Significance
The protein kinase fms-like tyrosine kinase 3 receptor (Flt3) has an important role during early B-cell development. Recent reports have shown that Flt3 is reexpressed on peripheral B cells during activation, however the function for Flt3 during B-cell activation is poorly understood. The present study describes a role for Flt3 during B-cell activation and antibody production. We show that Flt3 is reexpressed on activated germinal center B cells and that lack of functional Flt3 signaling in peripheral B cells results in a specific deficiency in production of the antibody subclass IgG1. Mechanistic studies indicate that Flt3 synergizes with the IL-4 receptor to promote proper activation of Stat6 and class switch to IgG1. This study provides evidence for a previously unknown function for Flt3 during peripheral B-cell activation.
Keywords: class-switch recombination, germinal center B cells, Flt3, IgG1, IL-4R
Abstract
Switched antibody classes are important for efficient immune responses. Aberrant antibody production to otherwise harmless antigens may result in autoimmunity. The protein kinase fms-like tyrosine kinase 3 receptor (Flt3) has an important role during early B-cell development, but the role of Flt3 in peripheral B cells has not been assessed before. Herein we describe a previously unappreciated role for Flt3 in IgG1 class-switch recombination (CSR) and production. We show that Flt3 is reexpressed on B-cell lymphoma 6+ germinal center B cells in vivo and following LPS activation of peripheral B cells in vitro. Absence of Flt3 signaling in Flt3 ligand-deficient mice results in impaired IgG1 CSR and accumulation of IgM-secreting plasma cells. On activated B cells, Flt3 is coexpressed and functions in synergy with the common-gamma chain receptor family. B cells from Flt3 ligand-deficient mice have impaired IL-4R signaling, with reduced phosphorylation of signal transducer and activator of transcription (Stat) 6, and demonstrate a failure to initiate CSR to IgG1 with low expression of γ1 germ-line transcripts, resulting in impaired IgG1 production. Thus, functional synergy between Flt3 and IL-4R signaling is critical for Stat-mediated regulation of sterile γ1 germ-line transcripts and CSR to IgG1.
Activation of B cells by foreign antigens and the subsequent formation of antibody-producing plasma cells are crucial steps in protective humoral immunity. The immune system responds to different invading pathogens by production of antibodies with unique effector functions. This is accomplished by class-switch recombination (CSR), where the rearranged variable region of an antibody heavy chain is joined with different constant regions (CH) (1). Impaired CSR can cause serious complications, such as hyper-IgM syndromes with increased susceptibility to bacterial infections (2), but also systemic or organ-specific autoimmunity (3).
During CSR, the Ig heavy chain CH exons coding for IgM (Cμ) are deleted and replaced with CH exons coding for either IgG (Cγ), IgE (Cε), or IgA (Cα). This process is accomplished by joining two DNA sequences, switch regions, which are located upstream of each CH gene. CSR requires the expression of activation-induced cytidine deaminase (AID), which deaminates deoxycytosines in switch (S)-region DNA, yielding deoxyuracils. During the removal of deoxyuracil bases, double-stranded DNA breaks occur in the upstream (donor) and downstream (acceptor) S-regions. This activates a DNA damage response, which promotes long-range recombination. Eventually the double-stranded DNA breaks in Sμ and the downstream target S-region are joined to enable expression of a new antibody isotype (1, 4).
CSR is initiated through transcription from isotype-specific intronic promoters that continues through the intronic exon, the adjacent S-region, and the CH exons, creating a germ-line transcript (GLT). GLTs are noncoding but are thought to initiate CSR by rendering the S-region accessible for AID. In addition to B-cell receptor signals, primary and secondary stimuli control CSR in B cells. Whereas T-cell–dependent (i.e., CD40L) or T-cell–independent (i.e., TLR) primary stimuli induce expression of AID, secondary stimuli such as IL-4 (IgG1, IgE), IFN-γ (IgG2c), and TGF-β (IgA) are needed for directing the class switch to a specific antibody isotype through the induction of GLT (5).
During T-cell–dependent responses, CSR mainly occurs within germinal centers (GCs) (6). IgG1 production is dependent on GC formation and the type I cytokine IL-4 (7). Binding of IL-4 to the IL-4 receptor (IL-4R) leads to phosphorylation of signal transducer and activator of transcription (Stat) 6 by Janus kinase (8). Phosphorylated Stat6 binds the promoter region of γ1, inducing GLT and subsequent CSR to IgG1 (8). IL-4 is produced by follicular T cells (TFH) that are specialized B-helper T cells involved in GC establishment and function (9).
The protein kinase fms-like tyrosine kinase 3 receptor (Flt3) is a tyrosine kinase receptor expressed on early hematopoietic and lymphoid progenitors in the bone marrow (BM) (10). Flt3 is activated by Flt3-ligand (FL) binding, promoting survival and differentiation (11–13). FL is expressed in multiple cell types including BM stroma cells and activated T cells, either in a membrane-bound form or as a soluble protein (14, 15). Generally, FL has a weak stimulatory effect on its own and acts in combination with other cytokines (16). For example, Flt3 induces responsiveness to IL-7 in B-cell progenitors by driving expression of the IL-7R. Furthermore, Flt3 signaling is suggested to potentiate IL-7–induced phosphorylation of Stat5 during early B-cell differentiation (17–23). Despite the block in early B-cell development, Flt3- and FL-targeted mice have normal numbers of peripheral B cells, antibody levels, and responses toward T-dependent immunization (16, 24). Surface expression of Flt3 is lost when developing B cells acquire CD19 expression (25).
Recently, Flt3 was found to be reexpressed on splenic B cells after in vitro activation with LPS or anti-CD40 and IL-4 (26). Furthermore, Flt3+ B cells have been described in the peripheral blood of healthy individuals, whereas increased serum levels of FL have been measured in patients suffering from antibody-mediated autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and Sjögren’s syndrome (27–29). Also, protective properties of FL are identified in mouse models of chronic asthma, a disease associated with aberrant antibody production toward otherwise harmless antigens (30–32). Stimulation of human B cells with FL in vitro potentiate anti-IgM–induced proliferation and survival (29). Also, FL has potent adjuvant effects in vivo, enhancing protective IgA immunity in streptococcal pneumonia (33). Taken together, these results suggest that Flt3 may participate in processes associated with B-cell activation.
Herein we investigate the role of Flt3 on mature peripheral B cells and show that Flt3 is reexpressed on activated GC B cells and that signals through Flt3 are essential for proper IL-4–induced activation of Stat6 and IgG1 production.
Results
Activation of Peripheral B Cells Induces Reexpression of Surface Flt3.
The Flt3 receptor is reexpressed on mature peripheral B cells following LPS or anti-CD40 and IL-4 in vitro stimulation (26). We decided to further characterize B cells expressing Flt3 after in vitro LPS activation and during T-cell–dependent antigen challenge in vivo.
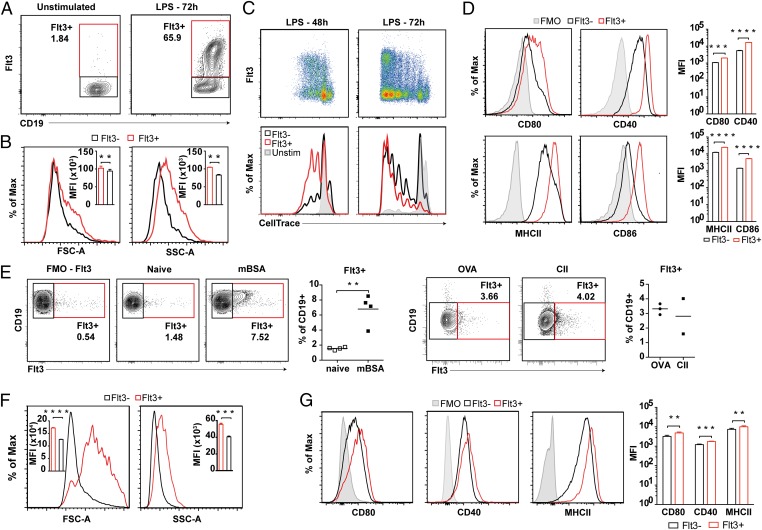
In vitro activation of naïve WT splenocytes with LPS resulted in reexpression of Flt3 on B cells (Fig. 1A). Flt3 expression was observed on proliferating B cells, with increased cell size and granulation compared with Flt3− B cells and elevated expression of activation markers CD80, CD86, MHCII, and CD40 (Fig. 1 B–D). In vivo, immunization with three different T-cell–dependent antigens [methylated bovine serum albumin (mBSA), ovalbumin (OVA), and collagen type II (CII)] resulted in a significant increase in Flt3+ B cells in lymph nodes (LNs) (Fig. 1E). As observed in vitro, Flt3+ B cells were increased in size and granulation and had increased expression of activation markers compared with Flt3− B cells (Fig. 1 F and G).
Fig. 1.
Flt3 is expressed on peripheral B cells after in vitro and in vivo activation. (A) Expression of Flt3 on LPS-stimulated B cells isolated from naïve mice. (B) Size (FSC) and granulation (SSC) of Flt3+ versus Flt3− B cells after 72 h of in vitro LPS stimulation. (C) Expression of Flt3 during LPS-induced B-cell proliferation after 48 h and 72 h. Cells are gated on CD19+-viable lymphocytes. (D) Expression of activation markers CD80, CD86, CD40, and MHCII (I-A/I-E) on Flt3+ versus Flt3− B cells after in vitro (LPS) activation. FMO, fluorochrome minus one staining control. (E) Presence of Flt3-expressing B cells in LNs of mBSA-, OVA-, and CII-immunized WT mice at day 10. (F) Size (FSC) and granulation (SSC) of Flt3+ versus Flt3− B cells found in vivo at day 10. (G) Expression of activation markers CD80, CD40, and MHCII (I-A/I-E) on Flt3+ versus Flt3− B cells found in vivo. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Statistics between Flt3+ and Flt3− B cells in the same sample were analyzed by paired Student’s t test.
Flt3 Is Reexpressed on GC B Cells.
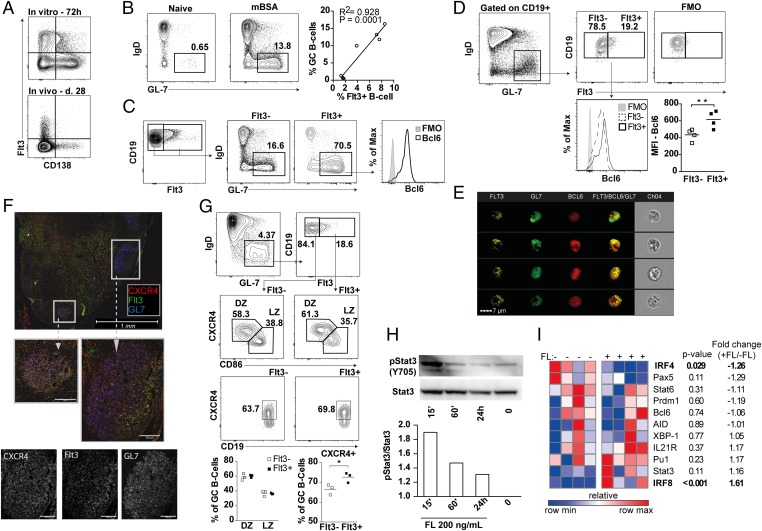
Antigen-mediated activation of B cells results in differentiation into antibody-secreting plasma cells (PC) (34). We found no coexpression of CD138 and Flt3 on activated splenocytes 28 d after immunization or after in vitro LPS stimulation (Fig. 2A and SI Appendix, Fig. S1), suggesting that Flt3+ B cells are activated cells that have yet to differentiate into PCs. Rather, the presence of Flt3+ B cells correlated with the frequency of GC B cells, suggesting that Flt3 might be expressed on GC B cells (Fig. 2B). Accordingly, Flt3+ B cells were CD19+GL-7+IgD− GC B cells that expressed increased B-cell lymphoma 6 (Bcl6) levels compared with Flt3− GC B cells (Fig. 2 C and D). Coexpression of Flt3, Bcl6, and GL7 was further visualized on LN B cells from OVA-immunized mice using imaging flow cytometry (Fig. 2E).
Fig. 2.
Flt3 is expressed on GC B cells. (A) Expression of Flt3 and CD138 on splenic lymphocytes from mBSA-immunized mice at day 28 in vivo and after in vitro LPS stimulation of splenocytes for 72 h. Cells are gated on a total mononuclear-viable population. FMO, fluorochrome minus one staining control. (B) Frequency of GC B cells (CD19+GL-7+IgD−) at day 10 in naïve and mBSA-immunized WT mice. The correlation between the frequency of Flt3+ and GC B cells in LNs at day 10 is shown. (C) Expression of Flt3 on GC B cells. (D) Level of Bcl6 expression in Flt3+ and Flt3− GC B cells. (E) Imaging flow cytometry illustrates expression of Bcl6 and Flt3 on GL-7+ GC B cells. (F) Immunofluorescent staining for Flt3 and CXCR4 on GL-7+ GC B cells in LNs of OVA-immunized mice. (G) Frequency of Flt3+ cells within the DZ (CXCR4+CD86lo) and the LZ (CXCR4−CD83HI) of GC. Expression of CXCR4 on the total Flt3+ and Flt3− GC B-cell population is shown. (H) Level of pStat3 (Y705) and total Stat3 after 15 min (15´), 60 min (60´), and 24 h of stimulation with 200 ng/mL rmFL. (I) Gene expression heat map of activated B cells stimulated with 200 ng/mL rmFL for 20 h. *P < 0.05, **P < 0.01, ***P < 0.001. Statistical analysis was performed using the unpaired Student’s t test.
Immunofluorescent staining of LNs from OVA-immunized mice showed that Flt3+ B cells could be visualized within the GL-7+ areas and colocalized with CXCR4+ cells (Fig. 2F). Because CXCR4 expression on B cells has been shown to be essential for migration of B cells between the light zone (LZ) and dark zone (DZ) area of GC (35, 36), we analyzed the presence of Flt3+ cells within these areas. The distribution of DZ (CXCR4+CD86lo) and LZ (CXCR4−CD86hi) GC B cells was identical between Flt3+ and Flt3− GC B cells, but the frequency of CXCR4+ cells was increased among the Flt3+ subset (Fig. 2G). Thus, Flt3 is expressed on a subset of GC B cells with high Bcl6 and CXCR4 expression, and it might be important for migration of B cells within GC.
FLs Induce Activation of Stat3 in Activated B Cells.
Flt3 signaling activates Stat3 in dendritic cell (DC) precursors (37). Because Stat3 also has an important role in terminal B-cell differentiation and formation of IgG-producing PC (38), we next examined if FL affected Stat3 activation in B cells. B cells were isolated and preactivated with LPS for 48 h to induce expression of Flt3 (SI Appendix, Fig. S2). Stimulation of these preactivated B cells with FL induced activation of Stat3, indicating functional Flt3 signaling (Fig. 2H). In respect to genes associated with B-cell differentiation, FL stimulation increased the transcription of interferon regulatory factor 8 (IRF8) in activated B cells, whereas it suppressed IRF4 (Fig. 2I). Transcription factor paired box protein 5 (Pax5), an essential repressor of Flt3, was down-regulated to a similar extent as IRF4 but did not reach statistical significance. The expression of other genes involved in PC differentiation such as PR domain zinc finger protein 1 (Prdm1) [coding for B lymphocyte-induced maturation protein-1 (Blimp-1)], Bcl6, Pu.1, AID, and IL-21R was not affected by FL stimulation (Fig. 2I).
FL Deficiency Does Not Affect the Formation of GCs.
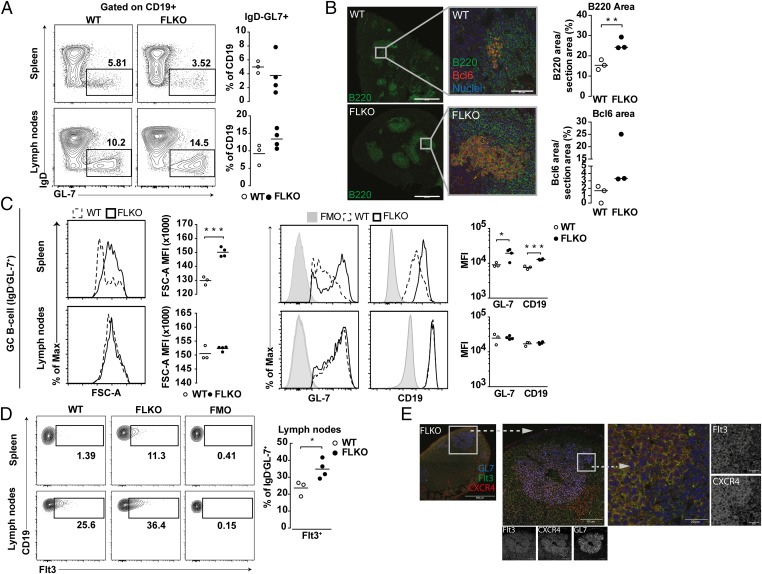
To study a potential role of Flt3 signaling during B-cell activation, we took advantage of FL-deficient (FLKO) mice. FLKO and WT mice were immunized with mBSA, and the formation of GCs in spleen and LNs was analyzed at day 14. We found normal formation of CD19+GL-7+IgD− GC B cells in FLKO mice (Fig. 3A). Interestingly, immunofluorescent staining revealed an enlargement of B-cell and Bcl6+ areas in the spleen of FLKO mice compared with WT (Fig. 3B), suggesting increased activation of GC B cells in FLKO mice. This was supported by increased expression of GL-7 and CD19 and an increased size of GC B cells in spleen from FLKO mice compared with WT cells (Fig. 3C).
Fig. 3.
FL deficiency does not affect formation of GCs. (A) Frequency of GC B cells (CD19+GL-7+IgD−) in spleen and LNs at day 14. (B) Immunofluorescent staining for Bcl6 (red) and B220 (green) in the spleen. Three individual spleens were stained, and areas were calculated as percent of total section area. (C) Expression levels of GL-7 and CD19 and size (FSC-A) of WT (dashed line) and FLKO (solid line) GC B cells (CD19+GL-7+IgD−) in the spleen and LNs. (D) Expression of Flt3 on GC B cells (CD19+GL-7+IgD−) in spleen and LNs. (E) Immunofluosecent staining for Flt3 and CXCR4 within the GL-7+ GC B cells in LNs of FLKO mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Statistical analysis was performed using the unpaired Student’s t test.
Next we examined the expression of Flt3 on FLKO versus WT GC B cells. Supporting a more activated phenotype of GC B cells from FLKO mice, splenic Flt3+ GC B cells were found in FLKO and not in WT mice (Fig. 3D). In LN, Flt3+ GC B cells were found in both strains, but the frequency was significantly increased in FLKO mice, presumably as a consequence of impaired Flt3 signaling (Fig. 3D). As in WT mice, the expression of Flt3 in GL7+ B-cell areas of FLKO mice colocalized with CXCR4 (Fig. 3E).
TFH supports formation of GC and aids in CSR during the GC reaction (9). FL deficiency did not affect the formation of TFH (CD4+ICOS+CXCR5+) cells or the expression of IL-21R, although an increase in IL-21 was observed (SI Appendix, Fig. S3).
Absence of Flt3 Signaling in Activated B Cells Results in Enhanced Plasma Cell Differentiation.
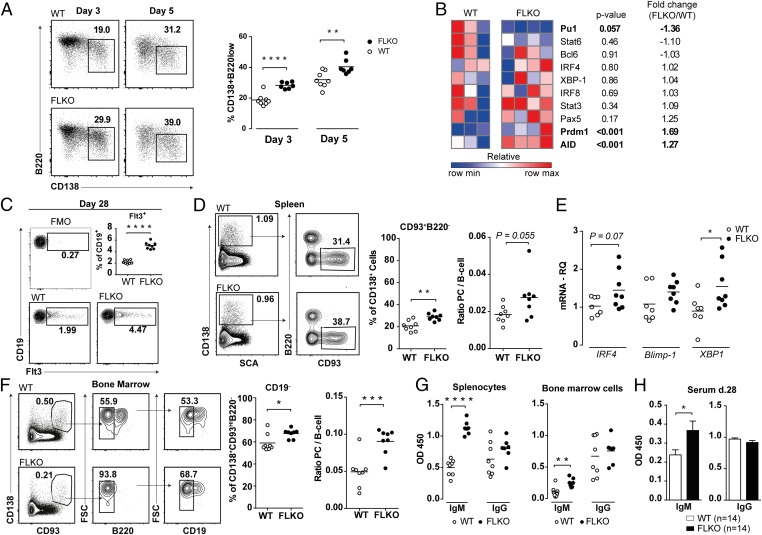
We next examined if PC differentiation is affected by Flt3 signaling. PC differentiation was first evaluated in LPS-stimulated WT and FLKO splenocyte cultures. We found an increased frequency of CD138+B220low plasmablastic cells in FLKO cultures both at day 3 and 5 (Fig. 4A). This was associated with increased transcription of PC-promoting genes AID and Prdm1 (Fig. 4B). The PC-suppressive gene Pu.1 was decreased, whereas Bcl6, Pax5, and IRF8 were equally expressed in WT and FLKO cultures (Fig. 4B). We measured production of soluble FL in cultures of LPS-activated WT splenocytes (29.85 ± 5.99 pg/mL, n = 8), confirming that Flt3 signaling was taking place during PC differentiation.
Fig. 4.
Absence of Flt3 signaling results in enhanced formation of IgM-producing plasma cells. (A) Frequencies of plasma cells after 3 and 5 d of in vitro LPS stimulation of splenocytes isolated from mBSA-immunized FLKO and WT mice at day 28. (B) Gene expression heat map of spleen cells from FLKO and WT mice at day 3 after in vitro LPS stimulation. Gene expression of target genes were normalized against GAPDH and compared with WT mice. (C) Frequency of Flt3+ B cells in the spleen of WT and FLKO mBSA immunized mice. (D) Absolute and relative frequency of splenic (CD138+CD93+B220−) plasma cells. The ratio was calculated by dividing the number of plasma cells by the number of B cells (CD19+CD3− cells). (E) mRNA levels of IRF4, Pdrm1, and XBP1 in the spleen. (F) Absolute and relative frequency of BM (CD138+CD93hiB220−CD19−) plasma cells. The ratio was calculated by dividing the number of plasma cells by the number of immature B cells (CD19+B220+IgM+ cells). (G) Spontaneous production of IgM and IgG by splenocytes and BM cells from mBSA-immunized mice cultured for 48 h without stimulation. (H) Serum levels of total IgM and IgG in FLKO (n = 14) and WT (n = 14) mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Statistical analysis was performed using unpaired Student’s t test. The figure presents the results of two independent mBSA-immunization experiments in vivo and in vitro.
We analyzed the formation of PCs in vivo after mBSA immunization. To identify PCs, we used the marker CD93 in combination with CD138, which identifies antibody-secreting plasma cells (39). FLKO mice had an enlarged population of splenic Flt3+ B cells at day 28 (Fig. 4C). This was associated with an increased formation of splenic and BM PCs in FLKO mice (Fig. 4 D and F) and an increased splenic expression of PC-promoting genes IRF4, Prdm1, and XBP1 (Fig. 4E). No difference was observed in the expression of Bcl6, Pax5, IRF8, and Pu.1 between FLKO and WT mice (SI Appendix, Fig. S4 A and B). Thus, PC differentiation is enhanced in the absence of Flt3 signaling.
Increased Formation of IgM-Producing Plasma Cells in FLKO Mice.
Next we assessed antibody production by culturing splenocytes and BM cells isolated from mBSA-immunized WT and FLKO mice. FLKO mice had increased production of IgM in both splenocyte and BM cultures compared with WT mice, whereas the levels of IgG were similar (Fig. 4G). An increased level of IgM was also found in the serum of FLKO mice at day 28 (Fig. 4H). Together this suggested that IgM-producing PCs were enriched in the absence of FL.
FL Deficiency Causes a Specific Impairment in IgG1 Production.
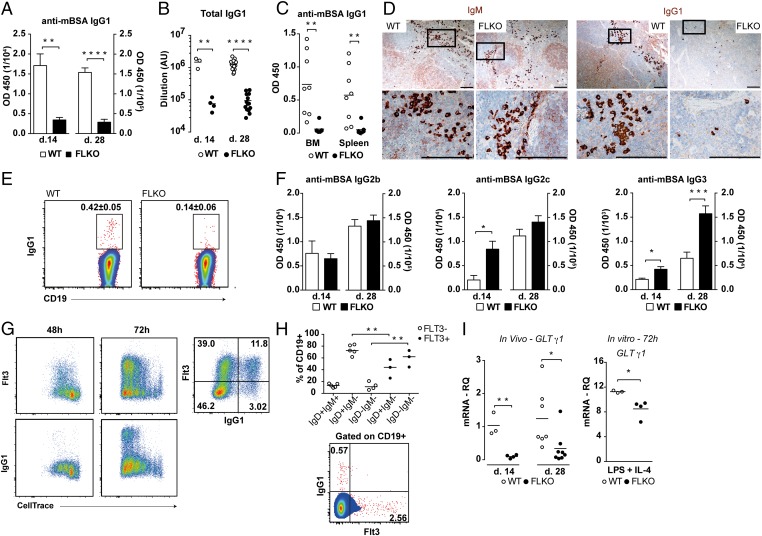
The combination of increased PC differentiation with increased IgM production led us to determine if IgG subclasses were produced normally in FLKO mice. Surprisingly, we observed a significant reduction in serum levels of total and mBSA-specific IgG1 antibodies at days 14 and 28 in FLKO mice (Fig. 5 A and B and SI Appendix, Fig. S5A). Similarly, the production of mBSA-specific IgG1 was reduced in supernatants from FLKO splenocytes and BM cultures (Fig. 5C). Additionally, few IgG1+ cells were found in spleens of mBSA-immunized FLKO mice at days 14 and 28, whereas staining for IgM showed no difference (Fig. 5 D and E). Immunized FLKO mice had sufficient levels of IgG2b, IgG2c, and increased IgG3 (Fig. 5F), potentially explaining the absence of differences in total IgG and total mBSA-specific IgG (Fig. 4H and SI Appendix, Fig. S5B). The increased levels of IgG3, a subclass mainly associated with marginal zone (MZ) B cells, could be associated with an altered distribution of MZ and follicular B cells in FLKO mice (SI Appendix, Fig. S6), which has also previously been reported (40). Measurement of baseline levels of IgG subclasses in naïve mice revealed a specific reduction in IgG1 in FLKO compared with WT mice, whereas levels of the other subclasses were similar between the strains (SI Appendix, Fig. S7). Taken together, these results indicate that Flt3 signaling is essential for IgG1 production.
Fig. 5.
Mice with impaired Flt3 signaling have a selective abrogation in IgG1 production. (A) Serum levels of anti-mBSA IgG1 antibodies at day 14 (WT, n = 3; FLKO, n = 4) and day 28 (WT, n = 14; FLKO, n = 14). (B) Total serum titers of IgG1 in mBSA-immunized mice at day 14 and day 28. (C) Spontaneous production of anti-mBSA IgG1 antibodies from splenocytes and BM cells isolated at day 28 and cultured for 48 h without stimulation. (D) Immunohistochemisty staining for IgM (brown) and IgG1 (brown) in the spleen of WT and FLKO mice at day 28. (Scale bar, 0.1 mm.) (E) IgG1+ B cells in the spleen at day 14. (F) Serum levels of anti-mBSA IgG2b, IgG2c, and IgG3 at day 14 (WT, n = 3; FLKO, n = 4) and day 28 (WT, n = 14; FLKO, n = 14). (G) Expression of Flt3 and IgG1 on proliferating splenic B cells after 48 and 72 h of in vitro LPS + IL-4 stimulation. (H) Surface expression of IgD, IgM, and IgG1 on Flt3+ and Flt3− B cells. Cells were gated on CD19+ cells. (I) GLT mRNA transcripts for γ1 in the spleen of WT and FLKO mice in vivo 14 and 28 d after mBSA immunization and in vitro after 72 h of stimulation with LPS and IL-4. Levels of GLT γ1 were normalized against Igβ. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Statistic analysis was performed using the unpaired Student’s t test.
B Cells from FLKO Mice Have Reduced Ability to Initiate IgG1 CSR in Vivo and in Vitro.
To evaluate a relationship between Flt3 and IgG1, CSR to IgG1 was induced in WT B cells in vitro by combined LPS + IL-4 stimulation. As expected, Flt3 and IgG1 expression was only detected on proliferating cells (Fig. 5G). Additionally, the expression of Flt3 preceded that of IgG1 and was detected already after 48 h, whereas IgG1 was first seen after 72 h. Also, IgG1 expression was mainly found on Flt3+ B cells (Fig. 5G), suggesting that Flt3 signaling was involved during initiation of IgG1 CSR. Both Flt3+ and Flt3− GC B cells in the LNs of OVA-immunized mice were mainly IgM−IgD−. However, assessment of surface Ig expression on total CD19+ B cells of OVA-immunized mice revealed an enrichment of IgD−IgM− cells within the Flt3+ subset, whereas IgD+IgM− cells were mostly Flt3− (Fig. 5H). Also, IgG1 and Flt3 were not coexpressed on B cells in vivo (Fig. 5H). Together, these results support the notion that Flt3+ GC B cells are undergoing CSR and that Flt3 is important in the early stages of CSR to IgG1.
A necessary step in CSR to IgG1 is the occurrence of GLT for γ1 (1). FLKO mice had significantly reduced splenic expression of γ1 GLT (Fig. 5I), but there was no difference in the levels of GLT for IgM or other IgG subclasses (SI Appendix, Fig. S8 A and B), supporting inability of FLKO mice to initiate CSR to IgG1. Similar reduced ability to initiate IgG1 CSR was also found when splenocytes were stimulated with LPS and IL-4 in vitro (Fig. 5I). Lastly, IL-4 significantly potentiated LPS-induced production of soluble FL (LPS, 8.9 ± 1.34 pg/mL vs. LPS + IL-4, 23.1 ± 2.84 pg/mL; P = 0.011, n = 3) from WT splenocytes, further supporting a potential direct role for Flt3 involvement in IgG1 CSR.
Impaired Activation of Stat6 in FLKO B Cells.
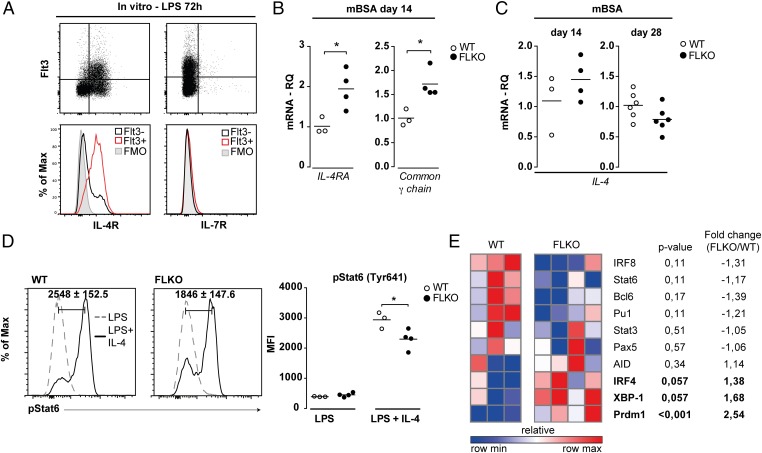
Deficiency of Stat3 in B cells does not affect CSR to IgG1 (38), indicating that FL affects other signaling pathways necessary for IgG1 GLT induction. Previous studies suggest that Flt3 aids in the expression and signaling through IL-7R in early B-cell progenitors (22). Due to functional similarities between the IL-4R and IL-7R, which both use the common γ-chain for signaling (41), we explored if Flt3 could synergize with IL-4R signaling in mature B cells. The LPS-activated Flt3+ B cells expressed IL-4R but not IL-7R (Fig. 6A). The expression of IL-4R and common γ-chain mRNA was increased in the spleen of immunized FLKO mice (Fig. 6B), suggesting that expression of IL-4R was not directly controlled by Flt3. Furthermore, similar levels of IL-4 mRNA were found in the spleen of FLKO and WT mice (Fig. 6C).
Fig. 6.
Deficiency of functional Flt3 signaling in activated B cells results in impaired IL-4–induced activation of Stat6. (A) Expression of the IL-4R and IL-7R on activated Flt3+ WT B cells (gated on CD19+ cells) after 72 h of LPS stimulation. (B) mRNA for IL-4R and the common γ-chain in the spleen of FLKO and WT mice. (C) IL-4 mRNA in the spleen of WT and FLKO mice. (D) Phosphorylation of Stat6 (Tyr641) in WT and FLKO B cells after 72 h stimulation with LPS (dashed line) or LPS + IL-4 (solid line). Cells are gated on B220+ lymphocytes. Histograms represent the difference between LPS and LPS + IL-4 simulated cells. (E) Gene expression heat map of spleen cells from FLKO and WT mice at day 3 after in vitro LPS + IL-4 stimulation. Gene expression of target genes was normalized against GAPDH- and LPS-stimulated cultures. *P < 0.05. Statistical analysis was performed using the unpaired Student’s t test.
Because FLKO mice had normal expression of IL-4 and IL-4R, we explored intracellular IL-4R signaling and evaluated activation of Stat6, the major downstream signaling molecule of IL-4R and the transcription factor responsible for induction of IgG1 CSR (8, 41). LPS and IL-4 stimulation induced phosphorylation of Stat6 in WT and FLKO B cells, however phosphorylation was significantly lower in FLKO B cells (Fig. 6D). This effect of LPS + IL-4 stimulation was not a consequence of impaired expression of IL-4R, as similar levels of both IL-4R and common γ-chain mRNA were found in FLKO and WT cultures (SI Appendix, Fig. S9).
The gene transcription analysis showed that this insufficient phosphorylation of Stat6 in FLKO splenocytes was associated with the sustained increase in IRF4 and Prdm1, which results in higher XBP-1 (Fig. 6E), whereas the important suppressors of Prdm1, IRF8, and Bcl6 were lower in FLKO cultures compared with WT. IL-4 is known to inhibit the transcription of Blimp-1 during in vitro stimulation (42). In turn, repression of Blimp-1 results in higher Stat6 levels and increased CSR to IgG1 (43). This sequence of events was clearly impaired in FLKO mice. Together, these findings suggest that functional Flt3 signaling is needed for proper activation of Stat6 and completion of the IL-4R–induced transcriptional program in B cells.
Discussion
Flt3 is expressed on the surface of B-cell progenitors and has an important role during B-cell development (16, 19). Although Flt3 is down-regulated early during B-cell differentiation, it reappears on peripheral B cells after in vitro activation (26). However, the functional role of Flt3 signaling in mature B cells has not been addressed in any detail before.
Herein we show that Flt3 expression defines an activated and proliferating B-cell population, which can be found in peripheral lymphoid organs and is associated with GC formation. Furthermore, by using FLKO mice, we discovered a previously unacknowledged function for the Flt3 receptor during mature B-cell responses. We show that lack of functional Flt3 signaling causes an enhanced PC differentiation and a specific IgG1 CSR deficiency, an effect of impaired IL-4–dependent transcriptional program in FLKO B cells.
DCs are important for T-cell activation and differentiation and subsequent IgG CSR (44, 45). FLKO mice have a significantly reduced number of DCs, and previous studies on the role for FL in mediating DC- and T-cell–dependent production of IgG have rendered contradictory results (16, 46). Here we demonstrate normal formation of TFH cells, production of IL-4, CSR to T-dependent IgG2c, and comparable levels of total anti-mBSA IgG in the absence of Flt3 signaling, all of which imply functional antigen presentation.
PC differentiation relies on a tightly regulated network of transcription factors. IRF4, Pu.1, and IRF8 have important functions in terminal B-cell differentiation. IRF4 promotes PC differentiation through induction of Blimp-1, whereas Pu.1 and IRF8 suppress PC differentiation by promoting transcription of Pax5 and Bcl6 and suppressing the function of IRF4 (47, 48). Pu.1 controls expression of Flt3, and FL has previously been shown to induce IRF8 in DC progenitors (49, 50). We show that FL affects the balance between IRF8 and IRF4 in activated B cells and that loss of FL causes increased expression of IRF4 and Prdm-1 (coding for Blimp-1), resulting in enhanced PC differentiation. We found this to be potentially regulated by FL through activation of Stat3. However, neither Stat3- nor IRF8-deficient B cells show any defects in CSR to IgG1 and could therefore not explain the IgG1 deficiency in FLKO mice.
The receptors for IL-7 and IL-4 (IL-7R and IL-4R) belong to the common γ-chain receptor family and have important roles in B-cell development and function (41, 51). In the BM, IL-7R signaling is critical for promoting early B-cell commitment and differentiation, but IL-7 has a limited function in mature B cells (52, 53). In contrast, IL-4R supports proliferation and CSR during peripheral B-cell activation but is dispensable in early development (41, 54). Flt3 has previously been suggested to regulate the expression of IL-7R on B-cell progenitors in the BM (22), but a connection between Flt3 and IL-4R has never been described. Herein, we show that Flt3 is coexpressed with the IL-4R on activated peripheral B cells, but in contrast to the IL-7R, we find no indications that IL-4R is affected by FL deficiency. Instead, increased splenic expression of IL-4R mRNA is associated with an increased frequency of Flt3+ B cells, suggesting that the two receptors are expressed concomitantly rather than in sequence and enabling functional synergy between these receptors during B-cell activation.
In addition to induction of IL-7R expression, Flt3 has been suggested to synergize with IL-7R signaling through promotion of Stat5 phosphorylation in early B-cell progenitors (17). Stat6 is the major downstream signaling molecule of the IL-4R and is required for the initiation of CSR to IgG1 by activating transcription of γ1 GLT (55). Accordingly, both Stat6- and IL-4–deficient mice show a specific abrogation in IgG1 production. Also, immunization of IL-4–deficient mice with a T-cell–dependent antigen induces high production of antigen-specific IgG2b, IgG2a, and IgG3, whereas IgG1 production is severely diminished (8, 54, 56). We observed a similar IgG subclass phenotype in FLKO mice characterized by a specific abrogation in IgG1-producing and -expressing B cells, whereas production of other IgG subclasses (IgG2b, IgG2c, and IgG3) was unaffected. This suggested that FLKO B cells might have a specific impairment in IL-4R signaling. Accordingly, we could show that FLKO B cells had reduced activation of Stat6 in response to IL-4, which resulted in reduced transcription of γ1 GLT and impaired production of IgG1.
CSR to IgG1 occurs during the GC reaction (57). In accordance with the proposed involvement of Flt3 in CSR to IgG1, we show that Flt3 was expressed on GC B cells and up-regulated before IgG1 CSR. We found no indications that the lack of FL affected the formation of GC B cells or TFH cells. This is in line with previous reports, in which FLKO mice have been found to respond normally to T-cell–dependent immunizations (16).
GC B cells are highly prone to apoptosis due to increased DNA damage during somatic hypermutation and CSR (58). FL promotes survival of hematopoietic stem cells and peripheral DCs (13) and has been suggested to promote survival of human B cells (29). These findings suggest that FL might regulate the survival of Flt3+ GC B cells during or after CSR to IgG1. However, the reduced levels of GLT for γ1 in FLKO mice argue against this, as this is the initial step in CSR to IgG1 that precedes AID-induced DNA damage (5).
FL-induced activation of Flt3 has previously been shown to regulate CXCL12/CXCR4-induced migration of hematopoietic stem cells. Whereas short exposure to FL promotes CXCL12/CXCR4-induced migration, prolonged exposure led to down-regulation of the CXCR4 receptor and reduced migration of hematopoietic stem cells (59). Because CXCR4 is important for the migration between LZ and DZ during the GC reaction, signaling through the Flt3 receptor could help to regulate shuttling through these two zones by affecting CXCL12/CXCR4-induced migration. This would support why Flt3+ B cells were equally distributed between the LZ and DZ of GC.
In conclusion, this study provides consistent experimental evidence for a previously unacknowledged role for Flt3 during activation of B cells. We show that functional synergy between Flt3 and the IL-4R is critical for proper activation of Stat6 and induction of GLT for γ1, CSR, and production of IgG1. We also show that Flt3 directly activates Stat3 and induces transcription of IRF8 during B-cell activation, which may be of importance for limiting premature PC differentiation.
Methods
Mice.
WT and FLKO mice, on a C57BL/6 background, were purchased from Taconic, and BALB/c mice were purchased from Charles River. Animals were housed at the animal facility of the Department of Rheumatology & Inflammation Research, supplied with continuous airflow, and fed laboratory chow and water ad libitum. The Ethics Committee of Gothenburg University approved all animal experiments.
Immunization Procedure.
Mice were immunized with mBSA (Sigma), CII, or OVA as previously described (60, 61). Briefly, the antigens were emulsified in complete Freund’s adjuvant (Sigma-Aldrich) s.c. on day 0 (200 μg per mouse) followed by a booster emulsified in incomplete Freund’s adjuvant on day 7 (100 μg per mouse). Mice were immunized with mBSA in four independent experiments and killed on days 10 (n = 1, 10 mice), 14 (n = 1, 8 mice), or 28 (n = 2, 16 mice). Immunization with CII or OVA was performed in one experiment, and mice (n = 6) were killed on day 14. Serum, spleen, LNs, and BM were collected.
In Vitro Stimulations and Induction of CSR.
Splenic B cells, purified using the EasySep Mouse B Cell Isolation kit (Stemcell), and total splenocytes isolated from naïve WT mice were stained with CellTrace Violet (Life Technologies) according to the manufacturer’s protocol and stimulated with 10 μg/mL LPS (Sigma-Aldrich) or 10 μg/mL LPS and 50 ng/mL IL-4 (Peprotech) for 48 and 72 h. PC differentiation was induced in splenocytes of mBSA-immunized mice by stimulation with 10 μg/mL LPS for 3 and 5 d, as previously described (47). For in vitro induction of Stat6 phosphorylation, splenocytes were isolated at day 14 and stimulated with LPS (10 μg/mL) or LPS (10 μg/mL) and IL-4 (50 ng/mL). For in vitro stimulation of Flt3+ B cells, WT B cells were stimulated for 48 h with 10 μg/mL LPS, washed, and incubated for 3 h without LPS before stimulation with recombinant mouse FL (rmFL) (200 ng/mL; Creative Biomart) for 15 min, 60 min, or 24 h.
Cell Preparation and Flow Cytometry.
Single-cell suspensions were preincubated with Fc block (BD Pharmingen) before antibody staining. Antibodies used for flow cytometry staining are listed in SI Appendix, Table S1. For staining of intracellular antigens, the FoxP3/Transcription Factor staining buffer set was used (eBioscience). Phosphorylated Stat6 (pY641) was stained according to the protocol adopted from BD Phosflow. Data collection was performed on a FACSCanto II equipped with FACSDiva software (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc.). The gating of cells was based on the isotype control and fluorochrome minus one (FMO) settings.
For the imaging flow cytometry, purified WT B cells were stimulated with 10 μg/mL LPS for 48 h. Cells were stained with FITC-conjugated anti-GL7, PE-conjugated anti-Flt3, and Alexa Fluor 647-conjugated anti–Bcl-6 antibodies, with the addition of DAPI (3 µM), to visualize cell nuclei. Samples (1 × 105 cells) were acquired on an imaging flow cytometer (ImageStreamX MkII, Amnis) and analyzed using IDEAS version 6.1 software (Amnis) for coexpression of GL7, Flt3, and Bcl-6 as described (61).
Immunhistochemical and Immunofluorescent Staining.
Freshly isolated groin LNs were put in a 4% (vol/vol) paraformaldehyde in PBS containing 10% (vol/vol) sucrose. Antigen retrieval was performed overnight in 30% (vol/vol) sucrose in PBS. LNs were transferred into optimum cutting temperature compound and frozen in isopentane cooled in liquid nitrogen. The tissue was sectioned and fixated for 10 min in 100% acetone. Unspecific binding was blocked using an Avidin/Biotin blocking kit (Vector Laboratories), Fc block (CD16/32), and 5% (vol/vol) goat serum. Sections were incubated in separate steps with rat anti-Flt3 antibody and secondary goat anti-rat antibody followed by the rat anti-GL7 and anti-CXCR4 antibodies. The tissue sections were then incubated with streptavidin and nuclear stain for 1 additional hour and mounted using Dako Fluorescence Mounting Medium (Dako).
Formalin-fixed mouse spleens were sectioned and deparaffinized, and antigen retrieval was performed in DIVA Decloaker buffer (Biocare Medical) using a Retriever 2100 (EMS). Endogenous peroxidase was inactivated by 3% (vol/vol) H2O2. For staining of IgM and IgG1, sections were blocked with 5% (vol/vol) goat serum and stained against IgG1 or IgM followed by VECTASTAIN ABC kit (Vector Labs). The reaction was completed using the ImmPACT AEC Peroxidase Substrate kit (Vector Labs). For immunofluorescent staining, sections were blocked with Fc block and 1% goat serum. Sections were then stained with mouse anti-Bcl6 and rat anti-B220. Next, sections were incubated in the presence of the goat anti-rat secondary antibody and nuclear stain before mounting with Dako Fluorescence Mounting Medium. Paraffin sections of formalin-fixed mouse LNs were also stained for Flt3, CXCR4, and GL7, as described above. Images were captured using LSM 700 confocal microscope and the software ZEN 2009 (Zeiss). Antibodies and reagents used for immunohistochemistry are listed in SI Appendix, Table S2.
Detection of Antibodies.
Levels of total and anti-mBSA Ig in serum and supernatants were measured using ELISA as described (60). In brief, plates were coated with mBSA (10ug/mL) or with goat F(ab´)2 fragments to mouse Igs (5 μg/mL). Binding of Igs to antigen was detected using biotin-conjugated antibodies specific for IgM, IgG, IgG1, IgG2b, IgG2c, and IgG3 followed by extravidin–HRP (Sigma) and 3,3′, 5,5′ Tetramethylbenzidine substrate. Absorbance was read at 450 nm. Measurements were performed using serial dilutions of serum and supernatants. Antibodies and reagents used for ELISA are listed in SI Appendix, Table S3.
Gene Expression Analysis.
RNA samples were prepared from cell lysates in RLT buffer or from tissues in RNAlater solution (Qiagen), by RNeasy Mini kit (Qiagen), as described (62). We used 100–400 ng RNA for cDNA synthesis using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR was performed using primers as indicated in SI Appendix, Table S4. The results are presented as a fold change compared with the expression level in control cells with the ΔΔCq method and presented as heat maps using Gene-e software (www.broadinstitute.org).
Protein Preparation and Western Blotting.
Cells for Western blot were lysed and sonicated in lysis buffer containing protease and phosphatase inhibitors (Complete Mini and PhosSTOP; Roche Diagnostics). Protein concentrations were measured using the Bicinchoninic Acid Protein Assay kit (Pierce). Proteins were separated using SDS/PAGE on 4–12% Bis·Tris gels (NuPAGE; Invitrogen) and transferred to polyvinylidene difluoride membranes (NuPAGE; Invitrogen), which were blocked with 5% BSA and incubated with anti–pStat3-Y705 (ab76315; Abcam) or anti-Stat3 (#4904; Cell Signaling) at 4 °C overnight. Detection was performed with peroxidase-conjugated anti-rabbit secondary antibody (NA934VS; GE Healthcare Life Sciences) using Amersham ECL Select substrate (GE Healthcare Life Sciences). Chemiluminescent signals were visualized and quantified using ChemiDoc equipment with Quantity One software (Bio-Rad Laboratories).
Statistical Analysis.
Data are presented as mean ± SEM, and the comparison between the groups was performed using unpaired t test. For comparisons between populations within the same sample, paired t test was used. All statistical evaluation was done using Prism version 6.00 (GraphPad Software). A P value < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This study was supported by the Medical Society of Gothenburg; Swedish Association Against Rheumatism Grants R-229521, R-313411, and R-477321; the King Gustaf V’s 80-Year Anniversary Foundation; Swedish Research Council Grants 521-2011-2414 and 521-2014-2637; EU Commission Grant FP7-HEALTH, 2010-261460; the Swedish Research Agency for Innovation Systems; Torsten Söderbergs Foundation Grant 1-2010; Ingabritt and Arne Lundberg’s Foundation; the University of Gothenburg; and the regional agreement on medical training and clinical research between the Western Götaland county council and the University of Gothenburg, Grants ALFGBG-138661 and ALFGBG-431141.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514191112/-/DCSupplemental.
References
- 1.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai K, et al. Hyper-IgM syndrome type 4 with a B lymphocyte-intrinsic selective deficiency in Ig class-switch recombination. J Clin Invest. 2003;112(1):136–142. doi: 10.1172/JCI18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durandy A, Kracker S. Immunoglobulin class-switch recombination deficiencies. Arthritis Res Ther. 2012;14(4):218. doi: 10.1186/ar3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honjo T. A memoir of AID, which engraves antibody memory on DNA. Nat Immunol. 2008;9(4):335–337. doi: 10.1038/ni0408-335. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: Induction, targeting and beyond. Nat Rev Immunol. 2012;12(7):517–531. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein U, Dalla-Favera R. Germinal centres: Role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Hatzi K, Melnick A. Lineage-specific functions of Bcl-6 in immunity and inflammation are mediated by distinct biochemical mechanisms. Nat Immunol. 2013;14(4):380–388. doi: 10.1038/ni.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000;19(21):2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- 9.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 10.Matthews W, Jordan CT, Wiegand GW, Pardoll D, Lemischka IR. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991;65(7):1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- 11.Hudak S, et al. FLT3/FLK2 ligand promotes the growth of murine stem cells and the expansion of colony-forming cells and spleen colony-forming units. Blood. 1995;85(10):2747–2755. [PubMed] [Google Scholar]
- 12.Muench MO, et al. FLK-2/FLT-3 ligand regulates the growth of early myeloid progenitors isolated from human fetal liver. Blood. 1995;85(4):963–972. [PubMed] [Google Scholar]
- 13.Veiby OP, Jacobsen FW, Cui L, Lyman SD, Jacobsen SE. The flt3 ligand promotes the survival of primitive hemopoietic progenitor cells with myeloid as well as B lymphoid potential. Suppression of apoptosis and counteraction by TNF-alpha and TGF-beta. J Immunol. 1996;157(7):2953–2960. [PubMed] [Google Scholar]
- 14.Lyman SD, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: A proliferative factor for primitive hematopoietic cells. Cell. 1993;75(6):1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Boddupalli CS, Borsotti C, Manz MG. Dendritic cell homeostasis is maintained by nonhematopoietic and T-cell-produced Flt3-ligand in steady state and during immune responses. Eur J Immunol. 2013;43(6):1651–1658. doi: 10.1002/eji.201243163. [DOI] [PubMed] [Google Scholar]
- 16.McKenna HJ, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95(11):3489–3497. [PubMed] [Google Scholar]
- 17.Li LX, Goetz CA, Katerndahl CD, Sakaguchi N, Farrar MA. A Flt3- and Ras-dependent pathway primes B cell development by inducing a state of IL-7 responsiveness. J Immunol. 2010;184(4):1728–1736. doi: 10.4049/jimmunol.0903023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray RJ, Paige CJ, Furlonger C, Lyman SD, Rottapel R. Flt3 ligand supports the differentiation of early B cell progenitors in the presence of interleukin-11 and interleukin-7. Eur J Immunol. 1996;26(7):1504–1510. doi: 10.1002/eji.1830260715. [DOI] [PubMed] [Google Scholar]
- 19.Sitnicka E, et al. Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis. J Exp Med. 2003;198(10):1495–1506. doi: 10.1084/jem.20031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitnicka E, et al. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17(4):463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 21.Nichogiannopoulou A, Trevisan M, Neben S, Friedrich C, Georgopoulos K. Defects in hemopoietic stem cell activity in Ikaros mutant mice. J Exp Med. 1999;190(9):1201–1214. doi: 10.1084/jem.190.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh H, Medina KL, Pongubala JM. Contingent gene regulatory networks and B cell fate specification. Proc Natl Acad Sci USA. 2005;102(14):4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeKoter RP, Lee HJ, Singh H. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity. 2002;16(2):297–309. doi: 10.1016/s1074-7613(02)00269-8. [DOI] [PubMed] [Google Scholar]
- 24.Mackarehtschian K, et al. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3(1):147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 25.Holmes ML, Carotta S, Corcoran LM, Nutt SL. Repression of Flt3 by Pax5 is crucial for B-cell lineage commitment. Genes Dev. 2006;20(8):933–938. doi: 10.1101/gad.1396206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallies A, et al. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity. 2007;26(5):555–566. doi: 10.1016/j.immuni.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Dehlin M, et al. Intra-articular fms-like tyrosine kinase 3 ligand expression is a driving force in induction and progression of arthritis. PLoS One. 2008;3(11):e3633. doi: 10.1371/journal.pone.0003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill MA, et al. Blood dendritic cells and DC-poietins in systemic lupus erythematosus. Hum Immunol. 2002;63(12):1172–1180. doi: 10.1016/s0198-8859(02)00756-5. [DOI] [PubMed] [Google Scholar]
- 29.Tobón GJ, et al. The Fms-like tyrosine kinase 3 ligand, a mediator of B cell survival, is also a marker of lymphoma in primary Sjögren’s syndrome. Arthritis Rheum. 2010;62(11):3447–3456. doi: 10.1002/art.27611. [DOI] [PubMed] [Google Scholar]
- 30.Edwan JH, Talmadge JE, Agrawal DK. Treatment with Flt3 ligand plasmid reverses allergic airway inflammation in ovalbumin-sensitized and -challenged mice. Int Immunopharmacol. 2005;5(2):345–357. doi: 10.1016/j.intimp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Edwan JH, Perry G, Talmadge JE, Agrawal DK. Flt-3 ligand reverses late allergic response and airway hyper-responsiveness in a mouse model of allergic inflammation. J Immunol. 2004;172(8):5016–5023. doi: 10.4049/jimmunol.172.8.5016. [DOI] [PubMed] [Google Scholar]
- 32.Shao Z, Bharadwaj AS, McGee HS, Makinde TO, Agrawal DK. Fms-like tyrosine kinase 3 ligand increases a lung DC subset with regulatory properties in allergic airway inflammation. J Allergy Clin Immunol. 2009;123(4):917–924.e2. doi: 10.1016/j.jaci.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuyama Y, et al. A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. J Immunol. 2011;186(4):2454–2461. doi: 10.4049/jimmunol.1002837. [DOI] [PubMed] [Google Scholar]
- 34.Tarlinton D, Radbruch A, Hiepe F, Dörner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008;20(2):162–169. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Victora GD, et al. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood. 2012;120(11):2240–2248. doi: 10.1182/blood-2012-03-415380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh P, et al. Blockade of prostaglandin E2 signaling through EP1 and EP3 receptors attenuates Flt3L-dependent dendritic cell development from hematopoietic progenitor cells. Blood. 2012;119(7):1671–1682. doi: 10.1182/blood-2011-03-342428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fornek JL, et al. Critical role for Stat3 in T-dependent terminal differentiation of IgG B cells. Blood. 2006;107(3):1085–1091. doi: 10.1182/blood-2005-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chevrier S, et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc Natl Acad Sci USA. 2009;106(10):3895–3900. doi: 10.1073/pnas.0809736106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolence JJ, et al. Cell extrinsic alterations in splenic B cell maturation in Flt3-ligand knockout mice. Immun Inflamm Dis. 2015;3(2):103–117. doi: 10.1002/iid3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 42.Knödel M, Kuss AW, Berberich I, Schimpl A. Blimp-1 over-expression abrogates IL-4- and CD40-mediated suppression of terminal B cell differentiation but arrests isotype switching. Eur J Immunol. 2001;31(7):1972–1980. doi: 10.1002/1521-4141(200107)31:7<1972::aid-immu1972>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 43.Shaffer AL, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17(1):51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39(4):722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams JW, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kingston D, et al. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114(4):835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- 47.Klein U, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7(7):773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 48.Carotta S, et al. The transcription factors IRF8 and PU.1 negatively regulate plasma cell differentiation. J Exp Med. 2014;211(11):2169–2181. doi: 10.1084/jem.20140425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carotta S, et al. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32(5):628–641. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Esashi E, et al. The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity. 2008;28(4):509–520. doi: 10.1016/j.immuni.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: Intelligent design. Nat Rev Immunol. 2007;7(2):144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 52.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201(8):1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sudo T, et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90(19):9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kühn R, Rajewsky K, Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 55.Suto A, et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100(13):4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 56.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to Freund’s complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26(9):2062–2066. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 57.Fukuda T, et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J Exp Med. 1997;186(3):439–448. doi: 10.1084/jem.186.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaudhuri J, Alt FW. Class-switch recombination: Interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4(7):541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 59.Fukuda S, Broxmeyer HE, Pelus LM. Flt3 ligand and the Flt3 receptor regulate hematopoietic cell migration by modulating the SDF-1alpha(CXCL12)/CXCR4 axis. Blood. 2005;105(8):3117–3126. doi: 10.1182/blood-2004-04-1440. [DOI] [PubMed] [Google Scholar]
- 60.Dehlin M, Andersson S, Erlandsson M, Brisslert M, Bokarewa M. Inhibition of fms-like tyrosine kinase 3 alleviates experimental arthritis by reducing formation of dendritic cells and antigen presentation. J Leukoc Biol. 2011;90(4):811–817. doi: 10.1189/jlb.1110640. [DOI] [PubMed] [Google Scholar]
- 61.Andersson KM, et al. Survivin co-ordinates formation of follicular T-cells acting in synergy with Bcl-6. Oncotarget. 2015;6(24):20043–20057. doi: 10.18632/oncotarget.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersson SE, et al. Activation of Fms-like tyrosine kinase 3 signaling enhances survivin expression in a mouse model of rheumatoid arthritis. PLoS One. 2012;7(10):e47668. doi: 10.1371/journal.pone.0047668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.