Significance
It is currently unclear how biodiversity loss affects ecosystem functioning. The concept of functional redundancy implies that species loss is compensated by other species contributing similarly to functioning. Functional redundancy can be revealed by the relationship between biodiversity and ecosystem functioning (e.g., biomass growth). The functioning of an ecosystem remains unaffected if redundant species are removed but decreases if the removed species have unique roles. Most research on biodiversity-ecosystem functioning (BEF) relationships hitherto was restricted to one single set of environmental conditions. Using a microbial model system, we explored this relationship in different environments. We found that the environmental conditions are pivotal when drawing conclusions about functional redundancy because BEF relationships drastically differ due to changing species roles and interactions.
Keywords: biodiversity-ecosystem functioning, functional redundancy, environmental change, microbial model system, species interactions
Abstract
Assessing the ecological impacts of environmental change requires knowledge of the relationship between biodiversity and ecosystem functioning. The exact nature of this relationship can differ considerably between ecosystems, with consequences for the efficacy of species diversity as a buffer against environmental change. Using a microbial model system, we show that the relationship can vary depending on environmental conditions. Shapes suggesting functional redundancy in one environment can change, suggesting functional differences in another environment. We find that this change is due to shifting species roles and interactions. Species that are functionally redundant in one environment may become pivotal in another. Thus, caution is advised in drawing conclusions about functional redundancy based on a single environmental situation. It also implies that species richness is important because it provides a pool of species with potentially relevant traits. These species may turn out to be essential performers or partners in new interspecific interactions after environmental change. Therefore, our results challenge the generality of functional redundancy.
Many ecosystems are currently in decline due to rapid loss of biodiversity and immense changes in species compositions (1). The impact of these changes on important ecosystem processes is still unclear (2, 3). Ecosystem functioning (e.g., in terms of biomass production) is often positively correlated with species richness (4–8). This correlation is typically explained by two types of mechanisms that are not mutually exclusive: selection and complementarity (9, 10). The former refers to the rising probability for species with particular traits (e.g., highly productive or resource-efficient species) to be included and achieve high abundance in species-rich communities. The latter refers to the rising potential for particular processes to operate in species-rich communities, such as niche partitioning (e.g., species use different resources or exploit limiting resources in different ways) or positive interactions (e.g., a species facilitates growth of another species). Complementarity can thus lead to species-rich communities performing better than any of the included species in monoculture (transgressive overyielding) (11, 12). As a consequence of these mechanisms, the biodiversity-ecosystem functioning (BEF) relationship is affected by the number and identities of species, their evenness within the community, their functional traits, and their interactions (13–17).
The BEF relationship also reflects whether species are functionally redundant and perform similar roles in given communities. Functioning increasing with species richness points to functional differences among the species (i.e., the added species have unique roles for ecosystem functioning). Saturation in functioning points to functional redundancy (i.e., the roles of the added species are already performed by other species) (18–20). Hence, stepwise inclusion of one species after another to experimental communities and observation of the associated functioning (e.g., in terms of biomass production) indicate the effect of each newly included species on the functioning in these particular communities.
Another factor may have an impact on the BEF relationship as well: the environmental context (3). The environmental context is important because contemporary ecosystems are facing multiple environmental changes of increasing intensity. To date, BEF relationships have mainly been investigated under a single environmental scenario leaving the effects of potential environmental changes unexplored. Only a few studies have tested the impact of disturbances (21), pathogens (22), drought (17), resource richness (23), and temperature fluctuations (24) on the BEF relationship. Thus, knowledge on the functioning of ecosystems under different environmental conditions after anticipated change is rare but urgently required (25).
Microbial model systems provide a formidable tool with which to address such theoretical ecological questions (26, 27). They offer various advantages: High numbers of experiments can be carried out in small laboratory microcosms, all external factors can be controlled and easily manipulated, and many generations of the focal organisms can be tested (26, 28–31). In contrast to such model systems, investigating natural microbial ecosystems may pose challenges, such as the difficulties in defining and estimating the vast number of bacterial species (32, 33). However, these limitations do not apply to laboratory systems with defined artificial communities containing well-characterized species.
In this study, we used microbial model systems to investigate how species richness, species identities, and potential species interactions affect ecosystem functioning under a different set of environmental conditions. We systematically assembled more than 800 artificial communities, with each containing between one and 12 bacterial species. As an indicator of ecosystem functioning, we observed the communities’ biomass production in three different abiotic environments of increasing abiotic stresses: low benzoate concentration (1 g/L, “environment 1”), high benzoate concentration (6 g/L, “environment 2”), and high benzoate concentration (6 g/L) with NaCl (15 g/L, “environment 3”). Each environment led to a differently shaped BEF relationship, and thus different levels of functional redundancy as well. Further, for analyzing the effects of particular compositions of species, we developed the concept of “minimal communities.” This concept allowed us to go beyond the BEF relationship curves and identify the roles of particular species and potential species interactions for ecosystem functioning.
Results and Discussion
Effect of Species Richness.
We found positive BEF relationships under all environmental scenarios. However, the functional form of these relationships (Box 1) was highly sensitive to the environmental conditions (compare black curves in Fig. 1). Its shape shifted from a saturation curve in environment 1 to a nearly linear curve in environment 2, to a rather sigmoidal curve in environment 3 [the separation between growing (blue) and nongrowing (red) communities under each environmental scenario is discussed in Materials and Methods, Data Analyses].
Box 1: Phenomenological BEF Model
The modified Michaelis–Menten model for BEF developed by Naeem (18):
was fitted to the communities’ biomass production using nonlinear regression [trust-region-reflective least squares algorithm in MATLAB (MathWorks)]. The ΔOD is the change in optical density as a metric of biomass production, estimated as the difference of OD between the start (0 h) and the end (72 h) of the experiment. S is the relative species richness as a metric of diversity, varying between 0 (no species) and 1 (12 species). Fmax represents the maximum biomass that could be built up under the given environmental conditions (“saturation biomass”). For fitting, we constrained the parameter Fmax to the highest ΔOD observed for any community in the respective environmental scenario. The coefficient of interaction represents additive (c = 1) or multiplicative (c > 1) effects of increasing species richness. High values of c indicate strong interaction between species. Smin is the minimum species richness for producing a “fair amount” of biomass. Small values express a high species equivalency (“functional redundancy”), and vice versa. For c = 1, Smin is the species richness required for producing half of Fmax.
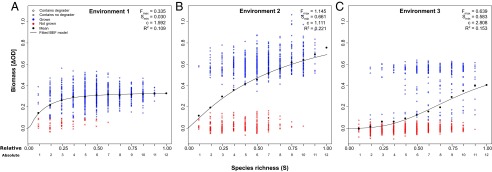
Fig. 1.
Biomass production (ΔOD) plotted against species richness in three different environmental scenarios: environment 1, 1 g/L benzoate (A); environment 2, 6 g/L benzoate (B); and environment 3, 6 g/L benzoate and 15 g/L NaCl (C). Communities are differentiated according to those communities containing no degrader (crosses) or at least one degrader (circles). Blue and red symbols indicate growing and nongrowing communities, respectively. For each species richness level, mean values (●) and modeled biomass production were calculated (Box 1). Model parameters include the theoretical maximum biomass (Fmax), functional redundancy (Smin), and coefficient of species interaction (c). Although the fitted BEF model is in good correspondence to the observations’ mean values, the R2 values underpin that functioning strongly depends on species composition in addition to species richness.
In environment 1, biomass production saturated at low species richness (relative species richness S ≈ 0.3; Fig. 1A) suggesting high functional redundancy. The early occupation of functional niches reduces the contributions of additional species (18, 19). In environment 2, mean biomass production increased almost linearly with species richness (in the considered range of 12 species) and no saturation occurred (Fig. 1B). This observation suggests that any species addition increases ecosystem functioning. However, this interpretation has to be taken with care because biomass growth under these conditions displayed particularly high variances: For all species richness levels, communities tended to separate toward either high or approximately no functioning, pointing to the importance of species identities (discussed below). In environment 3, mean biomass production showed an almost sigmoidal increase with species richness (Fig. 1C). Under these conditions, no species grew as a monoculture. Even for benzoate-tolerant degraders, at least one other species (benzoate degrader or not) was essential for growth and at least five species were needed to achieve growth of more than 50% of the experimental communities.
In comparison to environment 1, more biomass was built up by the communities in environment 2 and environment 3 [change in optical density as a metric of biomass production (ΔOD) at relative species richness S = 1 (i.e., 12 species): ≈0.33 at 1 g/L vs. ≈0.76 at 6 g/L and ≈0.41 at 6 g/L + NaCl, respectively] due to the sixfold higher substrate concentration. However, although access to minerals and space was provided, both environmental scenarios, on average, did not result in sixfold higher biomass, as might be suspected.
Our experimental results suggested fitting nonlinear BEF relationships that offer additional information (e.g., on functional redundancy) compared with studies considering the slopes of linear relationships (21, 22). Fitting model parameters (Box 1 and Fig. 1) resulted in the following values of the theoretical maximum biomass (Fmax): a relatively low Fmax for environment 1 as a result of a rather low benzoate concentration and a higher Fmax for environment 2 because species tolerating the toxicity benefitted from higher benzoate concentration (Fig. 1 A and B), although not proportionally to the additional resource amount. Accordingly, Fmax was reduced for environment 3 because all species suffered from the combined abiotic stresses (Fig. 1C). The value of functional redundancy (Smin; low in environment 1) increased considerably when abiotic stress was introduced (environment 2 and environment 3), suggesting that functional redundancy in the communities decreased. In fact, the experiments showed that the number of species capable of growing as monocultures was reduced by abiotic stress. Also, the coefficient of species interaction (parameter c) changed strongly, pointing to moderately multiplicative (environment 1), rather additive (environment 2), or highly multiplicative (environment 3) effects of species richness in the different environments.
Although some former studies already stressed the importance of the environmental context for the BEF relationships in general (17, 21, 22, 24), our analysis highlights the different interpretations concerning functional redundancy that may result from the differently shaped nonlinear BEF curves in different environments. The occurrence of nonsaturating BEF relationships under these regimes increases the pressure on conservation to avoid loss of species that may not be redundant after environmental change (34). Note that, in a similar way, the temporal context may become important because short-term and long-term shapes of BEF relationships may differ (35).
Effect of Species Identities.
We found a high variance around the mean BEF relationship under all environmental scenarios (blue and red points in Fig. 1), which indicates that functioning depends not only on species richness but also on species identities, that is, which species are present and interact in a community. Thus, at a given species richness level, certain (combinations of) species can make communities perform better or worse than average (24, 36, 37). We therefore investigated to what extent a community’s biomass production is increased or decreased due to the presence of a particular species by comparing communities with and without that species. Because this comparison was done for all available communities (see exemplary sequences in Fig. 2), we were able to estimate the average effect of each species on the communities’ biomass production in response to the environment (Fig. 3).
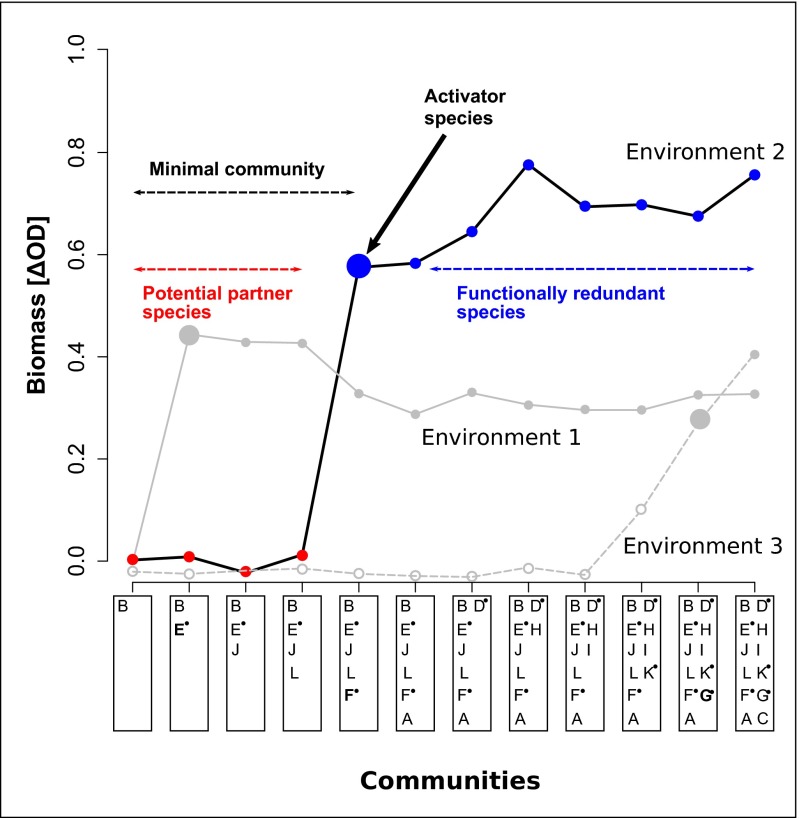
Fig. 2.
Concept of minimal communities. Each species’ effects on biomass production were estimated based on the ΔODDiff between communities including that species vs. excluding that species (exemplary changes in ΔOD along the sequence of communities are shown in the figure; species were coded with letters; benzoate degraders are marked with a small filled black circle; compare Table S1). If the community switched from nongrowing (red) to growing (blue) upon inclusion of a species, we call this species an activator species. The functioning was activated, and a positive effect on biomass production could be attributed to that particular species (e.g., species E in environment 1, species F in environment 2, species G in environment 3). The activator species can stimulate biomass production either autarkically as an essential biomass producer or by interacting with other species as a specific partner. In the first case, this species should also grow as a monoculture. In the second case, both partners can be the activator species, depending on whether the respective partner is already present in a particular community. The activator species, together with all species present in the activated community, is defined as a minimal community. Further addition of species to a minimal community may increase functional redundancy (e.g., species A in environment 2) or occasionally lead to minor changes in biomass growth (e.g., species H or C in environment 2), but these species are not required to ensure functioning. On the other hand, removal of a species from a minimal community may lead to loss of functioning if an essential biomass producer or partner for interactions is removed. We analyzed all minimal communities and related the cases in which a certain species’ inclusion initiated biomass growth to the identity of other species present in the corresponding minimal communities. This approach allowed us to identify potential specific partner species for each activator species.
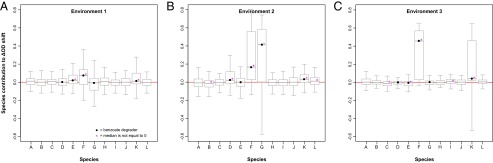
Fig. 3.
Distributions of species effects on biomass production for each of the 12 species (A–L) in environment 1 (A), environment 2 (B), and environment 3 (C). Species effects were calculated as the difference between biomass production in a community containing a species and biomass production of the corresponding community without that species (compare Materials and Methods, Data Analyses). Boxes widths span between the 25th and 75th percentiles [i.e., the interquartile range (IQR)], and whiskers extend to 1.5-fold times the IQR. Outliers beyond the whiskers are not plotted.
Under all environmental conditions and irrespective of species richness, the nondegrader species A, H, and J did not (on average) affect biomass production when in the system (Fig. 3). The four remaining nondegraders B, C, I, and L occasionally had significant minor negative or positive effects depending on environmental conditions. The degrading species F and, to a lesser extent, species K significantly increased biomass production in all environments. Species G and, to a much lesser extent, species D increased biomass production only in environment 2, but had no significant effects in the other two environments. Species E increased biomass production in environment 1, was neutral in environment 2, and had a significant minor negative effect on biomass production in environment 3. Thus, positive and negative effects of individual species appear to be conditional. Depending on the environmental conditions, the presence of such species may lead to an improvement or decline of ecosystem functioning. Moreover, the high variances for some species show that the strength of individual species’ effects on biomass production depends highly on other species present in a community (e.g., species G in Fig. 3B or species K in Fig. 3C).
Shifts in Species Interactions.
The number of communities that resulted in biomass growth decreased when abiotic stress was introduced (94% of all tested communities in environment 1, 73% in environment 2, and 31% in environment 3). However, also in environment 1, several communities that included a benzoate degrader did not grow (red circles in Fig. 1A). Thus, the mere presence of species with a decisive trait did not always guarantee the functioning of a system. Instead, this functioning depended additionally on the presence of other relevant species. Such dependencies were even more pronounced in the most extreme environment 3 (Fig. 1C).
To investigate potential species interactions, we developed the concept of “activator species” and minimal communities (Fig. 2). An activator species is defined as a species leading to biomass growth after being added to a particular previous nongrowing community. Including this activator results in a minimal community that does not require additional species to ensure functioning. We analyzed the experimental data based on this concept and found minimal communities ranging from single species growing as monocultures to communities of 12 species, where only the addition of the last remaining species from the given pool enabled biomass growth (Fig. 1C, red circle for species richness 11 but blue circle for species richness 12). Based on the minimal communities, potential specific partners for interactions were identified (Materials and Methods, Data Analyses).
As expected, in environment 1, all benzoate degraders (species D, E, F, G, and K) could grow as monocultures (Fig. 4A). Under these environmental conditions, species E, F, G, and especially species K were the most frequent activators of biomass growth. Other species, including all nondegraders, could induce biomass growth in the presence of partner species (solid circles in Fig. 4A). To a certain degree, these partner species were redundant for the interactions because almost no specific interactions between particular species could be identified from the minimal communities (only one arrow in Fig. 4A).
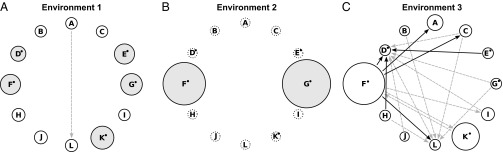
Fig. 4.
Species (A–L) interactions estimated from minimal communities ensuring biomass growth (details are provided in Materials and Methods, Data Analyses and Fig. 2) in environment 1 (A), environment 2 (B), and environment 3 (C). Benzoate degraders are marked with a small filled black circle. Filled gray circles indicate a species’ ability to grow under the given environmental conditions as a monoculture. Dashed circles indicate that a species never was the activator species. Otherwise, sizes of solid circles indicate how often a species was the activator species (relative number of all cases in the given environment). Arrows point from a potential partner species to an activator species if the respective partner species was present in either 100% (solid black arrows) or at least 80% (dashed gray arrows) of the corresponding minimal communities. This method reveals potential specific partners for species interactions, which differed strongly among the different environments.
Environment 2 gave a completely different picture (Fig. 4B). The mere presence of either one or both of the two benzoate degraders F and G was always sufficient for biomass growth, and the absence of these two species led to functional breakdown. Interactions or even specific partners for biomass growth could not be identified (dashed circles for all other species and no arrows in Fig. 4B). This finding is in line with the low coefficient of interaction (parameter c) derived from Fig. 1B and, together with the increase in functioning with species richness, suggests selection effects. Whether species F or G dominated when they co-occurred could not be estimated with the experimental design.
Under the harshest abiotic conditions (environment 3), species interactions were pronounced and no species grew as a monoculture (Fig. 4C). All benzoate degraders needed at least one partner to grow, and all species (also nondegraders) could take over the role of an activator species in particular communities (solid circles in Fig. 4C). As a result, a complex pattern of potential specific interactions between species developed (arrows in Fig. 4C) pointing to complementarity effects in this environment. Here, species F was almost always present in the minimal communities, and thus appears to have a unique role as a partner for interactions under these abiotic conditions (arrows in Fig. 4C).
Methodological Limitations and Transferability to Other Ecosystems.
As in comparable studies, our findings describe relationships between ecosystem functioning and species richness and composition of initially even communities (24). We assume that evenness changed during the experiments in many cases as an outcome of community dynamics under the different environmental conditions, resembling the situation in natural ecosystems. Because we could not measure the changes in each species’ relative abundance within the multispecies communities, we did not analytically separate selection and complementarity effects (9, 38). Nonetheless, we could interpret the BEF relationships (Fig. 1) and the discovered species interactions (Fig. 4) concerning the relevance of these effects.
In this study, we explicitly measured biomass production as a general proxy for ecosystem functioning. This proxy represents a robust measure of benzoate conversion into microbial biomass, be it direct or involving trophic interaction between species. An alternative could have been to measure benzoate degradation as a specific process of interest, but doing so would have blurred functioning of the microbial community because it might have included nonproductive transformation of the mother compound into dead-end metabolites, as well as complete mineralization and conversion into biomass. Given the observed considerable differences in species interactions and redundancy in the different environments, it is plausible to assume that the environmental context is similarly important for benzoate transformation as for biomass production from benzoate. The particular shapes of BEF relationships and the potential species interactions obtained from our analysis are specific for the microbial model system considered and the selected proxy for ecosystem functioning.
We cannot rigorously extrapolate this study to natural ecosystems containing other (and potentially many more) species from different domains of life. Our findings, however, that the environmental context may strongly determine the basic shape (nonlinearity) of BEF relationships and the extent of species interactions, and thus the assessment of functional redundancy, provide valuable hypotheses to be tested further with other species types and in different environments. They may also be interpreted as a general hint that species roles for ecosystem functioning can shift substantially after environmental change.
Another aspect of our system is the limited potential to mirror the highly complex structural and temporal context of natural communities. The design and establishment of long-term microbial model systems under natural environmental conditions (39) would be a future challenge. Integrating such systems with information on the microbial genomes, one could even assemble communities in such a way that specific traits and interactions may be more precisely monitored and interpreted.
The three selected environmental scenarios do not allow disentangling whether the decrease in functional redundancy and the importance of species interactions in environment 3 result from the presence of NaCl alone or from the combination of NaCl with a high benzoate concentration. However, making this distinction was not the focus of this study, and it does not affect our general findings that refer to different exemplary environments. These exemplary environments represent different abiotic conditions that can be seen as a result of environmental change. In our setup, we did not manipulate concentrations of benzoate or NaCl in the course of the single experiments. Observing transient dynamics under gradually changing environmental conditions is a potential next step to investigate the impact of environmental change on BEF relationships further.
Conclusions
Our results show that, under altered environmental conditions, high biodiversity can be beneficial to provide not only (i) a (set of) species with the relevant traits to thrive in the harsh environment but also (ii) potential partners that will allow these species to thrive. However, global environmental change also impairs biodiversity, and therefore may have severe consequences for ecosystem functioning. In particular, our finding that changes in interspecific interactions are essential for ecosystem functioning in different environments is an important step toward a more mechanistic explanation of biodiversity effects under different environmental conditions and suggests a careful interpretation of functional redundancy.
We found that the shape of the BEF relationship strongly depends on environmental conditions. Thus, initial conclusions concerning species’ functional redundancy in a given environmental setting and predictions on the loss of ecosystem functioning due to extinctions of species can be completely misleading when based on a BEF relationship for a given environment alone. Species loss can have an even stronger impact than anticipated when, additionally, the environmental conditions change. As shown here, such changes may result in modified interactions between species determining the functioning of an ecosystem and in an altered shape of the BEF relationship. Thus, taking into account the environmental context challenges the generality of functional redundancy and suggests that the potential of species-rich ecosystems for dynamically constituting positive interspecific interactions when necessary can be much more important than presently believed.
Vice versa, the extent to which the functioning of an ecosystem is maintained under altered environmental conditions strongly depends on its biodiversity. Diverse communities are more likely to contain species with traits pivotal for functioning in different environments. These species may benefit both selection and complementarity mechanisms, because they can be essential performers (e.g., biomass producers) or essential partner species for positive interspecific interactions. Species dismissed as functionally unimportant under present conditions may be required for future ecosystem functioning under altered conditions.
Our findings have potential consequences for the management of ecosystems in that they highlight the importance of diversity for maintaining ecosystem functioning, especially under altered, more extreme environmental regimes.
Materials and Methods
Experimental Design.
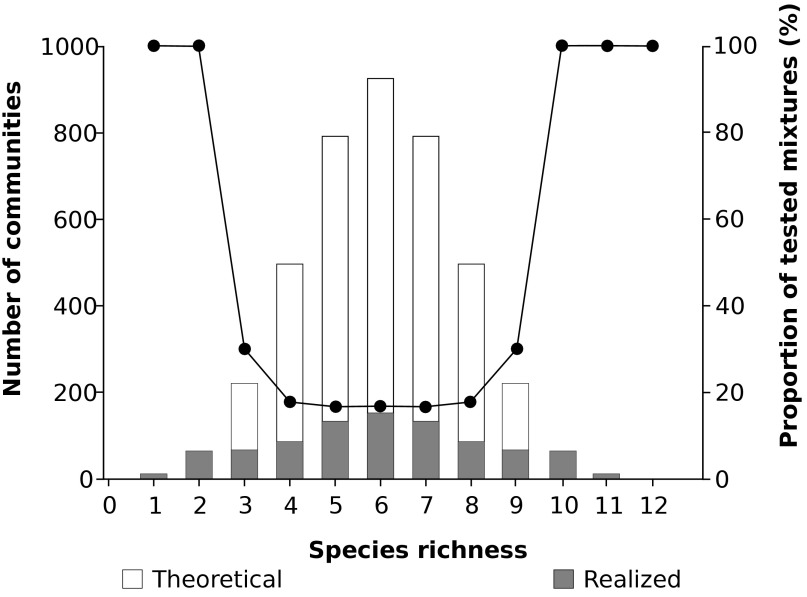
We conducted experiments with assemblages randomly drawn from a pool of 12 bacterial species (the species list is provided in Table S1). The species for the pool were selected such that they cover equal environmental requirements and growth performances, allowing for coexistence but also differences in certain specific metabolic characteristics. The pool included five species capable of directly using benzoate (which served as the sole carbon source; compare below), whereas seven species were incapable of directly using the resource provided. It cannot be clearly inferred from our experiments whether the latter species might potentially grow on intermediate metabolites of benzoate degraders. However, including these species enhances the potential for complementarity effects (24). We assembled 883 random communities (about 20% of all 4,095 theoretical assemblages possible) across all richness levels (ranging from single species monocultures to the 12-species combination) in multiwell plates. The number of random assemblages per richness level was chosen according to the respective maximum number (Fig. S1). The specific combination of species traits yielded random communities potentially covering a range from high functional redundancy to high, albeit undefined, functional differences. In each random community, total cell abundance was adjusted to be equal at the start of the experiment but most likely diverged during the incubation period. To avoid space limitations during growth, the chosen well volume was much larger than the volume required for the maximum possible build-up of biomass with the given amount of benzoate.
Table S1.
List of bacterial species names with abbreviation codes
| Phylum | Name | Strain | Code | Benzoate degrader |
| Firmicutes | Bacillus subtilis | ATCC 6633 | A | |
| Paenibacillus polymyxa | ATCC 842 | B | ||
| Brevibacillus brevis | ATCC 8246 | C | ||
| Betaproteobacteria | Comamonas testosteroni | ATCC 11996 | D | ● |
| Cupriavidus necator | JMP 134 | E | ● | |
| Variovorax paradoxus | ATCC 17713 | H | ||
| Acidovorax facilis | Isolate UFZ | J | ||
| Gammaproteobacteria | Pseudomonas putida | ATCC 17514 | F | ● |
| Pseudomonas fluorescens | DSM 6290 | G | ● | |
| Actinobacteria | Rhodococcus sp. | Isolate UFZ | I | |
| Rhodococcus ruber | BU3 | K | ● | |
| Alphaproteobacteria | Sphingobium yanoikuyae | DSM 6900 | L |
Fig. S1.
Overview of theoretical and realized number of communities for each species richness level.
To assess how identical communities behave in different environments, we used benzoate as the sole carbon source and systematically created the following external abiotic conditions to be applied to the whole set of random assemblages: (i) benzoate at a low concentration (1 g/L), causing high competition among degrading species for the limited resource (environment 1); (ii) benzoate at a high concentration (6 g/L), reducing resource competition due to the copious resource but simultaneously exerting toxic stress from the high benzoate concentration on some species (environment 2); and (iii) the same high benzoate concentration (6 g/L) with NaCl (15 g/L) exerting additional osmotic stress (environment 3).
Laboratory Setup.
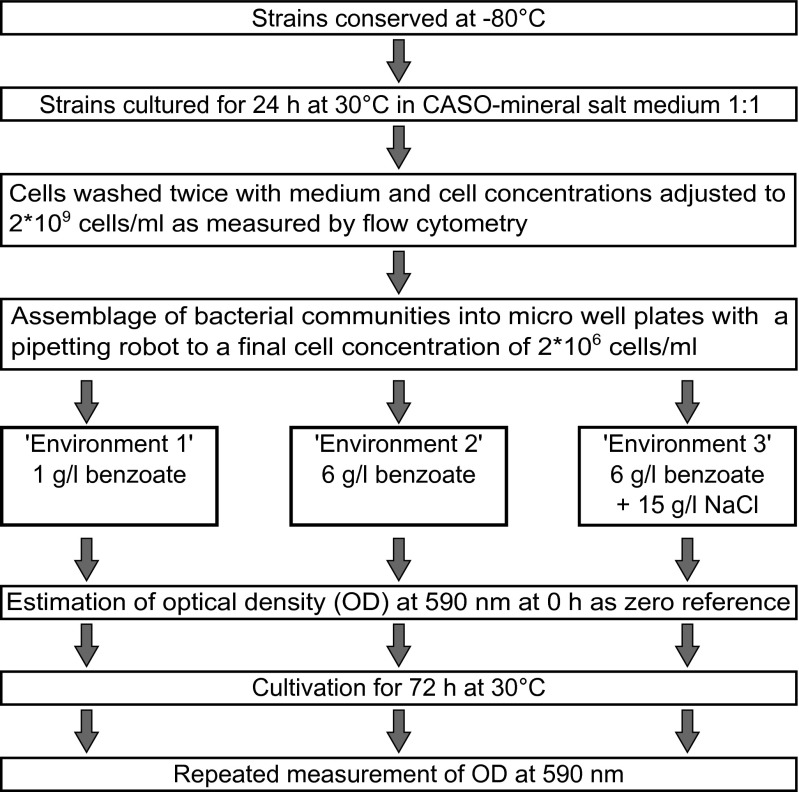
All 12 bacterial strains (the taxonomic names are provided in Table S1) were freshly prepared from −80 °C glycerol stocks. Fresh cultures were prepared by incubation for 24 h at 30 °C in CASO (casein-peptone soymeal-peptone)-mineral salt medium (Merck) (a detailed overview of the procedure is illustrated in Fig. S2). The final working suspensions were created by adjusting washed cells to 2 * 109 cells per milliliter of fresh medium by flow cytometry. Microcosms with random communities across all species richness levels were assembled from the working suspensions by a pipetting robot (BioMek 2000; Beckman Coulter) into microwell plates (96 wells with a 0.5-mL volume each) containing mineral salt medium, supplemented by the respective amounts of benzoate and NaCl for generating the different environments as described (compare Experimental Design). The communities’ initial total cell concentrations were 2 * 106 cells per milliliter for all wells and species combinations. Exactly the same experimental communities were established for each environmental scenario.
Fig. S2.
Experimental setup and treatment scheme.
Community biomass production (Fig. 1) was estimated through OD as a proxy for ecosystem functioning, indicating how well a community containing specific species with certain individual traits performs under a given environment. OD was determined spectroscopically measuring light absorbance at a wavelength of 590 nm with an automatic plate reader (BioTek) and used to calculate the ΔOD, the difference between initial OD and OD after 72 h of incubation for each experimental community. After 72 h, all communities had reached a stationary phase with no further change in biomass.
Data Analyses.
Differentiation between nongrowing (red in Fig. 1) and growing (blue in Fig. 1) communities was achieved separately for each environmental scenario, using a statistical two-component probability mixture model approach (with α < 0.05 for separation) (40, 41). Parameter values for the phenomenological BEF model (Box 1) were fitted using the trust-region reflective least squares algorithm in MATLAB, version 2013a (MathWorks), constraining Fmax to the maximum observed ΔOD in each environment.
For investigating a species’ (positive or negative) effect on biomass production (ΔODDiff) in a given experimental community, we calculated the difference in ΔOD values of the community with and the community without that particular species (compare Fig. 2). The species’ overall effects on biomass production (Fig. 3) were then assessed by collecting the species’ ΔODDiff values from all communities for which the corresponding community without that species existed in our set of investigated species assemblages (compare Materials and Methods, Experimental Design and Fig. S1). The estimation of positive, negative, or neutral overall effects (median above, below, or equal to zero) was based on the Wilcoxon test (with α < 0.05) and calculated with R, version 3.1.2 (40).
To elucidate species interactions, the concept of activator species and minimal communities was developed (a detailed explanation is provided in the legend for Fig. 2). In the minimal communities, we analyzed which other species (potential partners) were present in the previously nongrowing communities that resulted in growth after inclusion of a relevant activator species. To obtain specific partners for interactions on a reasonable basis, we only included cases where a species was the “activator” in at least five different minimal communities. For each of these activator species, other species occurring in 100% (strong indication for interaction; solid black arrows in Fig. 4) or in at least 80% (weaker indication for interaction; dashed gray arrows in Fig. 4) of the corresponding minimal communities were classified as potential specific partners. If no species occurred that often, then, obviously, several species could serve as partners for interactions with the activator species (Fig. 4A) or the activator species did not rely on interactions at all (species able to grow as monocultures in Fig. 4 A and B) in the given environment. Corresponding calculations were performed in MATLAB.
Acknowledgments
We thank Stuart Kininmonth for critical discussion and three anonymous reviewers for very helpful comments on the manuscript. Furthermore, we acknowledge Verena Jaschik and Anett Heidtmann for their technical assistance in the laboratory and for sharing their unfathomable practical knowledge with us. This work was supported by the Helmholtz Centre for Environmental Research–UFZ in the scope of the SAFIRA II Research Programme (Revitalization of Contaminated Land and Groundwater at Megasites, Project “Compartment Transfer”).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505587112/-/DCSupplemental.
References
- 1.Butchart SHM, et al. Global biodiversity: Indicators of recent declines. Science. 2010;328(5982):1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 2.Naeem S. Biodiversity: Biodiversity equals instability? Nature. 2002;416(6876):23–24. doi: 10.1038/416023a. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale BJ. Biodiversity improves water quality through niche partitioning. Nature. 2011;472(7341):86–89. doi: 10.1038/nature09904. [DOI] [PubMed] [Google Scholar]
- 4.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75(1):3–35. [Google Scholar]
- 5.Schmid B, et al. Consequences of species loss for ecosystem functioning: Meta-analyses of data from biodiversity experiments. In: Bunker DE, Hector A, Loreau M, Perrings C, editors. Biodiversity, Ecosystem Functioning, and Human Wellbeing: An Ecological and Economic Perspective. Oxford Scholarship Online; London: 2009. pp. 14–29. [Google Scholar]
- 6.Wagg C, Bender SF, Widmer F, van der Heijden MGA. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci USA. 2014;111(14):5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maestre FT, Castillo-Monroy AP, Bowker MA, Ochoa-Hueso R. Species richness effects on ecosystem multifunctionality depend on evenness, composition and spatial pattern. J Ecol. 2012;100(2):317–330. [Google Scholar]
- 8.Cardinale BJ, et al. Biodiversity simultaneously enhances the production and stability of community biomass, but the effects are independent. Ecology. 2013;94(8):1697–1707. doi: 10.1890/12-1334.1. [DOI] [PubMed] [Google Scholar]
- 9.Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412(6842):72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 10.Loreau M, et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294(5543):804–808.
- 11.Cardinale BJ, et al. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci USA. 2007;104(46):18123–18128. doi: 10.1073/pnas.0709069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmid B, Hector A, Saha P, Loreau M. Biodiversity effects and transgressive overyielding. J Plant Ecol. 2008;1(2):95–102. [Google Scholar]
- 13.Cardinale BJ, Palmer MA, Collins SL. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature. 2002;415(6870):426–429. doi: 10.1038/415426a. [DOI] [PubMed] [Google Scholar]
- 14.Wittebolle L, et al. Initial community evenness favours functionality under selective stress. Nature. 2009;458(7238):623–626. doi: 10.1038/nature07840. [DOI] [PubMed] [Google Scholar]
- 15.Botton S, van Heusden M, Parsons JR, Smidt H, van Straalen N. Resilience of microbial systems towards disturbances. Crit Rev Microbiol. 2006;32(2):101–112. doi: 10.1080/10408410600709933. [DOI] [PubMed] [Google Scholar]
- 16.De Roy K, et al. Environmental conditions and community evenness determine the outcome of biological invasion. Nat Commun. 2013;4:1383. doi: 10.1038/ncomms2392. [DOI] [PubMed] [Google Scholar]
- 17.Mulder CPH, Uliassi DD, Doak DF. Physical stress and diversity-productivity relationships: The role of positive interactions. Proc Natl Acad Sci USA. 2001;98(12):6704–6708. doi: 10.1073/pnas.111055298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naeem S. Ecosystem consequences of biodiversity loss: The evolution of a paradigm. Ecology. 2002;83(6):1537–1552. [Google Scholar]
- 19.Loreau M. Biodiversity and ecosystem functioning: A mechanistic model. Proc Natl Acad Sci USA. 1998;95(10):5632–5636. doi: 10.1073/pnas.95.10.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jax K. Function and “functioning” in ecology: What does it mean? Oikos. 2005;111(3):641–648. [Google Scholar]
- 21.Cardinale BJ, Palmer MA. Disturbance moderates biodiversity-ecosystem function relationships: Experimental evidence from caddisflies in stream mesocosms. Ecology. 2002;83(7):1915–1927. [Google Scholar]
- 22.Maron JL, Marler M, Klironomos JN, Cleveland CC. Soil fungal pathogens and the relationship between plant diversity and productivity. Ecol Lett. 2011;14(1):36–41. doi: 10.1111/j.1461-0248.2010.01547.x. [DOI] [PubMed] [Google Scholar]
- 23.Langenheder S, Bulling MT, Solan M, Prosser JI. Bacterial biodiversity-ecosystem functioning relations are modified by environmental complexity. PLoS One. 2010;5(5):e10834. doi: 10.1371/journal.pone.0010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langenheder S, Bulling MT, Prosser JI, Solan M. Role of functionally dominant species in varying environmental regimes: Evidence for the performance-enhancing effect of biodiversity. BMC Ecol. 2012;12(1):14. doi: 10.1186/1472-6785-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellnitz T, Poff NL. Functional redundancy in heterogeneous environments: Implications for conservation. Ecol Lett. 2001;4(3):177–179. [Google Scholar]
- 26.Jessup CM, Forde SE, Bohannan BJ. Microbial experimental systems in ecology. Adv Ecol Res. 2005;37:273–307. [Google Scholar]
- 27.Kreft J-U, et al. Mighty small: Observing and modeling individual microbes becomes big science. Proc Natl Acad Sci USA. 2013;110(45):18027–18028. doi: 10.1073/pnas.1317472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardinale BJ, et al. Effects of biodiversity on the functioning of ecosystems: A summary of 164 experimental manipulations of species richness. Ecology. 2009;90(3):854–854. [Google Scholar]
- 29.Saleem M, Fetzer I, Harms H, Chatzinotas A. Diversity of protists and bacteria determines predation performance and stability. ISME J. 2013;7(10):1912–1921. doi: 10.1038/ismej.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Roy K, Marzorati M, Van den Abbeele P, Van de Wiele T, Boon N. Synthetic microbial ecosystems: An exciting tool to understand and apply microbial communities. Environ Microbiol. 2014;16(6):1472–1481. doi: 10.1111/1462-2920.12343. [DOI] [PubMed] [Google Scholar]
- 31.Normand P, Duran R, Le Roux X, Morris C, Poggiale J-C. Biodiversity and microbial ecosystems functioning. In: Bertrand J-C, et al., editors. Environmental Microbiology: Fundamentals and Applications. Springer; Dordrecht, The Netherlands: 2015. pp. 261–291. [Google Scholar]
- 32.Prosser JI. Ecosystem processes and interactions in a morass of diversity. FEMS Microbiol Ecol. 2012;81(3):507–519. doi: 10.1111/j.1574-6941.2012.01435.x. [DOI] [PubMed] [Google Scholar]
- 33.Haegeman B, et al. Robust estimation of microbial diversity in theory and in practice. ISME J. 2013;7(6):1092–1101. doi: 10.1038/ismej.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardinale B. Ecology. Impacts of biodiversity loss. Science. 2012;336(6081):552–553. doi: 10.1126/science.1222102. [DOI] [PubMed] [Google Scholar]
- 35.Reich PB, et al. Impacts of biodiversity loss escalate through time as redundancy fades. Science. 2012;336(6081):589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- 36.Allison SD, Martiny JB. Colloquium paper: Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11512–11519. doi: 10.1073/pnas.0801925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salles JF, Poly F, Schmid B, Le Roux X. Community niche predicts the functioning of denitrifying bacterial assemblages. Ecology. 2009;90(12):3324–3332. doi: 10.1890/09-0188.1. [DOI] [PubMed] [Google Scholar]
- 38.Saleem M, Fetzer I, Dormann CF, Harms H, Chatzinotas A. Predator richness increases the effect of prey diversity on prey yield. Nat Commun. 2012;3:1305. doi: 10.1038/ncomms2287. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava DS, et al. Are natural microcosms useful model systems for ecology? Trends Ecol Evol. 2004;19(7):379–384. doi: 10.1016/j.tree.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 40.R Core Team 2014 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna) Available at www.R-project.org/. Accessed October 2014.
- 41.Benaglia T, Chauveau D, Hunter DR, Young D. mixtools: An R package for analyzing finite mixture models. J Stat Softw. 2009;32(6):1–29. [Google Scholar]