Abstract
Background:
Both diabetes mellitus (DM) and periodontitis are chronic diseases affecting large number of the population worldwide. Changes in human behavior and lifestyle over the last century have resulted in a dramatic increase in the incidence of diabetes in the world. This study was designed to evaluate the relationship between severity of periodontal disease and control of diabetes (glycated hemoglobin [HBA1c]) in patients with Type 1 DM in a hospital based study.
Materials and Methods:
Fifty patients (n = 50) with Type 1 diabetes were enrolled in the study. They were divided into three groups based on the degree of glycemic control by measuring HbA1c levels as: “Good” (HBA1c ≤7) Group A, fair (HBA1c = 7-8) Group B and poor (HBA1c >8) Group C. All enrolled patients underwent detailed history and dental checkup. Evaluation for periodontal disease was done by measuring dental plaque (plaque index), inflammation of gums (gingival index), probing pocket depth (PPD), and clinical attachment level.
Results:
Type 1 diabetics with poor glycemic control had increased gingival inflammation (P < 0.05), more dental plaque (P < 0.05), increased PPDs (P < 0.05) and attachment loss (P < 0.05) as compared to those with fair and good glycemic control, respectively.
Conclusion:
Severity of periodontal disease increases with poor glycemic control in patients with Type 1 DM.
Keywords: Glycated hemoglobin levels, periodontal disease, Type 1 diabetes mellitus
Introduction
Diabetes mellitus (DM) and periodontal diseases are common chronic diseases observed worldwide. DM is a heterogeneous chronic metabolic disorder principally characterized by persistent hyperglycemia resulting from defects in insulin action and/or insulin secretion.1 It affects more than 171 million individuals worldwide and has reached epidemic status.2 DM results from a malfunction of insulin-dependent glucose homeostasis. Classically, they present a triad of symptoms including polyuria, polyphagia, and polydipsia. These are the direct result of hyperglycemia and osmotic imbalance.3 The disease is characterized by an increased susceptibility to infection, poor wound healing, and increased morbidity and mortality associated with disease progression. Type 1, insulin-dependent DM is characterized by abrupt onset at any age, destruction of 80-90% of the pancreatic islet cells that produce insulin, and dependence on exogenous insulin to prevent ketosis and preserve life. The beta cell destruction is an autoimmune process which occurs in genetically susceptible individuals. The most frequent onset of new cases occurs around the age of puberty. About 15% of diabetics are Type 1.4 Long-term poor glycemic control in Type 1 DM patients leads to macrovascular and microvascular complications. Periodontal disease has been reported as the sixth complication of diabetes along with neuropathy, nephropathy, retinopathy, and micro and macrovascular diseases.5
Periodontal diseases are a group of inflammatory diseases that affect the periodontal attachment apparatus (gingiva, periodontal ligament, alveolar bone, and cementum). Gingivitis is defined as inflammation of the gingiva. It is characterized by redness and edema of gingival tissue, bleeding on provocation, changes in contour and presence of calculus or plaque with no radiographic evidence of crystal bone loss.6 Periodontitis is defined as an inflammatory disease of the supporting tissues of the teeth caused by specific microorganisms, resulting in progressive destruction of the periodontal ligament and alveolar bone with pocket formation, recession or both. The clinical feature that distinguishes periodontitis from gingivitis is the presence of clinically detectable attachment loss. This is often accompanied by periodontal pocket formation and changes in the density and height of subjacent alveolar bone.7
In Type 1 DM chronic hyperglycemia leads to formation of biologically active glycated proteins and lipids that promote inflammatory responses.8,9 Long-term poor control of DM in Type 1 patients leads to microvascular damage of the periodontium, alteration in composition of gingival crevicular fluid, host bacterial flora of the gingiva and impaired healing response of periodontium. Increased levels of inflammatory markers such as C-reactive protein, tumor necrosis factor-α, interleukin-6 (IL-6), IL-1, prostaglandin E2, and IL-10 observed in patients with diabetes may play a role in periodontal damage.10
The present study was designed to investigate the relationship between severity of periodontal disease and glycemic control in patients with Type 1 DM.
Materials and Methods
Fifty patients were recruited from the Department of Medicine, People’s College of Medical Sciences and Research Centre. Inclusion criteria were: Age between 12 and 25 years and with a diagnosis of Type 1 DM for more than 3 months duration. Patients were excluded from the study if they were non-Type 1 diabetics, undergoing active orthodontic treatment, patients with any chronic inflammatory diseases and on long-term medications that could influence the studied parameters such as antibiotics and antiepileptic immunosuppressive drugs. The individuals were classified according to their glycemic control as “good” (glycated hemoglobin [HBA1c] ≤7)11 Group A, fair (HBA1c = 7-8)11 Group B, and poor (HBA1c >8)11 Group C in this study. The periodontal status was examined by recording the following parameters: Plaque index (PI); Silness and Loe (1964)12, gingival index (GI); Loe and Silness (1963)12, probing pocket depth (PPD)13 and clinical attachment level (CAL)13 after adjusting for age, gender, frequency of prior dental visits and dental examiner.
Dental plaque is a soft, non-mineralized deposit that forms on the tooth surface. PI assessed the thickness of plaque at the gingival area of the tooth surface. A dental explorer was used to evaluate the cervical third of tooth. GI assessed the severity of gingivitis, by examining qualitative changes of gingival soft tissue. A blunt instrument such as periodontal probe was used to assess the bleeding potential of tissues. PPD and CAL measured the distance from the gingival margin to the base of gingival sulcus and distance from cementoenamel junction to base of sulcus respectively.12,13
PPD and CAL were obtained from six exploratory points for each tooth with a calibrated North Carolina probe, by a single examiner. The pockets were classified into two groups according to the depth: Medium pockets with depth 4-5 mm and deep pockets with depth >6 mm. The CAL was classified into three groups: CAL <2 mm (mild periodontitis), CAL 3-4 mm (moderate periodontitis), CAL ≥5 mm (severe periodontitis).13
Results
The study group consisted of 50 subjects diagnosed with Type 1 DM. The subjects were divided into three groups depending on the HbA1c levels: “Good” (HBA1c ≤7)11 Group A, “fair” (HBA1c = 7-8)11 Group B and “poor” (HBA1c >8)11 Group C in this study. The number and percentage of subjects in each group were Group A - 15 (30%), Group B - 16 (32%), and Group C - 19 (38%) (Table 1). The number and percentage of males and females in the study group were males - 32 (64%), females - 18 (36%). The mean and standard deviation of periodontal parameters PI, GI, PPD, and CAL in Group A (HBA1c ≤7) were 2.93 ± 0.59, 3.33 ± 0.48, 1.25 ± 0.20, and 1.25 ± 0.34, respectively. The mean and standard deviation of periodontal parameters PI, GI, PPD, and CAL in Group B (HBA1c = 7-8) were 3.81 ± 0.75, 4.43 ± 0.62, 1.82 ± 0.45, and 1.43 ± 0.33, respectively. The mean and standard deviation of periodontal parameters in Group C (HBA1c >8) were 5.31 ± 0.20, 6.15 ± 1.38, 2.39 ± 0.18, and 2.01 ± 0.29, respectively (Table 2). Comparison of periodontal parameters among the three groups was done by applying the ANOVA test. P < 0.05 was considered to be statistically significant for all analysis.
Table 1.
Number and percentage of individuals in each group.

Table 2.
Mean and standard deviation of periodontal parameters in Group A, B, C.
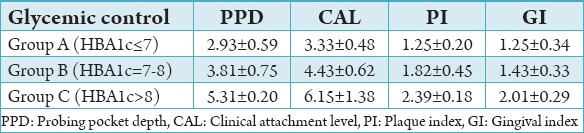
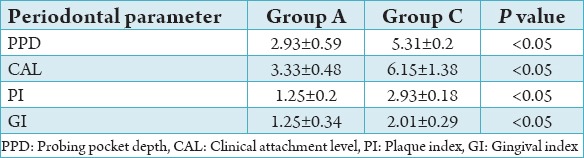
On comparison of the periodontal parameters PI, GI, PPD, and CAL between group A, B, and C the values were found to be significantly higher (P < 0.05) in Group C followed by Group B and lowest in Group A (Tables 3-5). The results of this study showed that there is increased severity of periodontal disease with poor glycemic control in Type 1 diabetics.
Table 3.
Comparison between Groups A and B.
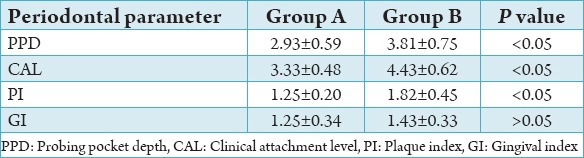
Table 4.
Comparison between Groups B and C.
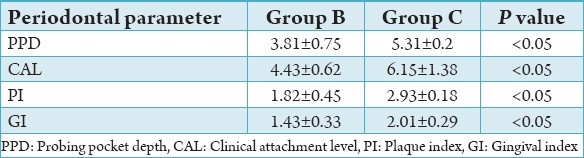
Table 5.
Comparison between Groups A and C.
Discussion
In this study, the patients with poor glycemic control (Group C) had deeper pockets and more attachment loss which was in accordance with the findings of studies conducted by Llambés et al. 2005, Silvestre et al. 2009,14,15 Tsai et al. 2002,16 and Seppälä et al. 1993, 1997.17,18 Tervonen and Oliver19 demonstrated this in a cross-sectional study into the association between long-term diabetic control and periodontal status. Diabetics were assessed using HbA1c and for plaque, calculus, probing depth, and attachment loss. Based on their control of blood glucose levels, they were grouped into “good control,” “moderate control,” and “poor control” of blood glucose levels. It was found that the prevalence of severe attachment loss increased with decreasing control of diabetes. This study found that 10% of well controlled and 27% of poorly controlled diabetics had loss of attachment >5 mm.19 These findings have been confirmed by other studies - both cross-sectional20 and longitudinal.17,21 The present study showed that subjects with poor glycemic control harbored more plaque and had more severe gingival inflammation as evident by the higher scores of GI and PI. Similar results were obtained in a study conducted by Lalla et al. 200722 who found higher PI scores and greater gingival inflammation in a large cohort of Type 1 diabetics as compared to non-diabetic controls. High blood glucose sustained over time appears to give rise to a situation of chronic inflammatory mediator secretion, and thus to an exaggerated periodontal response. However, from the opposite perspective, it could be postulated that the severity of PD could affect DM control despite adequate integral treatment and patient cooperation.15
Conclusion
Periodontal diseases can be prevented in susceptible individuals at an early stage23,24 if regular oral screenings and periodontal treatment programs are considered as a standard of care for young patients with Type 1 diabetes.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes. Diabetes Care. 2009;32:S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: An overview. Ann Periodontol. 2001;6(1):91–8. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- 4.Papapanou PN. Epidemiology of periodontal diseases: An update. J Int Acad Periodontol. 1999;1(4):110–6. [PubMed] [Google Scholar]
- 5.Lowe GD. The relationship between infection, inflammation, and cardiovascular disease: An overview. Ann Periodontol. 2001;6:1–8. doi: 10.1902/annals.2001.6.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Parameter on plaque-induced gingivitis. American academy of periodontology. J Periodontol. 2000;71:851–2. doi: 10.1902/jop.2000.71.5-S.851. [DOI] [PubMed] [Google Scholar]
- 7.Novak MJ. Classification of diseases and conditions affecting the periodontium. In: Newman MG, editor. Carranza’s Text Book of Clinical Periodontology. 10th ed. Missouri: Elsevier Publication; 2006. pp. 100–9. [Google Scholar]
- 8.Verma S. C-reactive protein incites atherosclerosis. Can J Cardiol. 2004;20(Suppl B):29B–31. [PubMed] [Google Scholar]
- 9.Iacopino AM. Periodontitis and diabetes interrelationships: Role of inflammation. Ann Periodontol. 2001;6(1):125–37. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 10.Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127–53. doi: 10.1111/j.1600-0757.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 11.Rose LF, Genco RJ, Cohen DW, Mealey BL. Periodontal Medicine. Hamilton: BC Decker Inc; 2000. pp. 130–42. [Google Scholar]
- 12.Carranza FA, Newman MG. Clinical Periodontology. 8th ed. Oxford: W B Saunders Co; 1996. pp. 64–8. [Google Scholar]
- 13.Newman MG, Takei HH, Klokkevold PR. Carranza’s Clinical Periodontology. 10th ed. Noida, India: Elsevier; 2006. pp. 551–3. [Google Scholar]
- 14.Llambés F, Silvestre FJ, Hernández-Mijares A, Guiha R, Caffesse R. Effect of non-surgical periodontal treatment with or without doxycycline on the periodontium of type 1 diabetic patients. J Clin Periodontol. 2005;32(8):915–20. doi: 10.1111/j.1600-051X.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 15.Silvestre FJ, Miralles L, Llambes F, Bautista D, Solá-Izquierdo E, Hernández-Mijares A. Type 1 diabetes mellitus and periodontal disease: Relationship to different clinical variables. Med Oral Patol Oral Cir Bucal. 2009;14(4):E175–9. [PubMed] [Google Scholar]
- 16.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30(3):182–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 17.Seppälä B, Seppälä M, Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol. 1993;20(3):161–5. doi: 10.1111/j.1600-051x.1993.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 18.Seppälä B, Sorsa T, Ainamo J. Morphometric analysis of cellular and vascular changes in gingival connective tissue in long-term insulin-dependent diabetes. J Periodontol. 1997;68(12):1237–45. doi: 10.1902/jop.1997.68.12.1237. [DOI] [PubMed] [Google Scholar]
- 19.Tervonen T, Oliver RC. Long-term control of diabetes mellitus and periodontitis. J Clin Periodontol. 1993;20(6):431–5. doi: 10.1111/j.1600-051x.1993.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 20.Tervonen T, Karjalainen K, Knuuttila M, Huumonen S. Alveolar bone loss in type 1 diabetic subjects. J Clin Periodontol. 2000;27(8):567–71. doi: 10.1034/j.1600-051x.2000.027008567.x. [DOI] [PubMed] [Google Scholar]
- 21.Seppälä B, Ainamo J. A site-by-site follow-up study on the effect of controlled versus poorly controlled insulin-dependent diabetes mellitus. J Clin Periodontol. 1994;21(3):161–5. doi: 10.1111/j.1600-051x.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 22.Lalla E, Cheng B, Lal S, Kaplan S, Softness B, Greenberg E, et al. Diabetes-related parameters and periodontal conditions in children. J Periodontal Res. 2007;42(4):345–9. doi: 10.1111/j.1600-0765.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 23.Mehta A. Risk factors associated with periodontal diseases and their clinical considerations. Int J Contemp Dent Med Rev. 2015;2015 Article ID: 040115. doi: 10.15713/ins.ijcdmr.31. [Google Scholar]
- 24.Nategh B, Moghaddam MA, Nodehi D, Sharbaf DA. Periodontitis and oral health. Int J Contemp Dent Med Rev. 2015;2015 Article ID: 020515. doi: 10.15713/ins.ijcdmr.76. [Google Scholar]


