Significance
Plasma lipoproteins carrying cholesteryl esters are taken up by low density lipoprotein receptors and delivered to lysosomes, where cholesterol is released and then exported for cellular use. Although cholesterol can partition freely between membranes, it does not freely partition out of lysosomes—Niemann-Pick type C 1 (NPC1) protein is required for cholesterol export. We show here that the requirement for NPC1 is decreased in cells containing lower levels of protein glycosylation. These data support a model in which NPC1 protein helps transfer cholesterol past the glycocalyx barrier that lines the interior of lysosomes.
Keywords: cholesterol, lysosomal glycoprotein, lysosomal storage disease, glycocalyx
Abstract
Lysosomes are lined with a glycocalyx that protects the limiting membrane from the action of degradative enzymes. We tested the hypothesis that Niemann-Pick type C 1 (NPC1) protein aids the transfer of low density lipoprotein-derived cholesterol across this glycocalyx. A prediction of this model is that cells will be less dependent upon NPC1 if their glycocalyx is decreased in density. Lysosome cholesterol content was significantly lower after treatment of NPC1-deficient human fibroblasts with benzyl-2-acetamido-2-deoxy-α-D-galactopyranoside, an inhibitor of O-linked glycosylation. Direct biochemical measurement of cholesterol showed that lysosomes purified from NPC1-deficient fibroblasts contained at least 30% less cholesterol when O-linked glycosylation was blocked. As an independent means to modify protein glycosylation, we used Chinese hamster ovary ldl-D cells defective in UDP-Gal/UDP-GalNAc 4-epimerase in which N- and O-linked glycosylation can be controlled. CRISPR generated, NPC1-deficient ldl-D cells supplemented with galactose accumulated more cholesterol than those in which sugar addition was blocked. In the absence of galactose supplementation, NPC1-deficient ldl-D cells also transported more cholesterol from lysosomes to the endoplasmic reticulum, as monitored by an increase in cholesteryl [14C]-oleate levels. These experiments support a model in which NPC1 protein functions to transfer cholesterol past a lysosomal glycocalyx.
Low-density lipoprotein-derived cholesterol is delivered to cells by receptor-mediated endocytosis and transport to lysosomes. Within lysosomes, cholesterol esters are hydrolyzed and cholesterol is exported to the cytoplasm for cellular use (1). Cholesterol export requires two lysosomal glycoproteins: NPC1 and NPC2 (2, 3). Patients carrying homozygous mutations in either of these proteins present with Niemann-Pick type C (NPC) disease, a neurological disorder that is associated with massive accumulation of unesterified cholesterol and glycosphingolipids in lysosomes (2–4).
NPC2 is a small, soluble, cholesterol binding protein that is thought to pick up cholesterol both from lipoprotein lipase and from the abundant, intralumenal membranes present within lysosomes (5, 6). NPC1 is a much larger glycoprotein that contains 13 transmembrane domains and three, relatively large, lumenally oriented domains (2). NPC1’s first (N-terminal), luminal domain binds cholesterol directly (7, 8); the second domain can bind NPC2 in a cholesterol-dependent manner and has been proposed to facilitate transfer of cholesterol from NPC2 onto NPC1’s N-terminal domain (9).
The requirement for NPC1 and NPC2 proteins for cholesterol export from lysosomes is somewhat enigmatic because cholesterol can generally partition freely into membrane bilayers (10). The limiting membrane of lysosomes is lined predominantly by densely packed, highly glycosylated, lysosomal membrane proteins; their oligosaccharides are thought to protect the phospholipid bilayer from hydrolytic destruction (11, 12). This glycoprotein coat can be visualized by electron microscopy and has an average thickness of about 8 nm (13). It has been proposed that NPC1 is needed to help cholesterol traverse this glycocalyx (8). A direct prediction of this model is that decreasing the lysosomal glycocalyx density should make cells less dependent on NPC1 function.
In this paper, we have used two approaches to modify the glycocalyx. Alteration of glycosylation decreases lysosomal cholesterol levels in NPC1-deficient Chinese hamster ovary (CHO) and human cells. These experiments support a model in which NPC1 protein functions to help cholesterol traverse the glycocalyx that lines the interior of lysosome limiting membranes.
Results and Discussion
LAMP1 and LAMP2 proteins represent the major glycoproteins of the lysosome membrane (12). These proteins are glycosylated on both asparagine residues (N linked) and serine or threonine residues (O linked), and their oligosaccharides contribute significantly to the structure of the lysosomal glycocalyx (11). We sought to alter glycosylation to explore the role of the glycocalyx in lysosomal cholesterol export. Inhibition of total N-linked glycosylation using deoxymannojirimycin treatment was toxic for cells that were also lacking NPC1 function. We thus used subtler means to modify the glycocalyx and investigated the contributions of O-linked glycans to glycocalyx function.
Mucin type, O-linked glycosylation is initiated by the attachment of GalNAc to serine or threonine residues by one of 20 UDP-GalNAc-polypeptidyl N-acetylgalactosaminyl-transferase enzymes to form the so-called Tn antigen; subsequent sugars are then attached, most commonly galactose or GlcNAc. These two core forms can be further modified by the addition of sugar units such as GlcNAc, galactose, fucose, and sialic acid (14).
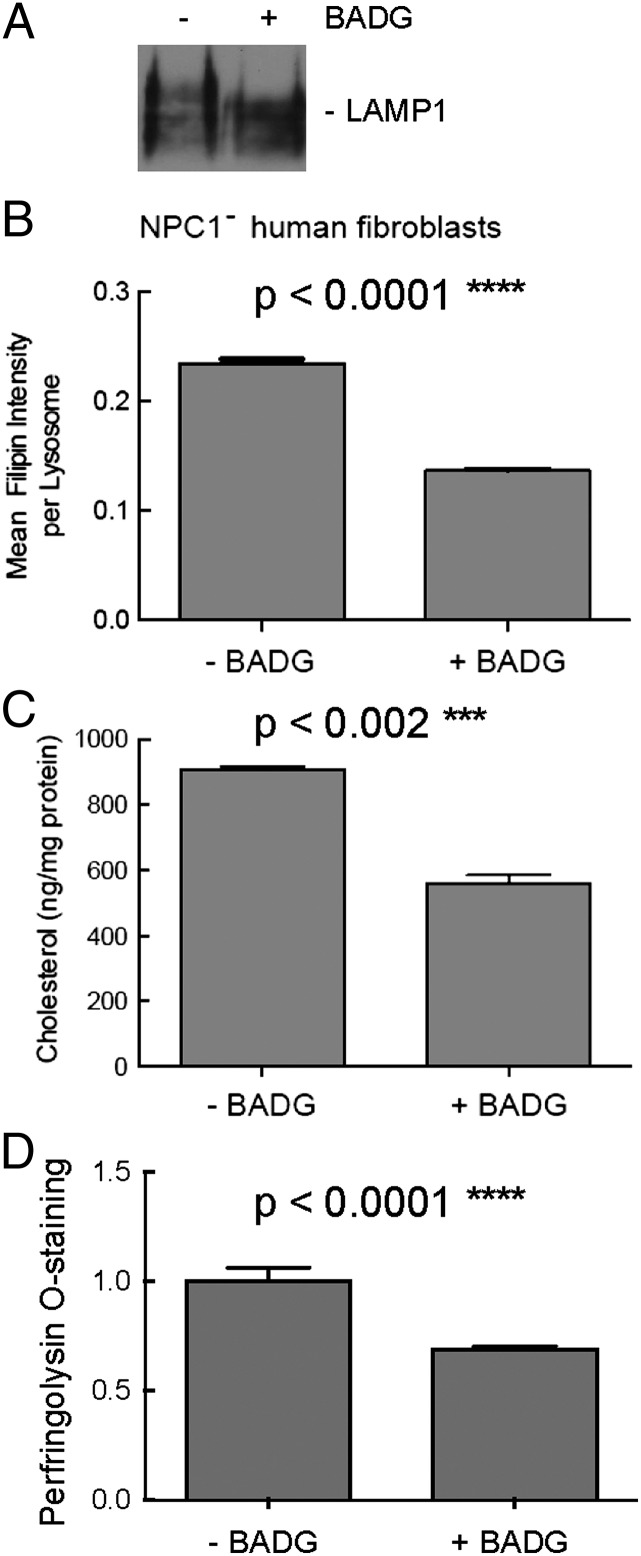
We first used benzyl 2-acetamido-2-deoxy-α-D-galactopyranoside (BADG), an analog of GalNAc-α-1-O-serine/threonine that acts as a competitive inhibitor to O-glycan chain extension (15, 16). Because some cell lines accumulate cytotoxic, nonphysiological aryl-glycans after many days of BADG treatment (17), we used shorter incubations and monitored for LAMP1 level increases that can indicate this type of accumulation (16, 17). BADG treatment altered the glycosylation of LAMP1 protein but did not affect its abundance, as monitored by immunoblot of NPC1-deficient, primary human fibroblasts (Fig. 1A). CellProfiler software (18) was next used in conjunction with fluorescence microscopy to quantify the amount of filipin staining within LAMP2-positive lysosomes. Upon BADG treatment, lysosome-specific filipin staining decreased 44% (Fig. 1B). To confirm that the staining observed represented cholesterol and not another potential filipin binding partner, we isolated a lysosome-enriched fraction from BADG-treated, NPC-deficient human fibroblasts and measured the cholesterol content directly using a cholesterol oxidase-based assay. Treatment of cells with BADG decreased cholesterol in isolated lysosomes (Fig. 1C), consistent with the findings obtained using filipin staining. Finally, the differences observed did not appear to be due to minor differences in LDL receptor function, because similar decreases were observed for cells cultured in lipoprotein deficient serum (LPDS) for a 3-d period, as monitored by staining with fluorescent perfringolysin O* (PFO*) that binds to free cholesterol (19) (Fig. 1D).
Fig. 1.
NPC1-deficient human fibroblasts harbor less cholesterol upon BADG treatment. (A) Immunoblot of LAMP1 protein after BADG treatment (48 h). (B) Filipin intensity over lysosomes quantified using CellProfiler. The results are expressed as an average of the mean intensity per lysosome ± SEM. A total of 1,483 lysosomes in 36 cells or 1,663 lysosomes in 44 cells were quantified without or with BADG treatment (48 h), respectively; unpaired, two-tailed t test P value <0.0001. (C) Cholesterol content of isolated lysosomes obtained from control or BADG-treated (72 h), NPC1-deficient human fibroblasts; unpaired, two-tailed t test P value <0.002. (D) Cholesterol content was monitored using flow cytometry of PFO*-stained cells treated 72 h in lipoprotein-deficient serum in the presence and absence of BADG. Error bars, SEM; n = 4 experiments, >4,000 cells per condition per experiment; unpaired, two-tailed t test P value <0.0001.
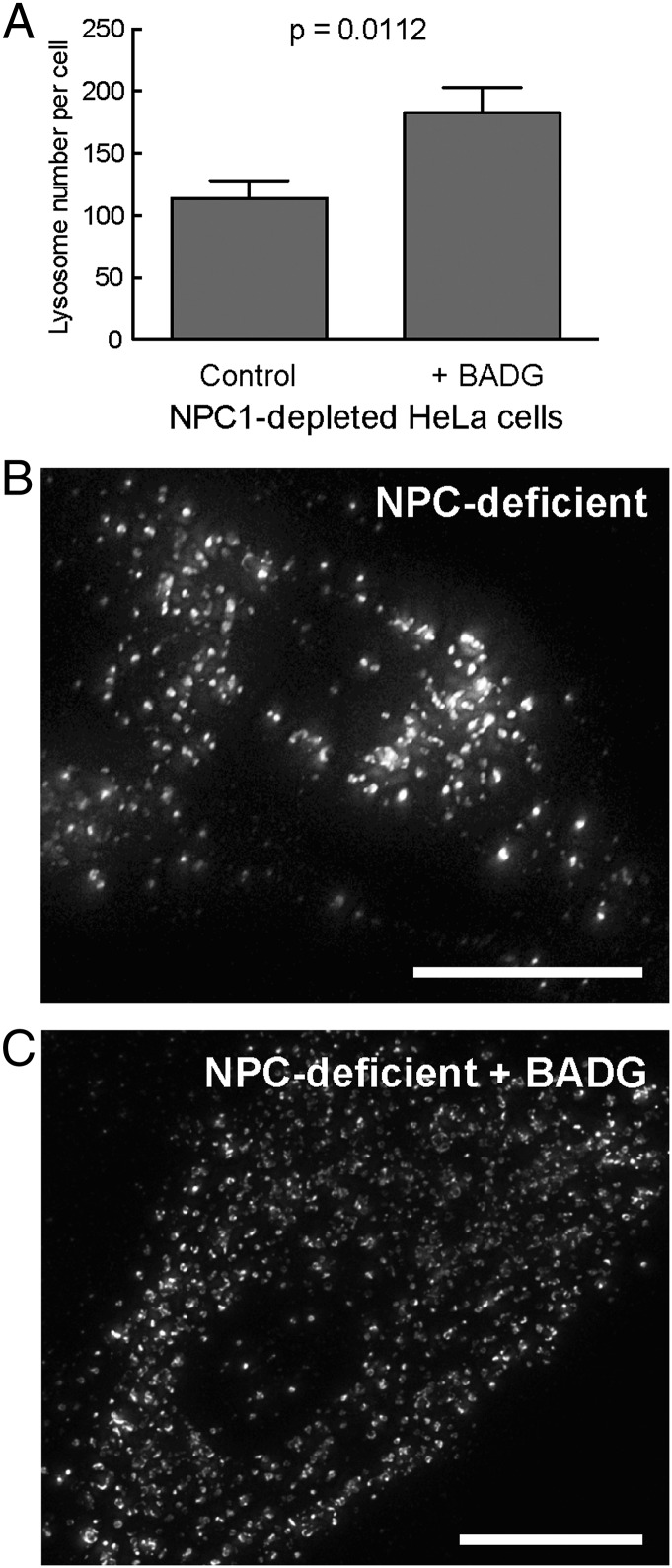
Cholesterol-filled lysosomes characteristic of NPC-deficient human fibroblasts show decreased motility and a more perinuclear localization (20–22). We noted that in HeLa cells depleted of NPC1 protein using siRNA (Fig. 2), BADG treatment increased lysosome number. In addition, BADG led to a less perinuclear distribution of lysosome-related structures in these cells as well as in fibroblasts from patients deficient in NPC1 function (Fig. 2 B and C). The change in lysosome number is consistent with restoration of more physiological levels of lysosomal cholesterol.
Fig. 2.
BADG treatment increases lysosome number in NPC1-depleted HeLa or NPC1-deficient human patient fibroblasts. Quantification of LAMP1 staining in NPC1-depleted HeLa cells (A) or NPC1-deficient human fibroblasts (B and C). Lysosome number is presented as the mean number of lysosomes per cell ± SEM. In A, 29 cells were counted ± BADG, respectively; unpaired, two-tailed t test P value <0.0001. (B and C) Micrographs of NPC1-deficient human patient fibroblasts stained with anti-LAMP1 antibody, ± BADG as indicated. (Scale bar, 20 μm.)
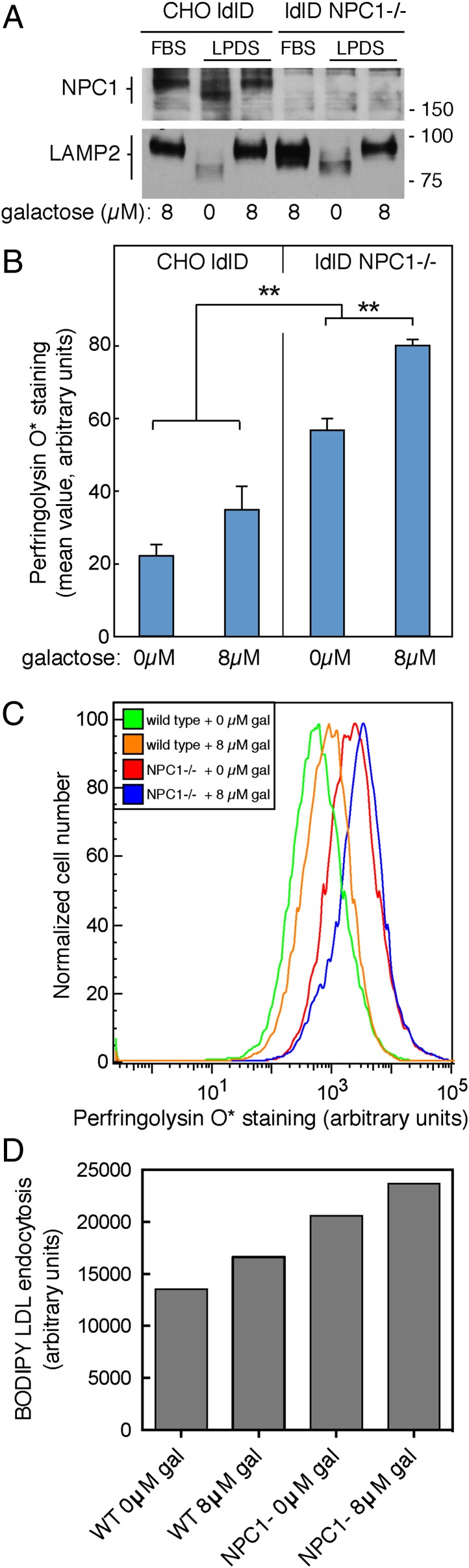
To verify our findings by an independent method, we made use of CHO ldl-D cells to control protein glycosylation. CHO ldl-D cells lack UDP-Gal/UDP-GalNAc 4-epimerase activity, and thus cannot synthesize normal amounts of UDP-galactose and UDP-N-acetylgalactosamine from the corresponding glucose precursors (23). Simple addition of galactose and GalNAc to the culture medium enables ldl-D cells to synthesize the UDP-sugar nucleotide precursors via salvage pathways and fully corrects all glycosylation defects (23). Note that this protocol will impact both N- and O-linked glycosylation, both of which rely on these sugar nucleotide precursors. For these experiments, cells were maintained in 8 µM galactose and 100 µM N-acetylgalactosamine, and galactose was either removed or readded for 3 d before analysis. To test the impact of the glycocalyx on NPC1 protein function, we used CRISPR technology to generate NPC1-deficient CHO ldl-D cells. Immunoblotting of cell extracts confirmed the absence of NPC1 protein in these cells, compared with the parental cell line (Fig. 3A).
Fig. 3.
Increased O-linked glycosylation leads to increased cholesterol accumulation in NPC1−/− CHO ldl-D cells. (A) Immunoblot of NPC1 and LAMP2 proteins in CHO ldl-D cells supplemented with 100 µM N-acetyl-galactosamine, ± galactose. CHO ldl-D cells in which the NPC1 gene was disrupted using CRISPR are shown in the right three lanes. The mobility of molecular weight markers is indicated at right in kilodaltons. Samples were from cells cultured in 10% (vol/vol) FBS or 5% (vol/vol) lipoprotein-deficient serum and the indicated amounts of supplemental galactose for 72 h. (B) Mean PFO* staining from CHO ldl-D cells ± 8 µM galactose. Over 4,000 cells were analyzed by flow cytometry per condition per experiment, error bars = SEM, n = 6 pooled experiments, unpaired t test P values between each condition <0.01 except for that between 0 and 8 µM galactose in the wild-type population. (C) Representative flow cytometry analysis as described in B. (D) LDL receptor activity monitored by endocytosis of BODIPY-LDL (10 µg/mL; Life Technologies) for 45 min followed by fixation and flow cytometry. Shown are mean fluorescence values, ∼19,000 cells per sample; shown is a representative example of an experiment carried out in duplicate.
Changes in glycosylation were readily detected in immunoblots of membrane glycoproteins from ldl-D cells grown with or without 8 µM galactose (Fig. 3A). Both NPC1 and LAMP2 proteins showed increased mobility upon SDS/PAGE in cells cultured in the absence of galactose (Fig. 3A) but returned to that of control cells when cells were supplemented with 8 µM galactose (Fig. 3A). Note that as expected, LAMP2 levels increased in FBS-cultured cells lacking NPC1 (24); LAMP2 levels were lower in cells in which glycosylation was inhibited (0 µM gal), likely due to protein destabilization.
Cholesterol accumulation was tracked in CHO ldl-D cells using PFO* staining and the differences in accumulation were first quantified by flow cytometry. As expected, cells lacking NPC1 accumulated more cholesterol as monitored by PFO* staining; less was present in control CHO ldl-D cells supplemented with 0 µM or 8 µM galactose. Moreover, decreasing the extent of glycosylation by removal of galactose led to a significant decrease in cholesterol accumulation in NPC1-deficient CHO ldl-D cells (Fig. 3 B and C). A slight decrease was also detected in wild-type ldl-D control cells starved of galactose, analyzed by flow cytometry; however, this trend was not adequately significant as the P value was 0.08 (Fig. 3B).
Again, the decrease in lysosomal cholesterol in NPC1-deficient cells did not appear to be due to a loss in LDL receptor activity because the experiment was carried out after three days of culture in LPDS. Nevertheless, we tested LDL receptor activity directly by monitoring the ability of CHO ldl-D wild-type and NPC1-deficient ldl-D cells to endocytose bodipy-LDL, with or without 3 d galactose supplementation; ∼20,000 cells were analyzed by flow cytometry for each condition. As shown in Fig. 3D, NPC1-deficient cells showed 13% less LDL uptake per cell when cultured in 0 µM galactose compared with 8 µM galactose; wild-type cells also showed a similar decrease. NPC1-deficient cells showed higher overall LDL receptor activity, as expected for cells deficient in cholesterol transport from lysosomes to the endoplasmic reticulum (ER) (23). This minor decrease in LDL uptake under glycosylation-deficient conditions is unlikely to explain the significantly reduced cholesterol storage observed.
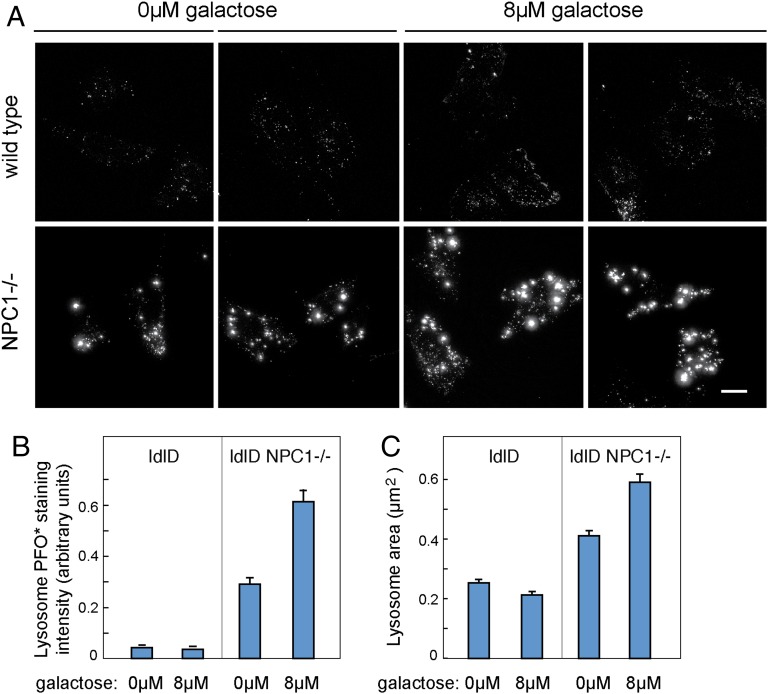
Cholesterol accumulation in CHO ldl-D cells was also monitored by light microscopy. As expected, PFO*-stained control cells displayed a punctate lysosomal staining pattern; NPC1-deficient cells showed significantly increased cholesterol content (Fig. 4A). Consistent with our earlier findings, quantitation of PFO* staining in cells visualized on coverslips confirmed that NPC1-deficient cells accumulated more cholesterol upon culture in 8 µM versus 0 µM galactose (Fig. 4 A and B); the levels of both were higher than those seen in the parental CHO ldl-D cells. No large difference was detected between control cells cultured ± galactose; however, it should be noted that the intensity of wild-type cell staining is at the lower limit of our range of sensitivity and many fewer cells were analyzed than in the flow cytometry experiments presented in Fig. 3. Finally, analysis of the PFO*-labeled area revealed that NPC1-deficient CHO ldl-D cells contained smaller puncta (lysosomes) after culture in the absence of galactose, consistent with their decreased cholesterol content and apparently healthier physiological state (Fig. 4C).
Fig. 4.
PFO* stained objects are brighter and larger in NPC1−/− CHO ldl-D cells in the presence of galactose. (A) Representative images of CHO ldl-D cells stained with PFO* under the indicated conditions. Images of wild-type cells (Top row) have been leveled five times brighter than they would appear compared with NPC1−/− cell levels. (Scale bar, 15 µm.) (B) Mean values of total intensity of PFO* objects per cell quantified using CellProfiler. (C) Mean values of the area of PFO* stained objects per cell in square micrometers as quantified by CellProfiler. Error bars, SEM. From Left to Right, 10, 9, 50, and 54 cells were analyzed with an average of more than 70 objects/cell/condition quantified. NPC1−/− populations were statistically different between themselves and compared with other populations with unpaired, two-tailed t test P values <0.001.
Cholesterol is exported from lysosomes to the ER, where it is esterified by acyl CoA: O-cholesterol acyltransferase (ACAT). LDL added to NPC1 mutant cells fails to increase ACAT-mediated cholesterol ester synthesis; cyclodextrin can overcome this block and permit cholesterol export to the ER (25, 26). To show directly that lysosomal cholesterol export is improved in NPC1-deficient ldl-D cells containing a less elaborate glycocalyx, we tested the ability of these cells to transfer LDL-derived cholesterol to the ER, as monitored by its availability for esterification by ER-localized ACAT.
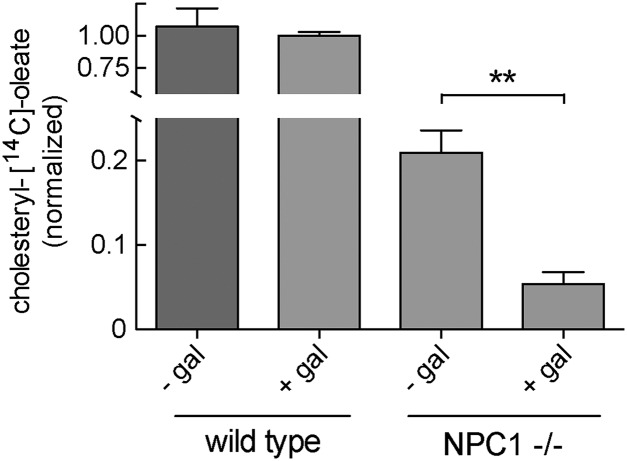
Cells grown for 3 d in LPDS were pulsed with LDL under conditions of cholesterol synthesis inhibition. As expected, NPC1-deficient CHO ldl-D cells grown in the presence of 8 µM galactose showed only ∼5% the level of cholesteryl [14C]-oleate production detected in wild-type CHO ldl-D cells cultured under similar conditions (Fig. 5). Remarkably, omission of galactose from the NPC1-deficient ldl-D cell cultures led to an approximately fourfold increase in cholesteryl ester formation due to cholesterol export from lysosomes to the ER (Fig. 5). This increase was seen despite a 13% decrease in LDL receptor activity, compared with NPC1-deficient cells grown in the presence of galactose (Fig. 3D). Absence of NPC1 activity was still evident and consistent with the cholesterol accumulation still detected under these conditions (Fig. 4).
Fig. 5.
Decreased glycosylation in NPC1−/− CHO ldl-D cells increases cholesterol transport from lysosomes to the ER. ACAT-catalyzed formation of cholesteryl [14C]oleate was measured ± galactose for NPC1+/+ (Left two bars) and NPC1−/− CHO ldl-D cells (Right two bars) as indicated. Data shown were combined from two independent experiments carried out in duplicate; error bars, SEM. P value (0.0025) was determined by unpaired, two-tailed t test.
In summary, we have shown here that altering the glycosylation of cellular glycoproteins influences the levels of cholesterol detected in lysosomes and its ability to be transported to the ER. Cells were less dependent on NPC1 function when glycosylation was modified by inhibition of O-GalNAc elongation or in the absence of UDP-Gal/UDP-GalNAc 4-epimerase activity.
Lysosomes are lined by a glycocalyx that seems to impede the spontaneous partitioning of cholesterol into the limiting membrane (11–13). The major glycoproteins that comprise the lysosome limiting membrane are highly glycosylated, and oligosaccharides on lysosomal glycoproteins contribute to their overall stability in cells. Our data suggest that altering the structure of this carbohydrate barrier facilitates the access of cholesterol to the membrane for export. Access could facilitate spontaneous transfer of cholesterol into the bilayer, or permit NPC2 protein to mediate direct delivery to lysosomal membranes.
At this stage, we cannot completely rule out other possible explanations for our findings. For example, Sugii et al. (27) showed that in NPC1 cells, acid lipase begins to act on LDL-derived cholesteryl esters in prelysosomal compartments. Perhaps conditions used here to thin the glycocalyx increase cholesteryl ester hydrolysis, permitting cholesterol to escape from compartments earlier in the endocytic pathway. Although formally possible, we consider this unlikely to explain much of the data presented here. In most experiments, cells were cultured without LDL for 3 d, and the glycocalyx could only be slowly thinned due to the very slow turnover of fully glycosylated lysosomal glycoproteins present at the start. Indeed, experiments monitoring the rate of glycosylation change of LAMP proteins showed that 3 d of culture was absolutely required to see complete glycosylation differences. Thus, the cholesterol changes monitored reflect cholesterol that had been endocytosed for at least 24 h—much more than enough time to reach the lysosome.
Another possibility is that under-glycosylation of an unknown lysosomal cholesterol transporter could enhance its activity. Note that such a hypothetical transporter would be inactive under conditions of normal glycosylation, or we (and others, refs. 25, 26) would have detected more cholesterol export to the ER in NPC1-deficient cells. Changes in the levels of sugar nucleotide glycosylation precursors could influence metabolism such that cells activate lysosome biogenesis. However, in the parental CHO ldl-D cells, we did not detect increases in the levels of LAMP2 or NPC1 proteins upon removal of galactose, inconsistent with this possibility. Finally, changes in the distribution or increased number of lysosomes may have in some way facilitated cholesterol export.
Kundra and Kornfeld (28) removed Asn-linked oligosaccharides from lysosomal membrane glycoproteins and found that Asn-linked glycans protect LAMP proteins from the action of proteases; they also found that LAMP1 and LAMP2 are not required for maintaining the acidic pH, density, membrane stability, or degradative capacity of lysosomes (28). Similar findings were obtained upon analysis of LAMP1/LAMP2 double-deficient fibroblasts (29). Lysosomes in double-deficient cells also accumulated significant amounts of lipid. Because of this abnormality, such cells could not be used to test the influence of LAMP1/2-specific oligosaccharides on NPC1 protein function. Why LAMP1/2-deficient cells lose their ability to support autophagy and also accumulate lipid represent important areas for future investigation. Growth of CHO ldl-D cells in the absence of galactose decreased LAMP2 levels and cholesterol accumulation. Because LAMP2 contributes significant glycan to the glycocalyx, these data are consistent with LAMP2 contributing to lysosomal cholesterol storage under these conditions. Although LAMP1 levels did not decrease in BADG-treated cells, these cells nevertheless showed decreased cholesterol accumulation coupled with LAMP1 under-glycosylation.
N-butyl-deoxynojirimycin (miglustat) is an iminosugar analog of glucose and is thought to inhibit glucosylceramide synthase that catalyzes the formation of glucosylceramide, the first step in glycosphingolipid biosynthesis. N-butyl-deoxynojirimycin has shown promise in stabilizing patients deficient in NPC1 protein function (4) and is thought to do so by decreasing glycosphingolipid accumulation in lysosomes. Another minor possibility is that the iminosugar may influence glycocalyx structure, thereby enhancing cholesterol export, albeit to a small extent. It will be of interest to monitor potential changes in the lysosomal glycocalyx upon miglustat treatment. In summary, these data suggest that glycosylation inhibitors may be of therapeutic value in patients deficient in NPC1 function. Future challenges include determining the mechanism by which cholesterol departs lysosomes after traversing the glycocalyx barrier and developing tools to specifically regulate lysosomal glycosylation.
Experimental Procedures
PFO* Purification.
PFO* plasmid, kindly provided by Arun Radhakrishnan (University of Texas Southwestern, Dallas), was used to purify PFO* (19). In brief, expression was induced in Rosetta 2 cells with 1 mM isopropyl β-p-thiogalactopyranside for 4 h at 37 °C. Cells were suspended in buffer A [PBS, 10% (vol/vol) glycerol, protease inhibitors] and disrupted by Emulsiflex C-5 (Avestin). Clarified lysate was incubated with Ni-NTA (McLab) for 1 h at 4 °C and after washing with buffer A + 50 mM imidazole was eluted with buffer A + 300 mM imidazole. The eluate was concentrated with an Amicon Ultra 10-kDa cutoff centrifugal filter (Millipore), exchanged into buffer A + 1 mm EDTA, and stored at 4 °C. PFO* was directly conjugated to NHS ester Alexa Fluor 647 dye as per the manufacturer (Life Technologies).
Cell Culture.
Wild-type “AG10803” fibroblasts and “GM30123” P237S/I1061T NPC1-deficient human fibroblasts were from Coriell Cell Repositories; CHO ldl-D cells (24) were from Bill Balch (The Scripps Research Institute, San Diego). NPC1 knockout CHO ldl-D clones were generated using CRISPR to the target sequence GGCCTTCTCATTACTTGCAGGGG in exon 4 found using CRISPy (30). Guide sequence cloned into PX458 was transfected into CHO ldl-D cells using FuGene 6 (Promega). Single cells positively transfected and expressing GFP were sorted by flow cytometry and after amplification were validated by immunoblot. HeLa cells were depleted of NPC1 using siRNA from Dharmacon at 50 nM for 72 h with N-Ter Nanoparticle siRNA transfection reagent (Sigma-Aldrich). Cell monolayers were maintained in DMEM or MEM α supplemented with 7.5% (vol/vol) FBS, 100 units/mL penicillin, 100 units/mL streptomycin in 5% CO2 at 37 °C.
Immunofluorescence.
Cells grown on coverslips were fixed with 3.7% (vol/vol) paraformaldehyde for 15 min at room temperature. [For PFO* labeling, they were permeabilized 4 min with 0.1% Triton X-100 + buffer B [PBS, 1% (vol/vol) BSA] and incubated in buffer B for an additional 60 min.] Cells were then stained with either 50 µg/mL filipin complex (Sigma) for 45 min or 8 µg/mL PFO* for 45 min. LAMP1 staining was performed with sequential incubation of rabbit anti-LAMP1 antibody (1:1,000, Cell Signaling) and an Alexa Fluor 555 goat anti-rabbit antibody (1:1,000, Invitrogen), each for 1 h in buffer B at room temperature. In filipin stained cells, nuclei were labeled with Topro-3 stain (1:1,000, Invitrogen) in PBS for 15 min. Coverslips were mounted using Mowiol or Vectashield with DAPI (Vector Laboratories) and imaged using a Leica SP2 confocal microscope and Leica software (for filipin-stained cells) or an Olympus IX70 microscope with a 60× 1.4 N.A. Plan Apochromat oil immersion lens (Olympus) and a charge-coupled device camera (CoolSNAP HQ, Photometrics). Maximum intensity projections were generated using softWoRx 4.1.0 software (Applied Precision) for PFO* stained cells.
Cholesterol Accumulation Quantification by Microscopy.
Images were acquired as described above. Quantification of cholesterol accumulation, indicated by filipin or PFO* staining, was done using CellProfiler (18). In brief, nuclear objects were segmented based on Topro-3 or DAPI staining using a “mixture of Gaussian” method or Otsu thresholding, respectively; lysosomal objects were segmented based on LAMP1 or PFO* staining using Otsu thresholding; cell objects were segmented using distance – B on previously detected nuclear objects. The mean background filipin intensity, measured from the cholesterol excluding nuclear objects, was subtracted from total filipin intensity values. The mean filipin intensity across lysosome objects, lysosomal size, total PFO* intensity in PFO* objects, and PFO* object size was measured and the mean of these is presented ± SEM.
Cell Sorting.
All steps were at room temperature. Cells were trypsinized and fixed as described above. Cells in PBS were then analyzed using a BD Bioscience LSRII UV flow cytometer (filipin) or FACScan Analyzer (PFO*).
Immunoblot Analysis.
Antibody binding was performed using 1 µg/mL rabbit anti-LAMP1, ∼1 µg/mL rabbit anti-NPC1 (Novus Biologicals), and mouse anti-CHO LAMP2 antibody culture supernatant (1B35, from Ira Mellman, Genentech, South San Francisco, CA). Bound antibodies were visualized using chemiluminescence (ECL Pierce Thermo Scientific or ECL Plus Amersham Bioscience) using a dilution of 1:4,000 anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase (Bio-Rad).
Determination of Cholesterol in Purified Lysosomes.
NPC1-deficient human fibroblasts were treated with DMSO or 3 mM BADG for 72 h. Cells were swollen in 10 mM Hepes, pH 7.4 for 10 min and broken by resuspension in 10 mM Hepes, pH 7.4, 150 mM NaCl, 1 mM ATP, 4 mM MgCl2, 1 mM EDTA, plus protease inhibitors followed by passage through a 22-gauge needle. Nuclei and unbroken cells were removed by centrifugation at 3,000 × g for 5 min. The supernatant was centrifuged in a Beckman TLA100.2 rotor for 15 min at 95,000 rpm; the pellet was resuspended in 10 mM Hepes, 2 M sucrose, pH 7.4, and layered onto a discontinuous sucrose gradient containing 500 μL 2 M, 750 μL 1.7 M, and 700 μL 0.5 M sucrose. Gradients were centrifuged in a TLS55 rotor for 1 h at 50,000 rpm; membranes at the 0.5 M and 1.7 M sucrose interface were collected as lysosomes. Total cholesterol content was determined using an Amplex Red Cholesterol Assay Kit (Invitrogen) according to the manufacturer and is reported per microgram of protein, determined using Advanced protein assay reagent (Cytoskeleton).
Cholesterol Ester Formation.
Wild-type and NPC1−/− CHO ldl-D cells were cultured in MEM alpha (Gibco) with 7.5% (vol/vol) FBS and 100 µM N-acetyl-galactosamine ± 8 µM galactose (8 × 104 cells/60-mm dish). After 24 h, cells were switched to the same medium with 5% (vol/vol) LPDS and assayed as described (31) with minor modification. After 72 h, 100 µg/mL LDL, 50 µM lovastatin, and 50 µM sodium mevalonate were added for 5 h. Cells were pulse labeled for 4 h with 0.1 mM sodium [1-14C]oleate (American Radiolabeled Chemicals)–albumin complex. Cells were washed two times with 2 mL 50 mM Tris, 150 mM NaCl, 2 mg/mL BSA, pH 7.4, followed by 2 mL 50 mM Tris, 150 mM NaCl, pH 7.4. Cells were extracted and rinsed with hexane-isopropanol (3:2), pooled, and evaporated. After resuspending each sample in 60 µl hexane, 4 µL of lipid standard containing 8 µg/mL triolein, 8 µg/mL oleic acid, and 8 µg/mL cholesteryl oleate was added. Samples were spotted onto a silica gel 60 plastic backed, thin layer chromatogram and developed in hexane. Cholesteryl oleate was identified with iodine vapor, scraped from chromatograms, and radioactivity determined by scintillation counting in 10 mL Biosafe II.
Acknowledgments
This research was funded by grants from the Ara Parseghian Medical Research Foundation and the National Institutes of Health (DK37332).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Carstea ED, et al. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science. 1997;277(5323):228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 3.Naureckiene S, et al. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290(5500):2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum AI, Maxfield FR. Niemann-Pick type C disease: Molecular mechanisms and potential therapeutic approaches. J Neurochem. 2011;116(5):789–795. doi: 10.1111/j.1471-4159.2010.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedland N, Liou HL, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc Natl Acad Sci USA. 2003;100(5):2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu S, Benoff B, Liou H-L, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem. 2007;282(32):23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Infante RE, et al. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283(2):1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 8.Kwon HJ, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137(7):1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deffieu MS, Pfeffer SR. Niemann-Pick type C 1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc Natl Acad Sci USA. 2011;108(47):18932–18936. doi: 10.1073/pnas.1110439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maxfield FR, van Meer G. Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol. 2010;22(4):422–429. doi: 10.1016/j.ceb.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilke S, Krausze J, Büssow K. Crystal structure of the conserved domain of the DC lysosomal associated membrane protein: Implications for the lysosomal glycocalyx. BMC Biol. 2012;10:62. doi: 10.1186/1741-7007-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwake M, Schröder B, Saftig P. Lysosomal membrane proteins and their central role in physiology. Traffic. 2013;14(7):739–748. doi: 10.1111/tra.12056. [DOI] [PubMed] [Google Scholar]
- 13.Neiss WF. A coat of glycoconjugates on the inner surface of the lysosomal membrane in the rat kidney. Histochemistry. 1984;80(6):603–608. [PubMed] [Google Scholar]
- 14.Schjoldager KT, Clausen H. Site-specific protein O-glycosylation modulates proprotein processing - deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim Biophys Acta. 2012;1820:2079–2094. doi: 10.1016/j.bbagen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Huet G, et al. Involvement of glycosylation in the intracellular trafficking of glycoproteins in polarized epithelial cells. Biochimie. 2003;85(3-4):323–330. doi: 10.1016/s0300-9084(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 16.Patsos G, et al. O-glycan inhibitors generate aryl-glycans, induce apoptosis and lead to growth inhibition in colorectal cancer cell lines. Glycobiology. 2009;19(4):382–398. doi: 10.1093/glycob/cwn149. [DOI] [PubMed] [Google Scholar]
- 17.Leteurtre E, et al. Induction of a storage phenotype and abnormal intracellular localization of apical glycoproteins are two independent responses to GalNAcalpha-O-bn. J Histochem Cytochem. 2003;51(3):349–361. doi: 10.1177/002215540305100310. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter AE, et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das A, Goldstein JL, Anderson DD, Brown MS, Radhakrishnan A. Use of mutant 125I-perfringolysin O to probe transport and organization of cholesterol in membranes of animal cells. Proc Natl Acad Sci USA. 2013;110(26):10580–10585. doi: 10.1073/pnas.1309273110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko DC, Gordon MD, Jin JY, Scott MP. Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events. Mol Biol Cell. 2001;12(3):601–614. doi: 10.1091/mbc.12.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, et al. Cessation of rapid late endosomal tubulovesicular trafficking in Niemann-Pick type C1 disease. Proc Natl Acad Sci USA. 2001;98(8):4466–4471. doi: 10.1073/pnas.081070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebrand C, et al. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002;21(6):1289–1300. doi: 10.1093/emboj/21.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingsley DM, Kozarsky KF, Hobbie L, Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- 24.Reddy JV, Ganley IG, Pfeffer SR. Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PLoS One. 2006;1:e19. doi: 10.1371/journal.pone.0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc Natl Acad Sci USA. 2009;106(46):19316–19321. doi: 10.1073/pnas.0910916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum AI, Zhang G, Warren JD, Maxfield FR. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc Natl Acad Sci USA. 2010;107(12):5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugii S, Reid PC, Ohgami N, Du H, Chang TY. Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2003;278(29):27180–27189. doi: 10.1074/jbc.M300542200. [DOI] [PubMed] [Google Scholar]
- 28.Kundra R, Kornfeld S. Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem. 1999;274(43):31039–31046. doi: 10.1074/jbc.274.43.31039. [DOI] [PubMed] [Google Scholar]
- 29.Eskelinen EL, et al. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol Biol Cell. 2004;15(7):3132–3145. doi: 10.1091/mbc.E04-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronda C, et al. Accelerating genome editing in CHO cells using CRISPR Cas9 and CRISPy, a web-based target finding tool. Biotechnol Bioeng. 2014;111(8):1604–1616. doi: 10.1002/bit.25233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]