Significance
Previous studies demonstrate that endogenous wingless-type MMTV integration site family (WNT)16 is a crucial regulator of cortical bone mass. Surprisingly, we demonstrate that overexpression of WNT16 increases mainly trabecular bone mass. Both estrogen and WNT16 are crucial regulators of bone mass, but the possible interaction between WNT16-signaling and estrogen-signaling is unknown. To determine the possible interaction between WNT16 and estrogen signaling in bone, we developed and used two genetically modified mouse models with either constantly high osteoblastic Wnt16 expression or no Wnt16 expression. We demonstrated that the bone-sparing effects of estrogen and WNT16 are independent of each other. As WNT16 signaling in bone does not require normal estrogen action, we propose that WNT16-targeted therapies might be useful for treatment of postmenopausal trabecular bone loss.
Keywords: WNT16, estrogen, cortical bone, trabecular bone, transgenic mice
Abstract
Wingless-type MMTV integration site family (WNT)16 is a key regulator of bone mass with high expression in cortical bone, and Wnt16−/− mice have reduced cortical bone mass. As Wnt16 expression is enhanced by estradiol treatment, we hypothesized that the bone-sparing effect of estrogen in females is WNT16-dependent. This hypothesis was tested in mechanistic studies using two genetically modified mouse models with either constantly high osteoblastic Wnt16 expression or no Wnt16 expression. We developed a mouse model with osteoblast-specific Wnt16 overexpression (Obl-Wnt16). These mice had several-fold elevated Wnt16 expression in both trabecular and cortical bone compared with wild type (WT) mice. Obl-Wnt16 mice displayed increased total body bone mineral density (BMD), surprisingly caused mainly by a substantial increase in trabecular bone mass, resulting in improved bone strength of vertebrae L3. Ovariectomy (ovx) reduced the total body BMD and the trabecular bone mass to the same degree in Obl-Wnt16 mice and WT mice, suggesting that the bone-sparing effect of estrogen is WNT16-independent. However, these bone parameters were similar in ovx Obl-Wnt16 mice and sham operated WT mice. The role of WNT16 for the bone-sparing effect of estrogen was also evaluated in Wnt16−/− mice. Treatment with estradiol increased the trabecular and cortical bone mass to a similar extent in both Wnt16−/− and WT mice. In conclusion, the bone-sparing effects of estrogen and WNT16 are independent of each other. Furthermore, loss of endogenous WNT16 results specifically in cortical bone loss, whereas overexpression of WNT16 surprisingly increases mainly trabecular bone mass. WNT16-targeted therapies might be useful for treatment of postmenopausal trabecular bone loss.
Both estrogen and wingless-type MMTV integration site family (WNT)16 are crucial regulators of bone mass in women (1–5). The bone-sparing effect of estrogen is primarily mediated via estrogen receptor-α (ERα) (6). Estrogen-deficiency leads to rapid bone loss and contributes significantly to the development of postmenopausal osteoporosis that can be prevented by estradiol treatment. However, this treatment is associated with side effects such as breast cancer and thromboembolism (7, 8).
The WNTs are a family of secreted glycoproteins that consists of 19 members in mammals, and which mediates autocrine and paracrine effects by binding to frizzled (Fzd) receptors and LDL-related protein 5/6 (LRP5/6) coreceptors (9). During the last decade, several lines of clinical and preclinical evidence have indicated that WNT signaling is critical in bone development and in the regulation of adult bone homeostasis (10–20) and modulation of WNT signaling has emerged as a promising strategy for increasing bone mass (21–23). Crosstalk and synergy between ERα signaling and the WNT pathways have been described (24–26). In the brain, estrogen signaling activates WNT by down-regulating dickkopf-1 (Dkk1), a WNT antagonist, to prevent neurodegeneration (27). In the uterus, estrogen prompts the canonical WNT signaling pathway in the uterine epithelium to induce uterine epithelial cell growth (28), and in breast cancer, ERα activation enhances cell growth via WNT signaling (29).
Human genetic studies followed by subsequent mechanistic studies have recently revealed that WNT16 is a key physiological regulator of cortical bone mass and nonvertebral fracture risk (4, 5, 30–33). We recently demonstrated that WNT16 is osteoblast-derived and inhibits osteoclastogenesis both directly by acting on osteoclast progenitors and indirectly by increasing expression of osteoprotegerin (Opg) in osteoblasts (3). WNT16 is highly expressed in cortical bone but, for unknown reasons, only moderately expressed in trabecular bone. Experiments using WNT16-deleted mouse models clearly show that absence of WNT16 results in reduced cortical but not trabecular bone mass (3, 4, 30, 34). However, the effects of pharmacologically increased WNT16 levels on the cortical and trabecular bone compartments are unexplored. Furthermore, the role of WNT16 for the bone-sparing effect of estrogen is unknown. Herein we first demonstrate that the expression of WNT16 is enhanced by estradiol treatment, and we therefore hypothesized that the bone-sparing effects of estrogen are WNT16-mediated. This hypothesis was tested in mechanistic studies using two different genetically modified mouse models in which estrogen does not have the capacity to regulate WNT16 levels. Contradicting our hypothesis, we demonstrate that the bone-sparing effects of estrogen and WNT16 are independent of each other. In addition, unexpectedly, we demonstrate that overexpression of WNT16 increases mainly trabecular bone mass.
Results
Overexpression of Wnt16 in Osteoblasts Substantially Increases Trabecular Bone Mass.
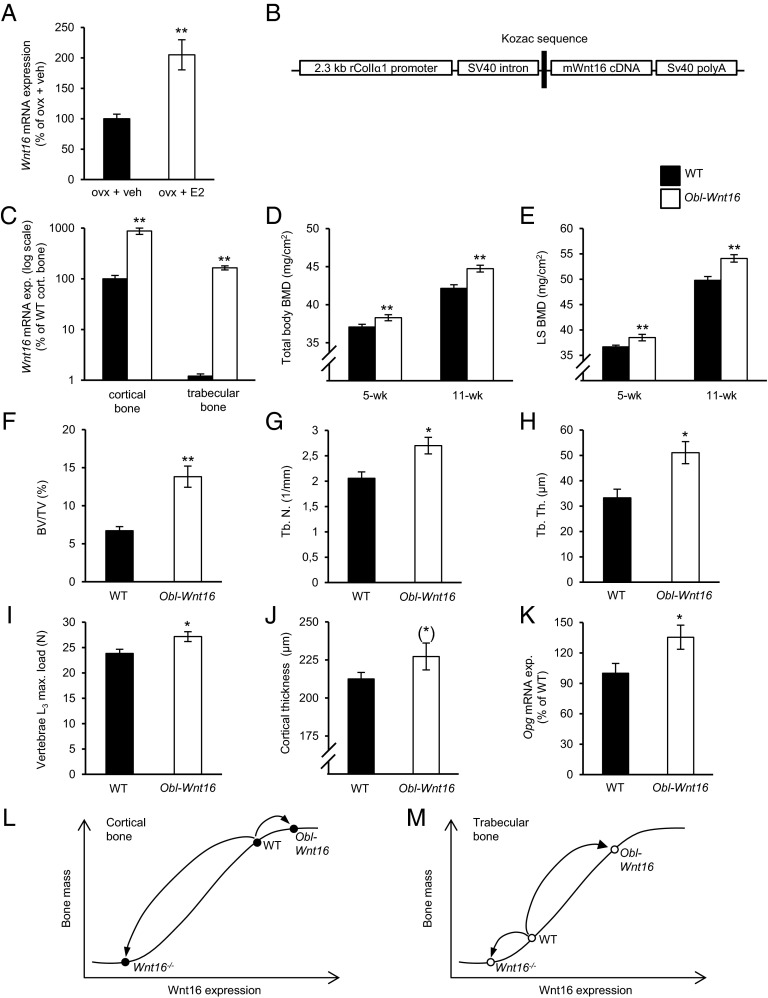
We initially observed that Wnt16 expression in bone is enhanced by estradiol treatment in ovx female mice (Fig. 1A) and, therefore, we hypothesized that the bone-sparing effects of estrogen are WNT16-dependent. We first aimed to test this hypothesis in a mouse model with constant high Wnt16 levels in bone. To this end we developed a transgenic mouse model (Obl-Wnt16) with osteoblast-specific Wnt16 overexpression under the control of a 2.3-kb fragment of the rat type I α 1 procollagen promoter (Fig. 1 B–K and Tables S1–S4). The Obl-Wnt16 mice displayed a specific and high overexpression of Wnt16 in bone (Fig. 1C). The endogenous moderate Wnt16 expression in trabecular bone was elevated 140-fold (P < 0.01), whereas the endogenous high Wnt16 expression in cortical bone was increased 9-fold (P < 0.01) in Obl-Wnt16 mice compared with WT mice, but still the Wnt16 expression was higher in cortical bone than in trabecular bone in Obl-Wnt16 mice (Fig. 1C). There was no change in Wnt16 expression in the uterus in the Obl-Wnt16 mice compared with WT mice (P = 0.3). The Obl-Wnt16 mice were born at a normal Mendelian distribution, were apparently healthy and had unaffected tibia length, body weight and weights of several visceral organs compared with WT mice (Fig. 2A and Table S1).
Fig. 1.
Overexpression of Wnt16 in osteoblasts substantially increases trabecular bone mass. (A) Wnt16 mRNA expression in humerus of 16-wk-old ovariectomized wild-type (WT) mice after 4 wk of estradiol (E2, 167 ng/mouse/d) or vehicle (veh) treatment. **P < 0.01; vs. ovx + veh using Student’s t test. (B) Structure of the transgene used to overexpress the mouse Wnt16 cDNA in the osteoblasts under the control of a 2.3-kb fragment of the rat Col-Iα1 promoter (Obl-Wnt16 mice). (C) Bone-specific overexpression of Wnt16 mRNA in 16-wk-old female Obl-Wnt16 mice compared with WT mice (n = 8). (D and E) Total body (D) and lumbar spine (LS; E) bone mineral density (BMD) as measured by DXA in 5- and 11-wk-old female Obl-Wnt16 mice and WT mice (n = 15–16). (F–H) Bone volume per total volume (BV/TV; F), trabecular number (Tb.N; G), and trabecular thickness (Tb.Th; H) of the distal metaphyseal region of femur in 16-wk-old female Obl-Wnt16 mice and WT mice as measured by histomorphometry (n = 8). (I) Maximal load at failure of compression test of L3 vertebrae of 16-wk-old female Obl-Wnt16 mice and WT mice (n = 15–19). (J) Cortical thickness of femur of 16-wk-old female Obl-Wnt16 mice and WT mice as measured by histomorphometry (*P = 0.17, n = 8). (K) Osteoprotegerin (Opg) mRNA expression in the trabecular bone of 16-wk-old female Obl-Wnt16 mice and WT mice as measured by real-time PCR. All values are given as mean ± SEM (C–K) **P < 0.01, *P < 0.05, Student’s t test compared with WT. (L–M) Proposed dose–response curve between Wnt16 expression and bone mass in cortical and trabecular bone, respectively.
Table S1.
Body characteristics
| Characteristic | WT | Obl-Wnt16 |
| Body weight, g | 20.6 ± 0.4 | 21.6 ± 0.4 |
| Liver, mg | 935 ± 24 | 986 ± 26 |
| Gonadal fat, mg | 265 ± 19 | 244 ± 27 |
| Uterus, mg | 59.9 ± 6.4 | 67.3 ± 9.6 |
Body characteristics of 16-wk-old female Obl-Wnt16 and wild-type (WT) mice (n = 8). Values are given as mean ± SEM.
Table S4.
Serum markers of bone metabolism
| Serum markers | WT | Obl-Wnt16 |
| Osteocalcin, ng/mL | 82.0 ± 7.4 | 70.3 ± 5.9 |
| CTX, ng/mL | 24.7 ± 1.5 | 29.3 ± 3.4 |
| TRAP5b, U/L | 7.6 ± 0.3 | 8.1 ± 0.3 |
Serum analyses of 16-wk-old female Obl-Wnt16 and wild-type (WT) mice (n = 8). Values are given as mean ± SEM. CTX, C-terminal type I collagen; TRAP5b, tartrate-resistant acid phosphatase 5b.
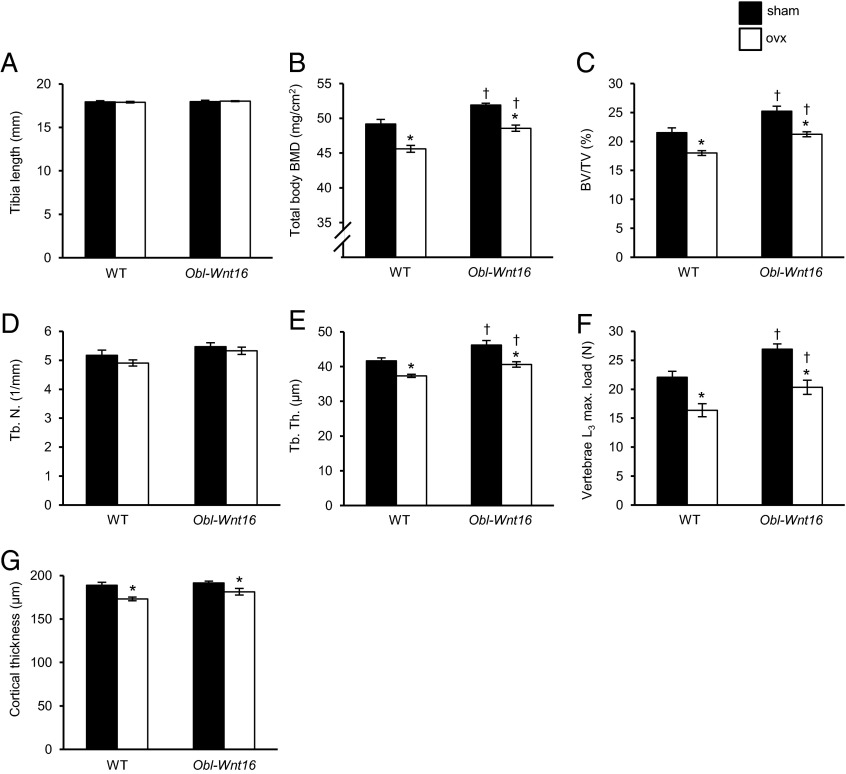
Fig. 2.
The bone-sparing effects of estrogen and WNT16 are mediated via independent mechanisms, as evaluated in the Obl-Wnt16 mouse model. (A and B) Tibia length (A) and total body bone mineral density (BMD; B) as measured by DXA. (C–E) Bone volume per total volume (BV/TV; C), trabecular number (Tb.N; D), and trabecular thickness (Tb.Th; E) of vertebrae L4 as measured by micro-CT. (F and G) Maximal load at failure of compression test of L3 vertebrae (F) and cortical thickness of femur (G), of 16-wk-old ovariectomized (ovx) female Obl-Wnt16 mice and wild-type (WT) mice compared with sham mice (n = 7–8). Values are given as mean ± SEM *P < 0.05 vs. sham; †P < 0.05 vs. WT using Student’s t test.
DXA analysis revealed that the Obl-Wnt16 mice had increased total body and lumbar spine (LS) BMD compared with WT mice both before (5-wk-old) and after (11- and 16-wk-old) sexual maturation (P < 0.05; Figs. 1 D and E and 2B). The trabecular and cortical bone compartments were analyzed separately at 16 wk of age using high-resolution µCT and histomorphometry. The trabecular bone volume fraction (BV/TV) was substantially increased (+106%, P < 0.01) in the distal metaphyseal region of femur in Obl-Wnt16 mice compared with WT mice and this was the result of both increased trabecular number and thickness (Fig. 1 F–H). The trabecular BV/TV was also increased in the vertebrae of Obl-Wnt16 mice (Fig. 2C). To evaluate whether the increased trabecular bone mass in Obl-Wnt16 mice resulted in increased bone strength, L3 vertebra was evaluated using a compression test, demonstrating that the maximal load at failure was significantly increased in Obl-Wnt16 mice compared with WT mice (Fig. 1I). In contrast, there was only a nonsignificant tendency of increased cortical bone thickness in Obl-Wnt16 mice compared with control mice (+6.9%, P = 0.17; Fig. 1J). Surprisingly, this demonstrates that overexpression of Wnt16 in osteoblasts increases bone mass mainly as a result of substantially increased trabecular bone mass.
The mRNA level of the anti-osteoclastogenic factor Opg was increased in the vertebral body of vertebrae L6, which is a bone with relatively high trabecular bone content, in Obl-Wnt16 mice compared with control mice (P < 0.05, Fig. 1K). However, no consistent significant effect on bone formation or bone resorption was observed in Obl-Wnt16 mice compared with WT mice when bone turnover was evaluated in thorough studies using (i) static histomorphometry of osteoclast number in trabecular bone of the distal femur metaphysis and vertebrae L5 (Table S2), (ii) dynamic histomorphometry of the trabecular bone in the distal femur metaphysis and vertebrae L5 (Table S2), and the cortical bone of the middiaphyseal region of femur (Table S3), and (iii) biochemical serum bone marker analyses (Table S4; osteocalcin, type I collagen fragments, tartrate-resistant acid phosphatase 5b). These findings suggest that, although adult WNT16 transgenic mice have substantially increased trabecular bone mass, a new steady state of normal bone turnover has been reached in the trabecular bone.
Table S2.
Trabecular bone parameters as analyzed by histomorphometry
| Bone parameters | WT | Obl-Wnt16 |
| Femur | ||
| Static histomorphometry | ||
| N.Oc/B.Pm, 1/mm | 2.97 ± 0.21 | 2.74 ± 0.31 |
| Dynamic histomorphometry | ||
| MS/BS, % | 31.2 ± 1.1 | 27.6 ± 1.5 |
| MAR, µm/d | 2.12 ± 0.1 | 2.36 ± 0.09 |
| BFR, µm2/µm/d | 0.65 ± 0.02 | 0.65 ± 0.04 |
| Vertebrae L5 | ||
| Static histomorphometry | ||
| N.Oc/B.Pm, 1/mm | 1.85 ± 0.15 | 1.81 ± 0.09 |
| Dynamic histomorphometry | ||
| MS/BS, % | 30.4 ± 1.7 | 28.4 ± 2.0 |
| MAR, µm/d | 1.70 ± 0.04 | 1.77 ± 0.05 |
| BFR, µm2/µm/d | 0.52 ± 0.04 | 0.50 ± 0.03 |
Static and dynamic histomorphometry analyses of trabecular bone of 16-wk-old female Obl-Wnt16 and wild-type (WT) mice (n = 8). Values are given as mean ± SEM. BFR, bone formation rate; N.Oc/B.Pm, number of osteoclasts/bone perimeter; MAR, mineral apposition rate; MS/BS, mineralizing surface/bone surface.
Table S3.
Femur cortical bone parameters as analyzed by histomorphometry
| Bone parameters | WT | Obl-Wnt16 |
| Static histomorphometry | ||
| Ct. Ar, mm2 | 0.82 ± 0.02 | 0.86 ± 0.02 |
| Ps. Pm, mm | 4.53 ± 0.03 | 4.53 ± 0.04 |
| Ec. Pm, mm | 3.19 ± 0.03 | 3.10 ± 0.07 |
| Dynamic histomorphometry | ||
| Periosteal | ||
| MS/BS, % | 30.6 ± 2.5 | 26.4 ± 2.4 |
| MAR, µm/d | 1.22 ± 0.08 | 1.06 ± 0.11 |
| BFR, µm2/µm/d | 0.38 ± 0.04 | 0.29 ± 0.05 |
| Endosteal | ||
| MS/BS, % | 80.0 ± 3.0 | 83.1 ± 4.2 |
| MAR, µm/d | 1.48 ± 0.05 | 1.56 ± 0.05 |
| BFR, µm2/µm/d | 1.18 ± 0.05 | 1.30 ± 0.09 |
Static and dynamic histomorphometry analyses of cortical bone of 16-wk-old female Obl-Wnt16 and wild-type (WT) mice (n = 8). Values are given as mean ± SEM. BFR, bone formation rate; Ct. Ar, cortical bone area; Ec. Pm, endosteal perimeter; MAR, mineral apposition rate; MS/BS, mineralizing surface/bone surface; Ps. Pm, periosteal perimeter.
The Bone-Sparing Effects of Estrogen and WNT16 Are Independent of Each Other When Evaluated in the Obl-Wnt16 Mouse Model.
We next used the Obl-Wnt16 mouse model to test our hypothesis that the bone-sparing effect of estrogen is WNT16-dependent in female mice. This mouse model has a constant high Wnt16 expression in bone. Twelve-week-old Obl-Wnt16 and WT mice were ovx or sham-operated. Four weeks after ovx, the total body BMD, trabecular BV/TV, trabecular thickness, vertebral compression bone strength, and cortical bone thickness were reduced significantly and to a similar extent in ovx Obl-Wnt16 and ovx WT mice compared with corresponding sham-operated mice (Fig. 2 B–G). This finding suggests that the bone-sparing effects of estrogens are not mediated via WNT16. In addition, the total body BMD, trabecular BV/TV, trabecular thickness, and vertebral compression bone strength were higher in the ovx Obl-Wnt16 mice compared with ovx WT mice, demonstrating that the pharmacological stimulation by elevated Wnt16 level on bone mass does not require estrogen action (Fig. 2 B–F). Notably, the total body BMD, trabecular bone volume fraction, trabecular bone thickness, and vertebral compression bone strength were similar in ovx Obl-Wnt16 mice and sham-operated WT mice, implying that WNT16 overexpression could restore these bone parameters in ovx mice (Fig. 2 B–F). Jointly, these findings demonstrate that the bone-sparing effect of estrogen and WNT16 are independent of each other.
The Stimulatory Effect of Estrogen on Bone Does Not Require WNT16 When Evaluated in the Wnt16−/− Mouse Model.
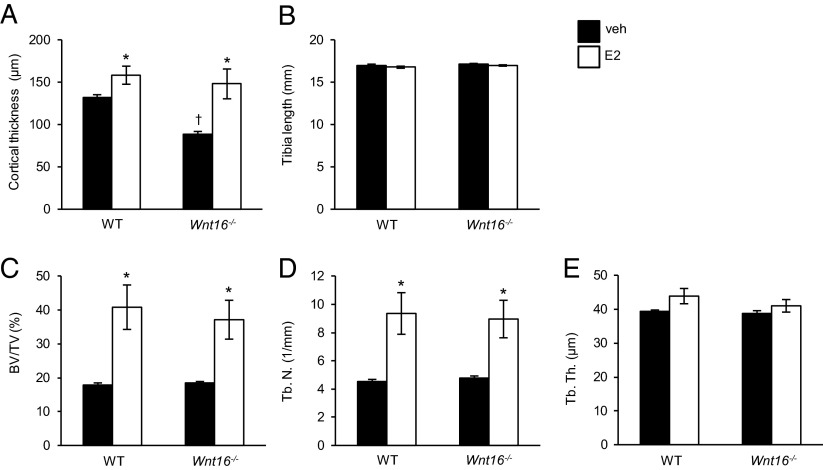
The role of WNT16 for the bone-sparing effect of estrogen was also evaluated in Wnt16−/− mice. In agreement with our previous observation (3), female Wnt16−/− mice had reduced cortical bone thickness (Fig. 3A), whereas the tibia length and trabecular bone mass was unaffected (Fig. 3 B–E) compared with WT mice. As adult Wnt16−/− mice develop spontaneous tibia fractures (3), we could not, due to ethical reasons, evaluate the potential effect of ovx on the bone strength in this mouse model. Instead, we treated female gonadal intact 4-wk-old WT and Wnt16−/− mice with a slightly supraphysiological dose of estradiol for four weeks, to further test if the stimulatory effect of estrogen on bone mass is dependent on WNT16. Estradiol treatment increased the trabecular BV/TV (Fig. 3C) as a result of increased trabecular number (Fig. 3D) both in WT and Wnt16−/− mice. In a similar manner, the cortical bone thickness in the middiaphyseal region of tibia was increased by estradiol treatment both in WT and Wnt16−/− mice (Fig. 3A). Thus, the effect of estradiol on bone does not require WNT16.
Fig. 3.
The stimulatory effect of estrogen on bone does not require WNT16 as evaluated in the Wnt16−/− mouse model. Cortical thickness (A), tibia length (B), bone volume/total volume (BV/TV; C), trabecular number (Tb.N; D), and trabecular thickness (Tb.Th; E) as measured by micro-CT of 8-wk-old female Wnt16−/− or wild-type (WT) mice treated for 4 wk with estradiol (E2) or vehicle (veh) (n = 5–6). Values are given as mean ± SEM *P < 0.05 vs. veh using Student’s t test.
Discussion
Although it was recently established that endogenous osteoblast-derived WNT16 is a crucial regulator of cortical bone mass and that the WNT16 locus associates with nonvertebral fracture susceptibility, the interaction and crosstalk between WNT16 signaling and other major regulators of bone mass is essentially unknown (3–5, 30, 34). To determine the possible interaction between WNT16 and estrogen signaling in bone, we developed and used two genetically modified mouse models with either constantly high osteoblastic Wnt16 expression or with no Wnt16 expression. We demonstrated that the bone-sparing effects of estrogen and WNT16 are independent of each other. In addition, overexpression of WNT16 unexpectedly increased mainly trabecular bone mass.
To evaluate the interaction between estrogen action and WNT16 signaling in bone, we first developed a transgenic mouse model with osteoblast-specific Wnt16 overexpression. Endogenous WNT16 is highly expressed in cortical bone but for unknown reasons only moderately expressed in trabecular bone (3). As we used an osteoblast-specific promoter, the overexpression of Wnt16 in the Obl-Wnt16 mouse model was bone-specific with several-fold increase of Wnt16 levels in both the trabecular and cortical bone. Indeed, Wnt16 levels in the trabecular bone of Obl-Wnt16 mice were significantly higher than in the cortical bone of WT mice. Obl-Wnt16 mice were apparently healthy but had increased total body BMD that was mainly caused by a prominent increase in trabecular bone mass both in the metaphyseal region of the long bones and in the vertebrae. These changes were further associated with improved compression strength of the vertebrae. In contrast to observations in the trabecular bone, Obl-Wnt16 mice displayed only a nonsignificant tendency for increased cortical bone mass. The unexpected finding that Wnt16 overexpression in osteoblasts results in an effect mainly in the trabecular bone is in sharp contrast to the specific reduction of cortical bone mass described in previous mouse models with either global or osteoblast specific inactivation of Wnt16 expression as well as the strong association observed between the Wnt16 locus and cortical bone thickness in human genetic studies (3, 4). Based on these data, we propose that the high endogenous expression of WNT16 in cortical bone of WT mice results in a cortical bone mass value in the upper end of a sigmoidal dose–response curve between WNT16 expression and bone mass, with a substantial loss of cortical bone mass in the absence of WNT16 (Wnt16−/− mice), whereas overexpression of WNT16 (Obl-Wnt16 mice) does not result in any major increase in cortical bone mass (Fig. 1L). In contrast, the relatively modest endogenous WNT16 expression in trabecular bone of WT mice results in a trabecular bone mass value that is at the lower end of this sigmoidal WNT16 dose–response curve. Thus, no major trabecular bone loss is observed in the absence of WNT16 (3), whereas overexpression of WNT16 markedly increases trabecular bone mass, as observed in the present study (Fig. 1M). These findings emphasize that pharmacological and physiological effects often differ, and we propose that enhancement of WNT16 signaling by pharmacological means would be useful for the treatment of trabecular bone loss. As we observed bone-site specific effects of WNT16, it is likely that osteoblast-derived WNT16, in a similar manner as observed for several other WNT proteins, acts as a short-range local cellular signal (35). This notion is further supported by the fact that WNT proteins are highly hydrophobic (36).
We have previously shown that osteoblast-derived WNT16 inhibits osteoclastogenesis, both directly and indirectly via increased production of Opg (3). This is supported by the reduced Opg levels in the Wnt16−/− mice. In the present study, the Obl-Wnt16 mice had increased Opg mRNA levels in trabecular bone, indicating that elevated Opg levels might have contributed to the increased trabecular bone mass. However, no significant alterations of bone resorption or bone formation markers were observed in the Obl-Wnt16 mice, suggesting that although adult WNT16 transgenic mice have substantially increased trabecular bone mass, a new steady state for the bone turnover has been reached.
Postmenopausal osteoporosis is caused by a pronounced drop in estrogen levels and ovariectomy of rodents has been widely used as an animal model of this disease (37, 38). Both estrogen and WNT16 are major regulators of bone mass (1–5), and in the first phase of the present study we demonstrated that estradiol treatment increases Wnt16 expression in bone of ovx mice. We, thus, hypothesized that the bone-sparing effects of estrogens in females could be WNT16-dependent. The hypothesis was first evaluated using our Obl-Wnt16 mouse model with constant several-fold increased Wnt16 expression in both trabecular and cortical bone. However, ovariectomy reduced the trabecular and cortical bone mass similarly in Obl-Wnt16 mice and WT mice, suggesting that the bone-sparing effects of estrogen are WNT16-independent. Conversely, total body BMD and trabecular bone mass were higher in the ovx Obl-Wnt16 mice compared with ovx WT mice, demonstrating that the stimulatory effects of WNT16-overexpression on bone mass do not require estrogen action. All together, these data establish that the bone-sparing effects of estrogen and WNT16 act via independent mechanisms. Notably, the total body BMD and trabecular bone mass were similar in ovx Obl-Wnt16 mice and sham operated WT mice, implying that WNT16 overexpression restores trabecular bone mass in ovx mice.
To further evaluate the interaction between the stimulatory effects of estrogen and WNT16 on bone mass, the role of WNT16 for the bone-sparing effects of estrogen was evaluated in Wnt16−/− mice. Estradiol treatment increased trabecular and cortical bone mass in both Wnt16−/− and WT mice, clearly demonstrating that the stimulatory effect of estrogen on bone mass does not require WNT16 expression.
In conclusion, using two different mouse models, we here demonstrate that, although WNT16 expression is stimulated by estrogen, the bone-sparing effect of estrogen and WNT16 are independent of each other. In addition, loss of endogenous WNT16 results specifically in cortical bone loss, whereas pharmacological overexpression of WNT16, surprisingly, increases mainly trabecular bone mass. As WNT16 signaling in bone does not require normal estrogen action, we propose that WNT16-targeted therapies might be useful for treatment of postmenopausal trabecular bone loss.
Materials and Methods
Development of an Obl-Wnt16 Transgenic Mouse Line.
To develop an osteoblast-specific WNT16 overexpressing mouse model (Obl-Wnt16), we generated a 4843 bp-long transgenic construct in pGL3 plasmid backbone. The transgene consists of the rat procollagen type I α 1 promoter (2.3 kb in length), simian virus 40 small-t intron as well as a full-length cDNA from mouse Wnt16 and a SV40 polyA signal (Fig. 1B). For microinjection, the construct was linearized and cleaved from the plasmid backbone by restriction enzyme digestion using NotI and BamHI. Transgenic mouse lines were generated in the C57BL/6 genetic background by microinjecting the DNA into pronuclei of fertilized oocytes using standard techniques. Integration of the transgene was verified by PCR screening using DNA isolated from tissue biopsies obtained during ear marking of the potential founder mice, and of the offspring thereafter. The 5′-AGGAGGCACACGGAGTGAGG-3′ and 5′-CTGCTCCCATTCATCAGTTC-3′ primers were used for genotyping of the Obl-Wnt16 mice.
Establishment of an Obl-Wnt16 Transgenic Mouse Line.
Obl-Wnt16 mice showed normal fertility. All experiments were carried out on mice born from crossing a male Obl-Wnt16 mouse with a female C57BL/6N mouse. WT littermates were used as control groups. The mice were fed standard phytoestrogen-free pellet diet ad libitum (R70, Lactamin AB). Ovariectomy (ovx) or sham operation were performed on 12-wk-old female mice and the mice were killed after 4 wk.
Mice with Total Inactivation of Wnt16.
The generation of Wnt16−/− mice has been described (3). Four-week-old female Wnt16−/− and WT littermate mice were treated with vehicle or estradiol (E2, 167 ng per mouse per day) for 4 wk using slow-release pellets inserted s.c. (Innovative Research of America).
All mice were housed in a standard animal facility under controlled temperature (22 °C) and photoperiod (12 h of light, 12 h of dark) and the animal care was in accordance with institutional guidelines. All animal experiments had been approved by the local Ethical Committees for Animal Research at the University of Gothenburg.
Quantitative Real-Time PCR Analysis.
Total RNA was prepared from humerus, middiaphyseal cortical bone of the tibia, vertebral body of vertebrae L6 (trabecular bone) and uterus using TRIzol reagent (Life Technologies) followed by the RNeasy Mini Kit (Qiagen). The RNA was reverse transcribed into cDNA, and real-time PCR analysis was performed using predesigned real-time PCR assays for Wnt16 (Mm00446420_m1), and osteoprotegerin (Opg; Tnfrsf11b, Mm0043545_m1) on the StepOnePlus Real-Time PCR system (Applied Biosystems). The mRNA abundance of each gene was adjusted for the expression of 18S.
Dual-Energy X-Ray Absorptiometry (DXA).
Analyses of total body areal BMD (aBMD) and lumbar spine (LS) BMD were performed by DXA using the Lunar PIXImus Mouse Densitometer (Wipro GE Healthcare).
High-Resolution Micro-CT (µCT).
High-resolution micro-CT analyses were performed on the lumbar vertebra L4 and tibia using an 1172 model micro-CT (Bruker micro-CT) (3). The vertebra and tibia were imaged with an X-ray tube voltage of 50 kV, a current of 201 μA, and with a 0.5-mm aluminum filter. The scanning angular rotation was 180°, and the angular increment was 0.70°. The voxel size was 4.49 mm isotropically. NRecon (version 1.6.9) was used to perform the reconstruction after the scans. In the vertebra, the trabecular bone in the vertebral body caudal of the pedicles was selected for analysis within a conforming volume of interest (cortical bone excluded) commencing at a distance of 4.5 µm caudal of the lower end of the pedicles, and extending a further longitudinal distance of 328 µm in the caudal direction. In the tibia, the trabecular bone distal to the proximal growth plate was selected for analyses within a conforming volume of interest (cortical bone excluded) commencing at a distance of 650 µm from the growth plate, and extending a further longitudinal distance of 134 µm in the distal direction. Cortical measurements were performed in the diaphyseal region of tibia starting at a distance of 5.2 mm from the growth plate and extending a further longitudinal distance of 134 µm in the distal direction.
Bone Histomorphometry.
For the measurement of dynamic parameters, the mice were double labeled with calcein, which was injected (intraperitoneally) into the mice 1 and 8 d before termination. Femur and L5 vertebrae were fixed in 4% paraformaldehyde, dehydrated in 70% EtOH and embedded in methyl methacrylate. Femur was dissected into two regions, one including proximal epiphysis and metaphysis and one including the cortical diaphyseal shaft. The distal femur and L5 vertebrae were sectioned longitudinally, whereas the femur shaft was sectioned in a transverse plane. Static parameters were determined in a 4-µm-thick section stained in Masson–Goldner’s Trichrome and dynamic parameters in an unstained 8-µm-thick section.
All parameters were analyzed using OsteoMeasure histomorphometry system (OsteoMetrics) following the guidelines of the American Society for Bone and Mineral Research (39). The analyses were performed by PharmaTest.
Mechanical Strength.
Intact L3 vertebrae were axially loaded after stabilizing them with a 1-mm-thick holder going through the vertebral foramen. The vertebral body was loaded with a press head of 2 mm in diameter. Loading speed were 0.155 mm/s using a mechanical testing machine (Instron 3366, Instron). Based on the computer recorded load deformation raw data curves produced by Bluehill 2 software v2.6 (Instron), the results were calculated with custom-made Excel macros.
Measurement of Serum Markers.
As markers of bone resorption, serum levels of C-terminal type I collagen fragments were assessed using an ELISA RatLaps kit (CTX, Immunodiagostic Systems) according to the manufacturer’s instructions and total amount of Trap5b was analyzed using a commercial TRACP 5b ELISA (Immunodiagostic Systems). Serum levels of osteocalcin (OCN), a marker of bone formation, were determined with a mouse osteocalcin immunoradiometric assay kit (Immutopics).
Acknowledgments
We are grateful to A. Hansevi, B. Aleksic, and C. Uggla for technical assistance. This study was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the ALF/LUA research grant from the Sahlgrenska University Hospital, the Lundberg Foundation, the Torsten and Ragnar Söderberg’s Foundation, the Novo Nordisk Foundation, the Swedish Cancer Society, the Cancer Prevention and Research Institute of Texas (Grant RP110444-P1), the Texas Emerging Technology Fund under Agreement 18 No. 300-9-1958, and the Robert A. Welch Foundation (E-0004).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520408112/-/DCSupplemental.
References
- 1.Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011;26(3):441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol. 2009;5(8):437–443. doi: 10.1038/nrendo.2009.112. [DOI] [PubMed] [Google Scholar]
- 3.Movérare-Skrtic S, et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med. 2014;20(11):1279–1288. doi: 10.1038/nm.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng HF, et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012;8(7):e1002745. doi: 10.1371/journal.pgen.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrada K, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanderschueren D, et al. Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–960. doi: 10.1210/er.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly E, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348(9033):977–980. doi: 10.1016/S0140-6736(96)07113-9. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 9.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y, et al. Osteoporosis-Pseudoglioma Syndrome Collaborative Group LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 11.Little RD, Recker RR, Johnson ML. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;347(12):943–944, author reply 943–944. doi: 10.1056/NEJM200209193471216. [DOI] [PubMed] [Google Scholar]
- 12.Little RD, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 14.Poole KE, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19(13):1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 15.Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balemans W, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loots GG, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15(7):928–935. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laine CM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368(19):1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda K, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18(3):405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 20.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102(9):3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24(4):578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 22.Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 23.Li X, et al. Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J Bone Miner Res. 2011;26(11):2610–2621. doi: 10.1002/jbmr.472. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, et al. Crosstalk between Wnt/β-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2013;8(12):e82436. doi: 10.1371/journal.pone.0082436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouzmenko AP, et al. Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem. 2004;279(39):40255–40258. doi: 10.1074/jbc.C400331200. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong VJ, et al. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007;282(28):20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28(34):8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18(12):3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim SK, Orhant-Prioux M, Toy W, Tan KY, Lim YP. Tyrosine phosphorylation of transcriptional coactivator WW-domain binding protein 2 regulates estrogen receptor α function in breast cancer via the Wnt pathway. FASEB J. 2011;25(9):3004–3018. doi: 10.1096/fj.10-169136. [DOI] [PubMed] [Google Scholar]
- 30.Medina-Gomez C, et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 2012;8(7):e1002718. doi: 10.1371/journal.pgen.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koller DL, et al. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. J Bone Miner Res. 2013;28(3):547–558. doi: 10.1002/jbmr.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Ibarbia C, et al. Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporos Int. 2013;24(9):2449–2454. doi: 10.1007/s00198-013-2302-0. [DOI] [PubMed] [Google Scholar]
- 33.Hendrickx G, et al. Variation in the Kozak sequence of WNT16 results in an increased translation and is associated with osteoporosis related parameters. Bone. 2014;59:57–65. doi: 10.1016/j.bone.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Wergedal JE, Kesavan C, Brommage R, Das S, Mohan S. Role of WNT16 in the regulation of periosteal bone formation in female mice. Endocrinology. 2015;156(3):1023–1032. doi: 10.1210/en.2014-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 36.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 37.Turner RT. Mice, estrogen, and postmenopausal osteoporosis. J Bone Miner Res. 1999;14(2):187–191. doi: 10.1359/jbmr.1999.14.2.187. [DOI] [PubMed] [Google Scholar]
- 38.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest. 1999;104(7):895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dempster DW, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]