Significance
In mammals, sialic acids are the most abundant terminal residues on cell-surface glycans and comprise “self-associated molecular patterns,” which protect the host from inappropriate activation of multiple immune pathways. In a striking illustration of molecular mimicry, a number of microbial pathogens recruit host sialic acids to decorate their surfaces and hide from the same immune responses. Prions are proteinaceous infectious agents, not conventional pathogens. This study found that sialylation of prions is enhanced upon their colonization of secondary lymphoid organs, via extracellular host sialylation machinery. Thus, prions may use strategies similar to other pathogens to camouflage themselves from the immune system, facilitating host invasion.
Keywords: prions, prion diseases, sialic acid, sialylated glycans, macrophages
Abstract
Sialylated glycans on the surface of mammalian cells act as part of a “self-associated molecular pattern,” helping the immune system to recognize “self” from “altered self” or “nonself.” To escape the host immune system, some bacterial pathogens have evolved biosynthetic pathways for host-like sialic acids, whereas others recruited host sialic acids for decorating their surfaces. Prions lack nucleic acids and are not conventional pathogens. Nevertheless, prions might use a similar strategy for invading and colonizing the lymphoreticular system. Here we show that the sialylation status of the infectious, disease-associated state of the prion protein (PrPSc) changes with colonization of secondary lymphoid organs (SLOs). As a result, spleen-derived PrPSc is more sialylated than brain-derived PrPSc. Enhanced sialylation of PrPSc is recapitulated in vitro by incubating brain-derived PrPSc with primary splenocytes or cultured macrophage RAW 264.7 cells. General inhibitors of sialyltranserases (STs), the enzymes that transfer sialic acid residues onto terminal positions of glycans, suppressed extrasialylation of PrPSc. A fluorescently labeled precursor of sialic acid revealed ST activity associated with RAW macrophages. This study illustrates that, upon colonization of SLOs, the sialylation status of prions changes by host STs. We propose that this mechanism is responsible for camouflaging prions in SLOs and has broad implications.
Prions are proteinaceous infectious agents that lack nucleic acids (1, 2). Mammalian prions replicate by recruiting the normal cellular form of the prion protein (PrP), denoted as PrPC, and converting it into the disease-associated form, denoted as PrPSc (3). Although the central nervous system (CNS) represents the main site of PrPSc replication and deposition, PrPSc is also found in peripheral organs, including cells and organs of the lymphoreticular system (4–7). Not only does PrPSc colonize secondary lymphoid organs (SLOs), the germinal centers of spleen and lymph nodes offer suitable environments for replicating PrPSc autonomously from the CNS (8–11). Prion colonization and replication in SLOs is important for several reasons. First, for prions acquired via peripheral routes, colonization of SLOs precedes invasion of the CNS (12–14). Second, SLOs are more permissive to prions acquired via cross-species transmission than the CNS (15). As such, the lymphoreticuar system represents a silent reservoir of infection, where prions could hide undetected while imposing a high risk of transmission through blood donation (16, 17). Finally, chronic inflammation could expand distribution of prions to inflamed tissues that are usually not affected by prions under normal conditions (18, 19).
PrPC is a sialoglycoprotein in which terminal sialic acids are attached to galactose residues of two N-linked glycans via α2–3 and α2–6 linkages (20–22). The ratio of diglycosylated to monoglycosylated and unglycosylated PrPC glycoforms increases with neuronal differentiation (23), yet the role of N-linked glycans and their sialylation for PrPC function is unknown. Upon conversion to PrPSc, the posttranslational modifications of PrPC, including its N-linked glycans, are carried over, giving rise to sialylated PrPSc (24, 25). The sialylation status of brain-derived PrPSc was found to closely resemble that of PrPC (25). In mammals, sialic acids are the most abundant terminal residues of cell membrane glycans. Sialic acids play an essential role in a broad range of cellular functions, but are especially important for neuronal plasticity and immunity (26). Among other functions, sialic acids on the surface of mammalian cells act as a part of a “self-associated molecular pattern,” helping the immune system to recognize “self” from “altered self” or “nonself” (27). A decline in sialic acid content represents one of the molecular signatures of “apoptotic-cell-associated molecular patterns” found in apoptotic or aging cells (28, 29). Removal of sialic acids from cell surface glycans exposes galactose residues that generate “eat me” signals for professional and nonprofessional macrophages. Examples include clearance of erythrocytes or platelets with reduced sialic acid residues by Kupffer cells (30, 31) or apoptotic neurons by microglia (32, 33). Pathogens without sialic acids are more likely to be detected as foreign, by virtue of recognition of their nonsialylated glycans that contribute to “pathogen-associated molecular patterns” and also because of the lack of the protective effects of sialic acids (34). In a striking illustration of molecular mimicry, a number of bacterial pathogens, including Escherichia coli K1 and group B Streptococcus, evolved enzymes to synthesize and express host-like sialic acids on their surfaces (34). Although ∼20% of the prokaryotic genomes sequenced evolved genes responsible for biosynthesis of sialic acids or their analogs (35), a number of pathogens, including Trypanosoma cruzi, Corynebacterium diphtheriae, Hemophilus influenzae, Niesseria meningitides, and others, recruit host sialic acid or its precursor to decorate their surfaces, presumably to hide from the immune system (34).
In the present study, we show that sialylation status of PrPSc is tissue-specific. Spleen-derived PrPSc was found to be more sialylated than brain-derived PrPSc. Enhanced sialylation of spleen-derived PrPSc occurred after conversion. Upon i.p. administration, the sialylation status of PrPSc sequestered in spleen changed within 6–24 h after injection. Enhanced sialylation of PrPSc could be recapitulated in vitro by incubating brain-derived PrPSc with primary splenocytes or cultured macrophage RAW cells. General inhibitors of sialyltranserases (STs), the enzymes that transfer sialic acid residues onto terminal positions, suppressed enhanced sialylation of PrPSc, suggesting that changes in PrPSc sialylation involve genuine ST activity. Application of a fluorescently labeled precursor of sialic acid revealed ST activity associated with RAW macrophages. Our data illustrate that, upon colonization of SLOs, prions alter their sialylation status by using host STs. We propose that this mechanism camouflages prions in SLOs and could account for the high permissiveness of SLOs to prions.
Results
Spleen-Derived PrPSc Is More Sialylated than Brain-Derived PrPSc.
PrPSc particles are multimers that range greatly in size (36). To compare sialylation states of spleen- and brain-derived PrPSc, scrapie materials were treated with proteinase K (PK) to remove PrPC, then denatured and analyzed by 2D gel electrophoresis (2D) (Fig. S1). In 2D, individual PrP molecules are separated according to their net charge in horizontal dimension. In PrP, each of the two N-linked glycans can carry up to five terminal sialic acid residues (21, 25), while an additional single sialic acid could be present on the glycosylphosphatidylinositol (GPI) anchor (37). Because individual PrP molecules contain from 0 to 10 sialic acids on their glycans, scrapie material is expected to display substantial charge heterogeneity (38). Indeed, PK-treated and denatured scrapie material showed multiple charge isoforms for both diglycosylated and monoglycosylated glycoforms (Fig. S1 and Fig. 1 A–E). Several charge isoforms were also seen for unglycosylated forms, a heterogeneity that is primarily attributed to the structural heterogeneity of the GPI-anchor (Fig. S1 and Fig. 1 A–E) (37). Nevertheless, as expected, the distribution of diglycosylated and monoglycosylated charged isoforms were shifted toward acidic pH relative to unglycosylated isoforms, and the heterogeneity of diglycosylated and monoglycosylated isoforms was much higher than that of unglycosylated forms. Both features are in agreement with glycan sialylation.
Fig. S1.
Schematic diagram illustrating 2D analysis of PrPSc. Scrapie materials are treated with PK to clear PrPC, denatured into monomers and then analyzed by 2D. In horizontal dimension of 2D, individual PrP molecules are separated according to their pI. Charge distribution of individual PrP molecules reports on contribution of sialoglycoforms in PrPSc particles. The charge distribution of monoglycosylated isoforms extends toward acidic pH beyond that of unglycosylated isoforms, and the charge distribution of diglycosylated isoforms extends toward acidic pH beyond that of monoglycosylated isoforms, according to the sialylation status of individual PrP molecules. For statistical analysis of sialylation status, diglycosylated charge isoforms are arbitrarily separated into two groups: hypersialylated (on the left of pI 7.5) and hyposialylated (on the right of pI 7.5). N-linked glycans are shown as blue lines, and terminal sialic acid residues are shown as red diamonds.
Fig. 1.
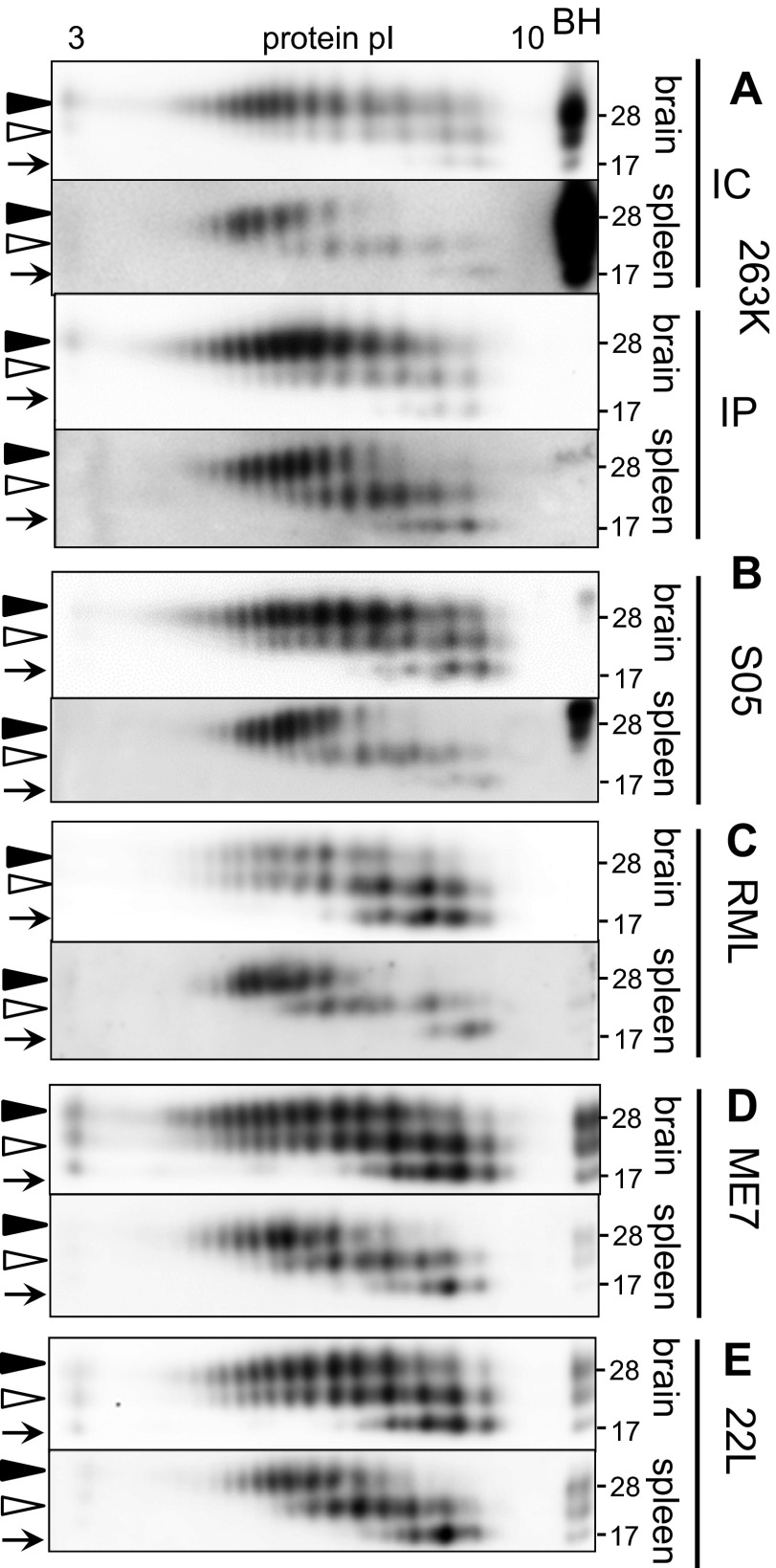
The 2D analysis of brain- and spleen-derived materials. Shown is 2D analysis of PK-treated brain- and spleen-derived materials from animals infected with hamster-adapted strain 263K via i.c. or i.p. routes (A) or synthetic hamster strain S05 (B), mouse-adapted strains RML (C), ME7 (D), or 22L (E), all inoculated via i.c. Western blots were stained with 3F4 antibody for hamster or ab3531 for mouse. Filled and open triangles mark diglycosylated and monoglycosylated forms, respectively, and arrows mark unglycosylated forms. A marker lane shows PK-treated scrapie brain homogenate (BH).
Animals were infected intracranially with mouse-adapted strains RML, ME7, and 22L; hamster-adapted strain 263K; or synthetic strain S05 (39), and their brain- and spleen-derived materials were analyzed by using 2D. Comparison of brain-derived materials revealed only minor strain- or species-specific differences in charged isoform distribution patterns (Fig. 1 A–E). Remarkably, regardless of the strain- or species-specific differences, brain-derived PrPSc glycoforms displayed a broader charge distribution than spleen-derived PrPSc, whereas spleen-derived diglycosylated glycoforms were shifted toward acidic pH relative to those of brain-derived glycoforms (Fig. 1 A–E). In contrast to diglycosylated glycoforms, unglycosylated glycoforms from spleen-derived material did not show acidic shift relative to the unglycosylated glycoforms from brain-derived material. With unglycosylated forms serving as internal references, acidic shift of diglycosylated isoforms indicates that the sialylation level of spleen-derived PrPSc was higher than that of brain-derived PrPSc. In animals inoculated intraperitoneally (i.p.), the sialylation patterns of spleen- or brain-derived PrPSc were very similar to those observed for animals inoculated intracranially, arguing that the spleen-specific sialylation pattern did not depend on the inoculation route (Fig. 1A). The spleen- and brain-specific 2D patterns were highly reproducible within animal groups inoculated with the same strains (Fig. S2).
Fig. S2.
The 2D analysis of 22L spleen- and brain-derived materials showing reproducibility of 2D gels. The 2D analysis of spleen- or brain-derived materials is shown. Three spleens and brains were from three animals inoculated i.c. with 22L. Western blots were stained ab3531. Filled and open triangles mark diglycosylated and monoglycosylated forms, respectively, and arrows mark unglycosylated forms.
Differences in lengths of PK-resistant cores of PrPSc deposited in brain and spleen could contribute to differences in tissue-specific patterns of charged isoforms. PK cleaves PrPSc at multiple sites located within residues 74–102 (40, 41). Although distribution of sites is largely dependent on strain-specific structure, other parameters such as cleavage conditions and PK concentration could also affect site distribution (41, 42). Because the pI values of unglycosylated forms were the same for brain- and spleen-derived materials (Fig. 1 A–E), it is unlikely that differences in pI distribution of diglycosylated and monoglycosylated forms were due to different PK cleavage sites. Nevertheless, to determine the extent to which distribution of charge isoforms could be affected by digestion conditions, 263K brain-derived material was treated with 2, 20, or 200 μg/mL PK. An increase in PK concentration did not change the distribution of charge isoform on 2D (Fig. S3A). This experiment argues that, even if differences in PK-cleavage sites between brain- and spleen derived materials exist, it is highly unlikely that such differences account for tissue-specific differences in 2D patterns.
Fig. S3.
Control experiments confirming that differences in charge distribution of brain- and spleen-derived PrPSc glycoforms are attributable to differences in sialylation. (A) The 263K brain-derived material was treated with 2, 20, or 200 μg/mL PK and analyzed by 2D. A marker lane shows PK-treated 263K brain homogenate (BH). (B) The 10% spleen homogenate from a noninfected animal was spiked with 0.2% of brain material from a S05-infected animal and analyzed by 2D. The samples were treated according to the procedure for spleen-derived PrPSc as described in Materials and Methods. Spleen- and brain-derived S05 materials are shown as references. (C) The 263K spleen- or brain-derived materials were treated with acetic acid or mock-treated and analyzed by 2D. (D) The 22L spleen- or brain-derived materials were treated with Arthrobacter ureafaciens sialidase or mock-treated and analyzed by 2D. All Western blots in A–C were stained with 3F4 antibody and in D with ab3531 antibody. Filled and open triangles mark diglycosylated and monoglycosylated forms, respectively, and arrows mark unglycosylated forms.
To make sure that differences in charge distribution of brain- and spleen-derived materials were not due to the procedure used for preparation of spleen-derived PrPSc, normal spleen homogenate was spiked with brain-derived PrPSc, treated according to the procedure for spleen-derived PrPSc. Incubation of brain-derived PrPSc with spleen homogenate did not alter the brain-specific sialylation pattern of PrPSc (Fig. S3B). Finally, to confirm that charge distribution on 2D reports on sialylation status, brain- and spleen-derived materials were treated with acetic acid (Fig. S3C) or Arthrobacter ureafaciens sialidase (Fig. S3D), according to the protocols that remove sialic acid residues chemically or enzymatically, respectively. As expected, the charge distributions shifted toward basic pH in both brain- and spleen-derived materials in both control experiments (Fig. S3 C and D). In summary, the above experiments established that brain- and spleen-derived prions are different with respect to their sialylation status.
PrPSc Sequestered by Spleen Undergoes Sialylation.
The higher level of sialylation of spleen- vs. brain-derived PrPSc could be due to (i) higher levels of sialylation of spleen- vs. brain-expressed PrPC, (ii) additional sialylation of PrPSc deposited in spleens, and/or (iii) fast metabolic clearance of PrPSc particles with low sialylation levels in SLOs (Fig. 2 A–C). The last hypothesis assumes that subpopulations of differentially sialylated PrPSc particles—i.e., particles sialylated above or below statistically averaged level—exist. The first hypothesis was not supported by previous data illustrating that spleen-expressed PrPC was equally or even less sialylated than brain-expressed PrPC (38). To test the second and third hypotheses, we asked whether the sialylation status of PrPSc changes upon colonization of SLOs. Wild-type mice were inoculated i.p. by using brain-derived 263K, and sialylation status of PrPSc sequestered by cells of the peritoneal cavity, spleen, and mediastinal lymph nodes was tested 6 or 24 h after injection. Hamster-adapted strain 263K was used for inoculating mice to avoid interference due to immediate replication of mouse prions in mouse SLOs (43).
Fig. 2.
Schematic diagram illustrating three alternative hypotheses. The higher level of sialylation of spleen- vs. brain-derived PrPSc could be due to the following reasons: Spleen-expressed PrPC is sialylated at higher levels than brain-expressed PrPC (A); PrPSc deposited in SLOs is sialylated by sialyltransferases (B); or, upon colonization of SLOs, PrPSc particles sialylated below the statistically average level are cleared faster than the PrPSc particles sialylated above the statistically average level (C). The third hypothesis assumes that differentially sialylated PrPSc particles exists. N-linked glycans are shown as blue lines, and terminal sialic acid residues are shown as red diamonds.
Relative to the charge isoform distribution of injected material, PrPSc recovered from all three sites (spleen, lymph nodes, and peritoneal cavity) showed isoform distribution shifted toward acidic pH on 2D by 24 h (Fig. 3). To analyze individual sialylation profiles, diglycosylated charge isoforms were separated arbitrarily into two categories, as described in Materials and Methods. Isoforms located toward acidic pH from pI 7.5 were designated as hypersialylated, and those toward basic pH were designated as hyposialylated (Fig. S1). The percentage of sum intensities of hypersialylated diglycosylated isoforms was used to estimate whether the shifts were statistically significant. PrPSc from spleen showed a more substantial shift than PrPSc sequestered by peritoneal cells or lymph nodes. Nevertheless, for all three sites, the shift toward acidic pH was statistically significant by 24 h after injection (Fig. 3). This result is in agreement with the hypothesis that PrPSc sequestered in SLOs is a substrate of STs and undergoes enhanced sialylation (Fig. 2B). However, this result cannot exclude the third hypothesis, according to which the acidic shift is due to faster clearance of PrPSc particles with low sialylation levels than particles with high sialylation levels (Fig. 2C).
Fig. 3.
PrPSc sequestered by SLOs undergoes sialylation. (Left) The 263K brain-derived material was administered i.p. to mice, and then scrapie material sequestered by cells of peritoneal cavity, lymph nodes, or spleen 6 or 24 h after injection was analyzed by 2D. (Top) The 2D analysis of the brain-derived 263K material used for IP injection is provided as a reference. (Right) Statistical analysis of sialylation status was performed as described in Materials and Methods. Data are presented as means ± SD. *P < 0.05 (n = 3). Western blots were stained with 3F4 antibody. Filled and open triangles mark diglycosylated and monoglycosylated forms, respectively, and arrows mark unglycosylated forms. Normal brain homogenate (BH) was loaded into the marker lane as a size reference.
If subpopulations of differentially sialylated PrPSc particles exist, they are expected to display a broad range of net charges. To test this, cation- and anion-exchange resins were used to isolate differentially charged and, therefore, differentially sialylated, PrPSc particles. Scrapie material was incubated with ion-exchange resins and then eluted by using increasing concentrations of NaCl (Fig. S4A). Peaks were eluted at 1.2 or 1.6 M NaCl from anion- or cation-exchange resins, respectively, and analyzed by using 2D. High concentrations of salt were required for eluting PrPSc, indicating that sorbtion of PrPSc to the resins was strong and depended on electrostatic interactions. Nevertheless, no differences with respect to the sialylation pattern were observed between materials eluted from cation- or anion-exchange resins on 2D (Fig. S4A). In previous studies, PrPSc produced in Protein Misfolding Cyclic Amplification using desialylated PrPC was found to be more sensitive to PK treatment than brain-derived PrPSc (38). We speculated that, if differentially sialylated PrPSc particles exist, they might have different physical properties, such as solubility or PK resistance. If this hypothesis is the case, fast proteolytic clearance of soluble, proteolytically sensitive PrPSc subfractions in SLOs could account for the acidic shifts on 2D. To test this hypothesis, 263K brain material was subjected to ultracentrifugation, and then the pellet and supernatant were analyzed by 2D. The distribution of charge isoforms was remarkably similar for PrPSc particles in both the pellet and supernatant (Fig. S4B). By using an alternative format, RML brain material was centrifuged according to the protocol that separates PK-sensitive and -resistant PrPSc particles (Fig. S4C). Again, no notable differences were observed in the distribution of charge isoforms between PK-sensitive and -resistant PrPSc (Fig. S4C). In summary, these results did not support the hypothesis that differentially sialylated PrPSc particles exist within brain material. Instead, these data suggest that individual PrPSc particles consist of PrP molecules with a broad range of sialylation levels.
Fig. S4.
The 2D analysis of brain-derived scrapie material fractionated according to charge, solubility, or PK-resistance. (A) The 263K brain-derived material was adsorbed to cation- or anion-exchanged resins and eluted by using increasing concentrations of NaCl (Top). Materials eluted by 1.6 or 1.2 M NaCl from cation- or anion-exchange resins, respectively, were analyzed by using 2D gels (Middle and Bottom). No differences in sialylation patterns were observed between the fractions eluted from cation- or anion-exchange resins. (B) The 263K brain-derived material was ultracentrifuged as described in Materials and Methods, and then pellet and supernatant were analyzed by 2D. No differences in sialylation patterns were observed between pellet and supernatant. (C, Left) PK-sensitive and -resistant PrPSc fractions were prepared by using RML brain-derived materials as described in Materials and Methods (Western blot). (C, Right) PK-res material treated with 20 µg/mL PK and PK-sen material treated with 2 µg/mL PK were analyzed by 2D. Western blots were stained with 3F4 antibody for hamster samples (A and B) and ab3531 for mouse samples (C). Filled and open triangles mark diglycosylated and monoglycosylated forms, respectively, and arrows mark unglycosylated forms. A marker lane shows PK-treated scrapie brain homogenate (BH).
Primary Splenocytes, Peritoneal Cells, and Cultured Macrophages Sialylate PrPSc.
To test whether PrPSc sialylation observed in vivo can be recapitulated in vitro, mouse splenocytes and peritoneal cells were isolated and cultured in vitro. Because macrophages are known to sequester and transport PrPSc to SLOs, the mouse macrophage RAW cell line was used in parallel experiments. Brain-derived PrPSc material was incubated with primary splenocytes, peritoneal cells, or RAW cells for 2 h, then cells were washed, medium was replaced (this time point is designated as 0 h), and the cells were cultured in fresh medium for 24 or 48 h. The distribution of PrPSc charge isoforms on 2D exhibited a notable, statistically significant shift toward acidic pH after 24 and 48 h in all cultured cells in comparison with the 0-h time points (Fig. 4 A–D). To make sure that this acidic shift was not due to a spontaneous “aging” or enzymatic activity that might be present in FBS, PrPSc was incubated in PBS alone or in the presence of FBS for 48 h in the absence of cells and assayed by 2D. No change in sialylation pattern was observed on 2D after 48 h of incubation in PBS or FBS (Fig. S5A). To rule out the possibility that PrPSc sialylation is due to sialyltransferases released by dead or spontaneously lysed cells, brain-derived PrPSc was incubated with cell medium collected after culturing confluent RAW cells for 48 h. Again, no change in sialylation pattern was observed on 2D (Fig. S5B). In another control experiment, brain-derived PrPSc materials were incubated with N2a cells, which are of neuronal origin, and probed by 2D. After 48 h of incubation, a very minor shift in the charge distribution was noticed, which was not statistically significant (Fig. S5C). These experiments established that the shift of PrPSc glycoforms toward acidic pH was due to cell-associated enzymatic activity and specific to splenocytes, peritoneal cells, or macrophages.
Fig. 4.
Primary splenocytes and cultured macrophages sialylate PrPSc. The 263K brain-derived material (A) was incubated with primary splenocytes (B), peritoneal cells (C), or RAW cultured cells (D) for 2 h, and then scrapie material was removed, cells were rinsed, and culture medium was replaced. (Left) The sialylation status of 263K material sequestered by cells was analyzed at 0, 24, or 48 h of culturing cells after replacing the medium. (Right) Statistical analysis of sialylation status was performed as described in Materials and Methods. Data are presented as means ± SD. *P < 0.05; **P < 0.005 (n = 3). All Western blots were stained with 3F4 antibody. Filled and open triangles mark diglycosylated and monoglycosylated forms, respectively, and arrows mark unglycosylated forms. Normal brain homogenate (BH) was loaded into the marker lane as a size reference.
Fig. S5.
Control experiments for testing whether changes is sialylation is attributed to cell-associated sialyltransferase activity. (A) The 263K brain-derived material was incubated in PBS or FBS for 48 h under the same dilution and temperature conditions as in experiments shown in Fig. 4 and analyzed by 2D. No differences in sialylation status were observed on 2D (Left) or by statistical analysis of sialylation status (Right; n = 3). (B) The 263K brain-derived material was incubated for 24 h in fresh cell culture medium or cell culture medium collected after culturing confluent RAW cells for 48 h and analyzed by 2D. No differences in sialylation status were observed on 2D (Left) or by statistical analysis of sialylation status (Right; n = 3). (C) The 263K brain-derived material was incubated with N2a neuroblastoma cell line for 2 h, and then scrapie material was removed, cells were rinsed, and culture medium was replaced. (C, Left) The sialylation status of 263K material sequestered by cells were analyzed at 0 or 48 h of culturing cells after replacing the medium using 2D. (C, Right) Statistical analysis of sialylation status in A–C was performed as described in Materials and Methods. Data are presented as means ± SD. #P > 0.05 (n = 3). All Western blots were stained with 3F4 antibody. Filled and open triangles mark diglycosylated and monoglycosylated forms, respectively, and arrows mark unglycosylated forms. Normal brain homogenate (BH) was loaded into the marker lane as a size reference.
To confirm that the acidic shift in distribution of PrPSc glycoforms was due to ST activity, two general ST inhibitors, CTP and peracetylated 3Fax-Neu5Ac (FPN), were used (44–49). In the absence of inhibitors, the distribution of PrPSc charge glycoforms showed statistically significant shift toward acidic pH after incubation with RAW cells or primary splenocytes for 24 h as before (Fig. 5 A and B). However, in the presence of CTP or FPN, the changes in glycoform distribution patterns were very minor, if any, and not statistically different in comparison with the distribution at the 0-h time point (Fig. 5 A and B). This experiment confirmed that the shift in PrPSc glycoform distribution depends on genuine sialyltransferase activity.
Fig. 5.
ST inhibitors suppressed PrPSc sialylation. The 263K brain-derived material was incubated with RAW cells (A) or primary splenocytes (B) for 2 h, and then scrapie material was removed, and cells were rinsed and cultured for additional 24 h in the absence or presence of CTP or FPN inhibitors. (Left) The sialylation status was analyzed by 2D. (Right) Statistical analysis of sialylation status is performed as described in Materials and Methods. Data are presented as means ± SD. *P < 0.05; #P > 0.05 (n = 3). All Western blots were stained with 3F4 antibody. Filled and open triangles mark diglycosylated and monoglycosylated forms, respectively, and arrows mark unglycosylated forms.
RAW Macrophages Exhibit Sialyltransferase Activity.
STs were traditionally thought to localize exclusively in Golgi (50). However, in a growing number of studies, ST activities were found on the surface of a wide variety of immune system cells (48, 49, 51–53). To test whether RAW cells exhibit surface ST activity, we used a fluorescently labeled precursor of sialic acid, CMP-5-FITC-neuraminic acid (CMP-5-FITC-NEU), which is a membrane-excluded and sialidase-resistant substrate (54). In the presence of CMP-5-FITC-NEU, RAW cells exhibited considerable fluorescence (Fig. 6). However, upon application of CTP, the fluorescence levels dropped more than fourfold (Fig. 6). When RAW cells were pretreated with bacterial neuraminidase, an enzyme that cleaves α2–3- and α2–6-linked sialic acid residues from cell surface glycoproteins and glycolypids, the cell-associated CMP-5-FITC-NEU fluorescence was sevenfold higher relative to the cells not treated with neuraminidase (Fig. 6). Remarkably, in the presence of CTP, the fluorescence level in neuraminidase-pretreated cells dropped by 30-fold from 80,220 to ∼2,603 (Fig. 6). Although some of the fluorescence in the presence of CMP-5-FITC-NEU could be attributed to uptake and use of CMP-5-FITC-NEU by intracellular sialyltransferases, the strong effect of the ST inhibitor and pretreatment with bacterial neuraminidase argues that RAW cells display genuine ST activity on the cell surface. This result is consistent with previous studies that attributed CMP-5-FITC-NEU fluorescence to ST activity on the cell surface (48, 49).
Fig. 6.
Flow cytometry analysis of ST activity associated with RAW cells. Autofluorescence of RAW cells in the absence of CMP-5-FITC-NEU (red), RAW cells incubated with CMP-5-FITC-NEU (blue), or ST inhibitor CTP and CMP-5-FITC-NEU (magenta); cells pretreated with neuraminidase and then incubated with CMP-5-FITC-NEU (orange); or cells pretreated with neuraminidase and then incubated with CTP and CMP-5-FITC-NEU (black). The experiments were repeated twice.
Discussion
The present study provides, to our knowledge, the first experimental illustration that the primary structure of PrPSc—in particular, its sialylation status—is changed after conversion. Direct structural analysis of the N-linked glycoforms from spleen-derived and in vitro-produced materials with enhanced sialylation will be needed in future studies to define the details of what exactly is occurring. Nevertheless, enhanced sialylation of PrPSc was observed in SLOs regardless of prion strain, host species, or inoculation route tested. The alternative explanations to the hypothesis on postconversion sialylation are (i) that spleen-expressed PrPC is sialylated at a higher level than the brain-derived PrPSc or (ii) fast metabolic clearance of PrPSc particles with low sialylation levels in SLOs (Fig. 2 A and C). The first alternative was not supported by the previous studies (38). The second alternative assumes that differentially sialylated PrPSc particles exist in brain-derived material. However, the experiments on fractionation of brain-derived PrPSc using three different methods did not support this hypothesis. Moreover, the model on differentially sialylated PrPSc particles assumes that PrPC molecules are sorted out during PrPSc replication according to their sialylation status, where hyposialylated PrPC would be recruited by hyposialylated PrPSc particles and hypersialylated PrPC would be recruited by hypersialylated PrPSc, which is highly unlikely. Finally, experiments with sialyltransferease inhibitors linked the acidic shift of charged isoform distribution to sialyltransferase activity. Because sialylation plays multiple roles in immunity (26, 34), the present finding that spleen-deposited PrPSc is sialylated at higher levels than brain-derived PrPSc has broad implications.
Prions are not conventional pathogens, yet sialylation of prions in SLOs displays remarkable parallels with molecular mimicry of microbial pathogens. A number of pathogens decorate their surfaces by using host sialic acid in an attempt to hide from the immune system (34). Surfaces of mammalian cells and glycoproteins in blood circulation are densely sialylated (26). Sialylation represents a part of the self-associated molecular pattern used by cells of the immune system for recognizing self from altered self or nonself (28, 34). Similar to molecular mimicry of microbial pathogens, sialylation of prions in SLOs might play a similar role. This mechanism could be particularly important for cross-species prion transmission between mammals and human. Because of an irreversible mutation in the gene encoding N-acetylneuraminic acid hydroxylase that occurred during evolution from primates to human, the type of sialic acid produced in peripheral tissues of humans (N-acetyl neuraminic acid; Neu5Ac) is different from the type synthesized by other mammalian species (N-glycolylneuraminic acid; Neu5Gc) (55). As a result, human-specific differences evolved in the binding sites of human Siglecs, the family of sialic acid-binding proteins, for selective recognition of Neu5Ac over Neu5Gc (55). It is not known whether differences in the type of sialic acid contribute to the species barrier of prion transmission between animals and human. Nevertheless, it is tempting to speculate that upon cross-species transmission of prions to humans, enhanced sialylation of PrPSc “humanizes” prions of nonhuman origin, helping them to deceive the immune system.
Transmission of prions between species is more difficult than within the same species because of the species barrier. Remarkably, lymphoid tissues were found to be more susceptible to cross-species prion transmission than nerve tissues (15). Although the mechanisms behind high permissiveness of lymphoid tissues to prions are not known, prions acquired via cross-species transmission can persist stably and silently in SLOs for a long time in the absence of neuroinvasion (15, 56, 57). Aged animals that are known to be less susceptible to prion infection than young animals can also accumulate prions within their lymphoid tissues, despite lack of prions in their brains (58). Moreover, the lymphoid tissues display a higher capacity than nerve tissues to replicate prions after low-dose infection (59). The subclinical disease acquired via cross-species transmission or with low prion doses might never lead to neuroinvasion or clinical disease. Nevertheless, colonization of SLOs by prions poses a real risk due to the possibility of silent spread of infection through donation of blood or organs or infected surgical instruments (16, 56, 57, 60). The higher permissiveness of lymphoid tissues than the CNS is very surprising, considering that the expression level of PrPC in lymphoid tissues is substantially lower than in brain (61–64) and that lymphoid cells are specialized in recognizing and degrading pathogens. Bearing in mind that sialylation status controls the lifetime of glycoclusters in blood circulation (65), our study suggests that enhanced sialylation of PrPSc in SLOs might contribute to the high permissiveness of lymphoid tissues to prions.
There are a number of mechanisms by which sialylation protects pathogens from the host (27). Among them is recruitment of factor H that dampens activation of alternative complement pathways by recognizing molecular patterns containing sialic acids on surfaces of host cells or pathogens (66, 67). Alternative mechanisms involve engaging inhibitory Siglecs (sialic acid recognizing Ig-like lectins) that likewise recognize sialic acid patterns and suppress innate immune cells (68, 69). In addition, sialylation can mask glycan ligands for Gal/GalNAc receptors or for galectins, a family of secreted proteins that recognize galactose and its derivatives (70). Finally, sialylation of glycans on the pathogen surface can inhibit production and/or binding of antibodies against asialoglycans (55). Of these four mechanisms, the first three could contribute to protection of prions due to enhanced PrPSc sialylation in SLOs.
Several types of cells—including macrophages, monocytes, B lymphocytes, and dendritic cells—capture prions at initial entry sites and transport them to SLOs (43, 71–73). In addition to trafficking of prions to SLOs, macrophages play a role in degrading prions (74–77). The present study suggests that, in addition to transporting and degrading prions, macrophages are also involved in sialylating PrPSc. Indeed, in vivo changes in sialylation patterns were observed in the peritoneal cavity, lymph nodes, and spleen—the sites with considerable populations of macrophages. Similar changes in PrPSc sialylation were also observed in vitro upon incubation of PrPSc with primary splenocytes or macrophage RAW cells. RAW cells showed significant ST activity on their surfaces. ST activity has also been found on the surface of dendritic cells and lymphocytes (48, 49). Considering that a number of cells of immune system—including B lymphocytes, monocytes, plasmacytoid dendritic cells, natural killer cells, and others—sequester and disseminate PrPSc (43, 72, 78), it is likely that sialylation of PrPSc is not limited to macrophages. Although degradation of PrPSc by macrophages has been well established, the present study suggests that macrophages might also play a protective role by enhancing sialylation of PrPSc in parallel to their role in degrading PrPSc. In a recent study, Siglec-1 (CD169) expressed on the surface of primary microphages was shown to be responsible for capturing sialylated viral pathogens and facilitating their intercellular transfer (79). It would be worth testing the possibility that enhanced sialylation of PrPSc in SLOs could also facilitate their intercellular transfer.
The present work suggests that enhanced sialylation of PrPSc occurs on the cell surface rather than intracellularly. Traditionally, STs were thought to localize exclusively within the intracellular secretory apparatus (50). A growing number of studies report ST activity in serum or on surfaces of the cells of immune system, including polymorphonuclear leukocyte, monocyte-derived dendritic cells, lymphocytes, and T cells (48, 49, 51–53, 80). Presence of ST on the cell surface or in serum allows rapid restoration or remodeling of the sialylation status of cells without de novo synthesis of cell surface glycoconjugates (48, 49, 81). ST expression and sialylation of cell surfaces are finely regulated during cell maturation, differentiation, and inflammation (48, 49, 81). Notably, systemic inflammatory response involves secretion of STs into serum, a process that modulates the inflammatory response (48, 81–83). It would be interesting to determine whether extracellular STs contribute to prion colonization of inflamed tissues via altering the sialylation status of PrPSc. Because the family of mammalian STs includes 20 enzymes that have redundant specificity (84), identifying those that are involved in enhancing sialylation of PrPSc would involve considerable effort.
If PrPSc is a subject of extracellular sialylation in SLOs, it is not clear why PrPC, which is expressed on a cell surface, is not targeted by extracellular STs to the same extent as PrPSc. In mouse-derived primary splenocytes, the half-life of PrPC was found to be only 1.5–2 h (85), which is much shorter than the half-life of PrPSc. In addition, the population of cells that express PrPC could be different from the population of cells that express cell surface STs. In spleen and lymph nodes, PrPC is primarily expressed by follicular dendritic cells (10), which are resident cells of germinal centers and different from dendritic cells of myeloid origin. It is not known whether follicular dendritic cells express cell surface STs.
Materials and Methods
Scrapie Materials.
Weanling Golden Syrian hamsters or C57BL/6NHsd mice were inoculated intracranially or i.p. under 2% O2/4 minimum alveolar concentration isoflurane anesthesia by using scrapie brain homogenates of hamster- or mouse-adapted strains, respectively. Animals were euthanized at the terminal stage of the disease, and organs were collected for 2D analysis. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (86). The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore (Assurance no. A32000-01; Permit no.: 0215002).
Preparation of Brain and Spleen Materials for 2D.
We prepared 10% (wt/vol) scrapie brain or spleen homogenates in PBS by using glass/Teflon homogenizers attached to a cordless 12-V compact drill (Ryobi) as described (87). For 2D electrophoresis of brain-derived material, an aliquot of 10% (wt/vol) homogenate was diluted with nine volumes of 1% (wt/vol) Triton X-100 in PBS, sonicated for 30 s inside Misonix S-4000 microplate horn (Qsonica), and treated with 20 µg/mL PK (New England BioLabs) for 30 min at 37 °C. For 2D electrophoresis of spleen-derived material, 250 µL of 10% (wt/vol) homogenate was diluted 1:1 with PBS, aliquotted into 0.2-mL thin-wall PCR tubes, sonicated for 30 s inside Misonix S-4000 microplate horn, and combined in one tube, which was subjected to a 30-min centrifugation at 16,000 × g at 4 °C. The pellet was resuspended in 25 µL of 1% (wt/vol) Triton in PBS and treated with 20 µg/mL PK for 30 min at 37 °C. Resulting brain or spleen samples were supplemented with 4× SDS loading buffer, heated for 10 min in a boiling water bath, and processed for 2D electrophoresis as described below.
Performance of 2D Electrophoresis.
Samples of 25-µL volume prepared in loading buffer as described above were solubilized for 1 h at room temperature in 200 µL of solubilization buffer [8 M urea, 2% (wt/vol) CHAPS, 5 mM TBP, 20 mM Tris, pH 8.0), alkylated by adding 7 µL of 0.5 M iodoacetamide, and incubated for 1 h at room temperature. Then, 1,150 µL of ice-cold methanol was added, and samples were incubated for 2 h at −20 °C. After centrifugation at 16,000 × g at 4 °C, supernatant was discarded, and the pellet was resolubilized in 160 µL of rehydration buffer [7 M urea, 2 M thiourea, 1% (wt/vol) DTT, 1% (wt/vol) CHAPS, 1% (wt/vol) Triton X-100, 1% (vol/vol) ampholyte, and trace amount of Bromophenol Blue]. Fixed immobilized precast IPG (immobilized pH gradient) strips [catalog (cat.) no. ZM0018; Life Technologies] with a linear pH gradient 3–10 were rehydrated in 155 µL of resulting mixture overnight at room temperature inside IPG Runner cassettes (cat. no. ZM0008; Life Technologies). Isoelectrofocusing (first dimension separation) was performed at room temperature with rising voltage (175 V for 15 min, then 175–2,000-V linear gradient for 45 min, and then 2,000 V for 30 min) on Life Technologies Zoom Dual Power Supply using the XCell SureLock Mini-Cell Electrophoresis System (cat. no. EI0001; Life Technologies). The IPG strips were then equilibrated for 15 min consecutively in (i) 6 M urea, 20% (vol/vol) glycerol, 2% SDS, 375 mM Tris⋅HCl, pH 8.8, 130 mM DTT, and (ii) 6 M urea, 20% (vol/vol) glycerol, 2% SDS, 37 5mM Tris⋅HCl, pH 8.8, and 135 mM iodoacetamide, and loaded on 4–12% Bis-Tris ZOOM SDS/PAGE precast gels (cat. no. NP0330BOX; Life Technologies). For the second dimension, SDS/PAGE was performed for 1 h at 170 V. Immunoblotting was performed as described elsewhere; blots were stained by using 3F4 (cat. no. SIG-39600; Covance) antibody for hamster and ab3531 antibody (Abcam) for mouse tissues. To determine a specific 2D pattern and to check reproducibility of the procedure, at least two brains or spleens from for each animal group were analyzed.
Desialylation of PrPSc with Acetic Acid.
PK-treated brain and spleen materials were prepared as for 2D electrophoresis. PK digestion was stopped by addition of 5 mM PMSF, then the samples were denatured by incubating for 10 min at 95 °C in the presence of 0.5% SDS and 40 mM DTT, followed by the addition of 1% (vol/vol) Nonidet P-40. Then the samples were supplemented with 1% (vol/vol) acetic acid and incubated at 100 °C for 1 h with 1,000 rpm shaking in Eppendorf thermomixer to achieve mild acid hydrolysis of sialic acids (80). Mock-treated samples were denatured by the same procedure, but not treated with acetic acid.
Desialylation of PrPSc with Sialidase.
We diluted 10% (wt/vol) scrapie brain and spleen materials 10-fold in 1× PBS supplied with 1% (vol/vol) Triton X-100 and supplemented with 25 μg/mL PK. After 30 min at 37 °C, PK digestion was stopped by the addition of 5 mM PMSF. Then the samples were denatured by incubating for 10 min at 95 °C. The subsequent treatment with Arthrobacter ureafaciens sialidase (cat. no. P0722L; New England Biolabs) was as follows. After addition of 10% (vol/vol) sialidase buffer GlycoBuffer1 supplied by the enzyme manufacturer, 200 units/mL sialidase were added, followed by incubation on a shaker at 37 °C for 10–12 h. Mock-treated samples were processed by the same procedure, but not supplied with the sialidase.
Ion-Exchange Chromatography.
We diluted 10% (wt/vol) 263K brain homogenates 10-fold in 25 mM Tris, pH 7.4, buffer supplied with 1% (wt/vol) Triton X-100 and sonicated for 30 s at 170 W energy output in the microplate horn (Misonix S-4000). The samples were then incubated with PK (10 µg/mL, 37 °C, 1 h) (cat. no. P8107S; New England Biolabs). The digestion was stopped by adding 2 mM PMSF. The samples were filtered through a 22-µm syringe filter (cat. no. SLGV033RB; Millipore). The cation or anion exchange mini columns (cat. no. 90008 and 90010, respectively; Thermo Scientific) were pre-equilibrated with Tris pH 7.4 and loaded with 400 µL of scrapie material prepared as described above, and centrifuged at 2,000 × g for 5 min. Then the columns were washed with 400 µL of Tris pH 7.4 buffer and centrifuged at 2,000 × g for 5 min. The columns were then washed consecutively with 400-µL volumes of the following concentrations of NaCl: 0.4, 0.8, 1.2, and 1.6 M prepared in Tris, pH 7.4. The material was collected by centrifugation, supplied with SDS sample buffer, heated for 10 min in a boiling water bath, and analyzed by SDS/PAGE or 2D electrophoresis.
Ultracentrifugation.
We solubilized 10% (wt/vol) 263K brain homogenates by addition of 9 volumes of 10% (wt/vol) sarkosyl in PBS and sonication for 30 s inside a Misonix S-4000 microplate horn. The samples were precleared by 2 min centrifugation at 500 × g in a tabletop centrifuge, and then 100 µL of the supernatant were diluted with 900 µL PBS and centrifuged for 20 min at 20 °C at 32,400 rpm by using a TLA 100.3 rotor in an Optima TLX microultracentrifuge (Beckman). The supernatant containing solubilized PrPSc and the pellet containing insoluble PrPSc were collected. The pellet was resuspended in 200 µL of 1% (wt/vol) sarkosyl in PBS, treated with 20 µg/mL PK for 30 min at 37 °C, and supplemented with 4× SDS sample buffer. A total of 60 µL of the supernatant were treated with 20 µg/mL PK for 30 min at 37 °C, and concentrated by precipitation with 4 volumes of ice-cold acetone, incubated overnight at −20 °C and subsequently centrifuged for 30 min at 16,000 × g. The resulting pellet was resuspended in 1× SDS loading buffer.
Separation of PK-Sensitive and -Resistant PrPSc.
We solubilized 10% (wt/vol) RML brain homogenates by addition of 9 volumes of 10% (wt/vol) sarkosyl in PBS and sonication for 30 s inside Misonix S-4000 microplate horn. The samples were precleared by 2 min centrifugation at 500 g in a tabletop centrifuge, and then 100 µL of the supernatant was diluted with 900 µL of PBS and centrifuged for 90 min at 16,000 × g at 4 °C. The supernatant containing solubilized PrPSc and the pellet containing insoluble PrPSc were collected. The pellet was resuspended in 50 µL of 1% (wt/vol) sarkosyl in PBS, treated with 20 µg/mL PK for 30 min at 37 °C, and supplemented with 4× SDS loading buffer. A total of 60 µL of the supernatant was treated with 2 or 20 µg/mL PK for 30 min at 37 °C, concentrated by precipitation with 4 volumes of ice-cold acetone, incubated overnight at −20 °C, and subsequently centrifuged for 30 min at 16,000 × g. The resulting pellet was resuspended in 1× SDS loading buffer.
IP Inoculation of Scrapie Material and Harvest of SLOs.
We diluted 10% (wt/vol) 263K brain homogenate by using sterile PBS to a final concentration of 4% (wt/vol). A total of 500 µL per animal was injected i.p. into 4-wk-old C57BL/6NHsd mice by using 1-mL syringes with a 26-gauge needle (cat. no. 309625; BD Biosciences). After 6, 24, or 48 h after inoculations, spleens, mediastinal lymph nodes, and peritoneal cavity cells were collected. Spleens were homogenized to make 10% (wt/vol) tissue suspension in PBS with protease inhibitors (cOmplete, Mini, EDTA-free; cat. no. 04693159001; Roche) using glass/Teflon homogenizers. A total of 200 µL of 10% (wt/vol) spleen homogenate was diluted twice in PBS, sonicated for 30 s using Misonix S-4000 microplate horn sonicator and centrifuged at 16,000 × g, for 30 min at 4 °C. The pellet was collected and resuspended in 50 µL of PBS with 1% (wt/vol) Triton X-100 and 1 mM EDTA, then digested with PK (20 µg/mL, 37 °C, 30 min) and precipitated by acetone overnight as described above. The pellet was collected and dissolved in 30 µL of 1xSDS loading buffer, heated for 10 min in a boiling water bath and analyzed by 2D. Cells from the peritoneal cavity were collected and lysed as 1 × 106 cells per 200 µL of Mammalian Protein Extraction Reagent (M-PER) lysis buffer (cat. no. 78501; Thermo Scientific); the lysate was digested with PK (20 µg/mL, 37 °C, 30 min) and concentrated threefold by acetone precipitation. The pellet was dissolved in 1× SDS loading buffer, heated for 10 min in a boiling water bath, and analyzed by 2D. For mediastinal lymph nodes, the residual connective tissue was removed, and nodes were homogenized in PBS, 1% (wt/vol) Triton X-100, 1 mM EDTA with protease inhibitors using bead beater (Model 2412PS-12W-B30; Biospec Products) and 0.1-mm glass beads (cat. no. 11079101; Biospec Products). This suspension was centrifuged at 200 × g for 1 min to remove beads; supernatant was collected and digested with PK (20 µg/mL, 37 °C, 30 min). PrPSc in lymph node samples was enriched sixfold by acetone precipitation, and the enriched lysates were denatured in 1× SDS loading buffer, heated for 10 min in a boiling water bath, and analyzed by 2D. The experiment was repeated twice using two mice for each time point in each experiment.
Isolation of Mouse Primary Splenocytes.
Healthy 5-wk-old female C57BL/6NHsd mice were euthanized with CO2 inhalation. Dissected spleens were transferred to a 10-cm cell culture dish containing 10 mL of cold PBS with 2 mM EDTA and homogenized by using a plunger of a 5 mL syringe. Cell suspensions were collected in 15-mL tubes and centrifuged at 1,500 × g for 5 min. Red blood cells from the pellet were lysed by using RBC lysis buffer (cat. no. R7757; Sigma) as described by the manufacturer, then the cells were resuspended in cell culture medium DMEM (cat. no. 10-013-CV; Corning-Cellgro) supplemented with 10% (vol/vol) FBS (cat. no. 10082-147; Life Technologies), 1% (vol/vol) Glutamax (cat. no. 35050-061; Life Technologies), and 1% (wt/vol) antibiotics (cat. no. 15240-062; Life Technologies), and transferred to 25-cm2 cell culture flasks (cat. no. SIAL0639; Sigma) for adherence. Nonadherent cells were removed; adherent cells were collected and checked for viability with trypan blue (cat. no. T8154; Sigma).
Culturing of RAW 264.7 Macrophages and Primary Splenocytes with PrPSc.
RAW 264.7 cells or primary splenocytes were seeded in six-well plates (cat. no. 3506; Corning Costar) at densities of ∼1.5 × 105 or ∼0.8 × 106 cells per well, respectively, and cultured overnight in culture medium [DMEM, 10% (vol/vol) FBS, 1% (vol/vol) Glutamax, and 1% (wt/vol) antibiotics] at 37 °C and 5% CO2 in humidified cell culture incubator. When RAW cells reached ∼50% confluence, the culture medium was changed to new medium containing 0.1% (wt/vol) 263K brain homogenate, cells were incubated for 2 h, then medium was removed, and cells were washed twice with prewarmed PBS to remove unbound PrPSc particles. Then, cells were cultured in culture medium [DMEM, 5% (vol/vol) FBS, 1% (vol/vol) Glutamax, and 1% (wt/vol) antibiotics] for 24 or 48 h. Cells were lysed in 200 µL of M-PER lysis buffer (cat. no. 78501; Thermo Scientific). A total of 90 µL of each lysate was treated with 20 µg/mL PK for 30 min at 37 °C under moderate shaking followed by precipitation overnight with acetone at −20 °C. Precipitated materials were centrifuged for 30 min at 16,000 × g at 4 °C. Pellets were air dried, dissolved in 30 µL of 1× SDS loading buffer, heated for 10 min in a boiling water bath, and analyzed by 2D.
In experiments with ST inhibitors, RAW 264.7 cells were incubated with 0.1% (wt/vol) 263K brain homogenate for 2 h and then washed as described above, and then CTP (cat. no. SIG-C1506; Sigma) or 3Fax-peracetyl Neu5Ac (cat. no. 566224; Millipore) was added to the medium to a final concentrations of 2 mM or 128 µM, respectively. Cells were cultured for 24 h in the presence of inhibitors and then harvested and analyzed as described above.
Flow Cytometry Analysis of ST Activity on RAW Cells.
A fluorescent precursor CMP-5-FITC-NEU was prepared and used as described with minor modifications (48, 49, 88). RAW cells were suspended at 5 × 105 cells per 100 µL of serum-free medium. For neuraminidase treatment, cells were suspended at 5 × 106 per mL into 300 µL of serum free medium and treated with 200 unit/mL Clostridium perfringens neuraminidase (α2–3,6,8 neuraminidase, cat. no. P0720L; New England Biolabs) for 90 min at 37 °C in CO2 incubator. After treatment, cells were washed with serum-free medium and divided into tubes containing 100 µL of serum-free medium at a density of 5 × 105 cells per tube. CMP-5-FITC-NEU was added to neuraminidase-treated or untreated cells to a final concentration of 10 µM, and cells were incubated for 2 h at 37 °C in a CO2 incubator with gentle intermittent shaking. In experiments with ST inhibitor, neuraminidase-treated or nontreated RAW cells were incubated with 5 mM CTP for 30 min before addition of CMP-5-FITC-NEU, and 5 mM CTP was maintained in culture medium during 2-h incubations with CMP-5-FITC-NEU. After incubation with CMP-5-FITC-NEU, 1 mL of cold serum-free medium was added to cells, and the cells were washed with serum-free medium to remove unbound fluorescent precursor. Cells were centrifuged at 1,000 × g for 3 min, and then the pellet was collected and suspended in 400 µL of PBS with 1% (wt/vol) BSA and 2 mM EDTA for FACS analysis. Cells, untreated with neither neuraminidase nor CMP-5-FITC-NEU, served as an autofluorescence control. The data were collected on FITC channel by using a BD FACS CANTO II instrument and analyzed by using Flow Jo software (Version 8.8.7).
Statistical Analysis of Sialylation on 2D.
The 2D Western blot signal intensity was digitized for densitometry analysis by using AlphaView software (ProteinSimple). The 2D blots were aligned horizontally, and a line drawn at pI 7.5 was used to arbitrarily separate diglycosylated charge isoforms into hypersialylated and hyposialylated (Fig. S1). In the software window, a rectangle was drawn to confine the spots of interest, and the densities were measured. The intensity of an equal background area from the same blot was subtracted before calculations were performed. The acquired spot ensemble intensities were used to calculate percentage of “hypersialylated” isoforms. Hypersialylated isoforms were defined operationally as all isoforms located to the left of the pI-7.5 line; the percentage of hypersialylated isoforms was calculated assuming the total intensity of all isoforms as 100%. Results are presented as the mean ± SD. Statistical significance (P) between groups were calculated by Student’s t test and is indicated in figures as * for significant (P < 0.05) and # for insignificant (P > 0.05) statistics.
Acknowledgments
We thank Pamela Wright for editing the manuscript. Flow-cytometry analyses were performed at the University of Maryland Marlene and Stewart Greenebaum Cancer Center Flow Cytometry Shared Service. This work was supported by National Institutes of Health Grants R01 NS045585 and R01 NS074998.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.V. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517993112/-/DCSupplemental.
References
- 1.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Legname G, et al. Synthetic mammalian prions. Science. 2004;305(5684):673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 3.Cohen FE, Prusiner SB. Pathologic conformations of prion proteins. Annu Rev Biochem. 1998;67:793–819. doi: 10.1146/annurev.biochem.67.1.793. [DOI] [PubMed] [Google Scholar]
- 4.Aguzzi A, Nuvolone M, Zhu C. The immunobiology of prion diseases. Nat Rev Immunol. 2013;13(12):888–902. doi: 10.1038/nri3553. [DOI] [PubMed] [Google Scholar]
- 5.Sigurdson CJ, et al. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80(Pt 10):2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 6.Hilton DA, Fathers E, Edwards P, Ironside JW, Zajicek J. Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease. Lancet. 1998;352(9129):703–704. doi: 10.1016/S0140-6736(98)24035-9. [DOI] [PubMed] [Google Scholar]
- 7.Andréoletti O, et al. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81(Pt 12):3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 8.McCulloch L, et al. Follicular dendritic cell-specific prion protein (PrP) expression alone is sufficient to sustain prion infection in the spleen. PLoS Pathog. 2011;7(12):e1002402. doi: 10.1371/journal.ppat.1002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujala P, et al. Prion uptake in the gut: Identification of the first uptake and replication sites. PLoS Pathog. 2011;7(12):e1002449. doi: 10.1371/journal.ppat.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown KL, et al. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat Med. 1999;5(11):1308–1312. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 11.Montrasio F, et al. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science. 2000;288(5469):1257–1259. doi: 10.1126/science.288.5469.1257. [DOI] [PubMed] [Google Scholar]
- 12.Glatzel M, Heppner FL, Albers KM, Aguzzi A. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron. 2001;31(1):25–34. doi: 10.1016/s0896-6273(01)00331-2. [DOI] [PubMed] [Google Scholar]
- 13.Mabbott NA, Mackay F, Minns F, Bruce ME. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat Med. 2000;6(7):719–720. doi: 10.1038/77401. [DOI] [PubMed] [Google Scholar]
- 14.Prinz M, et al. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature. 2003;425(6961):957–962. doi: 10.1038/nature02072. [DOI] [PubMed] [Google Scholar]
- 15.Béringue V, et al. Facilitated cross-species transmission of prions in extraneural tissue. Science. 2012;335(6067):472–475. doi: 10.1126/science.1215659. [DOI] [PubMed] [Google Scholar]
- 16.Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364(9433):527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 17.Hilton DA, et al. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol. 2004;203(3):733–739. doi: 10.1002/path.1580. [DOI] [PubMed] [Google Scholar]
- 18.Heikenwalder M, et al. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science. 2005;307(5712):1107–1110. doi: 10.1126/science.1106460. [DOI] [PubMed] [Google Scholar]
- 19.Seeger H, et al. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310(5746):324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 20.Turk E, Teplow DB, Hood LE, Prusiner SB. Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem. 1988;176(1):21–30. doi: 10.1111/j.1432-1033.1988.tb14246.x. [DOI] [PubMed] [Google Scholar]
- 21.Endo T, Groth D, Prusiner SB, Kobata A. Diversity of oligosaccharide structures linked to asparagines of the scrapie prion protein. Biochemistry. 1989;28(21):8380–8388. doi: 10.1021/bi00447a017. [DOI] [PubMed] [Google Scholar]
- 22.Stimson E, Hope J, Chong A, Burlingame AL. Site-specific characterization of the N-linked glycans of murine prion protein by high-performance liquid chromatography/electrospray mass spectrometry and exoglycosidase digestions. Biochemistry. 1999;38(15):4885–4895. doi: 10.1021/bi982330q. [DOI] [PubMed] [Google Scholar]
- 23.Novitskaya V, Makarava N, Sylvester I, Bronstein IB, Baskakov IV. Amyloid fibrils of mammalian prion protein induce axonal degeneration in NTERA2-derived terminally differentiated neurons. J Neurochem. 2007;102(2):398–407. doi: 10.1111/j.1471-4159.2007.04537.x. [DOI] [PubMed] [Google Scholar]
- 24.Stahl N, et al. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry. 1993;32(8):1991–2002. doi: 10.1021/bi00059a016. [DOI] [PubMed] [Google Scholar]
- 25.Rudd PM, et al. Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc Natl Acad Sci USA. 1999;96(23):13044–13049. doi: 10.1073/pnas.96.23.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011;21(9):1121–1124. doi: 10.1093/glycob/cwr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15(4):209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- 29.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2(12):965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 30.Aminoff D, Bruegge WF, Bell WC, Sarpolis K, Williams R. Role of sialic acid in survival of erythrocytes in the circulation: Interaction of neuraminidase-treated and untreated erythrocytes with spleen and liver at the cellular level. Proc Natl Acad Sci USA. 1977;74(4):1521–1524. doi: 10.1073/pnas.74.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen AJG, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbα metalloproteinase-mediated cleavage in mice. Blood. 2012;119(5):1263–1273. doi: 10.1182/blood-2011-05-355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linnartz B, Kopatz J, Tenner AJ, Neumann H. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J Neurosci. 2012;32(3):946–952. doi: 10.1523/JNEUROSCI.3830-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witting A, Müller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by microglia/brain macrophages in vitro: Involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J Neurochem. 2000;75(3):1060–1070. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- 34.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14(8):351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis AL, et al. Innovations in host and microbial sialic acid biosynthesis revealed by phylogenomic prediction of nonulosonic acid structure. Proc Natl Acad Sci USA. 2009;106(32):13552–13557. doi: 10.1073/pnas.0902431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silveira JR, et al. The most infectious prion protein particles. Nature. 2005;437(7056):257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahl N, et al. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry. 1992;31(21):5043–5053. doi: 10.1021/bi00136a600. [DOI] [PubMed] [Google Scholar]
- 38.Katorcha E, Makarava N, Savtchenko R, D’Azzo A, Baskakov IV. Sialylation of prion protein controls the rate of prion amplification, the cross-species barrier, the ratio of PrPSc glycoform and prion infectivity. PLoS Pathog. 2014;10(9):e1004366. doi: 10.1371/journal.ppat.1004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makarava N, et al. A new mechanism for transmissible prion diseases. J Neurosci. 2012;32(21):7345–7355. doi: 10.1523/JNEUROSCI.6351-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parchi P, et al. Genetic influence on the structural variations of the abnormal prion protein. Proc Natl Acad Sci USA. 2000;97(18):10168–10172. doi: 10.1073/pnas.97.18.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sajnani G, Pastrana MA, Dynin I, Onisko B, Requena JR. Scrapie prion protein structural constraints obtained by limited proteolysis and mass spectrometry. J Mol Biol. 2008;382(1):88–98. doi: 10.1016/j.jmb.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 42.Notari S, et al. Effects of different experimental conditions on the PrPSc core generated by protease digestion: Implications for strain typing and molecular classification of CJD. J Biol Chem. 2004;279(16):16797–16804. doi: 10.1074/jbc.M313220200. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Seoane R, et al. Plasmacytoid dendritic cells sequester high prion titres at early stages of prion infection. PLoS Pathog. 2012;8(2):e1002538. doi: 10.1371/journal.ppat.1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klohs WD, Bernacki RJ, Korytnyk W. Effects of nucleotides and nucleotide: Analogs on human serum sialyltransferase. Cancer Res. 1979;39(4):1231–1238. [PubMed] [Google Scholar]
- 45.Ortiz AI, Reglero A, Rodríguez-Aparicio LB, Luengo JM. In vitro synthesis of colominic acid by membrane-bound sialyltransferase of Escherichia coli K-235. Kinetic properties of this enzyme and inhibition by CMP and other cytidine nucleotides. Eur J Biochem. 1989;178(3):741–749. doi: 10.1111/j.1432-1033.1989.tb14505.x. [DOI] [PubMed] [Google Scholar]
- 46.Burkart MD, et al. Chemo-enzymatic synthesis of fluorinated sugar nucleotide: Useful mechanistic probes for glycosyltransferases. Bioorg Med Chem. 2000;8(8):1937–1946. doi: 10.1016/s0968-0896(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 47.Rillahan CD, et al. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. 2012;8(7):661–668. doi: 10.1038/nchembio.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rifat S, et al. Expression of sialyltransferase activity on intact human neutrophils. J Leukoc Biol. 2008;84(4):1075–1081. doi: 10.1189/jlb.0706462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabral MG, et al. Human dendritic cells contain cell surface sialyltransferase activity. Immunol Lett. 2010;131(1):89–96. doi: 10.1016/j.imlet.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Harduin-Lepers A, et al. The human sialyltransferase family. Biochimie. 2001;83(8):727–737. doi: 10.1016/s0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 51.Gross HJ, Merling A, Moldenhauer G, Schwartz-Albiez R. Ecto-sialyltransferase of human B lymphocytes reconstitutes differentiation markers in the presence of exogenous CMP-N-acetyl neuraminic acid. Blood. 1996;87(12):5113–5126. [PubMed] [Google Scholar]
- 52.Kaufmann M, et al. Identification of an alpha2,6-sialyltransferase induced early after lymphocyte activation. Int Immunol. 1999;11(5):731–738. doi: 10.1093/intimm/11.5.731. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz-Albiez R, Merling A, Martin S, Haas R, Gross HJ. Cell surface sialylation and ecto-sialyltransferase activity of human CD34 progenitors from peripheral blood and bone marrow. Glycoconj J. 2004;21(8-9):451–459. doi: 10.1007/s10719-004-5535-5. [DOI] [PubMed] [Google Scholar]
- 54.Gross HJ. Fluorescent CMP-sialic acids as a tool to study the specificity of the CMP-sialic acid carrier and the glycoconjugate sialylation in permeabilized cells. Eur J Biochem. 1992;203(1-2):269–275. doi: 10.1111/j.1432-1033.1992.tb19856.x. [DOI] [PubMed] [Google Scholar]
- 55.Varki A. Colloquium paper: Uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bishop MT, et al. Prion infectivity in the spleen of a PRNP heterozygous individual with subclinical variant Creutzfeldt-Jakob disease. Brain. 2013;136(Pt 4):1139–1145. doi: 10.1093/brain/awt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peden A, et al. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia. 2010;16(2):296–304. doi: 10.1111/j.1365-2516.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 58.Brown KL, Mabbott NA. Evidence of subclinical prion disease in aged mice following exposure to bovine spongiform encephalopathy. J Gen Virol. 2014;95(Pt 1):231–243. doi: 10.1099/vir.0.058958-0. [DOI] [PubMed] [Google Scholar]
- 59.Halliez S, et al. Accelerated, spleen-based titration of variant Creutzfeldt-Jakob disease infectivity in transgenic mice expressing human prion protein with sensitivity comparable to that of survival time bioassay. J Virol. 2014;88(15):8678–8686. doi: 10.1128/JVI.01118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wroe SJ, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: A case report. Lancet. 2006;368(9552):2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 61.Peralta OA, Eyestone WH. Quantitative and qualitative analysis of cellular prion protein (PrP(C)) expression in bovine somatic tissues. Prion. 2009;3(3):161–170. doi: 10.4161/pri.3.3.9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ford MJ, Burton LJ, Morris RJ, Hall SM. Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience. 2002;113(1):177–192. doi: 10.1016/s0306-4522(02)00155-0. [DOI] [PubMed] [Google Scholar]
- 63.Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M. A cellular form of prion protein (PrPC) exists in many non-neuronal tissues of sheep. J Gen Virol. 1995;76(Pt 10):2583–2587. doi: 10.1099/0022-1317-76-10-2583. [DOI] [PubMed] [Google Scholar]
- 64.Clouse MD, Shikiya RA, Bartz JC, Kincaid AE. Nasal associated lymphoid tissue of the Syrian golden hamster expresses high levels of PrPC. PLoS One. 2015;10(2):e0117935. doi: 10.1371/journal.pone.0117935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka K, et al. Noninvasive imaging of dendrimer-type N-glycan clusters: in vivo dynamics dependence on oligosaccharide structure. Angew Chem Int Ed Engl. 2010;49(44):8195–8200. doi: 10.1002/anie.201000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pangburn MK, Pangburn KL, Koistinen V, Meri S, Sharma AK. Molecular mechanisms of target recognition in an innate immune system: Interactions among factor H, C3b, and target in the alternative pathway of human complement. J Immunol. 2000;164(9):4742–4751. doi: 10.4049/jimmunol.164.9.4742. [DOI] [PubMed] [Google Scholar]
- 67.Kajander T, et al. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc Natl Acad Sci USA. 2011;108(7):2897–2902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113(14):3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao H, Crocker PR. Evolution of CD33-related siglecs: Regulating host immune functions and escaping pathogen exploitation? Immunology. 2011;132(1):18–26. [Google Scholar]
- 70.Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7(6):424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang FP, Farquhar CF, Mabbott NA, Bruce ME, MacPherson GG. Migrating intestinal dendritic cells transport PrP(Sc) from the gut. J Gen Virol. 2002;83(Pt 1):267–271. doi: 10.1099/0022-1317-83-1-267. [DOI] [PubMed] [Google Scholar]
- 72.Michel B, et al. Incunabular immunological events in prion trafficking. Sci Rep. 2012;2:440. doi: 10.1038/srep00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takakura I, et al. Orally administered prion protein is incorporated by m cells and spreads into lymphoid tissues with macrophages in prion protein knockout mice. Am J Pathol. 2011;179(3):1301–1309. doi: 10.1016/j.ajpath.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beringue V, et al. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J Pathol. 2000;190(4):495–502. doi: 10.1002/(SICI)1096-9896(200003)190:4<495::AID-PATH535>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 75.Carp RI, Callahan SM. In vitro interaction of scrapie agent and mouse peritoneal macrophages. Intervirology. 1981;16(1):8–13. doi: 10.1159/000149241. [DOI] [PubMed] [Google Scholar]
- 76.Sassa Y, Inoshima Y, Ishiguro N. Bovine macrophage degradation of scrapie and BSE PrPSc. Vet Immunol Immunopathol. 2010;133(1):33–39. doi: 10.1016/j.vetimm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 77.Sassa Y, Yamasaki T, Horiuchi M, Inoshima Y, Ishiguro N. The effects of lysosomal and proteasomal inhibitors on abnormal forms of prion protein degradation in murine macrophages. Microbiol Immunol. 2010;54(12):763–768. doi: 10.1111/j.1348-0421.2010.00272.x. [DOI] [PubMed] [Google Scholar]
- 78.Klein MA, et al. A crucial role for B cells in neuroinvasive scrapie. Nature. 1997;390(6661):687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 79.Erikson E, et al. 2015. Mouse Siglec-1 mediates trans-infection of surface-bound murine leukemia virus in a sialic acid N-acyl side chain-dependent manner. J Biol Chem, jbc.M115.681338, in press.
- 80.Nasirikenari M, Veillon L, Collins CC, Azadi P, Lau JT. Remodeling of marrow hematopoietic stem and progenitor cells by non-self ST6Gal-1 sialyltransferase. J Biol Chem. 2014;289(10):7178–7189. doi: 10.1074/jbc.M113.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crespo HJ, Lau JTY, Videira PA. Dendritic cells: A spot on sialic acid. Front Immunol. 2013;4:491. doi: 10.3389/fimmu.2013.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaplan HA, Woloski BM, Hellman M, Jamieson JC. Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta 1 leads to 4GlcNAc alpha 2 leads to 6 sialyltransferase from liver. J Biol Chem. 1983;258(19):11505–11509. [PubMed] [Google Scholar]
- 83.Jamieson JC, McCaffrey G, Harder PG. Sialyltransferase: A novel acute-phase reactant. Comp Biochem Physiol B. 1993;105(1):29–33. doi: 10.1016/0305-0491(93)90165-2. [DOI] [PubMed] [Google Scholar]
- 84.Takashima S. Characterization of mouse sialyltransferase genes: Their evolution and diversity. Biosci Biotechnol Biochem. 2008;72(5):1155–1167. doi: 10.1271/bbb.80025. [DOI] [PubMed] [Google Scholar]
- 85.Parizek P, et al. Similar turnover and shedding of the cellular prion protein in primary lymphoid and neuronal cells. J Biol Chem. 2001;276(48):44627–44632. doi: 10.1074/jbc.M107458200. [DOI] [PubMed] [Google Scholar]
- 86.Committee on Care and Use of Laboratory Animals 1996. Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 87.Makarava N, et al. Stabilization of a prion strain of synthetic origin requires multiple serial passages. J Biol Chem. 2012;287(36):30205–30214. doi: 10.1074/jbc.M112.392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brossmer R, Gross HJ. Fluorescent and photoactivatable sialic acids. Methods Enzymol. 1994;247:177–193. doi: 10.1016/s0076-6879(94)47014-6. [DOI] [PubMed] [Google Scholar]