Significance
Embryonic or pluripotent stem cells are unique in their ability to self-renew in culture and to generate all lineages of an adult organism, making them valuable tools for modeling early developmental processes and for developing regenerative medicine technologies. An important factor in controlling the expression of pluripotency genes is the Xeroderma pigmentosum complementation group C (XPC) DNA repair complex. This study presents, to our knowledge, the first complete structures of different XPC complexes by electron microscopy to establish an important framework for a molecular understanding of XPC’s two primary functions. In conjunction with our biochemical findings, we synthesize a model of how XPC performs both its evolutionarily conserved DNA repair function and its evolutionarily nonconserved transcription function.
Keywords: transcription, stem cells, DNA repair, structure, biochemistry
Abstract
The Xeroderma pigmentosum complementation group C (XPC) complex is a versatile factor involved in both nucleotide excision repair and transcriptional coactivation as a critical component of the NANOG, OCT4, and SOX2 pluripotency gene regulatory network. Here we present the structure of the human holo-XPC complex determined by single-particle electron microscopy to reveal a flexible, ear-shaped structure that undergoes localized loss of order upon DNA binding. We also determined the structure of the complete yeast homolog Rad4 holo-complex to find a similar overall architecture to the human complex, consistent with their shared DNA repair functions. Localized differences between these structures reflect an intriguing phylogenetic divergence in transcriptional capabilities that we present here. Having positioned the constituent subunits by tagging and deletion, we propose a model of key interaction interfaces that reveals the structural basis for this difference in functional conservation. Together, our findings establish a framework for understanding the structure-function relationships of the XPC complex in the interplay between transcription and DNA repair.
Genomes of living organisms serve two primary functions: as vehicles for hereditary information and as the template for gene products involved in an organism’s development and responses to environmental stimuli. Vital to maintaining the health of genomes in the face of intrinsic and extrinsic sources of DNA damage are a suite of DNA repair pathways, each dedicated to handling specific lesions. Similarly, proper use and expression of this essential genomic information is regulated by a host of transcription factors, chromatin remodelers, and epigenetic modifiers and readers (1). The Xeroderma pigmentosum complementation group C (XPC) protein complex performs crucial roles in both of these capacities by participating in nucleotide excision repair (NER) (2) and base excision repair (BER) (3), as well as transcriptional regulation (4) and other processes (5).
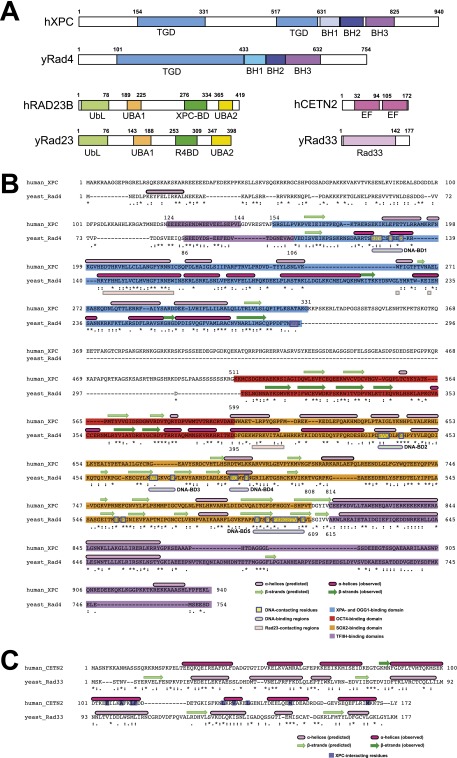
The XPC complex is one of seven XP complementation groups A–G and is composed of the 125-kDa XPC, the 58-kDa RAD23B (Rad23 homolog B; also known as HHR23B), and the 18-kDa CETN2 (Centrin2) subunits (2). RAD23B and CETN2 associate tightly with XPC and stabilize both its DNA repair (6–10) and stem cell coactivator functions (4). The XPC complex is the initiator and main DNA damage sensor in global genome nucleotide excision repair (GG-NER), one of two branches of the nucleotide excision repair pathway that repairs a wide array of bulky, helix-distorting lesions (2, 11); the second form of NER or transcription-coupled repair (TC-NER) targets lesions blocking transcription to reactivate proper gene expression (2, 12). Defects in GG-NER lead to photosensitivity and a predisposition to certain cancers in animal models and in human patients with Xeroderma pigmentosum (13). In conjunction with the UV-damage DNA-binding protein (UV-DDB) (2, 12), the XPC complex recruits >30 downstream factors, such as XPA (14), TFIIH (15, 16), and the endonucleases XPF and XPG to remove these adducts (2, 11). In addition to its role in GG-NER, XPC is also involved in base excision repair (BER). BER is responsible for removing primarily non-helix-distorting lesions from the genome (2). In BER, the XPC complex helps repair oxidative damage by stimulating the activities of DNA glycosylases such as OGG1 and TDG (3) to target lesions including 8-oxoguanine, independently of other downstream GG-NER factors (17).
More recently, the XPC complex has also been found to perform crucial duties in the regulation of gene transcription, the second primary function of the genome. In embryonic stem cells (ESCs), the XPC complex acts as a coactivator to enhance the expression of OCT4- and SOX2-driven pluripotency genes, most notably NANOG (4), buttressing the gene regulatory network that establishes and maintains the unique self-renewal and pluripotency properties of ESCs. The XPC complex performs its coactivator functions independently of DNA binding (4, 18), presumably by bridging interactions between the sequence-specific transcription factors OCT4 (octamer-binding transcription factor 4; also known as POU5F1) and SOX2 [SRY (sex-determining region Y)-box 2] and the general transcriptional machinery, such as TFIID and RNA pol II, thus following a mechanism reminiscent of that of other coactivator complexes such as Mediator and p300/CBP (19). In a separate study, XPC was found to localize to active but not to inactive RNA pol II-dependent gene promoters in the absence of exogenous genotoxic stress (11, 20).
Although both the DNA repair and transcriptional functions of mammalian XPC complexes have been characterized biochemically and genetically, 3D structural information of the holo-complex has been unavailable. The partial crystal structure of Rad4, the yeast homolog of the human XPC subunit, in complex with the Rad4-interaction domain of yeast Rad23, has provided information helpful toward an understanding of Rad4/XPC’s biochemical behavior and of certain phenotypic outcomes (18, 21). However, given XPC’s low sequence homology with the yeast homolog Rad4 (21, 22), the absence of key domains in the available crystal structure, and the divergence of requirements for transcriptional vs. repair activities (4, 15, 18, 23), structural information of the complete, three-subunit, native human XPC complex is much needed for an understanding of the functional versatility of the XPC complex. At present, no information has been reported on the overall architecture of the XPC holo-complex, the possible large-scale conformational rearrangements in XPC upon DNA-binding, or the extent of structural conservation of the human XPC complex with homolog complexes.
Here we address the lack of structural data on human XPC using 3D single-particle reconstruction by electron microscopy (EM) to characterize the overall architecture of XPC, as well as genetic tagging and computational docking to locate the relative positions of its constituent subunits. We also assess the conformational changes to the complex upon binding to DNA. Given the evolutionary conservation of GG-NER (24), we queried the extent of structural and functional conservation over evolutionary time by solving the structure of the complete yeast homolog Rad4 complex and testing whether the OCT4/SOX2 transcriptional coactivation function is supported by the yeast complex. Together with existing biochemical data (14–16, 23, 25), we sought to identify the approximate regions of contact between the XPC complex and its partner proteins OCT4, SOX2, XPA, and TFIIH.
Results
Reconstitution of the Human XPC Complex.
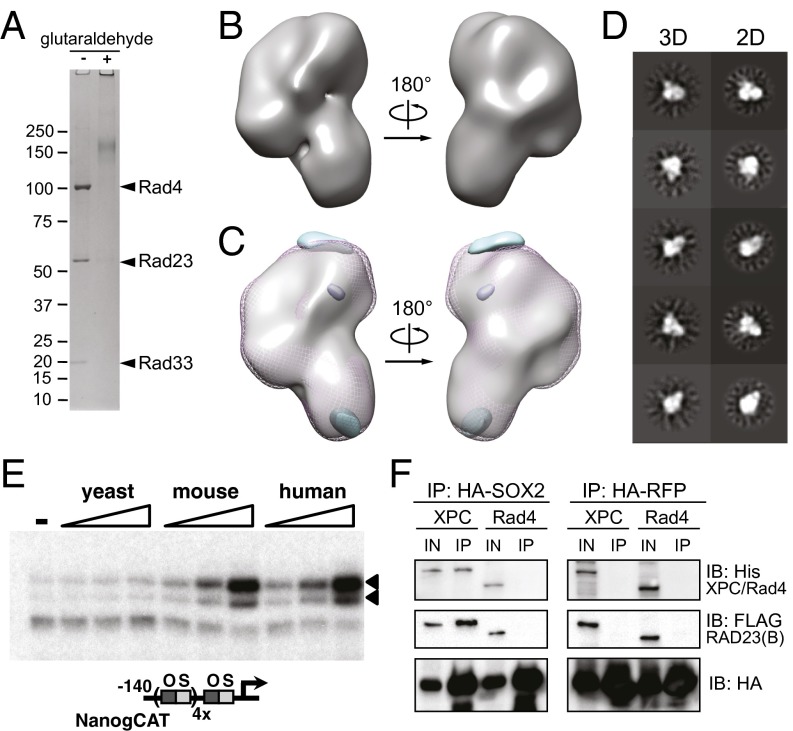
We purified the complete, three-subunit human XPC-RAD23B-CETN2 complex (Fig. 1A) expressed in Sf9 insect cells using a two-step affinity purification procedure (Fig. S1 A and B). SDS/PAGE analysis indicated that the purified complex was nearly homogeneous and stoichiometric (Fig. S1B, Left). This result is consistent with previous data showing that XPC and RAD23B interact in a 1:1 ratio (26) and that XPC and CETN2 also interact in a 1:1 ratio (27, 28). Furthermore, a 1:1:1 stoichiometry is consistent with the size of the ∼200-kDa product we observe for the cross-linked complex (Fig. S1B, Right).
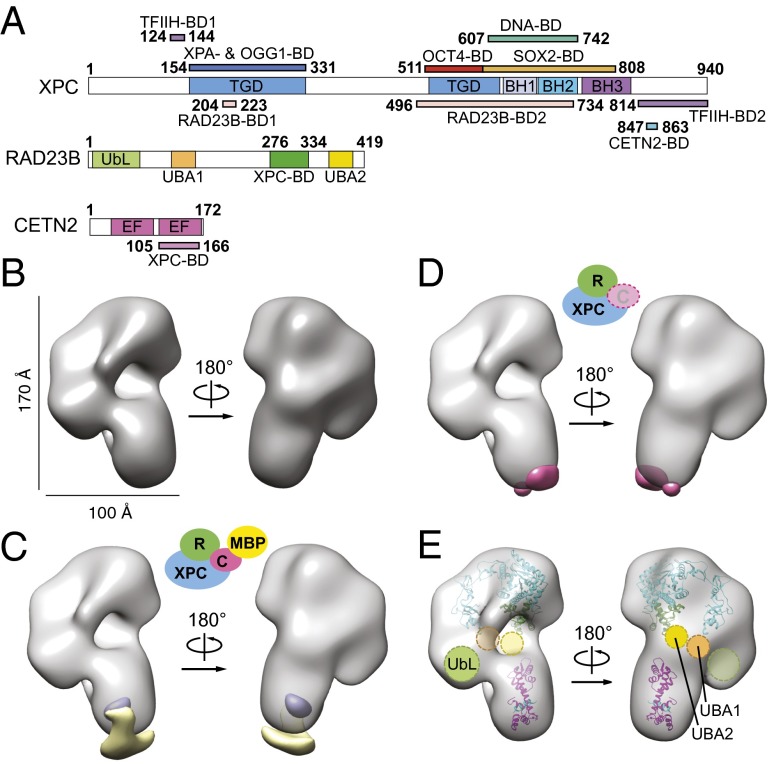
Fig. 1.
3D reconstruction of the human XPC complex and localization of subunits. (A) Schematic representation of the subunits and domains of the human XPC complex. Transglutaminase homology domain (TGD), β-hairpin domains 1–3 (BH), ubiquitin-like domain (UbL), ubiquitin-associated domains 1 and 2 (UBA), EF-hand domains (EF), and protein- and DNA-binding domains (BD) are indicated accordingly. (B) Front and back views of the XPC complex reconstructed in EMAN2 (49). Estimated dimensions are indicated. (C) Positive (yellow) and negative (purple) 3D difference densities at 4σ between the complex containing MBP-CETN2 and untagged complex. (D) Positive (pink) 3D difference density at 5σ between the full complex and the XPC-RAD23B subcomplex, indicating the likely position for the CETN2 subunit. No negative difference density was observed at this threshold. (E) Docking of the yeast Rad4/Rad23 [Protein Data Bank (PDB) ID 2QSF; cyan/green] into the model with the human CETN2 and XPC interaction peptide (PDB ID 2GGM; pink/cyan) by Situs (31) in a manner consistent with the difference density data in A and B. Shown are predicted approximate positions of the UbL, UBA1, and UBA2 domains of RAD23B based on positional information of the Rad23Rad4-BD (dark green) N and C termini in the crystal structure.
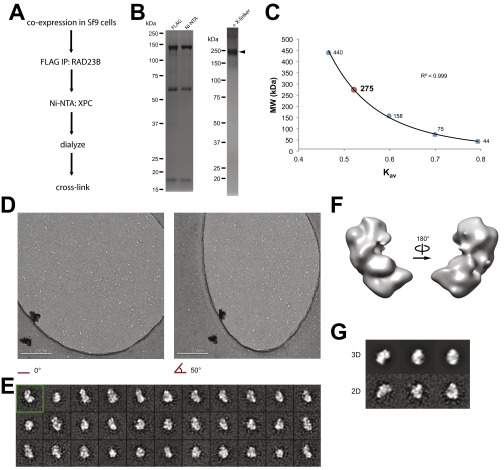
Fig. S1.
XPC complex sample preparation and generation of ab initio model by random conical tilt. (A) Purification and sample preparation strategy for EM for the XPC complex expressed recombinantly in Sf9 insect cells. (B) SDS/PAGE and Coomassie staining of the purified fractions of the XPC complex (Left) and the complex cross-linked with glutaraldehyde (Right). (C) Migration of the XPC complex at ∼275 kDa in size on a Superose 6 gel filtration column, as calculated by fitting to the Kav values (Kav = (Ve − Vo)/(Vt − Vo), where Ve = elution volume of the protein, Vo = column void volume, and Vt = total bed volume) of the molecular weight standards ferritin (440 kDa), aldoloase (158 kDa), conalbumin (75 kDa), and ovalbumin (44 kDa). (D) Representative tilt pair of images used for RCT. (Scale bar: 200 nm.) (E) Two-dimensional class averages of the RCT data set of 2,384 particles. The class average corresponding to the initial model selected for subsequent analysis is indicated by the green box. (F) Front and back views of the top RCT initial volume #1 selected for subsequent refinement. (G) Comparisons between its 3D reprojections and 2D class averages from the entire RCT data set.
Initial attempts at negative stain EM data collection were hampered by the extremely heterogeneous appearance of the particles in both size and shape (data not shown). These results suggested that the complex was either unstable during EM sample preparation and/or suffered from extreme conformational flexibility. To overcome these limitations, we optimized cross-linking conditions across temperature, incubation time, and cross-linker concentration to identify the minimum requirements for achieving complete subunit incorporation as detected by Coomassie staining (for more details, see SI Materials and Methods, Expression and Purification of XPC/Rad4 Complexes). These particular conditions were then used for subsequent negative stain sample preparation, followed by data collection and single particle analysis (Fig. S1B, Right and Fig. S2A).
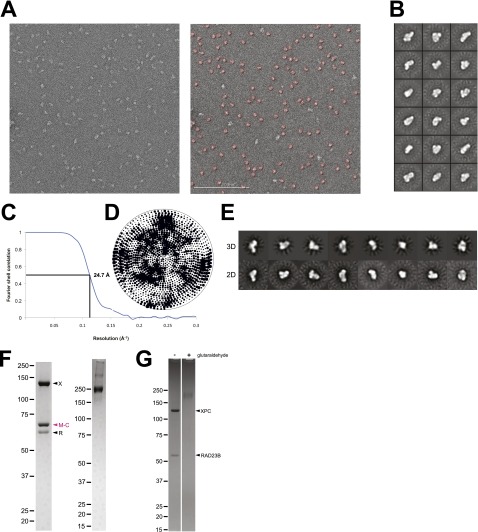
Fig. S2.
3D reconstruction of the apo human XPC complex and subunit labeling. (A) Representative image (Left) with DoG-picker (53) selected particles indicated in red (Right) of uranyl-formate stained sample taken at 80,000× magnification. (Scale bar: 200 nm.) (B) Representative 2D class averages of ∼46,000 total particles. (C) Fourier shell correlation (FSC) curve indicating the estimated resolution to be 24.7 Å using the FSC = 0.5 criterion (60). (D) Euler angular distribution plot. (E) Comparison of 3D reprojections of the refined model to their corresponding reference-free 2D class averages. (F) SDS/PAGE and Coomassie staining of purified XPC complex containing MBP-CETN2 (M-C) (Left) and the complex cross-linked with glutaraldehyde (Right). (G) SDS/PAGE and Coomassie staining of the purified and cross-linked XPC-RAD23B subcomplex.
Two-dimensional reference-free class averages (Fig. S2B) show C-shaped views, multilobed structures, and some very small, compact, globular shapes, with most of the particles having an elongated appearance. Such elongated shapes would be consistent with our observation that the XPC complex runs as a relatively broad peak centered at 275 kDa on a size-exclusion column, slightly larger than its mass of ∼200 kDa (Fig. 1A and Fig. S1 B and C).
Ab Initio 3D Reconstruction of the Human XPC Complex by Random Conical Tilt and Subunit Localization.
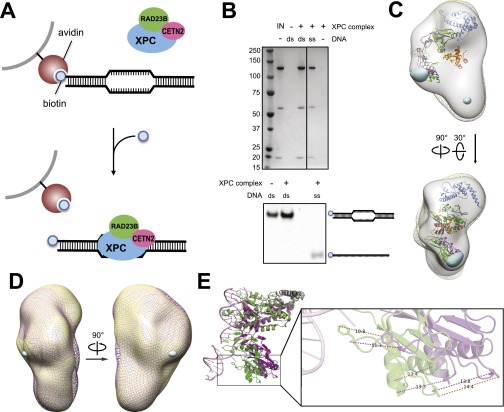
We used random conical tilt (29) (Fig. S1 D–G) to generate an ab initio 3D reconstruction of the human XPC complex (Fig. 1B and Fig. S2). Handedness and robustness of our EM reconstruction was supported by the results of the freehand test (30) using projection-matching of particle pairs at 0° and 30° tilt, which indicated that 40% of particles fall within 26 degrees of the expected tilt angle (Fig. S3D). The structure is ∼170 Å by ∼100 Å by ∼70 Å and roughly resembles the shape of a human ear (Fig. 1B). To localize the position of the CETN2 subunit within the reconstruction, we followed two parallel strategies: visualization of a complex that included a maltose-binding protein (MBP) tag at the N terminus of CETN2, and visualization of a complex lacking the CETN2 subunit (Fig. 1 C and D and Fig. S2 F and G). As seen in the 3D difference maps, the MBP density is localized primarily outside the shorter end or earlobe of the XPC complex, whereas the CETN2 density is localized primarily inside the earlobe (Fig. 1 C and D). Consistent with this localization of CETN2, the crystal structure of Rad4/Rad23Rad4-BD docked (31) into the upper end of the “ear” in an orientation such that the C terminus of Rad4 points downward toward the “earlobe.” In further agreement with this subunit organization, docking of the CETN2 was placed in the earlobe by objective, automatic docking using Situs (31) (Fig. 1E). Based on the orientation of the Rad23 termini observed in the Rad4/Rad23 crystal structure, we also indicate the predicted, approximate locations of the UbL, UBA1, and UBA2 subunits of RAD23B that are not observed in the Rad4/Rad23 crystal structure (Fig. 1E).
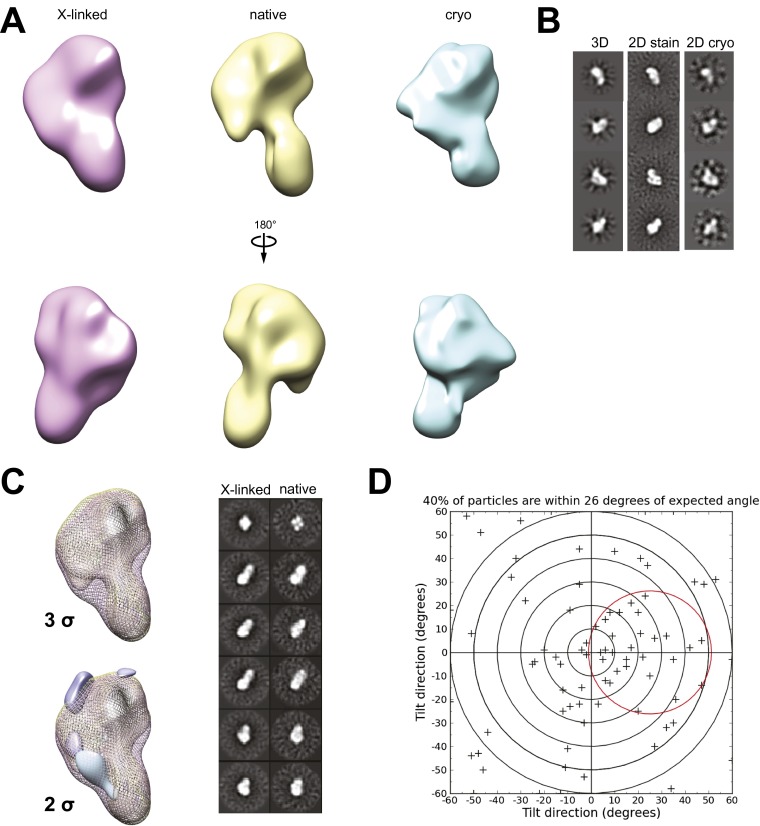
Fig. S3.
Model validation. (A) Front (Upper) and back (Lower) views of cross-linked (pink), uncross-linked (yellow), and cryo-EM models generated in RELION (32) using ∼20,000–30,000 particles each. (B) Negative stain 3D reprojections and cryo-EM reference-free 2D class averages corresponding to A. (C, Left) Positive (blue) and negative (purple) 3D difference maps at 3σ and 2σ between the cross-linked and native complexes. (C, Right) Corresponding 2D class averages of cross-linked (Left) and native (Right) particles. (D) Free-hand test of the XPC complex using 93 particles taken at a 0° and a 30° tilt.
To ensure that the cross-linking and the use of stain did not significantly compromise the integrity of the structure obtained, we also analyzed the native, uncross-linked complex and used cryo-electron microscopy (cryo-EM). The native complex in phosphotungstate stain and the cross-linked complex under cryo conditions were all consistent with the cross-linked complex under negative stain conditions (Fig. 1B and Fig. S3 A–C). Although the native complex appears to have better definition between the earlobe and the rest of the structure, this difference density is only present at 2σ and not at the statistically significant 3σ (Fig. S3C).
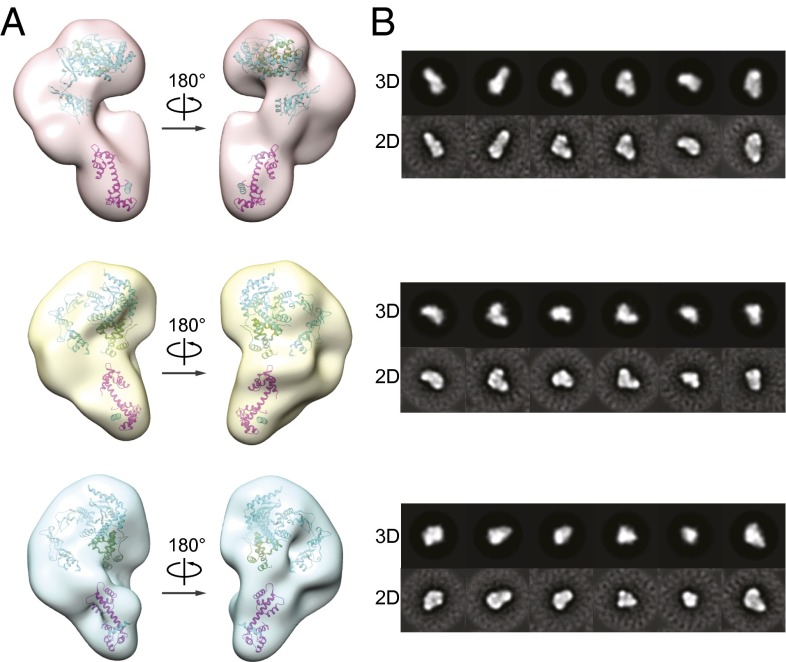
The low resolution of our reconstruction (∼25 Å) suggested that the XPC complex may be flexible and adopt multiple conformations. To gain an understanding of the possible range of conformational states, we used a large data set of ∼210,000 particles and performed 3D sorting and classification using RELION (32) to produce three distinct structures representing the range of XPC conformations (Fig. 2A), followed by Situs docking using available crystal structures. The XPC complex appears to be partitioned between a more elongated (Fig. 2A, Top) and more compact states (Fig. 2A, Middle and Bottom). Three-dimensional reprojections of these three models match reference-free 2D class averages (Fig. 2B).
Fig. 2.
The XPC complex adopts highly flexible conformations. (A) Three models of the XPC complex generated in RELION with Situs-based docking (31) of the yeast Rad4/Rad23 (PDB ID 2QSF; cyan/green) and the human CETN2/XPCCETN2-BD (PDB ID 2GGM; pink/cyan) crystal structures. (B) Comparison of 3D reprojections of the models to their corresponding reference-free 2D class averages.
Structural Changes Following DNA Binding.
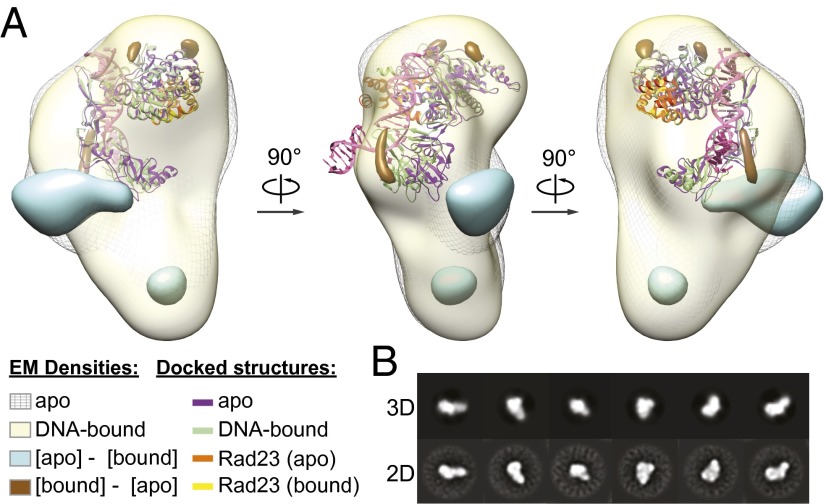
To visualize possible structural changes in the human XPC complex upon binding to DNA, we used a monomeric avidin and biotinylated-DNA affinity purification strategy to isolate only DNA-bound XPC molecules (Fig. S4 A and B). The 48-bp DNA bubble mismatch duplex was chosen from a validated EMSA probe (22) that demonstrated one of the strongest affinities for the XPC complex. Comparison of the reconstructions obtained from the apo vs. DNA-bound samples indicates that the addition of DNA primarily affected the density region immediately above the “earlobe” (Fig. 3A). Addition of DNA to XPC did not appear to lock the structure into a single conformation because the 2D class averages (Fig. 3B) and the resolution of ∼24 Å are similar to those we observed for the apo complex. The changes observed are consistent with a possible movement of the BH domains of XPC toward the region of DNA-binding. This assessment is based on comparisons between the changes imposed by DNA binding in the Rad4 crystal structure and the position of the crystal structure docked into the EM density either as the intact Rad4/Rad23 crystal structure (Fig. 3A) or with the C-terminal portion of the Rad4 TGD domain considered separately from the N-terminal portion to better reflect structural homology with the human XPC, which contains an insertion in its TGD domain (Figs. S4C and S5 A and B). A second interesting possibility is that certain regions of RAD23B become disordered upon DNA-binding, thus resulting in the observed loss of density; this is consistent with the finding that some regions of Rad23 that are ordered in the apo Rad4/Rad23 crystal structure becoming disordered upon binding DNA (21).
Fig. S4.
Purification of DNA-bound XPC complexes and alternate DNA-bound structural models. (A) Schematic of purification strategy of DNA-bound complexes. (B) SDS/PAGE and Coomassie staining of XPC-RAD23B-CETN2 copurifying with indicated DNA substrates (Upper); gel cropped for clarity. Native PAGE and SYBR Gold staining of DNA substrates eluted during sample preparation (Lower). IN = input before pull-down. (C) Alternative docking of the Rad4/Rad23 crystal structure (21) (PDB ID 2QSF) to better illustrate the proposed overlap between the primary difference density and the observed positional shift of Rad4 in the crystal structure. Shown are front and top-side views of the XPC complex bound to a mismatch bubble substrate generated in RELION (mesh yellow) with the apo structure (solid gray) and the [apo] – [DNA-bound] 3D difference density at 4σ (for clarity) in light blue. No [DNA-bound] - [apo] difference density was observed at this threshold. Here, the N- and C-terminal portions of the TGD domain (residues 99–295 and 298–433, respectively) are split to better reflect the human loop insertion (Fig. 1A and Fig. S5). Rad4TGD2-Rad23 structures were docked manually and are shown with their Rad23 domains aligned to one another. Rad4TGD1 was docked in the context of the full crystal structure using Situs (31) and Chimera (61). Rad4TGD1 is shown in blue, Rad4-TGD2apo is in purple, Rad23apo is in orange, Rad4-TGD2DNA-bound is in green, and Rad23DNA-bound is in solid yellow. (D) Front and side views of the ssDNA-bound XPC complex (mesh pink) shown with the mismatch-bound structure (solid yellow) with the [mismatch] – [ssDNA] difference density at 3σ shown in blue. No difference density is observed at 4σ. (E) Alignment of the apo (purple) and DNA-bound (green) Rad4 crystal structures by their TGD domain. Rad23 was omitted for clarity. Shown also is an enlarged view with distances in Ångstroms between indicated residues.
Fig. 3.
DNA binding by XPC is accompanied by large but localized conformational changes distal from the sites of presumed DNA contact. (A) Front, side, and back views of the XPC complex bound to a mismatch bubble substrate generated in RELION (32) (yellow) shown with the apo structure (mesh gray), with the 3D [apo] − [DNA-bound] difference density at 3σ (cyan), and with the [DNA-bound] − [apo] difference density (brown). Also shown is the Situs-based docking (31) of the yeast Rad4/Rad23 apo (PDB ID 2QSF; purple/orange) structure with the DNA-bound (PDB ID 2QSH; green/yellow) structure aligned to the apo via the Rad23 subunit. (B) Comparison of 3D reprojections of the model to their corresponding reference-free 2D class averages.
Fig. S5.
Conservation analysis via comparison of the human XPC complex and the yeast Rad4 complex. (A) Schematic representation of the subunits and domains of the human XPC complex and the yeast Rad4 complex. Transglutaminase homology domain (TGD), β-hairpin domains 1–3 (BH), ubiquitin-like domain (UbL), ubiquitin-associated domains 1 and 2 (UBA), EF-hand domains (EF), and protein- and DNA-binding domains (BD) are indicated accordingly. (B) Sequence alignment between human XPC and yeast Rad4. Predicted and observed α-helices (pink bars) and β-strands (green bars) are indicated. XPA-binding residues (blue), OCT4-binding residues (25) (red), SOX2-binding residues (25) (orange), and TFIIH-binding residues (15, 16, 44, 45) (violet) were designated based on sequence alignment between human XPC and yeast Rad4 and are indicated by color as noted. Regions of contact with Rad23 in the Rad4/Rad23 crystal structure (PDB ID 2QSF) are indicated with coral bars. Yellow boxes indicate DNA-binding residues in the DNA-bound Rad4/Rad23 crystal structure (PDB ID 2QSH) and light purple/aqua bars indicate these designated regions of contact with DNA. Secondary structure prediction was performed using JPred4 (62). (C) Sequence alignment between human CETN2 and yeast Rad33. Predicted and observed α-helices (pink bars) and β-strands (green bars) are indicated. XPC-binding residues are indicated in purple. Secondary structure prediction was performed using JPred4 (62).
The XPC complex has been demonstrated to bind other substrates as well, such as single-stranded DNA (33). Therefore, we also prepared ssDNA-bound XPC molecules using the same strategy of biotinylated-DNA pull-down (Fig. S4 A and B). Three-dimensional difference density analysis indicates that this structure is nearly indistinguishable from the mismatch-DNA-bound XPC complex (Fig. S4D).
Conservation of Structure and Function.
The human XPC complex (XPC, RAD23B, CETN2) and the yeast homolog Rad4 complex (Rad4, Rad23, Rad33; ref. 34) appear to function equivalently in nucleotide excision repair, given their similar binding properties to damaged DNA (22). These two complexes are also thought to be structurally similar based on strong sequence homology between human RAD23B and yeast Rad23 (6) and between human CETN2 and the purported yeast CETN2 homolog Rad33 (Fig. S5C). Despite low sequence conservation overall between XPC and Rad4, these two proteins share sequence homology of key domains (35) and very similar predicted secondary structures (Fig. S5 A and B). To examine this question of structural conservation, we obtained a 3D reconstruction at ∼23 Å resolution of the complete yeast Rad4 complex (Fig. 4 A and B). The overall architecture of the complexes from the two species is remarkably similar at both the 3D and 2D levels (Fig. 4 B–D, Right vs. Fig. 1B and Fig. S2 B and E), although there are small areas of difference between the human and yeast complexes, as seen in the 3D difference maps (Fig. 4C). We posit that the region of difference density in the earlobe may be due to structural differences between the CETN2 and Rad33 homologs (Fig. 1E and Fig. S5C).
Fig. 4.
Comparative studies of the human and yeast XPC/Rad4 complexes reveal divergence in function but not structure. (A) Purification and cross-linking of the homologous yeast Rad4-Rad23-Rad33 complex. (B) Three-dimensional model of the yeast Rad4 complex as solved by EMAN2 (49). (C) Three-dimensional difference density at 3σ between the human (mesh pink) and the yeast (solid gray) complexes. Positive difference density, or [human]-[yeast], is shown in cyan; negative difference density or [yeast] − [human] is shown in purple. (D) Comparisons of 3D reprojections of the yeast Rad4 complex with 2D class averages. (E) Titrations over a fourfold concentration range of yeast, mouse, and human XPC homolog complexes in in vitro transcription reactions of a NANOG promoter template engineered with four extra copies of the oct-sox composite binding element (bottom), performed in the presence of OCT4 and SOX2 protein. Transcripts are indicated with arrowheads. (F) Coimmunoprecipitation of human and yeast XPC/Rad4 complexes with HA-tagged SOX2 or RFP.
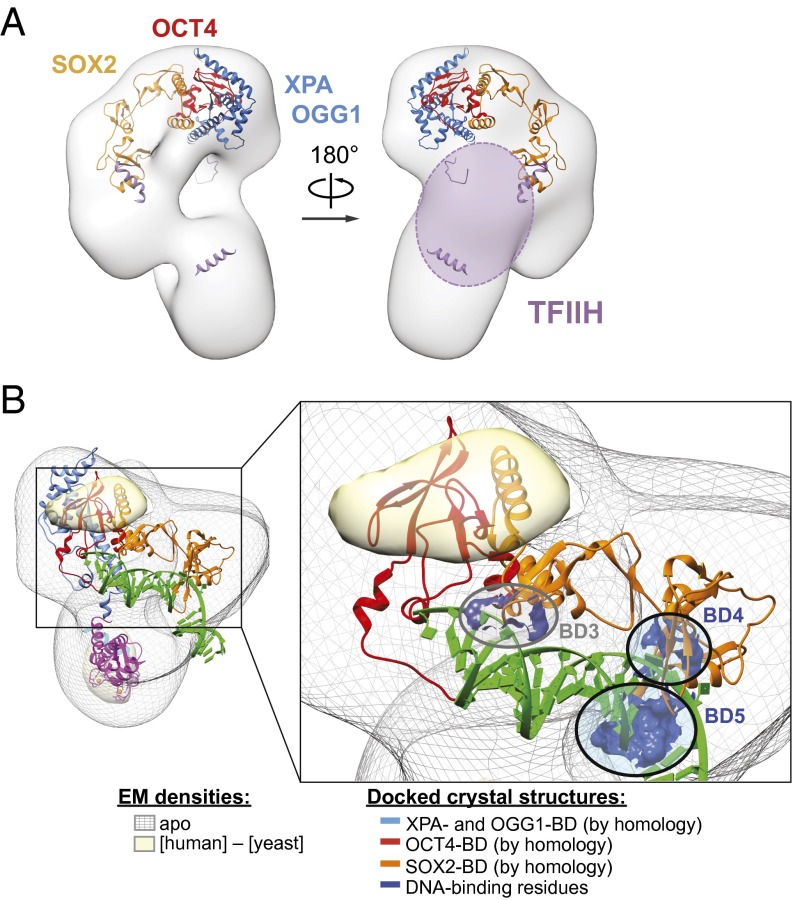
The structural similarity between the human and yeast XPC/Rad4 complexes suggested that other functions of the XPC complex, in particular its transcriptional roles in ES cells, might also be conserved. To our surprise, we observed that unlike the human and mouse XPC complexes, the Rad4 complex exhibited no coactivator activity in our in vitro transcription assay (Fig. 4E) and was completely incapable of forming a stable interaction with SOX2, the primary requisite activator for in vitro activation of NANOG gene transcription (4) (Fig. 4F). Using information on XPC’s interaction domains with partner proteins (14, 15, 25, 27, 36), sequence homology between yeast Rad4 and human XPC (Fig. S5), as well as the docking of the Rad4/Rad23 crystal structure, we were able to generate a model indicating the predicted locations of the interaction domains on the XPC complex (Fig. 5A). These interaction domains are clustered on the top of the ear. Intriguingly, when we superimpose the regions of difference density between the human and yeast EM maps with the predicted interaction domains, we note that one such region is located near the OCT4- and SOX2-binding interfaces but not the DNA-binding residues in regions DNA-BD3-5 (Fig. 5B), suggesting that these regions of difference may underlie the phylogenetic divergence in transcriptional activity between the human and yeast complexes.
Fig. 5.
Model of key interaction interfaces on the XPC complex. (A) Model of predicted interaction surfaces based on docking and sequence homology with the yeast Rad4/Rad23 crystal structure (PDB ID 2QSF). Indicated are residues involved in binding to XPA (14) (blue; yeast residues 101–296), OCT4 (25) (red; yeast residues 298–392), SOX2 (25) (orange; yeast residues 392–609), and TFIIH (15, 16, 44) (violet; human residues 847–863 and yeast residues 76–115 and 610–631). (B) Top back view of the XPC complex showing an area of positive difference density between the human and yeast structures (yellow) that coincides with the predicted OCT4- (red) and SOX2-binding domains (orange) but not the DNA-binding residues (dark blue residues and circles; BD3-5). The XPA binding domain has been omitted in the enlarged view for clarity.
SI Materials and Methods
Expression and Purification of XPC/Rad4 Complexes.
N-His6-tagged XPC, N-FLAG-tagged RAD23B, and untagged or N-MBP-tagged CETN2 sequences were amplified and cloned into pFastBac Dual vectors (Invitrogen). Recombinant bacmids were generated from pFastBac Dual constructs in Escherichia coli DH10Bac cells and transfected into Sf9 cells using Cellfectin II (Life Technologies) according to the manufacturer’s instructions for the generation of recombinant baculovirus, which was then twice-amplified in Sf9 cells. ES-Sf9 suspension cells (∼1.5–2 × 106 per mL) were then infected with amplified baculovirus, collected 48 h postinfection, and washed three times with ice-cold PBS. For biochemical analyses, complexes were purified as described (4). For EM samples, harvested cells were resuspended in 6 packed-cell volumes of high-salt lysis buffer HSNG [550 mM KCl, 50 mM Hepes, pH 7.6, 10% (vol/vol) glycerol, 3% (vol/vol) 1-propanol, 0.5% NP-40 alternative (EMD Millipore), 1 mM benzamidine, 1:1,000 aprotinin solution from bovine lung (Sigma-Aldrich), 0.5 mM PMSF, 1 mM TCEP, 1 mM DTT, and EDTA-free protease inhibitor tablet (Roche)] and lysed with a B dounce after a 10 min. incubation on ice. Lysates were cleared by centrifugation and were incubated for ∼2 h. with M2 anti-FLAG resin (Sigma-Aldrich), washed extensively with buffer HSNG, and subsequently equilibrated into and eluted in HSNG with no DTT supplemented with 0.3 mg/mL FLAG peptide (Sigma-Aldrich). The eluate was incubated with Ni-NTA agarose resin (Qiagen) in the presence of 10 mM imidazole. After washing with buffer HSNG – DTT + 10 mM imidazole, the resin was equilibrated into elution buffer B [300 mM KCl, 50 mM Hepes, pH 7.6, 0.1% NP-40 alternative, 10% (vol/vol) glycerol, 0.1 mM EDTA, 1 mM MgCl2, 1 mM TCEP] and eluted with buffer B + 250 mM imidazole. The sample was then dialyzed overnight to remove imidazole and to reintroduce 1 mM DTT before cross-linking with 0.2% glutaraldehyde for 15 min at room temperature. These conditions were chosen based on temperatures tested (on ice, room temperature, 37 °C), time of incubation (5 min, 15 min, 45 min), type of cross-linker (formaldehyde vs. glutaraldehyde), and percentage of glutaraldehyde (0.01%, 0.05%, 0.1%, 0.2%, 0.5%, 1%).
Protein analysis was performed using PageBlue Staining Solution (Life Technologies) and the gel filtration HMW calibration kit (GE Healthcare Life Sciences) on a Superose 6, 10/300 GL size exclusion column (GE Healthcare Life Sciences).
EM Preparation and Electron Microscopy.
For negative stain, 4 μL of sample diluted to ∼5–20 ng/μL or ∼25–100 nM were applied for 30–60 s to freshly glow-discharged continuous carbon films on 400 mesh copper grids, washed 2× in 40 μL of buffer G [300 mM KCl, 25 mM Hepes, pH 7.6, 3% (wt/vol) trehalose, 0.01% NP-40 alternative, 1 mM TCEP, 1 mM DTT, 0.1 mM EDTA, and 1 mM MgCl2] and 4 × 40 μL of 1% uranyl formate (UF) for 10 seconds per wash. Grids were then blotted dry with Whatman paper and allowed to air-dry rapidly in a chemical fume hood. For random conical tilt, negative stain samples were similarly prepared as described above with the exception of the use of a holey Formvar carbon film covered with a continuous thin-carbon support (51). For visualization of the native complex, 2% phosphotungstate (PTA) stain at pH 7.0–7.4 was used. For cryo-EM, 4 μL of sample were applied to glow-discharged continuous carbon supports covering a carbon-thickened C-flat grid (Protochips) with 2-μm holes spaced 2 μm apart, incubated for 30–60 s at 100% humidity and 22 °C, blotted for 4 s, and then plunge-frozen into liquid ethane using a Mark IV Vitrobot (FEI). Negative stain images were collected on a Tecnai F20 equipped with a field emission gun at an acceleration voltage of 120 kV on a Gatan UltraScan 4000 4k × 4k camera and at a nominal magnification of either 50,000× (2.29 Å/pixel at the specimen level) for random conical tilt images or 80,000× (1.51 Å/pixel at the specimen level) for untilted images with an electron dose of 20–30 e−/Å2. Cryo-EM images were collected as described for negative stain images except that images were taken at a nominal magnification of 100,000× (1.15 Å/pixel at the specimen level) with an electron dose of 20 e−/Å2 and a defocus range from −1.5 to −3.0. Leginon software (47) was used to collect all exposure images.
Ab Initio Random Conical Tilt Reconstruction.
We generated ab initio 3D volumes using random conical tilt reconstruction (29) (Fig. S1 D–G). Tilt pairs at 0° and 50° were collected using Leginon (52). Particles were picked using DoG picker (53) and correlated between tilt pairs using TiltPicker (53). Particle picks were then manually curated and extracted from the raw micrographs. Untilted particles were subjected first to iterative 2D classification and alignment using topology representing network classification (54) and multireference alignment in IMAGIC (55); these initial class averages were then used as templates for reference-based classification in SPIDER (56) after binning the stack of particles by a factor of 2. Ab initio models were generated for each of the 33 reference-free class averages calculated from the untilted classes (Fig. S1E) using the RCT module in the Appion pipeline (48) and back-projection in SPIDER (56). An elongated shape was evident in nearly all of the initial RCT reconstructions. One initial volume was selected as a representative model on the basis of the number of particles included, resolution, and sharpness of its structural features (Fig. S1F) and matching of model reprojections to reference-free class averages (Fig. S1G). This selected RCT volume was stringently low-pass filtered to 80-Å resolution and subjected to iterative projection-matching refinement in EMAN2 (49) using the untilted particle images with a total of 2,282 particles; 1,161 particles contributed to this initial reconstruction after applying a cross-correlation score of 0σ, representing the mean of all total cross-correlation scores, to discard ∼50% of the poorest-matching particles. The resulting 3D structure was again low-pass filtered to 60-Å resolution and used as the reference for projection matching refinement of larger data sets.
3D Reconstruction and Analysis.
Particles from all large data sets were automatically selected using DoG picker (53) and manually curated at the level of the raw images and the 2D class reference-free class averages to remove large aggregates or bad particles. Contrast transfer function (CTF) estimations were performed using CTFFIND3 (57), and CTF corrections via Wiener filtering were performed using ACE2 (58). All steps were performed within the Appion image processing environment (48).
Two-dimensional reference-free image analysis was performed within Appion using iterative rounds of multivariate statistical analysis and multireference alignment.
Three-dimensional reconstructions were performed using iterative projection-matching refinement in EMAN2 (49) and in RELION (32) on particles from Wiener-filtered images. Reconstructions performed in EMAN2 were conducted using the refined RCT model low-pass filtered to 60-Å resolution, described in Ab Initio Random Conical Tilt Reconstruction. Three-dimensional reconstruction was conducted using an iterative multireference projection-matching approach containing libraries from the EMAN2 and SPARX software packages (49, 59). Refinement began at an angular step of 25° and progressed down to 2° angular increments. For the human apo structure, a stringent, normalized cross-correlation cutoff value of 0σ, representing the mean of all total cross-correlation scores, was applied, thus discarding ∼50% of the data; the final particle number contributing to the final human XPC complex reconstruction was 22,777 out of the 46,219 preselected particles (by mean and SD filtering in XMIPP within the Appion package to remove bad particles). This reconstruction reached a final resolution of ∼25 Å based on the 0.5 Fourier shell correlation criterion (60) (Fig. S2C). The Euler plot shows a relatively even distribution of views (Fig. S2D). Comparison of reprojections of the 3D density with reference-free 2D class averages (Fig. S2E) shows good correspondence and supported the validity of our ab initio reconstruction.
Reconstructions obtained in RELION were conducted using the refined RCT model low-pass filtered to 60-Å resolution, 15° angular steps, a T regularization parameter value of 2 for the negative stain data sets and 4 for the cryo-EM data set, an offset search range of 10 pixels, and an offset search step of 4 pixels. A total of 30,008 particles contributed to the native, uncrosslinked apo complex; a total of 206,680 particles contributed to the three sorted models, with 75,567 contributing to model 1, 73,713 to model 2, and 57,382 to model 3. A total of 69,168 particles contributed to the dsDNA-bound reconstruction, 61,185 particles to the ssDNA-bound reconstruction, and 68,037 particles to the apo reconstruction computed as a comparison. 20,354 particles contributed to the cryo-EM reconstruction. A total of 12,879 particles out of 26,510 preselected particles contributed to the final yeast Rad4 reconstruction.
Three-dimensional difference densities were calculated using SPIDER (56). Volumes were first normalized identically and subtracted from one another as volume 1 − volume 2 (positive densities) and volume 2 − volume 1 (negative densities).
The data sets presented are summarized in Table S1.
Table S1.
Summary of data sets collected
| Sample | Method | Reconstruction method | No. particles (particles included after cutoff) | Other notes | Resolution, Å | Corresponding figure(s) |
| apo hXPC | Negative stain (UF) | EMAN2 | 2,282 (1,161) | RCT | 60 (filtered) | Not shown |
| apo hXPC | Negative stain (UF) | EMAN2 | 46,219 (22,777) | Single model | 24.7 | Figs. 1 B–E, 4C, and 5 |
| MBP-CETN2 hXPC | Negative stain (UF) | EMAN2 | 11,290 (9,693) | Single model | 21.0 | Fig. 1C |
| XPC-RAD23B | Negative stain (UF) | EMAN2 | 14,313 (12,572) | Single model | 24.8 | Fig. 1D |
| native apo hXPC | Negative stain (PTA) | RELION | 30,008 | Single model | 32.1 | Fig. S3A |
| apo hXPC (X-linked) | Negative stain (PTA) | RELION | 23,757 | Single model | 27.5 | Fig. S3A |
| apo hXPC | Negative stain (UF) | RELION | 206,680 total | Multimodel | 24.1 | Fig. 2A |
| model 1: 75,567 | ||||||
| model 2: 73,713 | ||||||
| model 3: 57,382 | ||||||
| apo hXPC | Cryo | RELION | 20,354 | Single model | 24.6 | Fig. S3A |
| apo hXPC | Negative stain (UF) | RELION | 68,019 | Single model | 24.1 | Fig. 3A and Fig. S4C |
| dsDNA-bound hXPC | Negative stain (UF) | RELION | 69,150 | Single model | 24.1 | Fig. 3A and Fig. S4 C and D |
| ssDNA-bound hXPC | Negative stain (UF) | RELION | 61,167 | Single model | 24.1 | Fig. S4D |
| apo yRad4 | Negative stain (UF) | EMAN2 | 26,510 (12,879) | Single model | 23.5 | Figs. 4 C and D and 5B |
Purification of DNA-Bound Complexes.
Oligonucleotides used were:
5′-biotin-CTATGGCGAGGCGATTATCAACCCATTGCAGTGGGTCTTCCGAACGAC and 5′- GTCGTTCGGAAGACCCTGACGTTGCCCAACTTAATCGCCTCGCCATAG (Integrated DNA Technologies).
For the dsDNA-bound complex, oligos were annealed in 40 mM Tris⋅HCl pH 7.5, 50 mM NaCl, and 10 mM MgCl2 by incubation at ∼95 °C in a water bath and cooling overnight. Annealed products were separated on an 8% 1× TBE native polyacrylamide gel, cut out via UV shadowing, electroeluted, and ethanol-precipitated. For the ssDNA-bound complex, the 5′-biotin-oligo was used at 100 μM upon resuspension.
The dsDNA or ssDNA substrates were then applied to monomeric avidin resin prepared according to the manufacturer’s instructions (catalog no. 20228, Thermo Scientific). Protein sample was then applied. After extensive washing of the resin, DNA-bound protein complexes were eluted with the addition of free biotin. Samples were then cross-linked for 15 min at room temperature with 0.2% glutaraldehyde and prepared for subsequent EM analysis. Eluted DNA was visualized by SYBR Gold staining (Life Technologies).
Coimmunoprecipitation Assays.
pCMV5a-Sox2-HA or control pLKO.1-FLAG-HA-RFP expression plasmids were transfected using calcium phosphate into HEK293T cells. A total of 2.5 × 106 cells were seeded on 10-cm dishes 24 h before transfection and harvested 48 h posttransfection in 1 mL of co-IP buffer per 10 cm dish (0.2 M KCl, 50 mM Hepes pH 7.6, 0.1% NP-40 alternative, 10% glycerol, 0.1 mM EDTA, 1 mM MgCl2, 1 mM DTT, 1 mM TCEP, 1 mM benzamidine, 1:1,000 aprotinin, 0.5 mM PMSF, 1× Roche Complete Protease Inhibitor tablet per 50 mL of co-IP buffer) by direct lysis on the plates and passing the lysate through a 25-gauge needle two times. Cleared lysates were incubated overnight at 4 °C with ∼50 μg of human His6-XPC:FLAG-RAD23B:CETN2 or yeast His6-Rad4:FLAG-RAD23:RAD33 prepurified from Sf9 cells using the two-step Ni-NTA and FLAG affinity purification described in ref. 4. Following the overnight incubation, anti-HA resin (Sigma-Aldrich) was added and incubated for an additional 4 h, followed by six washes in co-IP buffer. Immunoprecipitating proteins were specifically eluted with the addition of 0.2 mg/mL HA peptide (Sigma-Aldrich) and analyzed by Western blotting with HisProbe HRP (Thermo Scientific), anti-HA (ab9110, Abcam), and anti-FLAG M2 (Sigma-Aldrich) antibodies as indicated.
In Vitro Transcription Assays.
In vitro transcription reactions with the factors TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH, RNA polymerase II, OCT4, SOX2, fraction Q(4), and the human, mouse, and yeast homologs of the XPC complex were carried out as described (50), except that immuno-affinity purified TFIIH and RNA pol II were used. Plasmids encoding FLAG-tagged Oct4 or Sox2 were transiently transfected into HeLa cells using Lipofectamine 2000 (Invitrogen) and purified as described (4).
Discussion
Our single particle analyses reveal an ear-like shape for the human XPC complex and indicate the existence of a range of conformational states for this DNA repair and stem cell coactivator complex (Figs. 1 and 2). We show that the yeast homolog Rad4 holo-complex has a similar overall architecture but small regions of difference compared with the human XPC complex that may reflect their functional nonequivalence in biochemical assays (Fig. 4). Using labeling, mutational, and docking strategies, we localize the individual subunits of the complex within the structure (Fig. 1). The binding to the two distinct DNA substrates used in this study resulted in a similar overall conformational change in the left domain immediately above the earlobe, which is consistent with previous observations in the Rad4/Rad23 crystal structures (21) (Fig. 3).
Our study reveals the apparent flexibility of the XPC complex, in large part mirroring its functional versatility. The flexibility of the complex may stem, at least in part, from RAD23B, because certain regions of Rad23 were found to be disordered in the Rad4/Rad23 crystal structure (21). Part of the conformational heterogeneity seen in our EM structures may be due to variations in the interaction between the UbL and the equivalent UBA1 and UBA2 domains of RAD23B (Fig. 1A). Some of the conformational variability may also originate from the XPC subunit. Structure prediction analysis (14), limited proteolysis (14), and NMR (37) identified several highly disordered regions: the N terminus (residues 1–154), the C terminus (residues 816–940), and a loop inserted into the TGD domain comprising residues 331–517. Finally, CETN2 may also contribute to this overall flexibility, because it can adopt different conformations depending on its metal-binding state (38); however, the resolution of the XPC-RAD23B subcomplex was not markedly improved, suggesting that this contribution to complex flexibility is minor, as would be expected for its relatively small mass contribution to the complex. The recently described requirement of RNA for the XPC complex to interact with its transcription partner SOX2 (25) invokes the idea of low-complexity domains or regions, perhaps interspersed throughout XPC, linking their inherent flexibility to a critical aspect of the XPC complex’s function. The possibility that the mammalian-specific insertion within the TGD domain (residues 331–517; Fig. 1A and Fig. S5B) could be partly responsible for some of the observed structural heterogeneity is particularly interesting.
With the use of an MBP-tag as a labeling strategy, as well as the exclusion of CETN2 from the complex, we were able to localize CETN2 to the earlobe of the structure (Fig. 1 C and D). Extending these findings, we used rigid-body docking in an unbiased manner to place the Rad4/Rad23 crystal structure at the top of the ear and the CETN2 crystal structure in the earlobe (Fig. 1E). Attempts to tag the XPC and RAD23B subunits were not successful for a variety of reasons. The absence of CETN2 does not appear to impose large conformational rearrangements, as seen by the comparison between the full complex and the XPC-RAD23B subcomplex (Fig. 1 B and D). This observation is consistent with the lesser functional consequence of removing CETN2 than that of removing RAD23B in transcriptional coactivation assays, as well as with the inconsequential removal of the C-terminal CETN2-interaction domain on XPC (residues 814–940) (4).
The similarity between the dsDNA- and ssDNA-bound structures is consistent with the fact that the XPC complex is capable of binding a large suite of different DNA structures, including UV-induced thymine dimers (2), mismatch bubbles (22), ssDNA–dsDNA junctions (39), apurinic/apyrimidic (AP) sites (40), and even undamaged duplex and certain single-stranded DNA substrates (33). This similarity between different DNA-bound structures is also consistent with recent work describing a kinetic but not structural means of discrimination between damaged and undamaged DNA by Rad4 (41). The reduction of density in our DNA-bound structures compared with the apo XPC complex reflects a conformational change consistent with two phenomena observed in the Rad4 crystal structure: (i) the C-terminal portion of Rad4 shifting toward the DNA substrate, and in the context of our docking, away from the region of reduced density (Fig. 3A and Fig. S4 C and E); (ii) portions of Rad23 originally contributing to ordered density in the apo crystal structure becoming disordered upon binding to DNA (21). We are inclined to favor this latter observation to explain the DNA-induced changes to the XPC complex because the primary EM density difference we observe cannot easily be accounted for by the modest, ∼13- to 14-Å shift in Rad4 observed in the crystal structure (Fig. S5E). Therefore, we propose that DNA binding by the XPC complex induces specific conformational changes and disorder of certain domains, possibly such as the UbL domain (Fig. 1E).
The overall similarities between the human XPC structure and the yeast Rad4 structure, despite their divergent amino acid sequences, provide additional, indirect validation of the accuracies of the 3D models. Indeed, it seems quite remarkable that without requiring major changes to the overall evolutionarily conserved 3D shape and structure of the mammalian XPC complex, it has nevertheless adopted entirely new transcriptional coactivator functions in the context of ES cell regulatory pathways that are not relevant in yeast. The extent of structural conservation is consistent with their equivalence in repair (22). Regions displaying differences may reflect the divergence in functional capabilities that we observe in a transcriptional context (Fig. 4). Indeed, one of these regions at the top portion of the ear corresponds to residues homologous to those shown to interact with OCT4 and SOX2 (Fig. 5B, yellow). Importantly, this region is well separated from DNA-binding residues (Fig. 5B, circled BD3-5; key residues in dark blue). The only close-by DNA-binding residues (BD3) are in fact not conserved and are not found in the human sequence (Fig. S5B). This structural separation-of-function suggests that the local degree of structural conservation, even at modest resolution, is predictive of functional convergence or divergence. From an evolutionary point of view, a conserved process, such as nucleotide excision repair, would be expected to exhibit functional conservation between the yeast and human XPC homologs, whereas a nonconserved process, such as regulating genes expressed in mammalian embryonic stem cells, would not. Indeed, a number of XPC mutations that have differential effects in DNA repair vs. transcription support this idea. For instance, although deletion of the N-terminal UbL domain of yRad23 (42) and the W690S mutation of XPC (18, 43) have adverse consequences on nucleotide excision repair capabilities, respective mutations in XPC did not affect the ability to coactivate transcription (4) (Y.W.F., unpublished). Similarly, although the N and C termini of XPC are critical for recruitment and stimulation of TFIIH at sites of damage for global nucleotide excision repair (15, 16, 44, 45), the removal of the N- and C-terminal TFIIH-binding domains of XPC (residues 1–195 and 814–940, respectively) only impacts repair but not transcriptional activity (4, 25).
Reflecting the ever-expanding repertoire of reported XPC roles is the number of known physical and functional interactors of the XPC complex, e.g., TFIIH (15, 16), OGG1 (23), TDG (3), SOX2 (4, 25) (Fig. 4), and OCT4 (4, 25), among others (46). It is possible that the XPC complex serves as a coactivator not just for OCT4 and SOX2, especially given that its transcriptional activities do not appear to be cell-type-restrained (20); therefore, the list of XPC’s functional and physical partners is likely to grow. Although the residues on XPC through which some of these known interactions occur have been mapped, there is a degree of overlap between some of these regions, suggesting the need for more fine-tuned characterization and structural elucidation in the future (Fig. 1A). Our recent work describing the involvement of RNA in mediating the XPC-SOX2 interaction adds an additional and potentially intriguing dimension to future structural studies in this regard (25). Additionally, it would be interesting to explore whether structural changes are imposed on the XPC complex upon binding to its partner proteins; further biochemical and structural work to assemble such larger protein assemblies is required. In summary, this work provides a structural framework for integrating biochemical and structural information into a mechanistic understanding of the XPC complex’s undoubtedly complicated roles in DNA repair and transcriptional regulation.
Materials and Methods
Detailed methods can be found in SI Materials and Methods. XPC/Rad4 complexes were affinity purified from Sf9 cells. DNA-bound XPC samples were affinity purified using biotinylated DNA substrates. EM was performed using continuous carbon films and uranyl formate or phosphotungstate stain for negative stain. Leginon software (47) was used to collect images in a Tecnai F20 microscope equipped with a Gatan UltraScan 4000 camera. Data processing was performed primarily in the Appion pipeline (48). Three-dimensional reconstructions were performed using EMAN2 (49) and RELION (32). Coimmunoprecipitation assays were performed in HEK293T cells. In vitro transcription assays were performed essentially as described (50).
Acknowledgments
We thank G. Kemalyan, R. Louder, S. Howes, and D. W. Taylor for microscope and data processing guidance; T. Houweling for computer support; G. Dailey for help with expression constructs; and S. Zheng and C. Inouye for help with in vitro transcription components. We are grateful to M. Iadanza and T. Gonen for help with initial negative stain analysis. We thank R. Lesch and R. Schekman for yeast cells for cloning the yeast Rad4 complex. We thank C. Cattoglio, J. J. Ho, G. E. Katibah, D. C. Rio, A. Martin, and D. E. Wemmer for valuable discussion. This work was supported by the California Institute for Regenerative Medicine (CIRM) Research Grant RB4-06016 (to R.T.) and by the National Institute of General Medical Sciences (GM63072; to E.N.). E.T.Z. was a National Science Foundation Graduate Research Fellow and a University of California Berkeley Distinguished Fellow. Y.W.F. was a CIRM Scholar (Training Grant T1-00007). R.T. and E.N. are Howard Hughes Medical Institute (HHMI) Investigators. R.T. is the President of HHMI and the Director of the Li Ka Shing Center for Biomedical and Health Sciences.
Footnotes
The authors declare no conflict of interest.
Data deposition: Atomic coordinates and structure factors have been deposited in the Electron Microscopy Data Bank (EMDB), https://www.ebi.ac.uk/pdbe/emdb/ (accession nos. EMD-6495–EMD-6498).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520104112/-/DCSupplemental.
References
- 1.Weake VM, Workman JL. Inducible gene expression: Diverse regulatory mechanisms. Nat Rev Genet. 2010;11(6):426–437. doi: 10.1038/nrg2781. [DOI] [PubMed] [Google Scholar]
- 2.Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JHJ. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol. 2014;15(7):465–481. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu Y, Iwai S, Hanaoka F, Sugasawa K. Xeroderma pigmentosum group C protein interacts physically and functionally with thymine DNA glycosylase. EMBO J. 2003;22(1):164–173. doi: 10.1093/emboj/cdg016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong YW, et al. A DNA repair complex functions as an Oct4/Sox2 coactivator in embryonic stem cells. Cell. 2011;147(1):120–131. doi: 10.1016/j.cell.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krzeszinski JY, et al. XPC promotes MDM2-mediated degradation of the p53 tumor suppressor. Mol Biol Cell. 2014;25(2):213–221. doi: 10.1091/mbc.E13-05-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugasawa K, et al. HHR23B, a human Rad23 homolog, stimulates XPC protein in nucleotide excision repair in vitro. Mol Cell Biol. 1996;16(9):4852–4861. doi: 10.1128/mcb.16.9.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishi R, et al. Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein. Mol Cell Biol. 2005;25(13):5664–5674. doi: 10.1128/MCB.25.13.5664-5674.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Z, Liu S, Zhang Y, Wang Z. Roles of Rad23 protein in yeast nucleotide excision repair. Nucleic Acids Res. 2004;32(20):5981–5990. doi: 10.1093/nar/gkh934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortolan TG, Chen L, Tongaonkar P, Madura K. Rad23 stabilizes Rad4 from degradation by the Ub/proteasome pathway. Nucleic Acids Res. 2004;32(22):6490–6500. doi: 10.1093/nar/gkh987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki M, et al. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J Biol Chem. 2001;276(22):18665–18672. doi: 10.1074/jbc.M100855200. [DOI] [PubMed] [Google Scholar]
- 11.Le May N, Egly JM, Coin F. True lies: The double life of the nucleotide excision repair factors in transcription and DNA repair. J Nucleic Acids. 2010;2010(9):1–10. doi: 10.4061/2010/616342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petruseva IO, Evdokimov AN, Lavrik OI. Molecular mechanism of global genome nucleotide excision repair. Acta Naturae. 2014;6(1):23–34. [PMC free article] [PubMed] [Google Scholar]
- 13.Melis JPM, Luijten M, Mullenders LHF, van Steeg H. The role of XPC: Implications in cancer and oxidative DNA damage. Mutat Res. 2011;728(3):107–117. doi: 10.1016/j.mrrev.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunick CG, Miller MR, Fuller BE, Fanning E, Chazin WJ. Biochemical and structural domain analysis of xeroderma pigmentosum complementation group C protein. Biochemistry. 2006;45(50):14965–14979. doi: 10.1021/bi061370o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida A, et al. The carboxy-terminal domain of the XPC protein plays a crucial role in nucleotide excision repair through interactions with transcription factor IIH. DNA Repair (Amst) 2002;1(6):449–461. doi: 10.1016/s1568-7864(02)00031-9. [DOI] [PubMed] [Google Scholar]
- 16.Yokoi M, et al. The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA. J Biol Chem. 2000;275(13):9870–9875. doi: 10.1074/jbc.275.13.9870. [DOI] [PubMed] [Google Scholar]
- 17.Menoni H, Hoeijmakers JHJ, Vermeulen W. Nucleotide excision repair-initiating proteins bind to oxidative DNA lesions in vivo. J Cell Biol. 2012;199(7):1037–1046. doi: 10.1083/jcb.201205149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda G, et al. In vivo destabilization and functional defects of the xeroderma pigmentosum C protein caused by a pathogenic missense mutation. Mol Cell Biol. 2007;27(19):6606–6614. doi: 10.1128/MCB.02166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Näär AM, Lemon BD, Tjian R. Transcriptional coactivator complexes. Annu Rev Biochem. 2001;70(1):475–501. doi: 10.1146/annurev.biochem.70.1.475. [DOI] [PubMed] [Google Scholar]
- 20.Le May N, et al. NER factors are recruited to active promoters and facilitate chromatin modification for transcription in the absence of exogenous genotoxic attack. Mol Cell. 2010;38(1):54–66. doi: 10.1016/j.molcel.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Min J-H, Pavletich NP. Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature. 2007;449(7162):570–575. doi: 10.1038/nature06155. [DOI] [PubMed] [Google Scholar]
- 22.Krasikova YS, et al. Human and yeast DNA damage recognition complexes bind with high affinity DNA structures mimicking in size transcription bubble. J Mol Recognit. 2013;26(12):653–661. doi: 10.1002/jmr.2308. [DOI] [PubMed] [Google Scholar]
- 23.Bernardes de Jesus BM, Bjørås M, Coin F, Egly JM. Dissection of the molecular defects caused by pathogenic mutations in the DNA repair factor XPC. Mol Cell Biol. 2008;28(23):7225–7235. doi: 10.1128/MCB.00781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuck SC, Short EA, Turchi JJ. Eukaryotic nucleotide excision repair: From understanding mechanisms to influencing biology. Cell Res. 2008;18(1):64–72. doi: 10.1038/cr.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattoglio C, et al. Functional and mechanistic studies of XPC DNA-repair complex as transcriptional coactivator in embryonic stem cells. Proc Natl Acad Sci USA. 2015;112(18):E2317–E2326. doi: 10.1073/pnas.1505569112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masutani C, et al. Purification and cloning of a nucleotide excision repair complex involving the xeroderma pigmentosum group C protein and a human homologue of yeast RAD23. EMBO J. 1994;13(8):1831–1843. doi: 10.1002/j.1460-2075.1994.tb06452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popescu A, et al. Xeroderma pigmentosum group C protein possesses a high affinity binding site to human centrin 2 and calmodulin. J Biol Chem. 2003;278(41):40252–40261. doi: 10.1074/jbc.M302546200. [DOI] [PubMed] [Google Scholar]
- 28.Thompson JR, Ryan ZC, Salisbury JL, Kumar R. The structure of the human centrin 2-xeroderma pigmentosum group C protein complex. J Biol Chem. 2006;281(27):18746–18752. doi: 10.1074/jbc.M513667200. [DOI] [PubMed] [Google Scholar]
- 29.Radermacher M, Wagenknecht T, Verschoor A, Frank J. Three-dimensional reconstruction from a single-exposure, random conical tilt series applied to the 50S ribosomal subunit of Escherichia coli. J Microsc. 1987;146(Pt 2):113–136. doi: 10.1111/j.1365-2818.1987.tb01333.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J Mol Biol. 2003;333(4):721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Wriggers W. Using Situs for the integration of multi-resolution structures. Biophys Rev. 2010;2(1):21–27. doi: 10.1007/s12551-009-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheres SHW. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180(3):519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trego KS, Turchi JJ. Pre-steady-state binding of damaged DNA by XPC-hHR23B reveals a kinetic mechanism for damage discrimination. Biochemistry. 2006;45(6):1961–1969. doi: 10.1021/bi05196t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dulk den B, van Eijk P, de Ruijter M, Brandsma JA, Brouwer J. The NER protein Rad33 shows functional homology to human Centrin2 and is involved in modification of Rad4. DNA Repair. 2008;7(6):858–868. doi: 10.1016/j.dnarep.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Legerski R, Peterson C. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature. 1992;360(6404):610–610. doi: 10.1038/360610b0. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Lu X, Peterson C, Legerski R. XPC interacts with both HHR23B and HHR23A in vivo. Mutat Res. 1997;383(3):197–203. doi: 10.1016/s0921-8777(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 37.Miron S, Duchambon P, Blouquit Y, Durand D, Craescu CT. The carboxy-terminal domain of xeroderma pigmentosum complementation group C protein, involved in TFIIH and centrin binding, is highly disordered. Biochemistry. 2008;47(5):1403–1413. doi: 10.1021/bi701863u. [DOI] [PubMed] [Google Scholar]
- 38.Craig TA, et al. Metal-binding properties of human centrin-2 determined by micro-electrospray ionization mass spectrometry and UV spectroscopy. J Am Soc Mass Spectrom. 2006;17(8):1158–1171. doi: 10.1016/j.jasms.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 39.Sugasawa K, Shimizu Y, Iwai S, Hanaoka F. A molecular mechanism for DNA damage recognition by the xeroderma pigmentosum group C protein complex. DNA Repair (Amst) 2002;1(1):95–107. doi: 10.1016/s1568-7864(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 40.Pestryakov P, et al. Effect of the multifunctional proteins RPA, YB-1, and XPC repair factor on AP site cleavage by DNA glycosylase NEIL1. J Mol Recognit. 2012;25(4):224–233. doi: 10.1002/jmr.2182. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, et al. Kinetic gating mechanism of DNA damage recognition by Rad4/XPC. Nat Commun. 2015;6:5849. doi: 10.1038/ncomms6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortolan TG, et al. The DNA repair protein rad23 is a negative regulator of multi-ubiquitin chain assembly. Nat Cell Biol. 2000;2(9):601–608. doi: 10.1038/35023547. [DOI] [PubMed] [Google Scholar]
- 43.Maillard O, Solyom S, Naegeli H. An aromatic sensor with aversion to damaged strands confers versatility to DNA repair. PLoS Biol. 2007;5(4):e79. doi: 10.1371/journal.pbio.0050079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lafrance-Vanasse J, Arseneault G, Cappadocia L, Legault P, Omichinski JG. Structural and functional evidence that Rad4 competes with Rad2 for binding to the Tfb1 subunit of TFIIH in NER. Nucleic Acids Res. 2013;41(4):2736–2745. doi: 10.1093/nar/gks1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziani S, et al. Sequential and ordered assembly of a large DNA repair complex on undamaged chromatin. J Cell Biol. 2014;206(5):589–598. doi: 10.1083/jcb.201403096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lubin A, Zhang L, Chen H, White VM, Gong F. A human XPC protein interactome--a resource. Int J Mol Sci. 2014;15(1):141–158. doi: 10.3390/ijms15010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suloway C, et al. Automated molecular microscopy: The new Leginon system. J Struct Biol. 2005;151(1):41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Lander GC, et al. Appion: An integrated, database-driven pipeline to facilitate EM image processing. J Struct Biol. 2009;166(1):95–102. doi: 10.1016/j.jsb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang G, et al. EMAN2: An extensible image processing suite for electron microscopy. J Struct Biol. 2007;157(1):38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Ryu S, Zhou S, Ladurner AG, Tjian R. The transcriptional cofactor complex CRSP is required for activity of the enhancer-binding protein Sp1. Nature. 1999;397(6718):446–450. doi: 10.1038/17141. [DOI] [PubMed] [Google Scholar]
- 51.Bradley DE. 1965. The preparation of specimen support films. Techniques for Electron Microscopy (Blackwell Scientific Publications, Oxford, UK), pp 58–74.
- 52.Suloway C, et al. Fully automated, sequential tilt-series acquisition with Leginon. J Struct Biol. 2009;167(1):11–18. doi: 10.1016/j.jsb.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voss NR, Yoshioka CK, Radermacher M, Potter CS, Carragher B. DoG Picker and TiltPicker: Software tools to facilitate particle selection in single particle electron microscopy. J Struct Biol. 2009;166(2):205–213. doi: 10.1016/j.jsb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogura T, Iwasaki K, Sato C. Topology representing network enables highly accurate classification of protein images taken by cryo electron-microscope without masking. J Struct Biol. 2003;143(3):185–200. doi: 10.1016/j.jsb.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 55.van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996;116(1):17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 56.Frank J, et al. SPIDER and WEB: Processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116(1):190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 57.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol. 2003;142(3):334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 58.Mallick SP, Carragher B, Potter CS, Kriegman DJ. ACE: Automated CTF estimation. Ultramicroscopy. 2005;104(1):8–29. doi: 10.1016/j.ultramic.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Hohn M, et al. SPARX, a new environment for Cryo-EM image processing. J Struct Biol. 2007;157(1):47–55. doi: 10.1016/j.jsb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 60.van Heel M, Schatz M. Fourier shell correlation threshold criteria. J Struct Biol. 2005;151(3):250–262. doi: 10.1016/j.jsb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 62.Drozdetskiy A, Cole C, Procter J, Barton GJ. JPred4: A protein secondary structure prediction server. Nucleic Acids Res. 2015;43(W1):W389-94. doi: 10.1093/nar/gkv332. [DOI] [PMC free article] [PubMed] [Google Scholar]