Significance
Nitrogenase biosynthesis protein NifB catalyzes the radical S-adenosyl-L-methionine (SAM)-dependent insertion of carbide into the nitrogenase cofactor, M cluster, in a chemically unprecedented and biologically important reaction. The observation that two naturally “truncated” NifB homologs from Methanosarcina acetivorans (NifBMa) and Methanobacterium thermoautotrophicum (NifBMt) are functional equivalents of NifB from the diazotrophic organism, Azotobacter vinelandii, establishes the minimum sequence requirement for a functional NifB protein and reveals the species-dependent difference between members of this protein family; more importantly, it leads to the categorization of a distinct class of radical SAM methyltransferases that function in complex metallocluster assembly while opening up new avenues to study the structure and mechanism of NifB.
Keywords: nitrogenase, NifB, methanogens, radical SAM, homologs
Abstract
Nitrogenase biosynthesis protein NifB catalyzes the radical S-adenosyl-L-methionine (SAM)-dependent insertion of carbide into the M cluster, the cofactor of the molybdenum nitrogenase from Azotobacter vinelandii. Here, we report the identification and characterization of two naturally “truncated” homologs of NifB from Methanosarcina acetivorans (NifBMa) and Methanobacterium thermoautotrophicum (NifBMt), which contain a SAM-binding domain at the N terminus but lack a domain toward the C terminus that shares homology with NifX, an accessory protein in M cluster biosynthesis. NifBMa and NifBMt are monomeric proteins containing a SAM-binding [Fe4S4] cluster (designated the SAM cluster) and a [Fe4S4]-like cluster pair (designated the K cluster) that can be processed into an [Fe8S9] precursor to the M cluster (designated the L cluster). Further, the K clusters in NifBMa and NifBMt can be converted to L clusters upon addition of SAM, which corresponds to their ability to heterologously donate L clusters to the biosynthetic machinery of A. vinelandii for further maturation into the M clusters. Perhaps even more excitingly, NifBMa and NifBMt can catalyze the removal of methyl group from SAM and the abstraction of hydrogen from this methyl group by 5′-deoxyadenosyl radical that initiates the radical-based incorporation of methyl-derived carbide into the M cluster. The successful identification of NifBMa and NifBMt as functional homologs of NifB not only enabled classification of a new subset of radical SAM methyltransferases that specialize in complex metallocluster assembly, but also provided a new tool for further characterization of the distinctive, NifB-catalyzed methyl transfer and conversion to an iron-bound carbide.
Nitrogenase biosynthesis protein NifB is a radical S-adenosyl-L-methionine (SAM) enzyme that plays an essential role in the biosynthesis of the M cluster, a [MoFe8S9C-homocitrate] cluster that serves as the cofactor of the molybdenum (Mo) nitrogenase from Azotobacter vinelandii (1–7). Carrying a signature CxxxCxxC motif at its N terminus that houses the SAM-binding [Fe4S4] cluster (designated the SAM cluster), NifB also contains a number of additional ligands that could accommodate coordination of the entire complement of iron (Fe) atoms of the M cluster (Fig. S1). Moreover, it shares sequence homology with NifX, an accessory protein in M-cluster biosynthesis (8), toward its C terminus (Fig. S1). Characterization of the NifB protein from A. vinelandii had long been hampered by the instability of NifB in aqueous solutions until this protein was expressed as part of a NifEN-B fusion protein, wherein NifB was fused with and protected by NifEN, the biosynthetic apparatus immediately downstream of NifB along the M-cluster assembly pathway (9). Expression of the NifEN-B fusion protein in A. vinelendii was “modeled” after a naturally occurring NifEN-B fusion protein in Clostridium pasteurianum, which has the N terminus of NifB fused with the C terminus of NifN at a 1:1 molar ratio.
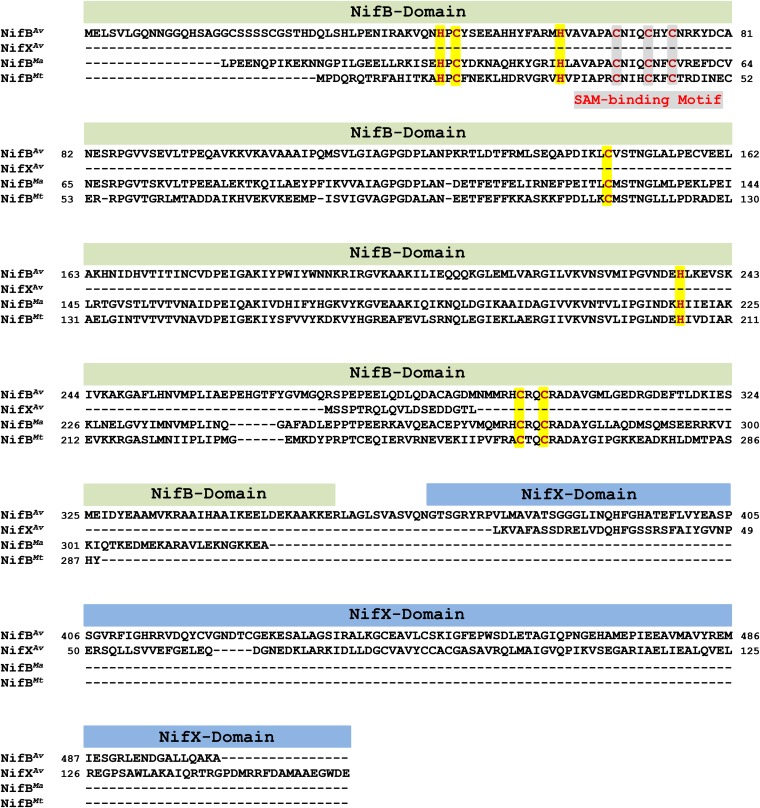
Fig. S1.
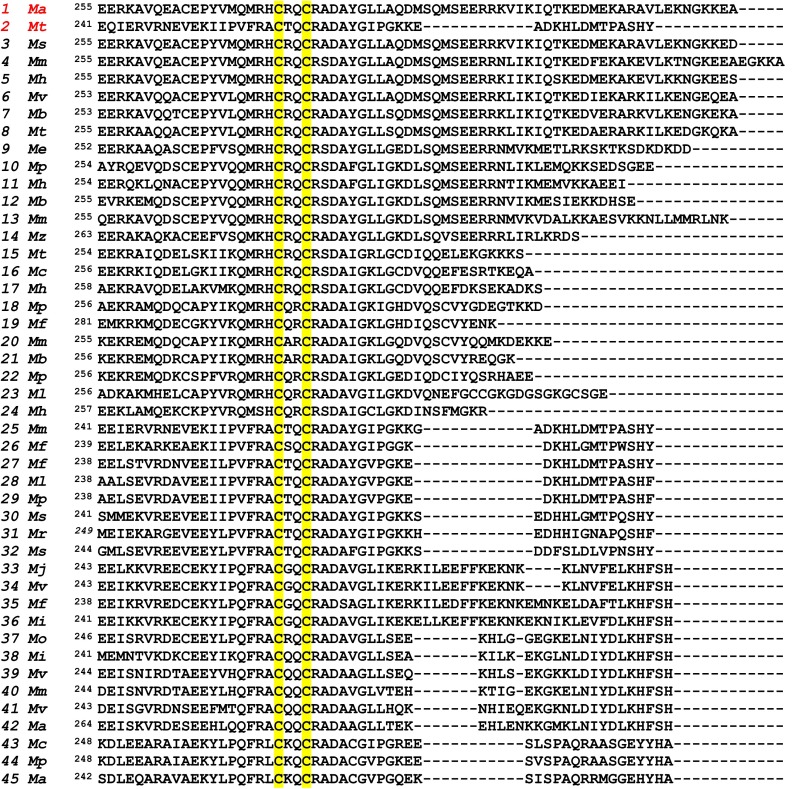
Sequence alignment of NifBAv, NifBMa, and NifBMt. All three sequences contain a signature CxxxCxxC domain (highlighted gray) that houses the SAM-binding [Fe4S4] cluster. Other conserved Cys and His residues (highlighted yellow) in the three sequences could accommodate an FeS precursor to the nitrogenase cofactor. The “NifB domain” toward the N terminus of the NifBAv sequence is also present in the sequences of NifBMa and NifBMt, whereas the “NifX domain” toward the C terminus of the NifBAv is notably missing from the sequences of both NifBMa and NifBMt.
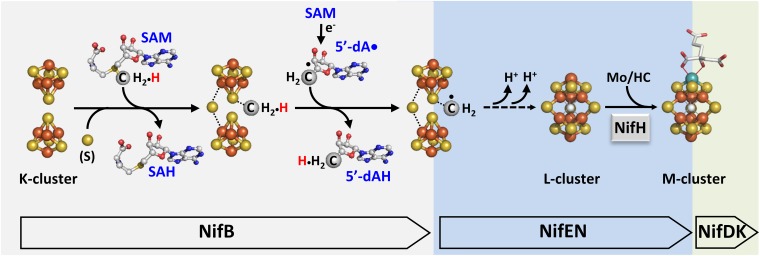
Subsequent studies of NifEN-B have demonstrated that, in the presence of SAM, NifB is capable of inserting a carbide concomitant with the coupling of a [Fe4S4]-like cluster pair (designated the K cluster) into an [Fe8S9C] cluster (designated the L cluster), which represents an all-iron core of the M cluster (9, 10). More excitingly, these studies have established the methyl group of SAM as the source of carbide, which is presumably transferred from SAM to the K cluster on NifB and processed initially by a hydrogen abstraction step that is enabled by a SAM-derived 5′-deoxyadenosyl radical (5′-dA•) (Fig. S2). The resultant methylene radical is further deprotonated into a carbide ion (C4−) concomitant with the radical-based rearrangement and coupling of the two [Fe4S4]-like modules of the K cluster into an [Fe8S9C] L cluster (Fig. S2) (11). The L cluster can then be converted into a mature M cluster on NifEN upon insertion of Mo and homocitrate by NifH (the reductase component of Mo ntirogenase), followed by delivery of the M cluster from NifEN to its target location in NifDK (the catalytic component of Mo nitrogenase) via direct protein–protein interactions (Fig. S2) (12–16).
Fig. S2.
Proposed pathway of carbide insertion. Carbide insertion begins with the transfer of a methyl group from one equivalent of SAM to the K cluster and the abstraction of a hydrogen atom from this methyl group by a 5-dA• radical that is generated upon homolytic cleavage of a second equivalent of SAM. The process continues with further deprotonation of the carbon-derived radical species concomitant with its incorporation into the L cluster as an interstitial carbide ion. Atoms of the clusters are colored as follows: Fe, orange; S, yellow; C, gray. The assembly proteins (Nif) housing these events are indicated.
Despite the success in characterizing the A. vinelandii NifB protein (designated NifBAv) in the form of NifEN-B fusion protein, the presence of NifEN entity in this protein has precluded an independent analysis of the subunit and cluster compositions of NifBAv. Moreover, the impact of the “NifX domain” on the functionality of NifBAv has not been evaluated to date. Interestingly, two naturally “truncated” NifB homologs, which do not have the “NifX domain” toward the C termini of their sequences, can be identified in two methanogenic, nitrogen-fixing organisms: one of them (designated NifBMa) is from the mesophilic organism, Methanosarcina acetivorans (17); whereas the other (designated NifBMt) is from the thermophilic organism, Methanobacterium thermoautotrophicum (also named Methanobacter thermoautotrophicus) (18). These NifB homologs were identified from the genomes of M. acetivorans C2A strain (Gene ID 638179084; Gene Symbol MA4195) and M. thermoautotrophicum Delta H strain (Gene ID 638156427; Gene Symbol MTH1871) at the website of Integrated Microbial Genomes (https://img.jgi.doe.gov/cgi-bin/w/main.cgi).
Whereas shorter in length, NifBMa and NifBMt share 69% and 64% sequence homology, respectively, with NifBAv (Fig. S1). More importantly, like NifBAv, they both contain the CxxxCxxC motif for coordination of the SAM cluster, as well as a number of conserved Cys and His residues for accommodation of an FeS precursor to the nitrogenase cofactor (Fig. S1). Such a simplified, NifX domain-free composition of NifBMa and NifBMt is appealing, as it not only enables assessment of the minimum sequence requirement for a functional NifB protein, but also facilitates heterologous expression of a stable form of NifB on its own, a feat that has not yet been accomplished in the case of NifBAv due to the presence of “extra” hydrophobic stretches of polypeptides in the primary sequence of this protein.
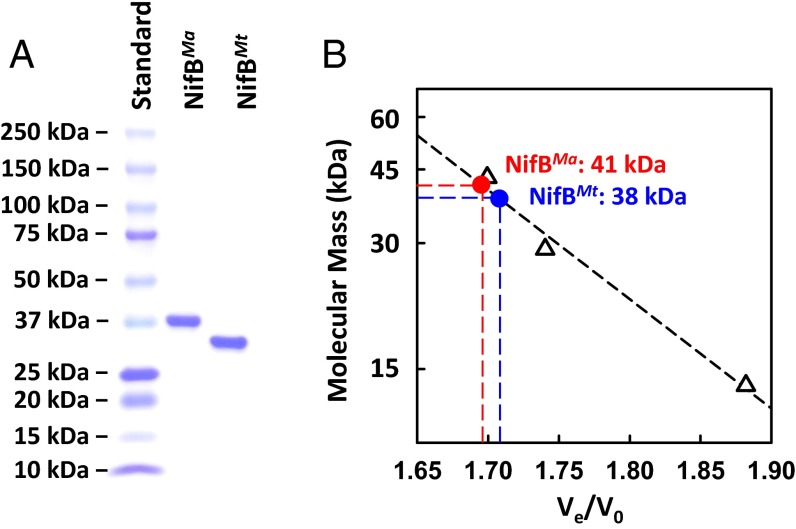
Indeed, His-tagged NifBMa and NifBMt were successfully coexpressed with the FeS assembly machinery, IscSUA, in Escherichia coli strain BL21(DE3) and purified at ∼350 and ∼180 mg/g wet cells, respectively, as intact, soluble proteins. The molecular masses of the subunits of NifBMa and NifBMt were confirmed as 38 kDa and 35 kDa, respectively, by SDS/PAGE analysis (Fig. 1A), whereas the apparent native molecular masses of NifBMa and NifBMt were determined as 41 kDa and 38 kDa, respectively, by gel filtration chromatography (Fig. 1B). These observations suggest a monomeric composition of both NifBMa and NifBMt, which correlate further with the 1:1 molar ratio between the NifEN and NifB entities in the NifEN-B fusion protein, a ratio that implies the action of NifB as a monomer by interacting in a one-on-one manner with the two αβ-dimers of NifEN (9). The in vitro reconstitution of NifBMa and NifBMt by FeCl3 and Na2S, followed by removal of excess Fe/S aggregates, resulted in a metal content of 14.0 ± 2.8 and 13.0 ± 2.2 mol Fe/mol protein, respectively, of NifBMa and NifBMt. Such an iron content would be consistent with the presence of three [Fe4S4] clusters in NifBMa or NifBMt, which could be assigned to one 4Fe SAM cluster and two 4Fe modules of the K cluster (Fig. S2). More importantly, it suggests that NifBMa and NifBMt contain all cluster species that are required to facilitate the K- to L-cluster conversion in the presence of SAM.
Fig. 1.
Molecular masses of NifBMa and NifBMt. (A) Coomassie blue-stained 4–15% SDS/PAGE (BioRad Mini-PROTEAN TGX SDS/PAGE) of NifBMa and NifBMt. Lanes from left to right: 10 μL of protein standards (BioRad Precision Plus Protein Kaleidoscope Standards), 2 μg of purified NifBMa, and 2 μg of purified NifBMt. (B) Determination of the native molecular masses of NifBMa and NifBMt by gel filtration. V0, void volume; Ve, elution volume. Protein standards (GE Healthcare Biosciences), shown in open triangles, are: ribonuclease A (13.7 kDa), carbonic anhydrase (29 kDa), and ovalbumin (43 kDa).
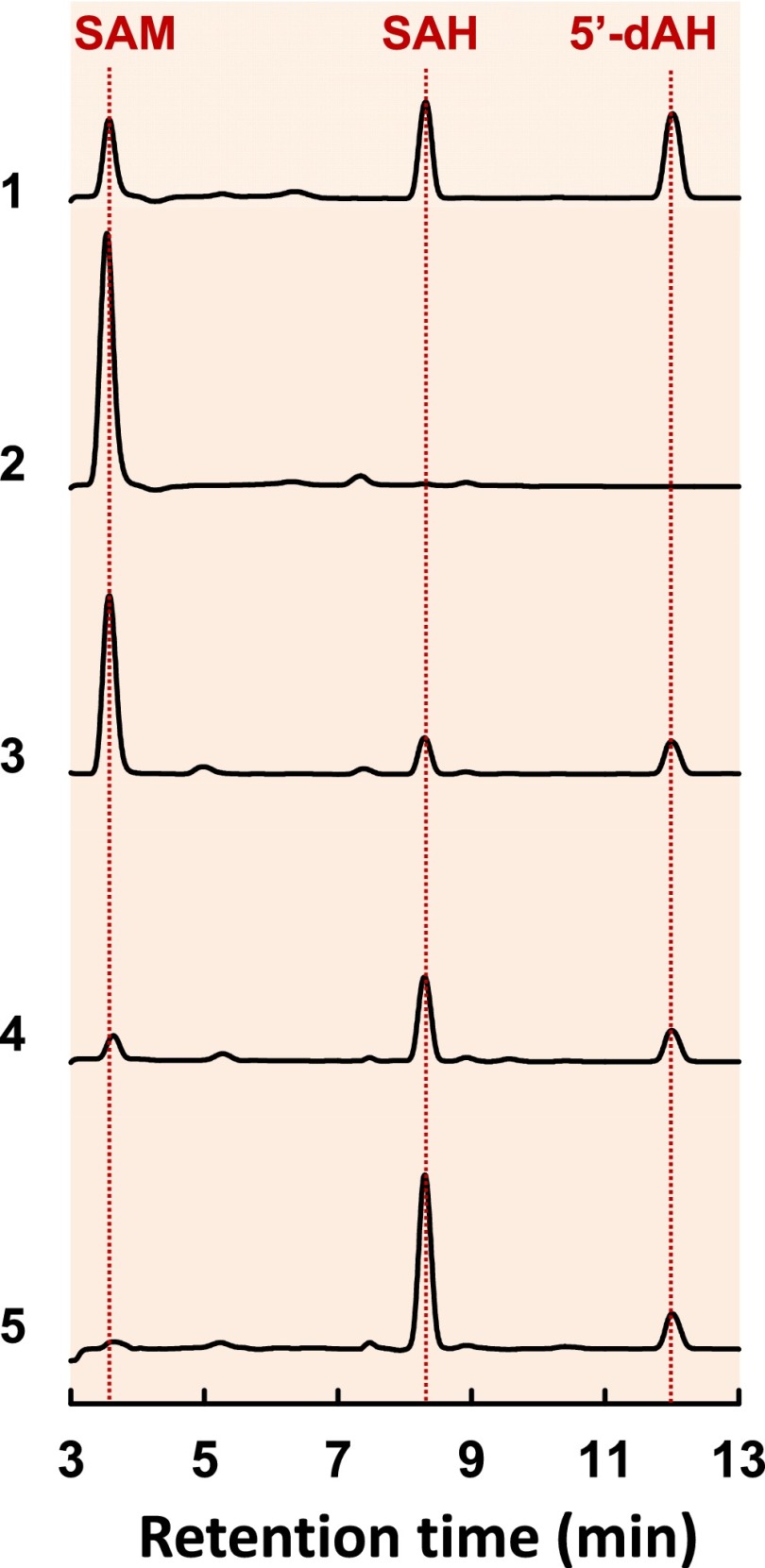
Consistent with this suggestion, high performance liquid chromatography (HPLC) analysis revealed that, like NifEN-B (Fig. 2, trace 3), NifBMa (Fig. 2, trace 4) or NifBMt (Fig. 2, trace 5) was capable of cleaving SAM into S-adenosyl-L-homocysteine (SAH) and 5′-deoxyadenosine (5′-dAH) in the presence of a reductant, dithionite. The observation of identical SAM cleavage products implies that NifBMa and NifBMt follow the same mechanism as that proposed for NifBAv in catalyzing the SAM-dependent reaction, mobilizing the methyl group of one equivalent of SAM and subsequently abstracting a hydrogen atom from this methyl group by a 5′-dA• radical that is derived from a second equivalent of SAM (Fig. S2). Moreover, formation of the same reaction byproducts by NifB proteins as those by radical SAM RNA methyltransferases RlmN and Cfr (19, 20) points to a similarity between NifB and these two well-characterized members of a larger subset of radical SAM enzymes that catalyze methylation reactions using SAM or other methyl donor molecules as cosubstrates (see Discussion). Interestingly, NifBMa and NifBMt appeared to be more efficient than NifBAv in cleaving SAM into SAH and 5′-dAH, as a substantial amount of SAM was left uncleaved when it was incubated with NifEN-B (Fig. 2, trace 3), but very little or almost no SAM was left uncleaved when it was incubated with NifBMa (Fig. 2, trace 4) or NifBMt (Fig. 2, trace 5) at an equimolar amount to that of NifBAv (in NifEN-B). Moreover, unlike NifBAv (in NifEN-B), which generated SAH and 5′-dAH at an approximate molar ratio of 1:1 (Fig. 2, trace 3), NifBMa or NifBMt generated much more SAH than 5′-dAH (Fig. 2, traces 4 and 5). The “asymmetric” formation of SAM cleavage products suggests that, compared with NifBAv, NifBMa and NifBMt catalyze the removal of methyl group from SAM at a much faster rate than the formation of 5′-dAH that results from hydrogen abstraction by SAM-derived 5′-dA• radical. This observation underlines certain species-dependent differences between different members of the NifB protein family.
Fig. 2.
SAM cleavage by NifBMa and NifBMt. HPLC elution profiles of (1) SAM, SAH, and 5′-dAH standards, (2) SAM alone, (3) SAM plus NifEN-B, (4) SAM plus NifBMa, and (5) SAM plus NifBMt. All samples contained dithionite. SAH was formed in the amounts of 0.20, 0.46, and 0.96 nanomole, respectively, per nanomole of NifEN-B, NifBMa, and NifBMt, whereas 5′-dAH was formed in the amounts of 0.18, 0.17, and 0.18 nanomole, respectively, per nanomole of NifEN-B, NifBMa, and NifBMt.
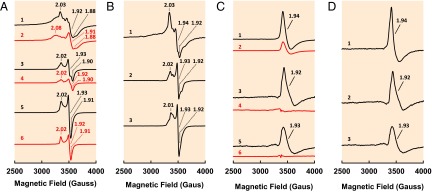
The close resemblance between the two NifB proteins from methanogens and their more complex counterpart in A. vinelandii is not only illustrated by the same SAM cleavage products they generate, but also highlighted by a highly similar spectroscopic response of their associated clusters to SAM treatment. Like NifEN-B (Fig. 3A, trace 1), both NifBMa (Fig. 3A, trace 3) and NifBMt (Fig. 3A, trace 5) displayed S = 1/2 EPR signals in the dithionite-reduced state, although the signal of NifEN-B was more complex than those of NifBMa and NifBMt due to the presence of additional cluster species in the NifEN entity of the fusion protein. Upon addition of SAM, there was a reduction in signal intensity in the cases of both NifBMa (Fig. 3A, trace 4) and NifBMt (Fig. 3A, trace 6), the same response to that observed in the case of NifEN-B (Fig. 3A, trace 2), which was associated with the disappearance of the K-cluster–originated, S = 1/2 signal following cluster conversion in the presence of SAM (9). Subtraction of the spectrum of the SAM-treated sample from that of the untreated sample in the dithionite-reduced state resulted in difference spectra of NifBMa (Fig. 3B, trace 2) and NifBMt (Fig. 3A, trace 3) with close resemblance to the difference spectrum of NifEN-B (Fig. 3A, trace 1), all displaying a similar line shape with g values of ∼2.02, ∼1.93, and ∼1.92. The observation that these difference spectra are composite S = 1/2 signals is consistent with the nature of K cluster as paired [Fe4S4]1+ clusters in the presence of dithionite (9). More excitingly, in the indigo disulfonate (IDS)-oxidized state, the untreated NifBMa (Fig. 3C, trace 4) and NifBMt (Fig. 3C, trace 6) were EPR silent, whereas the SAM-treated NifBMa (Fig. 3C, trace 3) and NifBMt (Fig. 3C, trace 5) displayed strong signals that are highly similar to each other. Subtraction of the spectrum of the SAM-treated sample from that of the untreated sample in the IDS-oxidized state resulted in difference spectra of NifBMa (Fig. 3D, trace 2) and NifBMt (Fig. 3D, trace 3) with close likeness to the difference spectrum of NifEN-B (Fig. 3D, trace 1), which centered at a g value of ∼1.93. The NifEN-B contains some L clusters that are “backed up” on its NifEN entity, as this fusion protein was expressed in a nifHDK-deletion background, which does not contain NifH (a protein factor required to mature the NifEN-bound L cluster into an M cluster) and NifDK (the terminal “acceptor” of M cluster from NifEN) (9). Upon incubation with SAM, more L clusters are generated on NifEN-B due to the conversion of K clusters to L clusters on the NifB entity of this protein (9). Such a signal has been previously determined as the signature EPR feature of the [Fe8S9] L cluster (12, 13), and the appearance of this signal in the spectra of SAM-treated NifBMa and NifBMt strongly suggests a K- to L-cluster conversion in these two proteins upon addition of SAM.
Fig. 3.
EPR properties of NifBMa and NifBMt. (A) EPR spectra of dithionite-reduced (1) NifEN-B, (2) NifEN-B plus SAM, (3) NifBMa, (4) NifBMa plus SAM, (5) NifBMt, and (6) NifBMt plus SAM. Spectra were collected in perpendicular mode at 50 mW and 10 K. (B) Difference spectra between untreated and SAM-treated (1) NifEN-B, (2) NifBMa, and (3) NifBMt in the dithionite-reduced state. All difference spectra were derived from the corresponding spectra in A and multiplied by a factor of 1.5 for better visualization of features. (C) EPR spectra of IDS-oxidized (1) NifEN-B plus SAM, (2) NifEN-B, (3) NifBMa plus SAM, (4) NifBMa, (5) NifBMt plus SAM, and (6) NifBMt. The spectra of NifEN-B plus SAM (C, 1) and NifEN-B (C, 2) were multiplied by a factor of 0.5 for better adaptation to the size of the graph. The untreated NifEN-B contained some L clusters (C, 2) and, upon SAM treatment, more L clusters were generated in this protein (C, 1), as indicated by an increase in the magnitude of the L-cluster–specific, g = 1.94 signal. Spectra were collected in perpendicular mode at 50 mW and 15 K. (D) Difference spectra between untreated and SAM-treated (1) NifEN-B, (2) NifBMa, and (3) NifBMt in the IDS-oxidized state. All difference spectra were derived from the corresponding spectra in C, and the difference spectrum of NifEN-B (D, 1) was multiplied by a factor of 2. The g values are indicated.
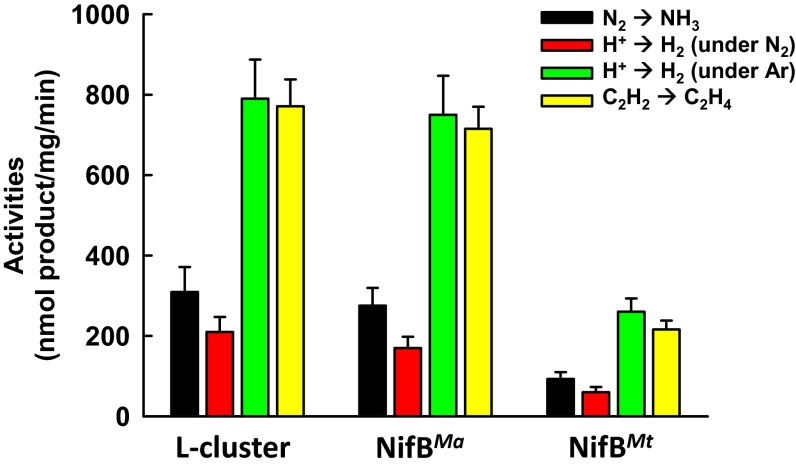
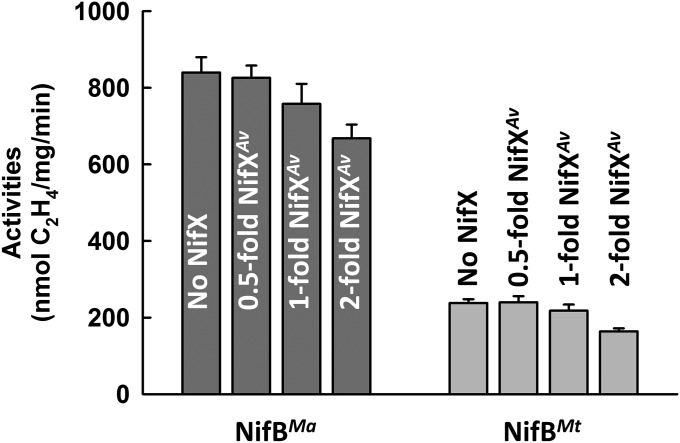
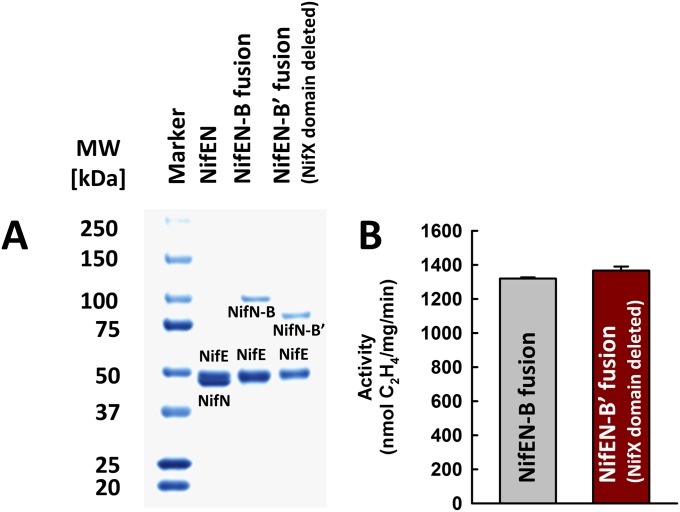
Direct proof in this regard came from the observation that SAM-treated NifBMa or NifBMt was capable of serving as a heterologous L-cluster donor to the assembly machinery of A. vinelandii in an in vitro cluster maturation assay. In this assay, SAM-treated NifBMa or NifBMt was incubated with dithionite, ATP, molybdate, homocitrate, NifHAv, apo NifENAv, and apo NifDKAv, which permitted transfer of the L cluster from NifBMa or NifBMt to apo NifENAv, maturation of the L cluster into an M cluster on NifENAv via NifHAv-mediated insertion of Mo and homocitrate, and transfer of the matured M cluster to apo NifDKAv that resulted in an active, reconstituted form of holo NifDKAv. Interestingly, the SAM-treated NifBMa was nearly as active as the solvent-extracted L cluster in this heterologous in vitro cluster maturation assay, whereas the SAM-treated NifBMt was ∼30% active compared with both NifBMa and the extracted L cluster (Fig. 4). Addition of NifXAv did not elevate the activities of NifBMa and NifBMt in the in vitro cluster maturation assays (Fig. S3), providing additional evidence that the NifX(-like) protein/domain is not essential for the functionality of NifB. This result is also consistent with the observation that a NifEN-B fusion protein containing a truncation of the NifX domain in NifB (designated NifEN-B′) was fully functional in the M-cluster maturation assay compared with the NifEN-B protein containing a full-length NifB entity (Fig. S4). The lower activity of NifBMt is likely due to the fact that this thermophilic protein does not work as efficiently as its methophilic NifBMa counterpart at the optimal assay temperature (30 °C), as well as a somewhat lower sequence homology between NifBAv and NifBMt than that between NifBAv and NifBMa (Fig. S1), which results in a less efficient transfer of the L cluster between NifBMt and apo NifENAv in the heterologous cluster maturation assay. Nevertheless, the results of these activity assays, together with those from the EPR analysis (see above), clearly demonstrate that the K- to L-cluster conversion is completed on NifB before the transfer of the L cluster to NifEN, a key sequence of events that could not be conclusively determined earlier through studies of the NifEN-B fusion protein. Moreover, the observation that the L cluster can be transferred from SAM-treated NifBMa or NifBMt to the assembly proteins of A. vinelandii points to the suitability to use this heterologous assay system to trace the fate of carbide from its origin (i.e., SAM), through the assembly intermediate (i.e., the L cluster), all of the way to the final cluster product (i.e., the M cluster).
Fig. 4.
Activities of NifBMa and NifBMt as L-cluster donors. Substrate-reducing activities of reconstituted NifDK in cluster maturation assays containing the extracted L cluster, NifBMa, and NifBMt as the respective L-cluster donors along with dithionite, ATP, molydate, homocitrate, NifH, apo NifEN, and apo NifDK.
Fig. S3.
Impact of NifXAv on the abilities of NifBMa and NifBMt to serve as L-cluster donors. Shown are substrate-reducing activities of reconstituted NifDK in in vitro cluster maturation assays containing NifBMa or NifBMt as the L-cluster donor along with dithionite, ATP, molybdate, homocitrate, NifH, apo NifEN, apo NifDK and, in some cases, NifXAv. Bars labeled with 0.5-, 1-, and 2-fold NifXAv represent activities of assays containing NifXAv at molar ratios of 0.5:1, 1:1, and 2:1, respectively, to NifB.
Fig. S4.
(A) SDS/PAGE and (B) M-cluster maturation assays of NifEN-B and NifEN-B′ fusion proteins of A. vinelandii. The NifX domain in NifB was deleted in NifEN-B′. The M-cluster maturation assays contained nonsaturated, equimolar amounts of NifEN-B or NifEN-B′. The activities of NifEN-B or NifEN-B′ in the M-cluster maturation assays were indistinguishable from each other. Data represent the average activities of proteins obtained from three independent purifications.
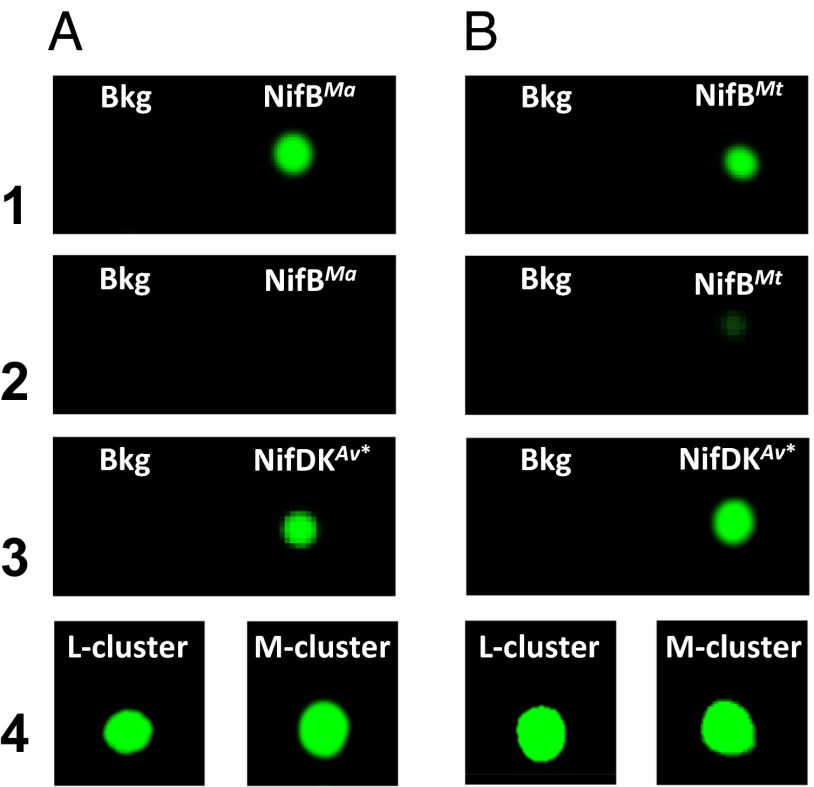
The carbide-tracing experiments were performed by using [methyl-14C] SAM as the initial carbon source. Consistent with the utilization of the SAM-derived methyl group for carbide insertion into the L cluster, the 14C label appeared in both NifBMa (Fig. 5A, 1) and NifBMt (Fig. 5B, 1) upon incubation with [methyl-14C] SAM. Following incubation with the heterologous cluster maturation components (see above) and reisolation of individual proteins from the incubation mixtures, however, the radiolabel disappeared from both NifB proteins (Fig. 5 A and B, 2) while appearing in the respective reconstituted NifDKAv proteins in these mixtures (Fig. 5 A and B, 3), suggesting that the 14C-labeled L cluster on NifB was maturated into a 14C-labeled M cluster and transferred to apo NifDK. In agreement with this suggestion, 14C-labeled L and M clusters could be extracted from the NifB proteins (Fig. 5 A and B, 4, Left) and the reconstituted NifDKAv proteins (Fig. 5 A and B, 4, Right), respectively, and their identities were further confirmed by the activity of the former (i.e., the L cluster) in the cluster maturation assay and the latter (i.e., the M cluster) in the apo NifDK reconstitution assay. The L-cluster maturation assay contains dithionite, L cluster, apo NifEN, MgATP, molybdate, homocitrate, NifH, and apo NifDK, whereas the apo NifDK reconstitution assay contains dithionite, M cluster, and apo NifDK. Together, these results provide compelling evidence that that NifBMa and NifBMt follow the same radical SAM-dependent mechanism as that proposed for NifBAv to facilitate carbide insertion into the M-cluster of nitrogenase (Fig. S2).
Fig. 5.
Carbide insertion by NifBMa and NifBMt. (1) His-tagged NifBMa (A) or His-tagged NifBMt (B) captured on affinity (IMAC) resin after incubation with [methyl-14C] SAM. (2) His-tagged NifBMa (A) or His-tagged NifBMt (B) captured on affinity (IMAC) resin after incubation with [methyl-14C] SAM, nontagged apo NifENAv, nontagged NifHAv, and nontagged apo NifDKAv. (3) Nontagged, reconstituted NifDKAv (designated NifDKAv∗) captured on anion-exchange (DEAE) resin after incubation with [methyl-14C] SAM, His-tagged NifBMa (A) or His-tagged NifBMt (B), His-tagged apo NifENAv, and His-tagged NifHAv. (4) Clusters extracted from the IMAC fraction in A, 1 and DEAC fraction in A, 3; and clusters extracted from the IMAC fraction in B, 1 and DEAE fraction in B, 3. Assays 2 and 3 also contained dithionite, ATP, molybdate, and homocitrate. Bkg, background.
It is interesting to note that, following cluster transfer, a small, background amount of radiolabel remained on NifBMt (Fig. 5B, 2). This observation led to an important question of whether the residual radiolabel on NifBMt originated from transfer of the 14C-labeled methyl group to a certain protein residue as an intermediary step of carbide insertion, which would suggest a reaction pathway somewhat analogous to the one used by RlmN and Cfr to facilitate methylation of an inert C-H bond (19, 20). To address this question, NifBMa and NifBMt were prepared in the absence and presence of SAM and subsequently analyzed for posttranslational modification (PTM). As was observed in the case of NifBAv (11), no unique amino acid methylation events could be detected in SAM-treated NifBMa and NifBMt samples. This result suggests that methyltransfer by these NifB proteins does not route via a methylated amino acid intermediate; rather, it proceeds directly from SAM to the K cluster (Fig. S2). The residual radiolabel on NifBMt, therefore, was likely a result of inefficient transfer of radiolabeled cluster from the thermophilic NifBMt to the mesophilic NifENAv at a temperature (i.e., 25 °C) below that which was optimal for NifBMt. This argument is supported by the absence of radiolabel on the mesophilic NifBMa following cluster transfer (Fig. 5A, 2), as well as the discrepancy between activities of NifBMt and NifBMa in the in vitro cluster maturation assays (Fig. 4).
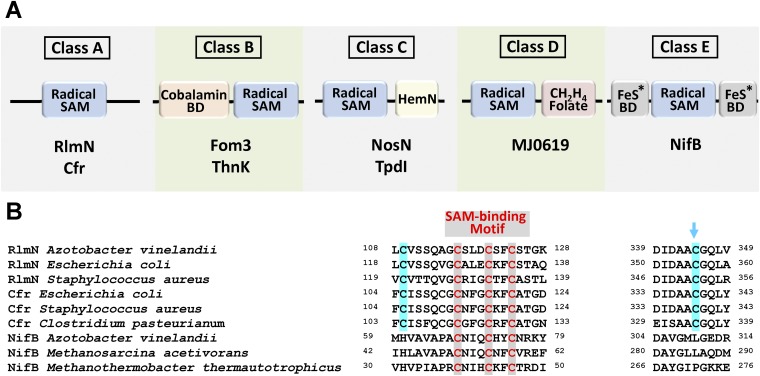
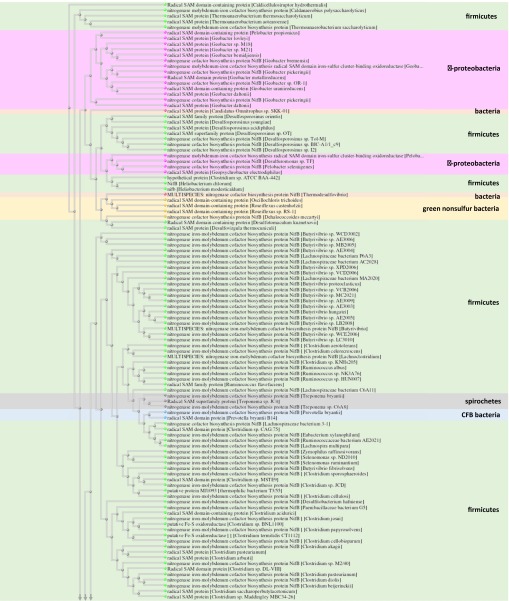
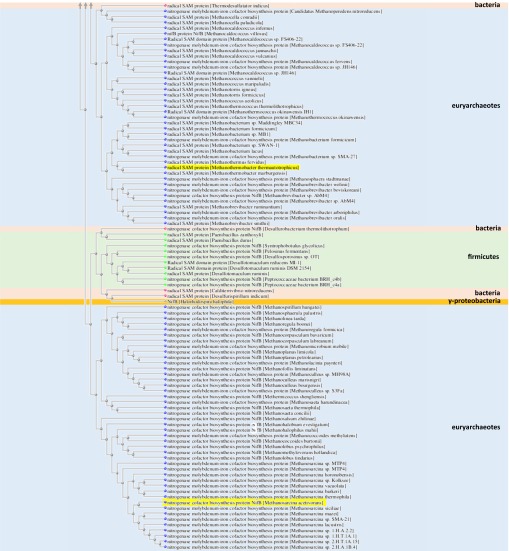
Subsequent sequence analysis provided further explanation for the inability of NifB to generate a methylated amino acid intermediate, demonstrating that a pair of conserved Cys residues in the sequences of RlmN and Cfr, including one that serves as the site of intermediary methylation (Fig. S5B, blue arrow), are absent from the sequences of NifBAv, NifBMa, and NifBMt. This feature sets the NifB proteins apart from the RlmN and Cfr proteins (Fig. S5A), which are emerging as a distinct class (class A) of a larger subset of radical SAM methyltransferases (RSMTs) that use conserved protein residues to facilitate methyltransfer (21, 22). The three NifB homologs are further distinguished from the other known classes of RSMTs (classes B–D), with classes B, C, and D carrying a cobalamin-binding domain, a HemN domain, and a methylenetetrahydrofolate domain, respectively, in addition to the canonical radical SAM domain (Fig. S5A). Excitingly, a BLAST search resulted in the identification of a large number of proteins with high sequence homology to NifBMa and NifBMt (over 300 proteins with a homology of higher than 70% over a range of 85% of the sequence). The organisms expressing these truncated NifB homologs (60% methanogenic organisms and 40% nonmethanogenic organisms) are widespread across the microbial biorealm; many of them are not nitrogen-fixing organisms, suggesting that the NifB proteins in these organisms carry out other functions that are yet to be identified. Sequence alignment of 45 closest matches of NifB homologs revealed the presence of the canonical radical SAM domain, as well as the same, conserved Cys and His residues as those identified in NifBMa and NifBMt, which potentially serve as FeS cluster-binding domains (Fig. S6). A new class of RSMTs (class E; Fig. S5A and Fig. S7) could be tentatively proposed based on this finding, which potentially specializes in radical SAM-based assembly of complex metallocenters.
Fig. S5.
(A) Five classes of radical SAM methyltransferases (RSMTs) and (B) partial sequence alignment of members of class A and class E. Class A proteins have a canonical radical SAM domain and two conserved Cys residues that facilitate methyl transfer; class B proteins have a radical SAM domain and an N terminus cobalamin binding domain, and they can use methyl-cobalamin in methylation reactions; class C proteins have a radical SAM domain and a C terminus HemN domain that assists with substrate binding; and class D proteins have a radical SAM domain and a C terminus methylenetetrahydrofolate domain and, unlike proteins of classes A, B, and C, they may use methylenetetrahydrofolate instead of SAM as the source of the methyl carbon (21, 22). Class E proteins have a radical SAM domain and conserved Cys and His residues that serve as FeS cluster-binding domains, and they are presumably specialized in complex metallocluster assembly. BD, binding domain. Representative members of all classes are shown in A. The blue arrow in B indicates the site of intermediary methylation in Rlm and Cfr.
Fig. S6.
Sequence alignment of 45 NifB proteins from methanogenic organisms. All sequences contain a signature CxxxCxxC domain (highlighted gray) that houses the SAM-binding [Fe4S4] cluster. Highly conserved Cys and His residues are highlighted yellow.
Fig. S7.
BLAST tree view of class E radical SAM methyltransferases. The tree contains proteins with 50% or higher identity to NifBMa and NifBMt.
Other than enabling the classification of a distinct subset of RSMTs, the successful identification of NifBMa or NifBMt as functional homologs of NifBAv is exciting, as it opens up new avenues to study the structure and mechanism of the NifB protein, both of which remain relatively uncharacterized and promise to reveal completely unprecedented chemical reactions catalyzed by biological systems. The fact that both methanogen NifB homologs can be expressed alone in E. coli as soluble, intact proteins permits structural and biochemical analysis of NifB without interference of its protein partner and associated metal centers, a feat that has not been achieved so far through investigations of the NifEN-B fusion protein; moreover, it suggests the possibility to express a truncated version of NifBAv in E. coli, which can then be used for comparative studies with its newly identified homologs in methanogens to shed light on the structure–function relationship of this important protein family. Novel mechanistic insights could be gained by studying these proteins side by side, and species-dependent differences revealed by these studies—such as a shift toward a higher SAH/5′-dAH ratio in the cases of NifBMa and NifBMt—could be explored to reveal differential mechanisms used by the NifB homologs to abstract hydrogen from the substrate-bound methyl group or identify additional functions of these proteins as methyltransferases in their native hosts. Additionally, important “snapshots” of carbide insertion pathway could be captured by mixing and matching the NifB protein from one organism with assembly components from another, which may back up certain intermediates on NifB due to a less efficient transfer of L clusters from NifB to its downstream assembly partner. All in all, the NifB homologs reported in this work provide a brand new tool in addition to the NifEN-B fusion protein (23) for further characterization of the distinctive methyl transfer and conversion to an iron-bound carbide by NifB, which is crucial for the unveiling of a chemically unique and biologically important reaction pathway.
Materials and Methods
Unless noted otherwise, all chemicals and reagents were obtained from Fisher Scientific or Sigma-Aldrich. Cell growth, protein purification, iron/sulfur reconstitution, molecular mass determination, iron determination, SAM cleavage assays, EPR analysis, cluster maturation assays, carbon-14 tracing experiments, cluster extraction, and PTM analysis were performed as described. See SI Materials and Methods for more information on these procedures.
Genes encoding the NifB homologs from M. acetivorans (NifBMa) and M. thermoautotrophicum (NifBMt) were codon optimized for E. coli expression and synthesized and cloned into the BamHI site of pET-3b and the NdeI site of pET-14b, respectively (GenScript USA). A short sequence encoding a 6xHis tag was inserted at the 5′-end of the gene encoding NifBMa before the cloning of this sequence into pET-3b, whereas the gene encoding NifBMt was placed behind a vector-derived sequence encoding a 6xHis tag when it was cloned into pET-14b. Each of these constructs was then cotransformed with a plasmid harboring iscSUA and hscABfdx genes—an ensemble of genes encoding Fe/S cluster assembly proteins (24–28)—into the E. coli strain BL21(DE3), resulting in strains overexpressing His-tagged forms of NifBMa (strain YM114EE) and NifBMt (strain YM127EE) upon induction by isopropyl β-d-1-thiogalactopyranoside (IPTG). The plasmid carrying iscSUA and hscABfdx genes was a generous gift from S. Leimkhüler (University of Potsdam, Potsdam, Germany). The gene encoding NifX from A. vinelandii (NifXAv) was PCR amplified from the genomic DNA using a pair of primers (forward primer: 5′-ATGGTAGGTCTCAAATGTCCAGCCC GACCCGACAATTG-3′; reverse primer: 5′-ATGGTAGGTCTCAGCGCTTTCGTCCCAGCCTTCGGCGG-3′) and subsequently cloned into the BsaI site of pASK-IBA3 (IBA). This construct was transformed into the E. coli strain BL21-CodonPlus, resulting in a strain expressing a C terminus strep-tagged form of NifXAv (strain YM300EE).
SI Materials and Methods
Unless noted otherwise, all protein work was performed under Ar gas at an O2 concentration of less than 5 ppm.
Cell Growth and Protein Purification.
E. coli strains expressing His-tagged NifBMa (strain YM114EE) and NifBMt (strain YM127EE) and strep-tagged NifXAv were grown in 10-L batches in Difco LB medium containing 100 mg/L ampicillin (BD Biosciences) in a BIOFLO 415 fermenter (New Brunswick Scientific) at 37 °C, with 200 rpm agitation and 10-liter-per-minute airflow. When OD600 reached 0.5, the temperature was lowered to 25 °C before expression of the NifB homologs and NifXAv was induced by addition of 25 µM IPTG and 200 µg/L anhydrotetracycline, respectively. Expression of protein was allowed to continue for 16 h before cells were harvested by centrifugation using a Thermo Fisher Scientific Legend XTR centrifuge. Subsequently, His-tagged NifBMa and NifBMt were purified by immobilized metal affinity chromatography (IMAC) using methods adapted from the purification of His-tagged nitrogenase proteins (9), whereas strep-tagged NifXAv was purified by affinity chromatography using a procedure provided by the manufacturer (manual version PR02-0007; IBA BioTagnology). A. vinelandii strain YM101A expressing a NifEN-B fusion protein containing a truncation of the NiX domain (amino acids 156–503 of NifB), designated NifEN-B′, was constructed as described for the full-length NifEN-B fusion protein earlier (9). A. vinelandii strains expressing ΔnifB NifEN (apo NifEN), ΔnifB NifDK (apo NifDK), NifH, NifEN-B, and NifEN-B′ proteins were grown at 30 °C in 180-L batches in a 200-L fermenter (New Brunswick Scientific) in Burke’s minimal medium supplemented with 2 mM ammonium acetate. Growth rates were monitored by measuring cell density at 436 nm using a Spectronic 20 Genesys spectrometer (Spectronic Instruments). Once ammonia was depleted, cells were allowed to derepress for 3 h before being harvested by a flow-through centrifugal harvester (Cepa). Published methods were used to purify ΔnifB NifEN (apo NifEN), ΔnifB NifDK (apo NifDK), and NifH proteins (9).
Fe/S Reconstitution.
The purified NifBMa and NifBMt proteins were subjected to in vitro reconstitution of [Fe4S4] cluster species in a procedure adapted from published methods (28). This procedure involved sequential addition of 10 mM DTT, 1 mM FeCl3, 1 mM Na2S, and 42 µM NifBMa or NifBMt in a buffer containing 25 mM Tris⋅HCl (pH 8.0) and 5% (vol/vol) glycerol, resulting in a total volume of ∼64 mL reaction mixture. Subsequently, the reaction mixture was incubated on ice with gentle stirring for 1 h before it was diluted fourfold with a buffer containing 25 mM Tris⋅HCl (pH 8.0) and 10% (vol/vol) glycerol and subsequently passed through the IMAC Sepharose resin. The IMAC resin-bound, reconstituted protein was then washed three times with four column volumes of a buffer containing 25 mM Tris⋅HCl (pH 8.0), 2 mM dithionite (Na2S2O4), 10% (vol/vol) glycerol, 500 mM NaCl, and 40 mM imidazole before it was eluted in a buffer containing 25 mM Tris⋅HCl (pH 8.0), 2 mM Na2S2O4, 10% (vol/vol) glycerol, 500 mM NaCl, and 250 mM imidazole. To eliminate Fe/S aggregates, the Fe/S-reconstituted NifBMa and NifBMt were heated at 65 °C for 3 and 15 min, respectively, followed by removal of excess soluble Fe and S ions by passing the samples through a G25 desalting column (GE Healthcare).
Molecular Mass Determination.
The native molecular masses of NifBMa and NifBMt were determined by gel filtration on a column packed with Ultrogel ACA 34 (Pall Life Science; ID: 1.5 cm, length: 1.4 m) at a flow rate of 0.5 mL/min. Protein standards (GE Healthcare) used for molecular mass determination were ribonuclease A (13.7 kDa), carbonic anhydrase (29 kDa), and ovalbumin (43 kDa).
Iron Determination.
The iron contents of NifBMa and NifBMt were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) using a Thermo Scientific iCAP7000. Stock solutions of elemental iron (1 mg/mL, Thermo-Fisher Scientific) were diluted to make standard solutions for calibration. Each protein sample was mixed with 100 µL concentrated sulfuric acid (H2SO4) and 100 µL concentrated nitric acid (HNO3) and heated at 250 °C for 30 min. This procedure was repeated until the solutions became colorless. Before sample analysis, the solutions were cooled to room temperature and then diluted to a total volume of 7.5 mL with 2% HNO3.
SAM Cleavage Assays.
The S-adenosyl-L-methionine (SAM) cleavage assays contained, in a total volume of 0.5 mL, 25 mM Tris⋅HCl (pH 8.0), 5% glycerol, 16 µM sodium dithionite (Na2S2O4)-free NifBMa or NifBMt, and 10 µM SAM. Assays were incubated at 25 °C for 30 min with intermittent mixing, before they were terminated by filtration through Amicon Ultra 30,000 MWCO centrifugal filters. Samples were then supplemented by trifluoroacetic acid (TFA) to a concentration of 0.14% before being analyzed by a Thermo Scientific Dionex Ultimate 3000 UHPLC system equipped with an Acclaim 120 C18 column (4.6 × 100 mm, 5-µm particle size). The flow rate of buffer was 0.5 mL/min and the column was kept at 30 °C. The column was equilibrated with 98% buffer A (50 mM KH2PO4, pH 6.6) and 2% buffer B (100% methanol) before each injection of a 100-µL sample. After sample injection, a linear gradient of 2–60% buffer B was applied over 20 min, followed by 8 min of isocratic flow with 60% buffer B and a linear gradient of 60–2% buffer B over 4 min. Elution of products was monitored at a UV wavelength of 254 nm. After each run, the column was equilibrated for 5 min with 2% buffer B before the injection of the next sample.
EPR Analysis.
For electron paramagnetic resonance (EPR) analysis, samples were prepared in a Vacuum Atmospheres glove box with less than 5 ppm O2 and flash frozen in liquid nitrogen before analysis. Reduced samples contained 25 mM Tris⋅HCl (pH 8.0), 10% (vol/vol) glycerol, 250 mM imidazole, 2 mM Na2S2O4, and, in some cases, 40 mM SAM. Oxidized samples were prepared by incubating reduced samples with excess indigo disulfonate (IDS) for 5 min. EPR spectra were recorded by an ESP 300 Ez spectrophotometer (Bruker) interfaced with an ESR-9002 liquid-helium continuous-flow cryostat (Oxford Instruments) using a microwave power of 50 mW, a gain of 5 × 104, a modulation frequency of 100 kHz, and a modulation amplitude of 5 G. Five scans of perpendicular-mode EPR were recorded at 10 K (reduced samples) or 15 K (oxidized samples) using a microwave frequency of 9.62 GHz.
Cluster Maturation Assays.
NifBMa or NifBMt was first incubated with 25 mM Tris⋅HCl (pH 8.0) in the presence or absence of 10 mM SAM at 37 °C (NifBMa) and 60 °C (NifBMt), respectively, for 30 min and then transferred to an assay mixture containing, in a total volume of 1 mL, 25 mM Tris⋅HCl (pH 8.0), 20 mM Na2S2O4, 0.45 mg ΔnifB NifDK, 1.4 mg NifH, 0.3 mM homocitrate, 0.3 mM sodium molybdate, 0.8 mM ATP, 1.6 mM MgCl2, 10 mM creatine phosphate, and 8 units of creatine phosphokinase. This mixture was incubated at 30 °C for 30 min before it was examined for enzymatic activities as described previously (13). The impact of NifXAv on the capacity of NifBMa or NifBMt to serve as an L-cluster donor was examined by performing the same assay as above except for addition of NifXAv at a molar ratio of 0.5:1, 1:1, and 2:1, respectively, to NifBMa or NifBMt. Maturation assays containing NifEN-B or NifEN-B′ were carried out as described earlier (9).
Carbon-14 Tracing Experiments.
The 14C-labeling experiments were performed as described previously (11). Each reaction contained, in a total volume of 32 µL, 20 mM Na2S2O4, 100 mM Tris⋅HCl (pH 8.0), 250 µM [methyl-14C] SAM (American Radiolabeled Chemicals) and (i) 10 µM NifBMa or NifBMt alone or (ii) 10 µM NifBMa or NifBMt, 12 µM nontagged apo NifDK, 26 µM nontagged NifH, 0.3 mM homocitrate, 0.3 mM Na2MoO4, 0.8 mM ATP, 1.6 mM MgCl2, 10 mM creatine phosphate, and 8 units of creatine phosphokinase. Reactions were incubated for 30 min at 25 °C with intermittent mixing and then run over IMAC Sepharose resin (10 µL packed volume; GE Healthcare) that was equilibrated with a buffer containing 25 mM Tris⋅HCl (pH 8.0), 10% (vol/vol) glycerol, 500 mM NaCl, and 2 mM Na2S2O4. The IMAC Sepharose resin was washed three times with 800 µL buffer containing 25 mM Tris⋅HCl (pH 8.0), 10% (vol/vol) glycerol, 500 mM NaCl, 40 mM imidazole, and 2 mM Na2S2O4. The flow-thru of IMAC Sepharose resin was diluted fourfold with a buffer containing 25 mM Tris⋅HCl (pH 8.0) and 2 mM Na2S2O4 before being loaded onto DEAE cellulose resin (20 µL packed volume; GE Healthcare). The DEAE cellulose resin was washed three times with 800 µL of the same buffer. Samples captured on the IMAC Sepharose and DEAE cellulose resins were resuspended in the wash buffer and applied onto Whatman 3 MM chromatography paper. Dried blots were exposed to a GE Healthcare Storage Phosphor Screen GP (20 × 25 cm) for 48 h before imaging was performed on a GE Healthcare Typhoon Trio+ variable mode imager.
Cluster Extraction.
Protein samples that were captured on IMAC Sepharose and DEAE cellulose resins were extracted in a small scale as described previously (11, 29, 30). Extracted clusters were analyzed for radioactivity by the same method as described above in “Carbon-14 Tracing Experiments.”
PTM Analysis.
The 200-µM reconstituted NifBMa or NifBMt protein sample was first incubated at 25 °C for 30 min with or without 10 mM SAM in a buffer containing 25 mM Tris⋅HCl (pH 8.0) and then separated from other components in the incubation mixture by 12% SDS/PAGE. Gels were stained with Coomassie Brilliant Blue R-250 (BioRad) dye in a solution containing 45% (vol/vol) methanol, 10% (vol/vol) acetic acid, and 45% (vol/vol) H2O, and destained with the same solution without dye. Gel slices containing single protein bands were excised and stored in H2O until further sample processing and analysis. Each sample was prepared in triplicate gel slices. PTM analysis was performed by Majid Ghassemian (University of California, San Diego Biomolecular/Proteomics Mass Spectrometry Facility, La Jolla, CA). Each gel slice was washed repeatedly with a solution containing 50% (vol/vol) acetonitrile and 100 mM ammonium bicarbonate until all dye was removed. Unmodified protein residues were alkylated by incubating the gel slice containing the protein band first with a solution containing 10 mM DTT and 100 mM ammonium bicarbonate for 45 min, and then with a solution containing 55 mM iodoacetamide and 100 mM ammonium bicarbonate for 30 min. The gel slice was then washed with a 100 mM ammonium bicarbonate solution before acetonitrile was added at a concentration of 50% (vol/vol) to facilitate drying of the gel slice. Each dried gel slice was then treated with a solution containing 2 mM CaCl2, 50 mM ammonium bicarbonate, and 0.0125 µg/µL trypsin to allow in-gel trypsin digestion for 45 min at 4 °C and then overnight at 37 °C. Subsequently, trypsin was removed from the solution, followed by extraction of the trypsin-digested protein fragments from the gel slice with repeated washing by an aqueous solution containing 1% formic acid and 2% (vol/vol) acetonitrile. The extract of trypsin digest was then dried, suspended in 20 µL of a 5% (vol/vol) formic acid solution, and injected onto an LC/MS/MS (AB SCIEX TripleTOF 5600) for separation and mass analysis of protein fragments. Datasets were analyzed for unique methylation events by ProteinPilot software (AB SCIEX).
Acknowledgments
We thank Prof. Markus Ribbe [University of California, Irvine (UCI)] for helpful discussion and technical support of biochemical experiments related to the NifEN-B′ fusion protein. This work was supported by UCI startup funds and a Hellman Fellowship (to Y.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510409112/-/DCSupplemental.
References
- 1.Burgess BK, Lowe DJ. Mechanism of molybdenum nitrogenase. Chem Rev. 1996;96(7):2983–3012. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 2.Rees DC, et al. Structural basis of biological nitrogen fixation. Philos Trans A Math Phys Eng Sci. 2005;363(1829):971–984. doi: 10.1098/rsta.2004.1539. [DOI] [PubMed] [Google Scholar]
- 3.Ribbe MW, Hu Y, Hodgson KO, Hedman B. Biosynthesis of nitrogenase metalloclusters. Chem Rev. 2014;114(8):4063–4080. doi: 10.1021/cr400463x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey PA, Hegeman AD, Ruzicka FJ. The radical SAM superfamily. Crit Rev Biochem Mol Biol. 2008;43(1):63–88. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JB, Duffus BR, Duschene KS, Shepard EM. Radical S-adenosylmethionine enzymes. Chem Rev. 2014;114(8):4229–4317. doi: 10.1021/cr4004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster KM, et al. X-ray emission spectroscopy evidences a central carbon in the nitrogenase iron-molybdenum cofactor. Science. 2011;334(6058):974–977. doi: 10.1126/science.1206445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spatzal T, et al. Evidence for interstitial carbon in nitrogenase FeMo cofactor. Science. 2011;334(6058):940. doi: 10.1126/science.1214025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson MR, et al. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J Bacteriol. 1989;171(2):1017–1027. doi: 10.1128/jb.171.2.1017-1027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiig JA, Hu Y, Ribbe MW. NifEN-B complex of Azotobacter vinelandii is fully functional in nitrogenase FeMo cofactor assembly. Proc Natl Acad Sci USA. 2011;108(21):8623–8627. doi: 10.1073/pnas.1102773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancaster KM, Hu Y, Bergmann U, Ribbe MW, DeBeer S. X-ray spectroscopic observation of an interstitial carbide in NifEN-bound FeMoco precursor. J Am Chem Soc. 2013;135(2):610–612. doi: 10.1021/ja309254g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiig JA, Hu Y, Lee CC, Ribbe MW. Radical SAM-dependent carbon insertion into the nitrogenase M-cluster. Science. 2012;337(6102):1672–1675. doi: 10.1126/science.1224603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett MC, et al. Structural insights into a protein-bound iron-molybdenum cofactor precursor. Proc Natl Acad Sci USA. 2006;103(5):1238–1243. doi: 10.1073/pnas.0507853103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Y, Fay AW, Ribbe MW. Identification of a nitrogenase FeMo cofactor precursor on NifEN complex. Proc Natl Acad Sci USA. 2005;102(9):3236–3241. doi: 10.1073/pnas.0409201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, et al. FeMo cofactor maturation on NifEN. Proc Natl Acad Sci USA. 2006;103(46):17119–17124. doi: 10.1073/pnas.0602647103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y, et al. Nitrogenase Fe protein: A molybdate/homocitrate insertase. Proc Natl Acad Sci USA. 2006;103(46):17125–17130. doi: 10.1073/pnas.0602651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshizawa JM, et al. Optimization of FeMoco maturation on NifEN. J Am Chem Soc. 2009;131(26):9321–9325. doi: 10.1021/ja9035225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oelgeschläger E, Rother M. Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch Microbiol. 2008;190(3):257–269. doi: 10.1007/s00203-008-0382-6. [DOI] [PubMed] [Google Scholar]
- 18.Ferry JG. Enzymology of one-carbon metabolism in methanogenic pathways. FEMS Microbiol Rev. 1999;23(1):13–38. doi: 10.1111/j.1574-6976.1999.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 19.Grove TL, et al. A radically different mechanism for S-adenosylmethionine-dependent methyltransferases. Science. 2011;332(6029):604–607. doi: 10.1126/science.1200877. [DOI] [PubMed] [Google Scholar]
- 20.Boal AK, et al. Structural basis for methyl transfer by a radical SAM enzyme. Science. 2011;332(6033):1089–1092. doi: 10.1126/science.1205358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, van der Donk WA, Liu W. Radical-mediated enzymatic methylation: a tale of two SAMS. Acc Chem Res. 2012;45(4):555–564. doi: 10.1021/ar200202c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauerle MR, Schwalm EL, Booker SJ. Mechanistic diversity of radical S-adenosylmethionine (SAM)-dependent methylation. J Biol Chem. 2015;290(7):3995–4002. doi: 10.1074/jbc.R114.607044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiig JA, Hu Y, Ribbe MW. Refining the pathway of carbide insertion into the nitrogenase M-cluster. Nat Commun. 2015;11(6):8034. doi: 10.1038/ncomms9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazzon J, Dean DR. Formation of iron-sulfur clusters in bacteria: An emerging field in bioinorganic chemistry. Curr Opin Chem Biol. 2003;7(2):166–173. doi: 10.1016/s1367-5931(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 25.Frazzon J, Fick JR, Dean DR. Biosynthesis of iron-sulphur clusters is a complex and highly conserved process. Biochem Soc Trans. 2002;30(4):680–685. doi: 10.1042/bst0300680. [DOI] [PubMed] [Google Scholar]
- 26.Kriek M, Peters L, Takahashi Y, Roach PL. Effect of iron-sulfur cluster assembly proteins on the expression of Escherichia coli lipoic acid synthase. Protein Expr Purif. 2003;28(2):241–245. doi: 10.1016/s1046-5928(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 27.Cicchillo RM, et al. Escherichia coli lipoyl synthase binds two distinct [4Fe-4S] clusters per polypeptide. Biochemistry. 2004;43(37):11770–11781. doi: 10.1021/bi0488505. [DOI] [PubMed] [Google Scholar]
- 28.Lanz ND, et al. RlmN and AtsB as models for the overproduction and characterization of radical SAM proteins. Methods Enzymol. 2012;516:125–152. doi: 10.1016/B978-0-12-394291-3.00030-7. [DOI] [PubMed] [Google Scholar]
- 29.Burgess BK. The iron-molybdenum cofactor of nitrogenase. Chem Rev. 1990;90(8):1377–1406. [Google Scholar]
- 30.Shah VK, Brill WJ. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci USA. 1977;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]