Abstract
There are many medical and dental conditions which do not have complete treatment modalities in conventional ways. The botulinum toxin can be used as an alternative treatment modality working through chemo denervation method in many medical and dental conditions. An internet research was done for botulinum toxin used in dentistry and all articles and studies were selected, articles related to dentistry were extracted and summarized. This article explains the basic of botulinum toxin and some of its uses in dentistry. In next parts, the extensive details of its use in dentistry will be dealt with.
Keywords: Botox, botulinum toxin, bruxism, chemodenervation, cosmetics, dentistry, facial wrinkles, temporomandibular disorders
Introduction
Botulinum toxin used in dentistry for the treatment conditions, such as parafunctional clenching, extracapsular myogenic temporomandibular disorder, trismus, and the associated headaches, is a new option for symptom relief in patients in whom conventional treatments are not effective.1
Botulinum toxin which is available in the market is purified exotoxin of the anaerobic bacteria, clostridium botulinum. This neurotoxin is the cause of serious paralytic illness, botulism. Seven types of botulinum toxins have been isolated but only two, Types A and B, have been made commercially available. Initially, only botulinum toxin A was available commercially on prescription but more recently, Type B also available in the market.2
Botulinum Toxin Overview3
Botulinum toxin is a deadly poison produced by a Gram-positive bacterium called c botulinum. The bacteria produce 7 antigenically distinct toxins that are lettered A through G. Toxin A, however, has been the most extensively studied. The clinical syndrome of botulism occurs after ingestion of contaminated food, from colonization of the infant gastrointestinal tract, or from wound infection. When foods containing the toxin are ingested, the toxin spreads to peripheral cholinergic nerve endings and blocks acetylcholine release. This results in a bilaterally symmetric descending neuroparalytic illness. The incubation period after ingestion is 18-36 h. In human beings, botulism is mainly caused by Types A, B, E, and rarely F, whereas in animals, it is caused by Types C and D. The toxin is heat labile and denatured by cooking.
History3
The idea for a possible therapeutic use for botulinum toxin was first developed by the German physician Justinus Kerner (1786-1862). He deduced that the toxin acted by interrupting signal transmission within the peripheral sympathetic nervous system, leaving sensory transmission intact. He called the toxin a “sausage poison,” because it was observed that illness followed ingestion of spoiled sausage. In 1870, John Muller, another German physician, coined the name “botulism” (from the Latin root botulus, which means “sausage”). In 1949, Burgen was the first to discover that the toxin was able to block neuromuscular transmission. Scott et al. proved this fact by experimentally administering the Type A strain in monkeys. This strain was approved by the US Food and Drug Administration (FDA) in 1989 under the trade name Botox (Allergan, Inc, Irvine, Calif) for treating strabismus, blepharospasm, and hemifacial spasm in patients younger than 12-year-old. In the year 2000, Botox was approved for use in treating cervical dystonia (wry neck) and 2 years later for the temporary improvement of moderate to severe frown lines between the eyebrows (glabellar lines). Serotype B has been FDA approved for treating cervical dystonia, and serotype F is under investigation in patients who are resistant to serotypes A and B.
Mechanism of Action3,4
The botulinum toxin causes muscle paralysis by inhibiting acetylcholine release at the neuromuscular junction via 3 steps as shown in the Flow chart 1.
Flow chart 1.
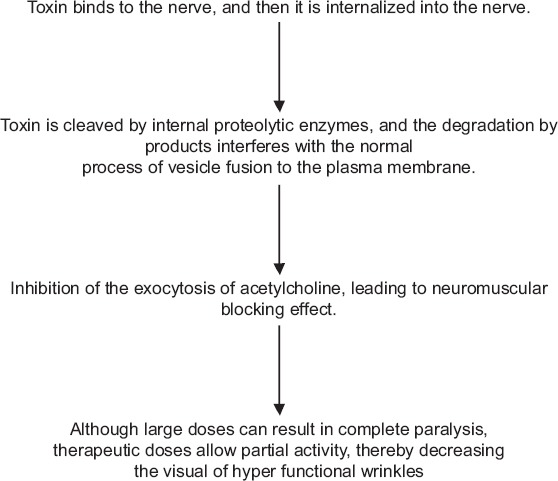
Mechanism of action of botulinum toxin.
Preparation3
Botox is prepared by laboratory fermentation of C botulinum, which lyses and liberates the toxin into the culture. The toxin is then harvested, purified, crystallized with ammonium sulfate, diluted with human serum albumin, lyophilized, bottled in vials, and sealed. Each vial contains 100 U of Botox. One unit is equal to the amount that will kill 50% of a group of 18 to 22 g Swiss Webster mice when injected intraperitoneally. The human lethal dose is estimated to be approximately 3,000 U. Botox dosages used for cosmetic purposes typically are less than 100 U. Optimal pH of the solution is between 4.2 and 6.8, and vials should be stored at or below −5°C.
Preparations should be reconstituted with 1-5 ml of saline without preservatives just before use. Because Botox is easily denatured via bubbling or agitation, the diluents should be gently injected into the inside wall of the vial. The reconstituted solution should be refrigerated at 2-8°C and used within 4 h. Botulinum toxin B is marketed under the trade name Myobloc (Elan Pharmaceutics, San Francisco, Calif). Its relative potency to Botox is 50-125 U of Myobloc to 1 U of Botox. This product does not require reconstitution and is stable for up to 21 months in a refrigerator.
Therapeutic Uses3
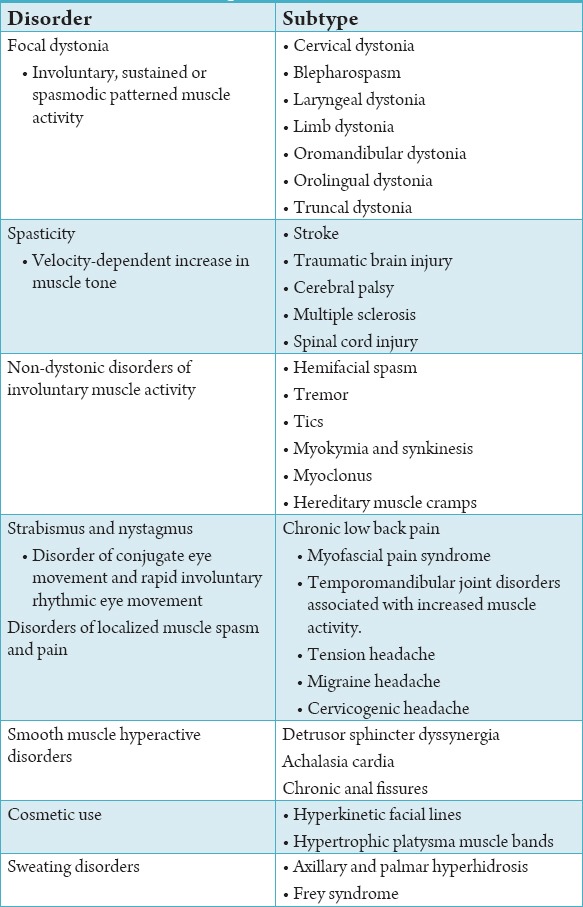
Botulinum toxin may be used for a variety of disorders ranging from pain management to the treatment of tremors and tics, to the improvement of the appearance of dynamic facial wrinkles. Table 1 summarizes the therapeutic uses of Botox.
Table 1.
Therapeutic uses of botulinum toxin.
Chronic Migraine5
Chronic migraine is a disorder with resulting reduced quality of life associated with discomfort. Botulinum toxin can be used to treat this disorder.
Dosage
The recommended dosage is dilution of 200 units/4 mL or 100 units/2 mL, with a final concentration of 5 units per 0.1 mL. It is administered intramuscularly using a sterile 30-gauge, 0.5 inch needle as 0.1 mL (5 units) injections per each site to treat chronic migraine. Injections should be divided across following 7 specific head/neck muscle areas. (1) Corrugator: 5 U each side. (2) Procerus: 5 U one side. (3) Frontalis: 10 U each side. (4) Temporalis: 20 U each side. (5) Occipitalis: 15 U each side. (6) Facial paraspinal: 10 U each side. (7) Trapezius: 15 U each side. It has to repeat for every 12 weeks.
Pathologic Clenching
Pathologic clenching is a disorder leading to chronic trauma to teeth, gingiva, and underlying tissues. Low doses of botulinum toxin Type A can potentially reduce this disorder. Because parafunctional clenching leads to periodontal trauma, limiting clenching before and after periodontal surgery can benefit healing.6
Mandibular Spasm
This type of muscular spasm results from spasm of all muscles of mastication and associated mandibular muscles. This disorder places limitations on completing the basic oral hygiene necessary to prevent oral disease.7 other impairments can include: Restrictions on dental treatment, difficulty with eating and diminished oral utility. Botulinum toxin treatment to the masticatory musculature diminishes the effects of hyperfunctional or spastic muscles.8
Bruxism
Botulinum neurotoxin has shown promise in decreasing the symptoms of bruxism.9 Ivanhoe et al.10 reported success with a 200 U dose of botulinum toxin Type A in a separate brain-injury case report. A long-term, open-label trial study with a history of severe bruxism who were refractory to medical and dental procedures, to them botulinum toxin Type A injections were given into the masseters (mean dose: 61.7 U/side; range 25 U to 100 U), which results in a total duration of therapeutic response of 19 weeks.11
Trigeminal Neuralgia12
It is a unilateral neurological disorder affecting orofacial muscles leading to acute severe pain. Botox can be used as an adjunctive treatment modality in these patients which acts on nerve endings, thereby reducing the severity of the pain.
Enhancing Facial Esthetics13
The facial wrinkles can be treated with Botox. But, the pathogenesis of wrinkles should be known first. The use of fillers in the lower face and the use of Botox for the upper face are advised. When the rhytid is primarily caused by muscular action deforming the overlying skin, Botox can be extremely effective treatment in the lower face.
Side Effects of Botox Therapy12
The muscles injected can be sore for a few days after the injections
Botox can cause temporary partial weakening of the muscles injected
When Botox is used for a long time, it may cause atrophy of the muscles injected. This atrophy is reversible if the therapy is discontinued
There have been reports of temporary side effects such as flu-like symptoms, palpitations, tingling sensations, or nausea. These side effects are rare and usually go away within 1-2 days.
Contraindications to Botox Therapy5
Hypersensitivity to any botulinum toxin preparation
Infection at the proposed injection site.
Conclusion
Chemodenervation using botulinum toxin is useful in many of the conditions of dentistry. The controlled use of this therapy is more important rather than its radical use.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Botulinum Toxin Frontline TMJ syndrome and Dental Therapeutic Treatment. Louis Makmacher. Academy of General Dentistry. 2013 May [Google Scholar]
- 2.David Mock. Botulinum Toxin and Dentistry. Ensuring Continued Trust, Royal College of Dental Surgeons of Ontario: Ensuring Continued Trust: Dispatch. 2009:1–4. [Google Scholar]
- 3.Dastoor SF, Misch CE, Wang HL. Botulinum toxin (Botox) to enhance facial macroesthetics: A literature review. J Oral Implantol. 2007;33(3):164–71. doi: 10.1563/0-835.1. [DOI] [PubMed] [Google Scholar]
- 4.Hallett M. One man’s poison - Clinical applications of botulinum toxin. N Engl J Med. 1999;341(2):118–20. doi: 10.1056/NEJM199907083410209. [DOI] [PubMed] [Google Scholar]
- 5.BOTOX. (Onabotulinum toxin A) Medication Guide: Initial U.S Approval. 1989 [Google Scholar]
- 6.Freund B, Schwartz M, Symington JM. Botulinum toxin: New treatment for temporomandibular disorders. Br J Oral Maxillofac Surg. 2000;38(5):466–71. doi: 10.1054/bjom.1999.0238. [DOI] [PubMed] [Google Scholar]
- 7.Andrew Blitzer, Paul E. Greene, Mitchell F.Brin, Stanley Fahn. Botulinum Toxin Injection for the treatment of Oromandibular Dystonia. Ann Otol Rhinol Laryngol February. 1989;98(no. 2):93–97. doi: 10.1177/000348948909800202. [DOI] [PubMed] [Google Scholar]
- 8.Cersósimo MG, Bertoti A, Roca CU, Micheli F. Botulinum toxin in a case of hemimasticatory spasm with severe worsening during pregnancy. Clin Neuropharmacol. 2004;27(1):6–8. doi: 10.1097/00002826-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Van Zandijcke M, Marchau MM. Treatment of bruxism with botulinum toxin injections. J Neurol Neurosurg Psychiatry. 1990;53(6):530. doi: 10.1136/jnnp.53.6.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanhoe CB, Lai JM, Francisco GE. Bruxism after brain injury: Successful treatment with botulinum toxin-A. Arch Phys Med Rehabil. 1997;78(11):1272–3. doi: 10.1016/s0003-9993(97)90343-9. [DOI] [PubMed] [Google Scholar]
- 11.Tan EK, Jankovic J. Treating severe bruxism with botulinum toxin. J Am Dent Assoc. 2000;131(2):211–6. doi: 10.14219/jada.archive.2000.0149. [DOI] [PubMed] [Google Scholar]
- 12.Sinha A, Hurakadli M, Yadav P. Botox and derma fillers: The twin face of cosmetic dentistry. Int J Contemp Dent Med Rev. 2015;2015 Article ID: 131214. DOI: 10.15713/ins.ijcdmr.27. [Google Scholar]
- 13.Suman T. Enhancing facial esthetics by other modalities. Int J Dent. 2011;2011:513957. doi: 10.1155/2011/513957. [DOI] [PMC free article] [PubMed] [Google Scholar]


