomic DNA digested with EcoRV and PstI. Chimera males were bred with C57BL/6 females and identified two independent clones that gave germline transmission of the targeted allele. To conditionally delete PELP1 in the forebrain neurons, we crossed PELP1loxp/loxp mice with CaMKIIα-Cre mice (Jackson Laboratory) that express Cre enzyme under the control of a Ca2+/calmodulin-dependent protein kinase II alpha (CaMKIIα) promoter that uniquely expresses Cre exclusively in forebrain excitatory neurons (59). Because of the potential for recombination in sertoli cells of CaMKIIα-Cre-positive mice (60), needed PELP1 FBKO mice were generated by breeding CaMKIIα-Cre-positive females (CaMKIIα-Cre+/−;PELP1loxp/loxp) with CaMKIIα-Cre negative males (PELP1loxp/loxp). Mouse genotyping was carried out using tail digestion, followed by genomic DNA PCR.
Global Cerebral Ischemia.
Four- to 6-mo-old FLOX and PELP1 FBKO were bilaterally ovariectomized 1 wk before performance of global cerebral ischemia. Placebo or E2 Alzet minipumps (Alzet osmotic minipumps; model 1007D, 7-d release; Durect Corporation) were implanted s.c. in the upper midback region at the time of ovariectomy. The dose of E2 used produces serum E2 levels of 10–15 pg/mL, which represents physiological low diestrus I levels of E2 (11). For cerebral ischemia, all animals (excepting sham control) underwent two-vessel occlusion global cerebral ischemia, as described previously (11). Briefly, animals were anesthetized using ketamine/xylazine, and both common carotid arteries of the mice were separated and occluded, using clips for 40 min. The clips were then removed, and the blood flow through the arteries was confirmed before the wound was sutured. Rectal temperature was maintained at 36.5–37.5 °C throughout the procedure, using a heat pad. The animals in the sham group underwent identical procedures, except the common carotid arteries were simply exposed, with no occlusion.
Preparation of Tissue Lysates and Western Blotting.
Mice were killed under anesthesia at various points, as detailed in the experiments. Whole brains were removed, and the hippocampal tissues were microdissected from both sides of the hippocampal fissure and snap frozen immediately in liquid nitrogen. For PELP1 knockout confirmation, hippocampus, cerebral cortex, and cerebellum were isolated. Tissues were homogenized in Tissue Protein Extraction Reagent (Thermo Scientific) containing protease and phosphatase inhibitors, according to the manufacturer's protocol. Protein concentrations were determined using the DC protein assay (BioRad), and the samples were stored at −80 °C until use. Equal amounts of total protein extracts were resolved using 4–12% Bis-Tris polyacrylamide gels (Biorad). The resolved proteins were transferred to a nitrocellulose membrane and probed with indicated antibodies and developed using an enhanced chemiluminescence kit (Thermo Scientific). Band intensities were quantified using ImageJ analysis software (version 1.30v; Wayne Rasband, National Institutes of Health), and band intensities for the indicated phosphoproteins were corrected for variations in loading and normalized to the corresponding band intensity for total protein signals, respectively. PELP1 antibodies were purchased from Bethyl Laboratories. p-GSK3β, GSK3β, p-ERK1/2, ERK, p-AKT, AKT, p-JNK1/2, JNK, p-β-catenin, β-catenin, β-Actin, and GAPDH were purchased from Cell Signaling Technology. The p745-PELP1-phospho antibody was custom generated and affinity purified by Open Biosystems (Thermo-Fisher Scientific), using phospho-PELP1 Thr-745 peptide sequence (LAPSGpTPPP).
Mass Spectrometry, Immunoprecipitation, and GST Pull-Down Assay.
Hippocampal tissues were collected 24 h after GCI reperfusion, and cytoplasmic fractions were isolated using a compartment protein extraction kit according to manufacturer’s instructions (EMD Millipore). Hippocampal lysates were immunoprecipitated with IgG or PELP1 antibodies, and PELP1 interacting proteins were analyzed by mass spectrometry (UTHSCSA core facility). Forebrain lysates were prepared using TritonX100-lysis buffer (50 mM Tris⋅HCl at pH 7.5, 0.2% Triton X-100, 0.3% Nonidet P-40, 150 mM NaCl, 25 mM NaF, 0.1 mM sodium orthovanadate) containing phosphatase and protease inhibitor mixture. For confirming PELP1-GSK3β interaction, lysates were immunoprecipitated with PELP1 antibody, followed by Western blot analysis. For GST pulldown assays, lysates were incubated with various PELP1-GST deletion fragments, bound proteins were isolated by GST-pull-down assay, and the interaction of PELP1 with GSK3β was analyzed by Western analysis.
GSK3β Phosphorylation Assay.
In vitro kinase assay was performed using the kinase buffer (60 mM Hepes⋅NaOH at pH 7.5, 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT), 10 μCi [γ32-P] ATP, 100 μM cold ATP, and 100 ng GSK3β enzyme (New England Biolabs). GST- or GST-tagged PELP1 deletions, as well as full-length PELP1, were used as substrates (∼2 μg) for the in vitro GSK3β kinase assays. Each reaction was carried out for 30 min at 30 °C and was stopped by the addition of 10 μL of 4× SDS sample buffer. Phosphorylation was analyzed by a phosphoimager.
Duolink Proximity Ligation Assay.
Tissue sections were prepared as described previously (11). Tissue sections were blocked in 5% (vol/vol) goat serum for 1 h and incubated overnight with appropriate primary antibodies at 4 °C. Then, slides were incubated with Duolink PLA Rabbit MINUS and PLA Mouse PLUS proximity probes for 1 h at 37 °C. Ligation and amplification were carried out using the Duolink in situ detection reagent kit (Sigma-Aldrich) according to the manufacturer’s protocol. Images were captured under confocal microscope.
Histological Analysis.
Histological examination of the ischemic brains was carried out as described previously (11). Briefly, 6 d after the GCI, mice were deeply anesthetized with isoflurane and subjected to transcardial perfusion with 0.9% saline followed by 4% (wt/vol) paraformaldehyde. Brains were isolated and further fixed in paraformaldehyde overnight, followed by incubation in 30% (wt/vol) sucrose until they sank and were embedded in OCT compound. Frozen sections of 20-µm thickness each were cut in series, using cryotome in the coronal plane of the dorsal hippocampus (∼2.5–4.5 mm posterior from the bregma). NeuN staining was performed using a mouse anti-NeuN monoclonal antibody (1:500; Millipore Bioscience), as described previously (11). Images were captured on an LSM510 Meta confocal laser microscope (Carl Zeiss). The intact NeuN-positive pyramidal cells with round nuclei were counted as surviving neurons. Quantitative analysis was performed by counting the number of surviving neurons per 250-µm length of medial CA1 pyramidal cell layer bilaterally in three to five representative sections per mouse. The cell counts from the right and left hippocampus were then averaged to provide a single value for each section, and the counts of five sections were averaged to provide a single value for each animal. Mean ± SE was calculated from the data in each group.
RNA Sequencing and qRT-PCR.
Total RNA was isolated from ovariectomized FLOX and PELP1 FBKO mice (implanted with E2 mini pumps) that were subjected to GCI for 40 min, followed by 24 h reperfusion, using an RNAesy mini kit (Qiagen) according to manufacturer’s instructions. Illumina TruSeq RNA Sample Preparation was performed following manufacturer’s protocol. Samples were run on an Illumina HiSEq. 2000 in duplicate. The combined raw reads were aligned to UCSC hg19, and genes were annotated by Tophat (61). Genes were annotated and quantified by HTSeq-DESeq pipeline (62). Differential expression analysis was performed by DEseq, and significant genes with at least twofold change with P < 0.01 were chosen for analysis. Interpretation of biological pathways using RNA-seq data were performed with ingenuity pathway analysis software, using all significant and differentially expressed genes. RNAseq data have been deposited in the Gene Expression Omnibus database under accession number GSE72136. To validate the selected genes, reverse transcription reactions were performed by using SuperScript III First Strand kit (Invitrogen) according to manufacturer’s protocol. Real-time PCR was done using SybrGreen on an Illumina Real-Time PCR system with the following primers:
SFRP5 F: CACTGCCACAAGTTCCCCC, R: TCTGTTCCATGAGGCCATCAG
WNT2 F: CTCGGTGGAATCTGGCTCTG, R: CACATTGTCACACATCACCCT
GREM1 F: CTGGGGACCCTACTGCCAA, R: TTTGCACCAATCTCGCTTCAG
GJA1 F: AATTTACTGGGTGGCATCCTAGA, R: GGGAAAGCATCATCGTAACAGAT
SDC1 F: CTTTGTCACGGCAGACACCTT, R: GACAGAGGTAAAAGCAGTCTCG
CXCL5 F: GTTCCATCTCGCCATTCATGC, R: GCGGCTATGACTGAGGAAGG
CXCR4 F: GACTGGCATAGTCGGCAATG, R: AGAAGGGGAGTGTGATGACAAA
IL1RN F: GCTCATTGCTGGGTACTTACAA, R: CCAGACTTGGCACAAGACAGG
CTSE F: GACATCAGTCCCTTCGGAAGA, R: AGGGGTTCATTGACACTCGAATA
JUB F: TTAGGGGAGAAAGCCAGTCGT, R: GGCCGGTTCCAAAGGTTCAT
MAP3K6 F: GCCTCTCAGTGTGGTCTACG, R: CGTCGCAAAGGGTAGGCTG
ITGAM F: ATGGACGCTGATGGCAATACC, R: TCCCCATTCACGTCTCCCA
PLTP F: CGCAAAGGGCCACTTTTACTA, R: GCCCCCATCATATAAGAACCAG
PLCB2 F: TGCTGATCGAAAACGGGTGG, R: AGCTTTAGAGTGGTAGGAAGTGA
β-ACTIN F: GTGGGCCGCTCTAGGCACCAA, R: CTCTTTGATGTCACGCACGATTTC
Immunofluorescence Staining.
Briefly, tissue sections were incubated with blocking solution [10% (vol/vol) normal donkey serum in PBS containing 0.1% Triton X-100] for 1 h at room temperature, followed by overnight incubation with appropriate primary antibodies. Sections were then incubated with respective Alexa Fluor 594/647/488 donkey anti-mouse/rabbit/goat secondary antibodies (1:500; Invitrogen) for 1 h at room temperature. After washes, sections were mounted with water-based mounting medium containing antifading agents. Images were captured on an LSM510 Meta confocal laser microscope (Carl Zeiss), using either a 5× or 40× oil immersion Neofluor objective (NA, 1.3) with the image size set at 1,024 × 1,024 pixels. The captured images were viewed and analyzed using LSM510 Meta imaging software. At least five representative sections per animal were used for immunostaining.
Barnes Maze.
Cognitive deficits in spatial learning and memory were tested with the Barnes maze test, as previously described (40). The acquisition period (learning phase) involved three trials per day, with an intertrial interval of 25 min for 5 consecutive days. For all trials, the test mouse was placed individually under an opaque start box in the center of the maze for 10 s, which was then removed to initiate the acquisition process. Each mouse was allowed to freely run on the platform for 3 min, which was recorded by the digital video camera from overhead. Each trial ended when the test mouse entered the burrow via the target hole, or after completion of 3 min. At the end of trial, the hole was covered and the mouse was allowed to remain in a hole for 1 min. If the test mouse did not find the hole by the end of the trial, it was gently guided into the burrow and left undisturbed for 1 min. The maze platform was cleaned with 70% (vol/vol) ethanol after the completion of each trail between mice. Probe trial was conducted 24 h after the final day of acquisition trail. During probe trial, the escape burrow was removed and was replaced with an identical false bottom, and each mouse was allowed to explore the maze platform for 90 s. The primary latency (time taken to reach the target hole) and path length (distance) traveled to find the correct target hole, and duration of the probe trial spent in each quadrant, were measured using Ethovision XT tracking software (Noldus). Sampling distribution (errors) was measured manually by a trained observer.
Statistics.
GraphPad Prism 6 software (GraphPad Software) was used to analyze all data. All the data represented in bar graphs shown as mean ± SEM t-test were performed for all pairwise comparisons in the behavioral and Western blot quantitations. A value of P < 0.05 was considered statistically significant. The multiple groups’ statistical data were analyzed with one-way ANOVA. RNA-seq data were analyzed using ingenuity pathway analysis software.
Acknowledgments
We thank Dr. Sujit S. Nair and Dr. Binoj C. Nair for their help in the initial establishment of floxed mouse line. We thank core facilities at the University of Texas Health Science Center at San Antonio for completing RNA-seq and mass spectrometry analysis. This research was supported by Research Grant NS050730 from the National Institutes of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE72136).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516729112/-/DCSupplemental.
References
- 1.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids. 2007;72(5):381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: Multiple cellular pathways of neuroprotection. Steroids. 2009;74(7):555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpkins JW, Singh M, Brock C, Etgen AM. Neuroprotection and estrogen receptors. Neuroendocrinology. 2012;96(2):119–130. doi: 10.1159/000338409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16(1):17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 5.Alkayed NJ, et al. Gender-linked brain injury in experimental stroke. Stroke. 1998;29(1):159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SJ, McCullough LD, Smith JM. Stroke in the female: Role of biological sex and estrogen. ILAR J. 2004;45(2):147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- 7.Niewada M, Kobayashi A, Sandercock PA, Kamiński B, Członkowska A. International Stroke Trial Collaborative Group Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24(3):123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 8.Di Carlo A, et al. European BIOMED Study of Stroke Care Group Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: Data from a multicenter multinational hospital-based registry. Stroke. 2003;34(5):1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 9. Merchenthaler I, Dellovade TL, Shughrue PJ (2003) Neuroprotection by estrogen in animal models of global and focal ischemia. Ann N Y Acad Sci 1007:89–100. [DOI] [PubMed]
- 10.Simpkins JW, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87(5):724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 11. Zhang QG, Wang R, Khan M, Mahesh V, Brann DW (2008) Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-inducedneuroprotection and attenuation of tau phosphorylation. J Neurosci 28(34):8430–8441. [DOI] [PMC free article] [PubMed]
- 12.Spencer JL, et al. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebesgue D, et al. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5(1):e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146(7):3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- 15.Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: Regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA. 2003;100(5):2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010(1-2):22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 17.Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16(2-3):140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- 18.Hammes SR, Levin ER. Extranuclear steroid receptors: Nature and actions. Endocr Rev. 2007;28(7):726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 19.Yang LC, et al. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One. 2010;5(5):e9851. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jover-Mengual T, et al. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res. 2010;1321:1–12. doi: 10.1016/j.brainres.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: A unified mechanism of estrogen action. J Neurosci. 2006;26(37):9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 23.Tang H, et al. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol Cell Endocrinol. 2014;387(1-2):52–58. doi: 10.1016/j.mce.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103(6):843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 25.Brann DW, Zhang QG, Wang RM, Mahesh VB, Vadlamudi RK. PELP1--a novel estrogen receptor-interacting protein. Mol Cell Endocrinol. 2008;290(1-2):2–7. doi: 10.1016/j.mce.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vadlamudi RK, et al. Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem. 2001;276(41):38272–38279. doi: 10.1074/jbc.M103783200. [DOI] [PubMed] [Google Scholar]
- 27.Gonugunta VK, et al. The social network of PELP1 and its implications in breast and prostate cancers. Endocr Relat Cancer. 2014;21(4):T79–T86. doi: 10.1530/ERC-13-0502. [DOI] [PubMed] [Google Scholar]
- 28.Girard BJ, Daniel AR, Lange CA, Ostrander JH. PELP1: A review of PELP1 interactions, signaling, and biology. Mol Cell Endocrinol. 2014;382(1):642–651. doi: 10.1016/j.mce.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair SS, et al. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004;64(18):6416–6423. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- 30.Nair SS, et al. PELP1 is a reader of histone H3 methylation that facilitates oestrogen receptor-alpha target gene activation by regulating lysine demethylase 1 specificity. EMBO Rep. 2010;11(6):438–444. doi: 10.1038/embor.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan MM, et al. Cloning, expression, and localization of MNAR/PELP1 in rodent brain: Colocalization in estrogen receptor-alpha- but not in gonadotropin-releasing hormone-positive neurons. Endocrinology. 2005;146(12):5215–5227. doi: 10.1210/en.2005-0276. [DOI] [PubMed] [Google Scholar]
- 32.Llorens-Martín M, Jurado J, Hernández F, Avila J. GSK-3β, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. 2014;7:46. doi: 10.3389/fnmol.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valerio A, et al. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J Neurochem. 2011;116(6):1148–1159. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- 34.Chuang DM, Wang Z, Chiu CT. GSK-3 as a Target for Lithium-Induced Neuroprotection Against Excitotoxicity in Neuronal Cultures and Animal Models of Ischemic Stroke. Front Mol Neurosci. 2011;4:15. doi: 10.3389/fnmol.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutherland C. What Are the bona fide GSK3 Substrates? Int J Alzheimers Dis. 2011;2011:505607. doi: 10.4061/2011/505607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C, Kim NG, Gumbiner BM. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle. 2009;8(24):4032–4039. doi: 10.4161/cc.8.24.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer U, Preiss F, Brauns-Schubert P, Schlicher L, Charvet C. GSK-3 - at the crossroads of cell death and survival. J Cell Sci. 2014;127(Pt 7):1369–1378. doi: 10.1242/jcs.138057. [DOI] [PubMed] [Google Scholar]
- 38.Sehara Y, et al. Survivin Is a transcriptional target of STAT3 critical to estradiol neuroprotection in global ischemia. J Neurosci. 2013;33(30):12364–12374. doi: 10.1523/JNEUROSCI.1852-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang QG, et al. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci USA. 2011;108(35):E617–E624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, et al. Cognitive dysfunction precedes the onset of motor symptoms in the MitoPark mouse model of Parkinson’s disease. PLoS One. 2013;8(8):e71341. doi: 10.1371/journal.pone.0071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petit-Paitel A. [GSK-3beta: A central kinase for neurodegenerative diseases?] Med Sci (Paris) 2010;26(5):516–521. doi: 10.1051/medsci/2010265516. [DOI] [PubMed] [Google Scholar]
- 42.Zhou C, et al. Delayed ischemic postconditioning protects hippocampal CA1 neurons by preserving mitochondrial integrity via Akt/GSK3β signaling. Neurochem Int. 2011;59(6):749–758. doi: 10.1016/j.neuint.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 44.Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci. 2004;25(3):363–373. doi: 10.1016/j.mcn.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Pérez-Álvarez MJ, Maza MdelC, Anton M, Ordoñez L, Wandosell F. Post-ischemic estradiol treatment reduced glial response and triggers distinct cortical and hippocampal signaling in a rat model of cerebral ischemia. J Neuroinflammation. 2012;9:157. doi: 10.1186/1742-2094-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol. 2006;69(4):1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- 47.Ballif BA, Villén J, Beausoleil SA, Schwartz D, Gygi SP. Phosphoproteomic analysis of the developing mouse brain. Mol Cell Proteomics. 2004;3(11):1093–1101. doi: 10.1074/mcp.M400085-MCP200. [DOI] [PubMed] [Google Scholar]
- 48.Choi YB, Ko JK, Shin J. The transcriptional corepressor, PELP1, recruits HDAC2 and masks histones using two separate domains. J Biol Chem. 2004;279(49):50930–50941. doi: 10.1074/jbc.M406831200. [DOI] [PubMed] [Google Scholar]
- 49.Varea O, et al. Interaction of estrogen receptors with insulin-like growth factor-I and Wnt signaling in the nervous system. Steroids. 2010;75(8-9):565–569. doi: 10.1016/j.steroids.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Mann M, Zou Y, Chen Y, Brann D, Vadlamudi R. PELP1 oncogenic functions involve alternative splicing via PRMT6. Mol Oncol. 2014;8(2):389–400. doi: 10.1016/j.molonc.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becker KJ. Modulation of the postischemic immune response to improve stroke outcome. Stroke. 2010;41(10) Suppl:S75–S78. doi: 10.1161/STROKEAHA.110.592881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamel H, Iadecola C. Brain-immune interactions and ischemic stroke: Clinical implications. Arch Neurol. 2012;69(5):576–581. doi: 10.1001/archneurol.2011.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nadkarni S, McArthur S. Oestrogen and immunomodulation: New mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol. 2013;13(4):576–581. doi: 10.1016/j.coph.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Petrone AB, Simpkins JW, Barr TL. 17β-estradiol and inflammation: Implications for ischemic stroke. Aging Dis. 2014;5(5):340–345. doi: 10.14336/AD.2014.0500340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Briones TL, Woods J, Wadowska M. Chronic neuroinflammation and cognitive impairment following transient global cerebral ischemia: Role of fractalkine/CX3CR1 signaling. J Neuroinflammation. 2014;11:13. doi: 10.1186/1742-2094-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Ceulemans AG, et al. The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypothermia. J Neuroinflammation. 2010;7:74. doi: 10.1186/1742-2094-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gan Y, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci USA. 2014;111(7):2704–2709. doi: 10.1073/pnas.1315943111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mirabelli-Badenier M, et al. CC and CXC chemokines are pivotal mediators of cerebral injury in ischaemic stroke. Thromb Haemost. 2011;105(3):409–420. doi: 10.1160/TH10-10-0662. [DOI] [PubMed] [Google Scholar]
- 59.Tsien JZ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87(7):1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 60.Latapy C, Rioux V, Guitton MJ, Beaulieu JM. Selective deletion of forebrain glycogen synthase kinase 3β reveals a central role in serotonin-sensitive anxiety and social behaviour. Philos Trans R Soc Lond B Biol Sci. 2012;367(1601):2460–2474. doi: 10.1098/rstb.2012.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trapnell C, Pachter L, Salzberg SL. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106–R111. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]