Abstract
Background:
The behavior of salivary myoepithelial cells (MEC) during chronic irradiation exposure is unknown. This study aimed to investigate the response of MEC to prolonged radiation exposure.
Materials and Methods:
16 rabbits and four controls were irradiated with either 10 Gy, 20 Gy, 30 Gy or 40 Gy (Gray units) of direct axial beam radiation. Parotid and submandibular glands were removed and examined using immunohistochemical double staining. Proliferating MEC were semi-quantified using alpha smooth muscle actin antibodies and proliferating cell nuclear antigen (PCNA) antibodies.
Results:
MEC proliferative activity increased after radiation in both submandibular (P = 0.037) and parotid groups (P = 0.006) compared to controls. Hyper-proliferation was seen only in parotid glands which was almost dose-dependent. Mean percentage MEC proliferation did not correlate with the clinical grading or recovery from oral mucositis (P = 0.47).
Conclusions:
Parotid glands are more sensitive to radiation compared to submandibular glands. Further research is needed to determine the role of MEC proliferative activity in response to radiation.
Keywords: Experimental, mucositis, myoepithelial cells, proliferating cell nuclear antigen, salivary
Introduction
Head and neck cancers are relatively uncommon accounting globally for only 4% of all cancers.1,2 However, 5 years survival within developing nations remains disproportionately inferior.3 This may be due to inadequate public awareness and late presentation. Radiotherapy is an established treatment for head and neck cancers.4 Radiotherapy causes xerostomia, which is the subjective feeling of oral dryness. It is caused by an inadequate or absence of saliva flow. It can also be caused by changes to the saliva composition.5 Prolonged xerostomia can cause mucositis which is the most commonly reported complication following radiation for head and neck cancer.6 Severe mucositis causes significant morbidity, reduced quality of life, and in some cases, interruption to treatment schedules.4-8
Salivary glands are composed mainly of parenchymal cells (acini) and myoepithelial cells (MECs). MECs play an important role in supporting the morphogenesis, differentiation and polarization of salivary acini.9-12 A reduction in saliva flow is seen almost immediately after radiation exposure.8 The long-term exposure to radiation may induce permanent gland damage, inflammation, and fibrosis. The precise pathophysiology of this process is unknown. On a cellular level, several pathways have been implicated.8 During inflammatory conditions like sialadenitis, an increase in MEC proliferative activity is observed which can be up to 10-fold higher than that seen in normal salivary tissue.13-15 Conversely, a decrease in the number of MECs after a single dose of radiation has also been reported.16 This would suggest that post-radiation inflammation induces a different MEC behavior than that seen in general inflammatory conditions. The behavior of MECs during chronic radiation exposure is unknown. Understanding these pathways are important as it may help to develop novel therapies which inhibit cellular processes which induce salivary gland damage.
In order to characterize the response of salivary MEC’s to the full radiation exposure the following study was conducted. It also aimed to determine MEC proliferative activity post-radiation correlated with the clinical grading of mucositis in an animal model.
Materials and Methods
Study design
All experiments were conducted in strict accordance to the recommendations laid out by National Institutes of Health and Research guidance on the “Care and Use of Laboratory Animals” guidelines.17 The study protocol was approved by the University of Damascus Animals in Science Ethics Committee (Approval ref: 528/2012). 6-month-old female New Zealand rabbits (n = 20) with an average weight of 2.5 kg were used in this study. The animals were bred in the Damascus University Animal Centre. All experiments were performed, while the rabbits were under general anesthesia following sodium pentobarbital infusion through the femoral vein. The rabbits were exposed to 12 h-12 h light-dark cycles and had free access to water and standard diet.
A protocol developed and validated by Hakim et al. was followed.16,18 In brief 16 rabbits were divided into four groups (n = 4 per group). Rabbits from each group received either 10 Gray units (Gy) (Group A), 20 Gy (Group B), 30 Gy (Group C) or 40 Gy (Group D) of ionizing radiation using a treatment schedule of 2 Gy/day over 5 days. Treatment schedules ranged between 5 days (Group A) and 20 days (Group D). Control group (n = 4) received no radiation. The protocol aimed to mimic typical radiation schedules given to patients with head and neck cancers. Radiation doses are increased in gradual fractions as described above. An equivalent dose of 40 Gy confers a 100% risk of mucositis.19 The study design hence simulated high (Group D), moderate (Group B and C), and low radiation (Group A) exposure.
Radiation was delivered using the ALCYON II telecobalt therapy device (Georges Speicher, France). An axial beam was directed toward the head of the rabbit at a extending from the retro-auricular region to the tip of the nose after a bolus delivered from 0.5 cm (Figure 1). A radiation field size of 5 cm × 10 cm was created, which allowed all salivary glands to be irradiated. All procedures were performed by a single researcher (RO). Rabbits were irradiated daily for 5 min. An experienced radiotherapist from the Nuclear Medicine Hospital in Damascus University Hospital was used for consultation and advice to ensure correct radiation dose delivery. The oral mucosa of animals was examined by an oral medicine specialist (OK) prior to execution and graded according to the oral mucositis assessment scale (OMAS).20
Figure 1.

The rabbits in set-up position using the ALCYON II telecobalt therapy device.
Following their respective radiation regimens all animals were killed immediately. Controls were killed at the end of 20 days. Parotid and submandibular glands from all animals were removed and fixed in 10% neutral buffered formalin for 24 h. Specimens were paraffin-embedded and sectioned for hematoxylin and eosin staining. Histopathological analysis was performed by a blinded histopathologist (NK).
An immunohistochemical double staining technique was used to quantify proliferating MECs. MECs were double-stained using antibodies against α-smooth muscle actin (α-SMA) while active proliferation was quantified using antibodies against proliferating cell nuclear antigen (PCNA). 4 µm formalin fixed sections were placed on poly-lysine-coated glass slides. Sections were dewaxed and rehydrated in standard serial dilutions in ethanol. Sections were then incubated with 200 µl of dual endogenous enzyme block for 5 min and then washed three times in 0·1 mol/L phosphate-buffered saline (PBS). Slides were incubated for 1 h at room temperature with PCNA primary mouse monoclonal antibody (Monoclonal anti-PCNA, clone PC10, DakoCytomation, Denmark) diluted 1:100 in antibody diluent (50 ml/PBS, 0.5 ml goat serum and 0.5 g bovine serum albumin). Slides were then washed in PBS and incubated in 200 µl of polymer/HRP for 5 min. After further PBS washes, slides were incubated in 200 µl DAB for 5 min followed by further PBS washes and incubation in PBS buffer for 1 h at room temperature. A 200 µl Doublestain block (EnVision™ DuoFLEX Doublestain System, DakoCytomation, Denmark) was applied for 5 min, then washed in PBS. Slides were incubated with 1:100 α-SMA second antibody (Monoclonal anti-α-SMA, Clone 1A4, DakoCytomation, Denmark) for 1 h at room temperature. The slides were then washed three times with PBS each for 3 min, followed by incubation with 200 µl Rabbit/Mouse (LINK) for 5 min. The slides were then washed 3 times with PBS each for 3 min, followed by incubation with 200 µl polymer/AP for 5 min. After a further three washes in PBS, the sections were incubated with 200 µl permanent red working solution (EnVision™ DuoFLEX Doublestain System, DakoCytomation, Denmark) for 15 min. Slides were washed with deionized water and placed in a distilled water bath for 5 min. Slides were counterstained for 2-3 s with hematoxylin. A negative control experiment was carried out on random sections from each gland, in which the primary antibody was omitted.
Statistical analysis
The labeling index of PCNA in MECs was calculated. MECs were counted under a microscope at ×400 magnification by two pathologists simultaneously (NK, RO) using eight randomly selected fields. The percentage of cycling (PCNA-positive) MECs was calculated by dividing the number of PCNA positive cells with the number of α-SMA positive cells from within the eight randomly selected fields. An average was then calculated
Data are presented as mean (standard deviation). To evaluate statistical differences between groups, a non-parametric U-test according to Wilcoxon, and Mann–Whitney was used, with P < 0.05 considered to be statistically significant. Statistical analysis was done using Statistical Package for Social Sciences (SPSS) software version 22.0 (SPSS®: Inc., Chicago, IL, USA).
Results
All animals tolerated radiation and continued to eat and drink. There were no premature deaths or weight loss.
Oral mucositis was observed in all animals (100%) following radiation (Table 1). The severity of mucositis directly correlated with strength of radiation with the greatest severity observed in Group D and the least in Group A. The severity scores ranged from 3 to 18 with a mean score of 10 across the groups. Mucositis was observed on average 2.25 days after the first dose of radiation and took on average 3 days to heal. Rabbits in Group D took the longest to recover from the mucositis (range 3-8 with a mean of 5 days). The OMAS score did not correlate with mean PCNA activity for any subgroup (P = 0.47). Histological H and E sections demonstrated mild to moderate inflammatory cell infiltration in all radiation groups. The greatest number of inflammatory cells was seen in Group D. In this group, parotid glands showed higher inflammatory infiltrates compared to submandibular glands (Figure 2).
Table 1.
Correlation of OMAS scale and response to different radiation doses.
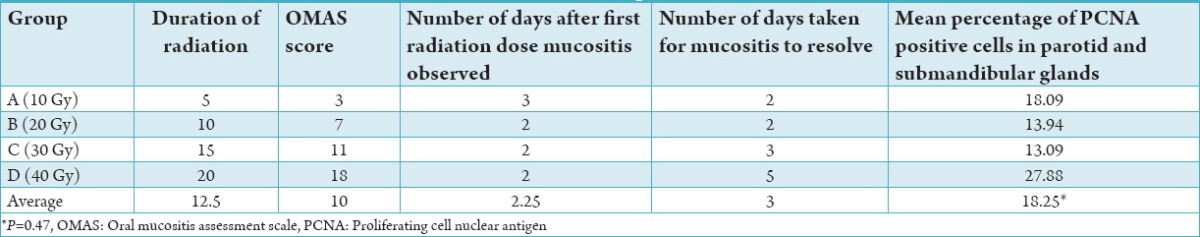
Figure 2.
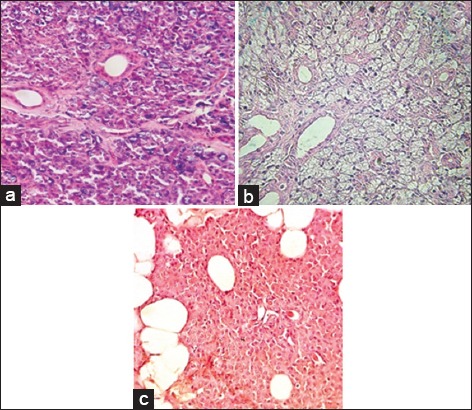
Haematoxylin and eosin sections of control (normal parotid gland) (a) and irradiated submandibular (b) and parotid 40 Gy (c) glands (40 Gy), (×200).
Graph 1 illustrates the mean percentage of PCNA-positive MECs in normal and irradiated salivary glands. Controls showed low mean MEC proliferative activity in both submandibular and parotid salivary glands; 9.56% and 8.36% respectively (P = 0.945).
Graph 1.
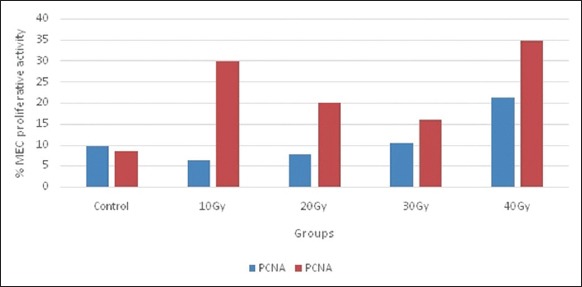
Mean percentage of proliferating cell nuclear antigen (PCNA) positive myoepithelial cells (MECs) in normal and irradiated salivary glands. MEC proliferative activity calculated as PCNA +ve cells/alpha smooth muscle actin antibodies +ve cells.
The highest mean percentage of PCNA-positive MECs was found in parotid salivary glands in Groups A and D (Figure 3) which were statistically different to other subgroups (P = 0.015) and normal parotid salivary tissue (P = 0.006).
Figure 3.
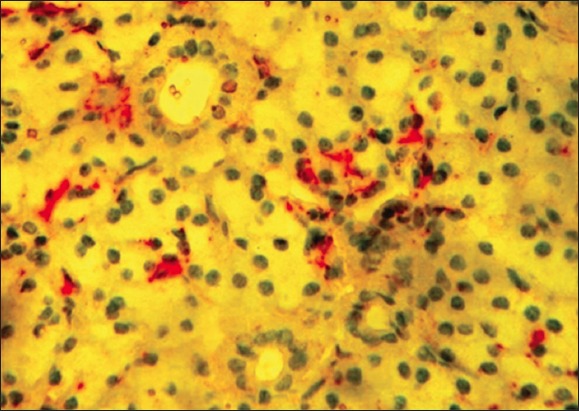
Double immunohistochemistry for proliferating cell nuclear antigen (brown) and actin (red) in the irradiated parotid gland (40 Gy) (×400).
Parotid Group D (40 Gy) showed higher mean% PCNA-positive MECs than other parotid subgroups (10, 20, 30 Gy). In addition, PCNA activity in parotid glands was significantly higher than submandibular glands, particularly in subgroups A (10 Gy; P = 0.001), B (20 Gy; P = 0.001), and D (40 Gy; P = 0.003).
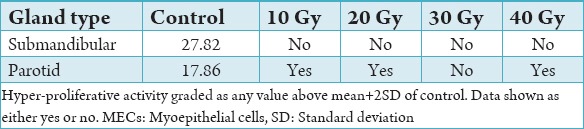
Hyper-proliferation of MECs was graded as a value higher than mean plus two standard deviations of controls. With this definition, hyper-proliferation of MECs was not observed in submandibular glands irrespective of radiation dose (Table 2a and b). Hyper-proliferation of MECs was observed in all parotid sub-groups except Group C (30 Gy).
Table 2a.
Mean proliferative activity of MECs against radiation doses.
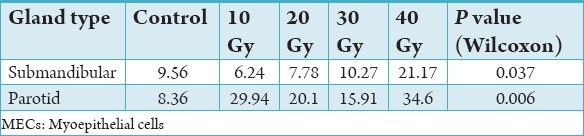
Table 2b.
Mean hyper-proliferative activity of MECs against radiation activity.
Discussion
Head and neck cancer patients receiving radiotherapy report poor quality of life. Salivary gland dysfunction and consequential mucositis hampers speech, causes difficulties with food mastication, swallowing, and taste.5,6,21 These impaired functions arise from direct acinar and ductal salivary cell damage. The present study suggests that salivary MECs play a limited or no role in the pathogenesis of radiation-induced mucositis but increase their proliferation activity which may highlight recovery processes. Despite holding integral roles in salivary gland function, the role of MECs after ionizing radiation is not well understood. We aimed in this study to evaluate the proliferative activity of these cells in a well-known animal model using PCNA antibodies as markers of proliferation. This approached was justified as the immunohistochemistry of PCNA has shown to be equivalent to Western blotting as a semi-quantitative method of assessing MEC proliferative activity.22 Furthermore, our protocol had been validated previously by Hakim et al.16
Our findings demonstrate that salivary MECs increase their proliferative activity after radiation. Moreover, MECs proliferative activity correlated with an increase in inflammation as shown through inflammatory infiltrates. This, however, did not correlate with the degree of mucositis or recovery from mucositis except at higher (40 Gy) doses. MEC hyper-proliferation was observed only in parotid glands. Though the MEC proliferative activity was dose-dependent, the proliferative activity in parotid glands at 30 Gy was <10 Gy and 20 Gy, with 10 Gy demonstrating higher activity than 20 Gy. However, at 40 Gy the mean proliferative activity increased once again. This observation may be explained by suggesting that the initial response of parotid MECs to ionizing radiation is to increase activity, however, as the radiation duration increases, the cells begin to trigger recovery processes. At larger doses, this recovery is hampered which increases proliferative activity further. The pro-inflammatory role of MECs has been characterized in chronic inflammatory conditions.23-25 It is unclear whether MEC proliferative activity was responsible for inducing a pro-inflammatory environment. As inflammatory infiltrates were seen in both gland types after ionizing radiation where only parotid glands significantly increased proliferative MEC activity; it would seem that proliferating MECs were not responsible for inducing inflammation.
We found hyper-proliferation of MECs in parotid glands only after exposure to radiation doses 10 Gy, 20 Gy, and 40 Gy. Parotid glands contain a higher number of serous cells which are more radiation-sensitive.26 Submandibular glands, on the other hand, contain a higher density of mucous cells.14 The results suggest that submandibular glands are more resistant to radiation. As parotid glands are more sensitive to radiation, increased MEC proliferate may act to compensate for the gross inflammation. The role of anti-inflammatories on MEC proliferative activity would be an interesting further study.
Mucositis was graded according to the OMAS scale by a blinded oral medicine specialist. Though the examiner was experienced in grading mucositis in humans, he lacked full training in grading this in rabbits. Furthermore, the OMAS scale has not been validated in animals. Using two blinded veterinary surgeons with experience in grading mucositis in rabbits would have added to the strength of the study. However, as no rabbits lost weight and continued to eat and drink throughout the study, it is likely that the recording of mucositis was accurate particularly as the main conclusions were that all rabbits recovered within 5 days.
Conclusion
Parotid MECs increase their proliferative activity after exposure to ionizing radiation which is almost dose-dependent. This observation is absent in submandibular glands. Oral mucositis is seen after radiation exposure which occurs as a result of inflammation. Recovery from mucositis is possible after radiation irrespective of dose. The pathogenesis of mucositis after radiation is not related to MECs proliferative activity.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Silva P, Homer JJ, Slevin NJ, Musgrove BT, Sloan P, Price P, et al. Clinical and biological factors affecting response to radiotherapy in patients with head and neck cancer: A review. Clin Otolaryngol. 2007;32(5):337–45. doi: 10.1111/j.1749-4486.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 5.Dirix P, Nuyts S, Van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: A literature review. Cancer. 2006;107(11):2525–34. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- 6.Deboni AL, Giordani AJ, Lopes NN, Dias RS, Segreto RA, Jensen SB, et al. Long-term oral effects in patients treated with radiochemotherapy for head and neck cancer. Support Care Cancer. 2012;20(11):2903–11. doi: 10.1007/s00520-012-1418-7. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JB, Emerton S, Kolbinson DA, Le ND, Phillips N, Stevenson-Moore P, et al. Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck. 1999;21(1):1–11. doi: 10.1002/(sici)1097-0347(199901)21:1<1::aid-hed1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: From animal models to therapies. J Dent Res. 2009;88(10):894–903. doi: 10.1177/0022034509343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malouf JG, Aragon C, Henson BS, Eisbruch A, Ship JA. Influence of parotid-sparing radiotherapy on xerostomia in head and neck cancer patients. Cancer Detect Prev. 2003;27(4):305–10. doi: 10.1016/s0361-090x(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SC, Wu VW, Kwong DL, Ying MT. Assessment of post-radiotherapy salivary glands. Br J Radiol. 2011;84(1001):393–402. doi: 10.1259/bjr/66754762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res. 1997;3(11):1949–58. [PubMed] [Google Scholar]
- 12.Savera AT, Zarbo RJ. Defining the role of myoepithelium in salivary gland neoplasia. Adv Anat Pathol. 2004;11(2):69–85. doi: 10.1097/00125480-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ. Myoepithelial cells: Their origin and function in breast morphogenesis and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10(3):261–72. doi: 10.1007/s10911-005-9586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ianez RF, Buim ME, Coutinho-Camillo CM, Schultz R, Soares FA, Lourenço SV. Human salivary gland morphogenesis: Myoepithelial cell maturation assessed by immunohistochemical markers. Histopathology. 2010;57(3):410–7. doi: 10.1111/j.1365-2559.2010.03645.x. [DOI] [PubMed] [Google Scholar]
- 15.Ihrler S, Blasenbreu-Vogt S, Sendelhofert A, Rössle M, Harrison JD, Löhrs U. Regeneration in chronic sialadenitis: An analysis of proliferation and apoptosis based on double immunohistochemical labelling. Virchows Arch. 2004;444(4):356–61. doi: 10.1007/s00428-003-0964-2. [DOI] [PubMed] [Google Scholar]
- 16.Hakim SG, Kosmehl H, Lauer I, Nadrowitz R, Wedel T, Sieg P. The role of myoepithelial cells in the short-term radiogenic impairment of salivary glands. An immunohistochemical, ultrastructural and scintigraphic study. Anticancer Res. 2002;22(6C):4121–8. [PubMed] [Google Scholar]
- 17.National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 18.Hakim SG, Benedek GA, Su YX, Jacobsen HC, Klinger M, Dendorfer A, et al. Radioprotective effect of lidocaine on function and ultrastructure of salivary glands receiving fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;82(4):e623, 30. doi: 10.1016/j.ijrobp.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Houweling AC, Philippens ME, Dijkema T, Roesink JM, Terhaard CH, Schilstra C, et al. A comparison of dose-response models for the parotid gland in a large group of head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2010;76(4):1259–65. doi: 10.1016/j.ijrobp.2009.07.1685. [DOI] [PubMed] [Google Scholar]
- 20.Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH, Jr, Mulagha MT, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer. 1999;85(10):2103–13. doi: 10.1002/(sici)1097-0142(19990515)85:10<2103::aid-cncr2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):213–25. doi: 10.1177/154411130301400306. [DOI] [PubMed] [Google Scholar]
- 22.Sternlicht MD, Barsky SH. The myoepithelial defense: A host defense against cancer. Med Hypotheses. 1997;48(1):37–46. doi: 10.1016/s0306-9877(97)90022-0. [DOI] [PubMed] [Google Scholar]
- 23.Burgess KL, Dardick I. Cell population changes during atrophy and regeneration of rat parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):699–706. doi: 10.1016/s1079-2104(98)90038-5. [DOI] [PubMed] [Google Scholar]
- 24.Barsky SH, Karlin NJ. Myoepithelial cells: Autocrine and paracrine suppressors of breast cancer progression. J Mammary Gland Biol Neoplasia. 2005;10(3):249–60. doi: 10.1007/s10911-005-9585-5. [DOI] [PubMed] [Google Scholar]
- 25.Gudjonsson T, Rønnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scovassi AI, Prosperi E. Analysis of proliferating cell nuclear antigen (PCNA) associated with DNA. Methods Mol Biol. 2006;314:457–75. doi: 10.1385/1-59259-973-7:457. [DOI] [PubMed] [Google Scholar]


