Abstract
Neuromotor therapy after spinal cord or brain injury often attempts to utilize activity-dependent plasticity to promote functional recovery. Neuromuscular electrical stimulation that activates paralyzed or paretic muscles may enhance passive assistance therapy by activating more muscle mass and enriching the sensory pattern with appropriately timed muscle spindle activation. To enable studies of activity-dependent plasticity, a rodent model for stimulation-assisted locomotor therapy was developed previously. To be effective, however, such a system must allow lengthy sessions of repetitive movements. In this study, we implemented an adaptive pattern generator/pattern shaper (PG/PS) control system for a rodent model of neuromotor therapy and evaluated its ability to generate accurate and repeatable hip movements in lengthy sessions by adjusting the activation patterns of an agonist/antagonist muscle pair. In 100-cycle movement trials, the PG/PS control system provided excellent movement tracking (<10% error), but stimulation levels steadily increased to account for muscle fatigue. In trials using an intermittent movement paradigm (100 sets of five-cycle bouts interspersed by 20-s rest periods), excellent performance (<8% error) was also observed with less stimulation, thus indicating reduced muscle fatigue. These results demonstrate the ability of the PG/PS control system to utilize an agonist/antagonist muscle pair to control movement at a joint in a rodent model. The demonstration of repeatable movements over lengthy intermittent sessions suggests that it may be well suited to provide efficient neuromotor therapy.
Index Terms: Activity-dependent plasticity, adaptive neural network control, intramuscular electrodes, locomotor therapy, neuromuscular stimulation, spinal cord injury (SCI)
I. Introduction
Physical therapy that uses passive movements or treadmill training has been shown to promote activity-dependent plasticity in spinal circuitry, thus enhancing motor recovery after neuromotor damage caused by spinal cord injury (SCI) [1]–[4], stroke [5], or traumatic brain injury [6]. Evidence suggests that appropriately timed neuromuscular electrical stimulation of paralyzed or paretic muscles can also enhance the speed and efficiency of walking in people with incomplete SCI [7]–[9]. Rehabilitative movement therapy can affect change from the cellular to the systems level [3]. Spinal cord and cortical circuitry changes are observed with use of electrical stimulation to elicit movement patterns [10], and electrical stimulation of peripheral nerves can trigger upregulation of cellular neurotrophic factors that promote axonal regeneration [4].
The rodent model, which has been widely used in a broad range of studies on neurotrauma and assessment of role of exercise therapy in enhancing sensorimotor recovery [11]–[14], could play an important role in gaining a better understanding of the neurophysiological mechanisms underlying neuromuscular stimulation-assisted therapy and could help to determine the most effective protocols for this therapeutic paradigm. In a previous study, we had developed a rodent model for neuromuscular stimulation-assisted therapy [15], [16] that used an open-loop feed forward controller to stimulate the hip flexor and extensor muscles in awake incomplete SCI rodents to produce bilateral coordinated movement of unloaded hindlimbs. The usefulness of the therapy system was limited, however, because the open-loop control system was not able to generate an accurate desired movement pattern nor was it able to adjust to account for muscle fatigue. In extended neuromotor retraining sessions, it will be particularly important to generate a suitable movement pattern in a repeatable fashion. In the current paper, we addressed these shortcomings by implementing an adaptive control system in the rodent model.
The adaptive pattern generator/pattern shaper (PG/PS) control system was developed to control cyclic movements using neuromuscular stimulation [17], and prior studies in human subjects demonstrated its ability to control cyclic limb movements in a paradigm in which a single muscle acts against gravity [18], [19]. For the proposed application in motor retraining, it will also be necessary for the control system to effectively utilize agonist/antagonist pairs of muscles and generate repeatable movements over extended trials with intermittent rest periods, since this is the paradigm that is most likely to be used in therapy sessions either in the clinic or in the research laboratory. Furthermore, since this study is focused on the development of the rodent model for this type of therapy, it is important to document the ability of the PG/PS system to control movements by activating lower limb muscles using implanted electrodes.
Therefore, in this paper, we have implemented the PG/PS control system in a rodent model and evaluated its ability to use agonist/antagonist muscle activation to control cyclic movements of the hip. Furthermore, we have characterized the ability of the controller to work in an intermittent movement paradigm (groups of movement cycles interspersed with rest periods), which will enable its use in lengthy neuromotor retraining sessions. In the evaluations, we have also implemented an open-loop stimulation paradigm because it is currently the most widely used system in the clinic and research laboratories and because it provides a context in which the performance of the adaptive controller can be characterized.
II. Methods
A. Surgical Preparation and Experimental Setup
We report data obtained from six hindlimbs from four intact, adult, female Long–Evans rats weighing 250–300 g (Table I). All procedures followed guidelines established by the Institutional Animal Care and Use Committee of Arizona State University. Prior to surgery, animals were anesthetized by administering sodium pentobarbital (35 mg/kg, i.p.) supplemented with 1%–1.5% isoflurane. The level of anesthesia was monitored by toe pinch, observation of respiration rate, and eye blink, and was maintained with 1%–1.5% isoflurane for the duration of the experiment. Although the effects of anesthesia on muscle strength remain controversial [20], in our study, we did not observe discernible effects of anesthesia on the ability to stimulate muscle and the stimulated responses were qualitatively similar to those observed in awake rodents and human subjects in other studies in our laboratories. Two cubic centimeters of 0.9% sodium chloride was also administered every 2 h to avoid dehydration. Monopolar intramuscular stimulating electrodes were implanted adjacent to the motor points of the hip extensor [biceps femoralis–hip (BFh )] and hip flexor [iliapsoas (IL)] muscles. In the rodent, BFh (anterior head) arises from the last sacral and the first caudal vertebrae, and inserts into the distal end of the femur; thus, it is a uniarticular hip extensor. The electrode wire was anchored in place using the retaining disks, and both disks were sutured to the surface of the muscle membrane using 5-0 absorbable suture (Dexon II; United States Surgical, Tyco Healthcare, Mansfield, MA) and the skin was sutured. A needle electrode was inserted under the skin in the back for use as a reference electrode. The stimulating electrodes were custom-made using Teflon-coated stainless steel wire (AS632-50-μm multistranded, Cooner Company, Chatsworth, CA), nonabsorbable polyurethane suture, and retaining disks. The electrode was based on previous designs that have been described in greater detail [21].
Table I.
Summary of Stimulation and Output Settings From Experiments
| Hindlimb ID | Animal ID | Side | Initial Minimum Twitch Threshold (mA) | Range of Desired Movements (Degrees) | ||||
|---|---|---|---|---|---|---|---|---|
| ‘Continuous’ movement paradigm | ‘Intermittent’ movement Paradigm | |||||||
| Flexor | Extensor | Flexion | Extension | Flexion | Extension | |||
| A | 1 | Left | 0.45 | 1.25 | 10 | 4 | 7 | 4 |
| B | 2 | Left | 1.2 | 1 | 10 | 5 | 10 | 5 |
| C | 2 | Right | 1 | 0.3 | 8 | 3 | 8 | 3 |
| D | 3 | Left | 0.3 | 0.5 | 7 | 5 | 7 | 5 |
| E | 3 | Right | 0.8 | 0.4 | 7 | 7 | 12 | 6 |
| F | 4 | Right | 0.55 | 0.4 | 10 | 8 | 10 | 8 |
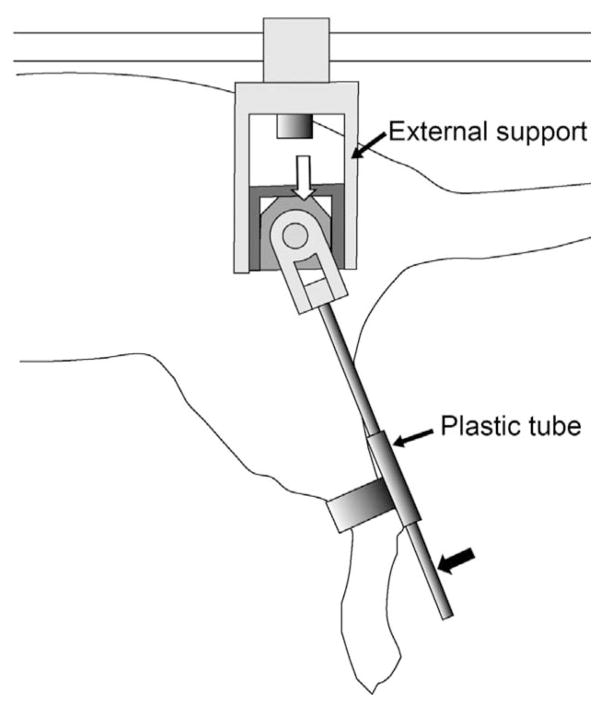
The rats were then placed on a horizontal base plate that was mounted on a custom frame placed on a table. The free ends of the implanted electrodes were connected to an isolated pulse stimulator (FNS16-CWE, Inc., Ardmore, PA) that was controlled by a PC using custom-designed software (LabVIEW, National Instruments, Inc., Austin, TX). In order to measure leg angle, an external rotary angle sensing potentiometer (Digikey, PVS1A103A01) was used. A small holder was custom-designed and built to attach the potentiometer and a rotating rod to the suspended hindlimb, as shown in Fig. 1. For real-time measurements of sagittal plane leg angle, the center of the potentiometer was aligned with the hip joint and the rotating rod passed through a plastic sleeve that was attached proximal to the ankle of the hindlimb. In this arrangement, hip flexion–extension movements caused rotation of the potentiometer and the sliding movement of the rod within the sleeve allowed for pure hip measurements. The potentiometer offered little resistance to rotation and components attached onto the leg weighed approximately 1 g, and therefore, were unlikely to constitute a significant load on the leg. The potentiometer was configured as a voltage divider and the output voltage was sampled, on request by the controller (at 25 Hz), by a PC using a DAQ board (model PCI-MIO-16E-1, National Instrument, Inc.) to provide real-time measurements of leg angle. In previous experiments [15], fused contractions on stimulation of rat hindlimb muscles were observed at 75 Hz stimulus frequency within a pulsewidth range of 10–100 μs. Therefore, electrical stimulation consisted of a cathodic-first, charge-balanced, 40 μs per phase, constant-current biphasic waveform delivered at 75 Hz. The adaptive control system used in this study to activate hip movements updated the stimulation current pulse amplitude at 25 Hz. Thus, the stimulation current input value was updated at every three pulse intervals. This reduction in update rate was used to accommodate the timing lag in serial communication between the computer and the stimulator. The measurement of leg angle was also done at 25 Hz.
Fig. 1.
Schematic drawing of the external angle sensor attached to a rodent hindlimb. A small rotary potentiometer (indicated by white arrow) is encased in an external support. A rigid pole (indicated by solid arrow) rotates about the center of the potentiometer that is aligned with the hip joint and connected to the hindlimb through a plastic tube attached just above the ankle. The rotating pole has an additional rotational degree of freedom (rotating in the coronal plane) to allow for unrestricted abduction/adduction movements.
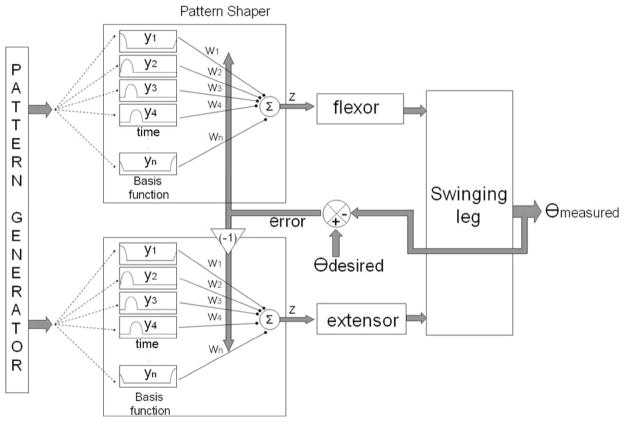
B. PG/PS Controller
The operation of the PG/PS control system, which has been described in detail in previous papers [17], [22], is summarized here. The control system consists of two major components: the PG and the PS, as shown in Fig. 2. The role of the PG is to provide general timing information for producing a given cyclic movement, i.e., it provides an oscillatory signal at the desired movement frequency. This signal is adaptively customized by the PS to determine the specific stimulation pattern used to activate the muscle. In this study, a fixed-frequency desired cyclic movement has been defined; hence, the PG is the same for all the animals, and therefore calculations for the PG were not performed in real time [23]. The PS is a single-layer neural network with each neuron output in the shape of a raised cosine waveform, thus forming a set of basis functions. The number of basis functions (neurons in the neural network) was set to be equal to the number of time steps across a desired movement period and the basis function outputs were time-shifted with respect to each other (Fig. 2). The network parameter na determines the width of the individual basis functions; each basis function was set to have activity spread (nonzero values) over na time steps. In this study, na was set to 3, 5, or 7 time steps. No performance differences were apparent between the three time-step settings. In this coarse-coding technique, each basis function contributes to the stimulation levels at more than one time step. Since the period of a single desired cyclic movement was set to 960 ms and the controller update frequency was 25 Hz (time step T = 40 ms), the number of basis functions was set to be 24 (=960/40) so that one basis function reached its peak activity level during each time step in a movement period. The controller output of the PS at each time step, which is denoted by z(t), is a weighted summation of the basis functions
| (1) |
where yj (t) is the output of the basis function j at time t, m is the number of basis functions, and wj (t) is the output weight for basis function j. The network output value z(t)—which has an output range from 0 to 1—is scaled by the maximum stimulation intensity (stimulus current amplitude) for each channel.
Fig. 2.
Diagram of the adaptive PG/PS control system. The controller determines the stimulation output current based on the error signal (θdesired – θmeasured ) to adapt the output weights in the PS neural network. The basis function outputs are raised cosine waveforms, time-shifted with respect to each other and spread over na time steps (where na = 3, 5, or 7). In this coarse-coding technique, each basis function contributes to the stimulation level at more than one time step. The number of basis functions was set to the number of time steps across a desired movement period. The error term that went to the antagonist was negative of the error term for the agonist. The PG was not implemented in real time in this study, and therefore, the PS basis function outputs were periodic and stored in a lookup table for access during the PS calculations of each controller iteration.
The PS modifies the output weights wj (t) in order to scale the contribution of each basis function to the PS output time series. Given the inherent delays between muscle stimulation and movement, an error in movement pattern at a given time can be attributed to the errors in past stimulation values. Therefore, the PS learning algorithm uses the error at the current time step to change the weights on basis functions that were active at prior time steps
| (2) |
where Δwj (t) is the change in output j of the PS network, η is a constant learning rate, e(t) is the error measured between the desired and measured angle, T is the controller update period, and n is the number of past activation values used by the algorithm. In this study, η = 0.002 and n was set at either 7 or 9. These values were guided by a previous simulation study [22]. Since this study required control of two muscles (agonist and antagonist), the learning algorithm was implemented with one PS per muscle to be stimulated. The error signal for the antagonist was defined as the negative of the error signal for the agonist, since their effects on movement are in opposite directions.
C. Experimental Protocol and Data Analysis
In separate trials, an open-loop and an adaptive PG/PS control system were each used to control cyclic movement of the swinging hindlimb. A template movement pattern for the angular hip rotation was developed based on prior data on hip joint angle kinematics obtained during treadmill walking by age- and weight-matched intact rodents [24], but the excursion range of the template pattern was adjusted for different hindlimbs based on initial measurements of peak movement amplitude. The period of one complete cycle of movement was set at 960 ms to approximate a slow walking pace for the rat. Initially, the minimum amplitudes of 40-μs monophasic current pulses required to elicit a twitch in the BFh, and IL muscles were determined. Swinging movements for five testing cycles were generated using an open-loop control system by stimulating the BFh, and IL muscles with pulse trains at 1.5 or 1.8 times the minimum twitch threshold current value for that channel. The maximum range of angular excursion was recorded during these testing cycles, and a desired movement pattern for each leg was created by scaling the template pattern by the recorded maximum angular value. Table I summarizes the initial twitch thresholds and the amplitudes of the desired pattern for each leg tested in this study. The stimulation current amplitudes delivered to the BFh, and IL muscles when testing the five cycles were termed the “open-loop amplitudes” of BFh, and IL, respectively. While the adaptive PG/PS control system automatically customized the stimulation current pulse amplitudes, the open-loop control system used the fixed-amplitude settings. The open-loop control system was set to alternate activation of the hip flexors and extensors by stimulating the IL muscle for 550 ms and the BFh, muscle for 410 ms during a trial.
Each hindlimb was tested in two types of stimulation paradigms: 1) an uninterrupted movement paradigm with 100 cycles of alternating flexor/extensor stimulation (96 s of movement with no rest periods) and 2) an intermittent movement paradigm with 500 cycles divided into groups of five cycles of alternating flexor/extensor stimulation (4.8 s of movement) interspersed with 20-s rest periods (for a total of 480 s of stimulation in a trial that lasted 2460 s).
This second paradigm was designed to mimic a paradigm that could be useful in the clinic to achieve long therapeutic sessions by providing intermittent rest periods to delay the onset of muscle fatigue, since stimulated muscles tend to fatigue very rapidly [25]. In a stimulation-assisted neuromotor retraining paradigm, this fatigue could limit the length of each therapy session, and therefore, may reduce the efficacy of the treatment. In the clinic, therapists often intersperse rest periods between bouts of movement therapy in an attempt to administer sufficient number of movement repetitions. Therefore, the intermittent movement paradigm was designed to evaluate the ability of the adaptive PG/PS control system to generate cyclic movements in a therapeutic paradigm that included longer trials with rest periods interspersed. The motivation of this evaluation was to examine how much the rest periods in an intermittent trial would disrupt tracking performance during the first few movement cycles after the rest period. The intermittent pattern of five-cycle movement bouts and 20-s rest periods was selected after initial experiments demonstrated that this paradigm did not induce the rapid fatigue observed in the uninterrupted movement paradigm, thus allowing a larger number of movements cycles within a training session.
Both paradigms were tested with the open-loop and the adaptive PG/PS control systems. Open-loop control trials always preceded the PG/PS control trials, and testing with an uninterrupted paradigm always preceded the intermittent paradigm testing. By using this sequence, the results from the uninterrupted paradigm were the least affected by cumulative muscle fatigue. There was also a 1-h rest period before every trial to allow the muscles to recover from cumulative fatigue. In the intermittent movement paradigm, a desired movement pattern was created such that the starting point of the pattern was matched with the neutral (free hanging) leg position. During the cyclic movements generated by electrical stimulation, the hip joint angles and stimulation current pulse amplitudes were recorded. Open-loop control systems are often used in the clinic and research laboratories to provide electrical stimulation for movement therapy; we compared the performance of the PG/PS control system with an open-loop control system used in a similar intermittent paradigm.
In order to evaluate tracking performance, the percent rms error between the actual joint angle and the desired movement pattern was calculated as a percent of the movement range. To quantify the level of stimulation input during a given movement cycle, the electric charge (in coulombs) delivered to muscles per cycle of movement was calculated as the total summation of the product of width and amplitude of the cathodic phases of the pulses during a cycle.
III. Results
A. Hip Movements Evoked by Electrical Stimulation Using Open-Loop or Adaptive PG/PS Control System
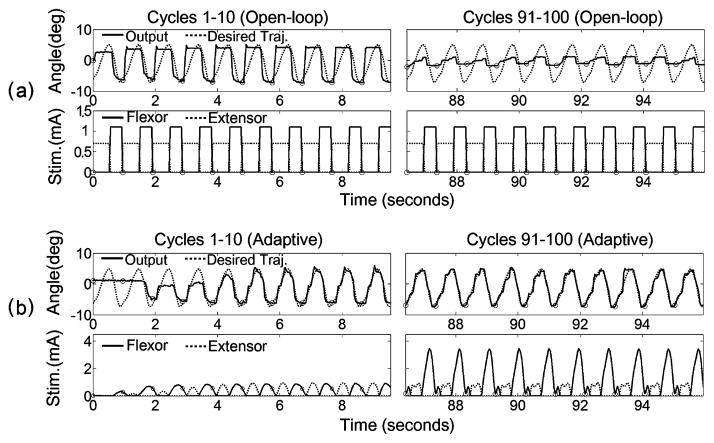
Fig. 3 shows the first and last ten cycles of movement in a single 100-cycle trial evoked by the open-loop and adaptive control systems in the same hindlimb. The envelopes of the stimulation current pulse amplitudes delivered to the hip flexor and extensor muscles during the first and the last ten cycles are also shown. As shown in Fig. 3(a), the open-loop control system, which used fixed stimulation current pulse amplitudes, poorly approximated the desired movement, but it successfully elicited the full range of desired output at the beginning of the trial. However, as the trial progressed, the movement range lowered substantially, presumably due to muscle fatigue. Unlike the open-loop control system, the adaptive PG/PS control system customized the stimulation current pulse amplitudes based on the error between desired and measured output angles. Consequently, the PG/PS control system was able to track the desired movement pattern well after an initial adaptation period, and this tracking performance was maintained throughout the remaining cycles of the trial [Fig. 3(b)]. Note that while the PG/PS control system optimally adjusted the stimulation current pattern to obtain the desired movement pattern, it automatically increased the stimulation current pulse amplitudes in order to maintain the same degree of contraction as the muscles fatigued.
Fig. 3.
Example of open-loop and PG/PS adaptive control outputs and stimulation levels for hindlimb D. (a) Open-loop stimulation pattern (bottom) and movement pattern (top) during the first ten cycles and the last ten cycles of a single 100-cycle trial. (b) PG/PS adaptive control system stimulation pattern (bottom) and movement pattern (top) during the first ten cycles and the last ten cycles of the 100-cycle trial. The top rows of (a) and (b) show desired (dotted) and measured angle output (solid) trajectories and the bottom rows show stimulus current pulse amplitude patterns delivered to the muscles. The horizontal axis shows time (in seconds); the vertical axis shows joint angle (in degrees) with hip extension defined as positive. The small circles indicate the onset of a cycle. The stimulation current pulse pattern is the envelope of the stimulation current pulse amplitude updated by the controller at 25 Hz for the PG/PS control system, whereas the open-loop control system used predetermined stimulus current pulse amplitude envelopes. These plots demonstrate that the open-loop control system achieved good movement excursion at the beginning of the trial, but this was substantially reduced toward the end; the adaptive control system quickly learned to achieve very good movement tracking, and this was maintained through the end of the trial by automatically adjusting the stimulation patterns.
B. Tracking Performance of Adaptive Control System
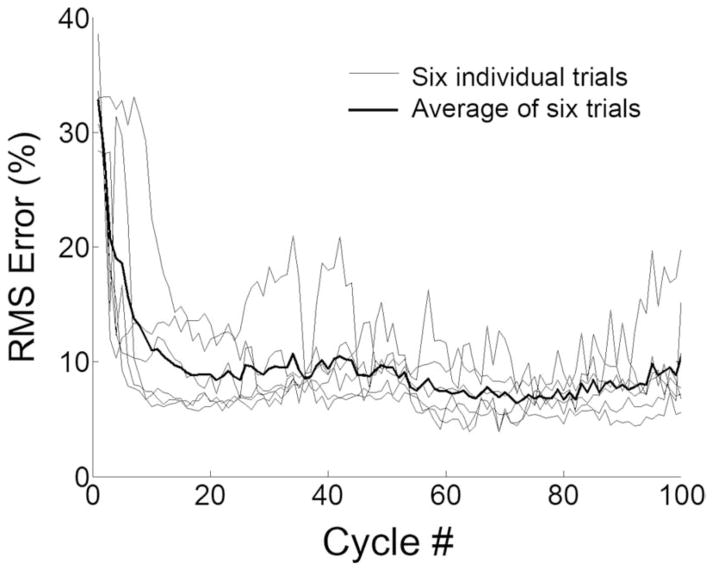
Fig. 4 shows the percent rms error (per cycle) from the six hindlimbs when tested for the 100-cycle stimulation paradigm using the adaptive PG/PS control system. On average, the PG/PS control system rapidly adjusted the stimulation to achieve tracking errors below 10% by approximately cycle 15, and then maintained or improved upon that performance throughout the rest of the trial.
Fig. 4.
Tracking performance of the PG/PS adaptive control system obtained from all animals. The percent rms error of angle output from each cycle of all six trials for the PG/PS control system. The mean percent rms error across these trials is indicated by the thick line. The horizontal axis shows cycle number. This plot demonstrates that the adaptive control system rapidly achieved and maintained good performance (low tracking error) in each of the six trials.
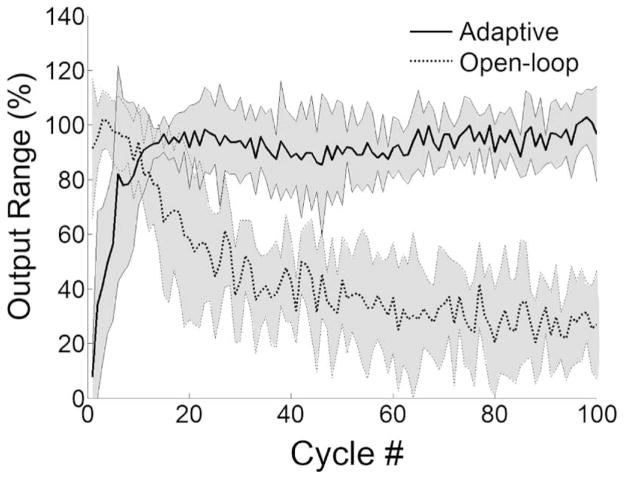
The movement range per cycle averaged for all hindlimbs (±1 standard deviation) for the open-loop and the adaptive control are shown in Fig. 5. As the trial progressed, the open-loop control system was not able to generate sufficient contraction. Thus, there was a relatively quick reduction in movement range during the first 30 cycles followed by a gradual reduction to less than 30% by the end of trial, while the PG/PS control system maintained good movement range throughout the trial.
Fig. 5.
Changes in movement range per cycle ± 1 standard deviation averaged across all trials for the PG/PS adaptive and open-loop control systems. The output range is given as a percent of the maximum range of the desired movement pattern. The horizontal axis shows cycle number. This plot demonstrates that the open-loop control system achieved good movement excursion at the beginning of the trial, but it rapidly decreased to less than half of the desired movement range; the adaptive control system rapidly learned to achieve a good movement range and maintained this performance throughout the trial.
C. Intermittent Movement Paradigm for Extended Stimulation
Fig. 6 shows the movement pattern during the first, the middle, and the last set of five cyclic movements for a single intermittent trial. These plots demonstrate that after adapting over the first few sets of cycles, the adaptive PG/PS control system was able to accurately produce the desired movement pattern throughout the entire duration of the intermittent trial.
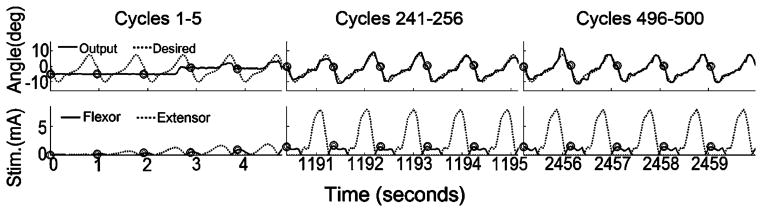
Fig. 6.
Example of PG/PS control system output (hindlimb F) in an intermittent training paradigm: angle output trajectories (top) and stimulus current amplitude patterns (bottom). Plot details as in Fig. 3. The first column shows output for the first set of five movement cycles (cycles 1–5), the second column shows a middle set (cycles 241–245), and the third column shows the 100th set (cycles 496–500). The intermittent training paradigm used sets of five movement cycles (4.8 s stimulation) interspersed with 20 s of rest. These plots demonstrate that the adaptive control system achieved and sustained good movement tracking throughout the trial.
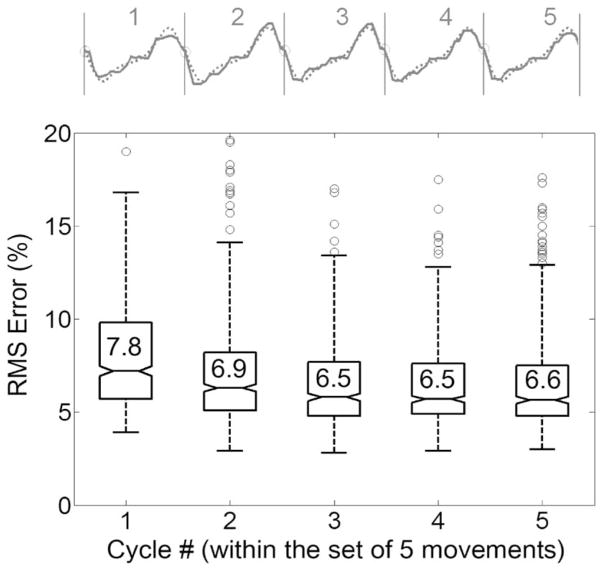
For the intermittent movement paradigm, the adaptive PG/PS control system retained the neural network weights during the rest interval. This feature of the controller may be advantageous in tracking a desired movement pattern when the next set of movement cycles is initiated. However, muscle properties could change during the stimulation rest period, thus leading to poor movement tracking when stimulation is resumed. Fig. 7 shows the percent rms error of each cycle (1–5) within a set of intermittent movements, averaged across all sets of five movements and also across all six intermittent training trials (from six hindlimbs). Although the rms error for the first cycle of the intermittent sets tended to be larger than those for the remaining cycles, the mean rms error was maintained at lower than 8% during the course of intermittent trials. These data indicate that the rest periods utilized in the intermittent trials did not affect the ability of the PG/PS control system to accurately control cyclic movements.
Fig. 7.
Tracking performance of the PG/PS adaptive control system for the intermittent training paradigm. Box plots of the percent rms error resulting from each cycle within the sets of five cycles during the intermittent movement trial are shown. The raw movement data (solid) and desired movement (dotted) from one representative set of five cycles are also presented. The rms error values for each cycle were averaged across all five-cycle sets except for the first four sets of each of the six intermittent trials. The mean rms error for each cycle number within the five-cycle set is indicated inside the box. The box represents the interquartile range that contains 50% of the values and a line across the box indicates the median. The whiskers represent 1.5× the interquartile range and outliers (data beyond the ends of the whiskers) are represented as circles. The rms error for the first cycle of the movement sets (i.e., the one just after the rest period) tended to be larger than those for the remaining cycles. The mean difference in the rms errors between the first cycle of the next set and the last cycle of the previous set was 1.1% ± 2.7%.
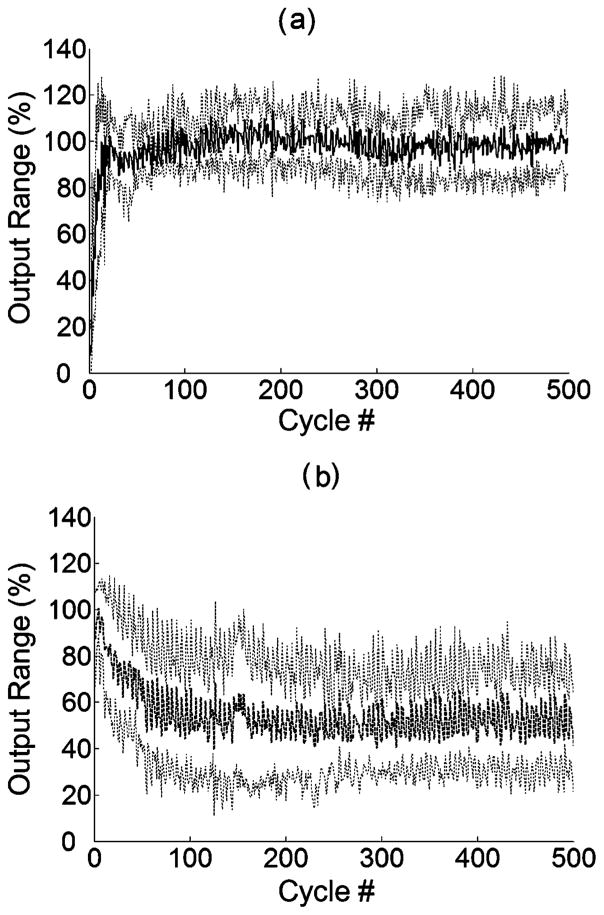
The intermittent movement paradigm was also tested with the open-loop control system. Fig. 8(a) and (b) shows the changes in mean maximum output range for six trials (± 1 standard deviation) from the adaptive PG/PS and the open-loop control systems, respectively. While the PG/PS system still achieved the maximum range of the desired movement pattern throughout the trial, the open-loop system using the fixed stimulation current amplitudes was not able to produce the desired movement range as the trial progressed.
Fig. 8.
Changes in maximum output range of cyclic movement ±1 standard deviation averaged across all trials for (a) the PG/PS adaptive and (b) the open-loop control system during a 500-cycle movement from an intermittent training paradigm. The intermittent movement trial consists of 100 sets of stimulation of five movement cycles (4.8 s stimulation) interspersed with 20 s of rest (500 cycles, 8 min stimulation in total). The horizontal axis shows only the successive stimulating cycle number and the output range is given as a percent of the maximum range of the desired movement pattern.
D. Stimulation Levels Delivered by PG/PS Control System
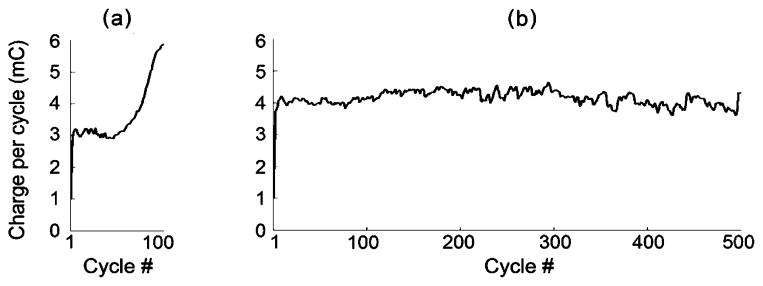
Fig. 9 shows an example of the electrical charge per cycle delivered to the flexor as a function of cycle number for the continuous and the intermittent movement paradigm from hindlimb B using the adaptive PG/PS control system. While the charge per cycle steadily increased throughout the continuous trial, the charge per cycle remained constant during the latter stages of the 500 movement cycles in the intermittent trials. Although the intermittent trials consisted of five times as many movement cycles, they utilized less stimulation per cycle than in the continuous stimulation paradigm (25% less in the trials shown). The plateau in this plot from the intermittent trial indicates that the adaptive control system was able to repeatedly generate the desired movement without inducing further fatigue. This feature of the adaptive control system when used in an intermittent movement paradigm could enable long and effective therapy sessions.
Fig. 9.
Example of charge delivered per cycle by the adaptive control system to the flexor (hindlimb B) during (a) continuous (100 cycles—ON) and (b) intermittent movement trials (100 sets of five movement cycle bouts with 20-s rest periods interspersed). The charge per cycle was calculated as the total summation of the product of width and amplitude of the cathodic phases of the pulses during a cycle. The maximum stimulation current pulse amplitudes at the end of the continuous and intermittent trials were 5.2 and 3.9 mA, respectively. Note that the charge per cycle steadily increased throughout the continuous trial, but reached a plateau and was sustained during the intermittent trials. These plots suggest that fatigue increased throughout the latter half of the continuous movement trial but that the muscle fatigue had reached a plateau during the intermittent trial.
IV. Discussion
The most important results of this study are: 1) the ability of the adaptive PG/PS system to utilize an agonist/antagonist pair of muscles to control cyclic movements, which expands the repertoire of movements that this controller can achieve; 2) the ability of the adaptive PG/PS system to control movements during an intermittent training paradigm, which increases its potential utility in the clinic and in research laboratories; and 3) the ability of the adaptive PG/PS system to use implanted electrodes to control movements in the rodent model, which makes it a potentially useful tool in animal studies designed to investigate the mechanisms that underlie electrical stimulation-assisted neuromotor therapy. Furthermore, this study provides additional evidence from studies on physiological systems of the ability of this adaptive control system to automatically determine an appropriate stimulation pattern to generate a specified movement in an individual and automatically adjust stimulation patterns to account for muscle fatigue. Each of these points is further discussed below.
In prior tests on human subjects, the PG/PS control system had been used to control isometric muscle force [23] and cyclic limb movements by stimulating a single muscle that acted against gravity [18], [19]. In the present study in the rodent model, the controller utilized an agonist/antagonist pair of muscles to generate leg movements. This capability of the PG/PS system is particularly important, since most activities involve reciprocal activation of agonist/antagonist pairs of muscles. Such a reciprocal activation pattern is similar to the pattern observed under physiological activation [24]. The controller iteratively establishes a customized mapping for each muscle acting at the joint by adapting the weights of its PS unit. The asymmetry in agonist/antagonist activation patterns is evident in the stimulation profiles shown in the bottom row of plots in Fig. 3(b). At the end of the trial, the PG/PS control system generated the movement by providing higher levels of stimulation to the hip flexor than the hip extensor. Furthermore, these results also demonstrate that the PG/PS control system can account for fatigue when using the agonist/antagonist arrangement. This feature is particularly important because muscles fatigue at a higher rate with electrical stimulation than with physiological activation, primarily due to the fact that the larger more fatigable fibers have a lower activation threshold when using electrical stimulation [26] and that electrical stimulation frequencies are typically higher than physiological rates in order to achieve a fused contraction with synchronous muscle fiber activation. With open-loop control, even if the specified stimulation levels were capable of generating the desired movement excursion at the beginning of the trial, movement range rapidly degraded [as shown in Figs. 3(a), 5, and 8(b)], presumably due to muscle fatigue. Although the rate of muscle fatigue is different between the continuous and intermittent trials [Figs. 5 and 8(b)], this rapid reduction in performance observed for all hindlimbs may also be attributed to the fact that in the rat, the stimulated hindlimb muscles are composed mainly of fast twitch (fast fatigable) fibers [27]. The PG/PS control system accounted for the muscle fatigue by adjusting agonist/antagonist stimulation levels to maintain good movement range throughout the trials.
In the clinic and the research laboratory (both in animal and human studies), exercise or therapy sessions typically involve bouts of activity interspersed with rest periods [2], [8], [13], [28]. The rest periods are introduced to reduce the overall rate of fatigue, and therefore, increase the total number of repetitions that can be completed within a session. This exercise/therapy paradigm with intermittent rest periods could possibly interfere with the accurate tracking performance during the first few cycles following the rest period because the alternation of fatigue/recovery could produce oscillatory changes in muscle properties that could induce instabilities in the adaptation process. In particular, if adaptation is too fast (i.e., if the learning rate is too high), the control system may overreact to changes in system properties in a manner that would be compounded by alternating fatigue/recovery. The results shown here indicate that the controller always responded in a stable manner and that performance was only minimally affected by the rest periods of the intermittent paradigm [Figs. 7 and 8(a)]. The rms error from the first cycle of every set tended to be only slightly higher than the error from the last cycle of the previous set (Fig. 7). This change in rms error is presumably in part due to slight changes in muscle properties during the resting intervals. However, the degradation in performance was minimal (1.1%), thus demonstrating that the rest periods introduced in the intermittent movement paradigm did not adversely affect the adaptive processes and that the PG/PS control system can function properly in this paradigm. In addition, as shown in Fig. 9(b), while the charge per cycle steadily increased throughout the shorter continuous trial, the total amount of charge per cycle was contained for the longer intermittent trial, hence indicating the absence of significant cumulative fatigue in the intermittent paradigm trials.
In prior studies controlling cyclic movements, the PG/PS control system was tested in a paradigm that used transcutaneous electrical stimulation (surface electrodes) to activate the quadriceps to generate movements of the lower leg against gravity. When used in a chronic rodent model of electrically stimulated neuromotor therapy, there are differences that could affect control system performance: 1) implanted electrodes must be used, which typically have recruitment characteristics that are different from surface electrodes; 2) the dynamic response of smaller rat muscles are different from the large muscles of the human lower extremity; and 3) the inertial loads of the rat hindlimb are different from the inertial properties of the human lower leg. The results of the study reported here are an important extension of prior research in that this study demonstrates the ability of the PG/PS control system to account for the nonlinear recruitment properties and the dynamic properties of the muscle/limb in the rodent model with implanted electrodes. The study has focused on the hip because it is the most proximal joint, and therefore, plays an important role in controlling limb dynamics and because sensory feedback from the hip has been shown to be a critical component of sensorimotor integration during locomotion [29]. Future studies will address the issue of activating muscles that act at the knee and ankle to achieve coordinated limb control.
For practical use in the clinic, the primary advantage of the PG/PS control system is its ability to automatically customize stimulation parameters for an individual and automatically adjust these parameters to account for fatigue [19]. The results presented here build upon this study and provide additional evidence of the adaptive capabilities of the PG/PS system. The adaptive control system was able to generate the specified movement pattern in a repeatable manner in all of the legs tested without altering controller parameters other than the maximum stimulation level (Figs. 3(b) and 5). The PG/PS control system was thus able to automatically account for differences in muscle strength, muscle fiber recruitment nonlinearities, and the activation/contraction dynamics of the muscle that was stimulated [18]. The practical implication of this demonstration is that in the research laboratory or the clinic, the movement therapy can readily be performed on an individual in a manner that does not require special training or experience by the operator. In addition, unlike other controllers using neural network paradigms [30], [31], the PG/PS adaptive control system is customized to individual muscles using online training without the need for offline iterations. As shown in Fig. 5, on average, the controller learned the appropriate stimulation patterns within approximately 15 cycles. This rapid automatic customization with sustained performance makes this controller an attractive option for use in providing daily movement therapy to multiple animals in the research laboratory or a variety of humans in the clinic.
In this paper, we used an open-loop control paradigm as a basis upon which to demonstrate the performance capabilities of the PG/PS control system. There are many options for feedback or adaptive control that would outperform an open-loop system, but the open-loop paradigm was chosen for comparison because it is often used in the clinic and research laboratories and because it provides a readily implemented, repeatable comparative base. Although an extensive comparison of various feedback or adaptive control strategies may be informative, this was not the objective of the study reported here.
One potential disadvantage of using an adaptive (or feedback) approach is the need for sensors to provide feedback to the control system. In this study, we used a custom-designed goniometer system attached to a brace, while other studies have utilized implanted, body-mounted, or laboratory-based systems. Each of these sensor options provides added complexity and potential sources of system failure. Based on the results of this study and previous PG/PS control system implementations, the advantages provided by this control system may outweigh the disadvantages associated with the sensor system.
As an alternative to directly stimulating motor neurons, electrical pulses can also be used to stimulate sensory fibers to elicit a reflex response, and with repeated activation, this type of stimulation can be used as a therapeutic modality to induce plasticity in sensorimotor circuits. For example, stimulation of the flexion withdrawal reflex can assist a person with an incomplete SCI in bringing their leg forward during the swing phase of gait. When used as therapy, the technique has resulted in a carryover of increased functional mobility and speed, decreased effort, and improved intralimb coordination during unstimulated over-ground locomotion [28]. In this case, patterned nerve stimulation that mimics sensory afferent stimulation during walking rather than unpatterned stimulation leads to beneficial adaptation of the spinal H-reflex [10], i.e., functional plasticity in sensorimotor circuits was observed when the timing of the sensory stimulation was coordinated with the ongoing movement pattern. Such context-dependent learning of motor responses to sensory stimulation has also been demonstrated in spinal-cord-transected rodents [32]. The PG/PS paradigm presented here used direct activation of motor neurons to generate repeatable movement patterns. Although the stimulation pulses may not have directly elicited reflex responses, the resulting sensory pattern may be sufficient to promote spinal learning. The coordinated activation of muscle spindle afferents, joint proprioceptors, and cutaneous receptors may provide sufficient multimodal feedback to elicit meaningful context-dependent learning, and therefore, lead to improved motor recovery after neurotrauma such as SCI.
V. Conclusion
In this study, we have extended the investigation of the efficacy of PG/PS control system to a rodent model for neuromuscular stimulation therapy that uses agonist/antagonist control. The results of this study showed that the adaptive PG/PS control system was able to track the desired hip movement pattern by automatically adjusting stimulation levels delivered to hip flexor and extensor muscles and also that tracking performance remained consistent when utilized in an intermittent movement paradigm. These results suggest that the adaptive PG/PS control system may be well suited for generating cyclic joint movements using neuromuscular electrical stimulation therapy over lengthy sessions. Further animal experiments and translational studies are in progress to evaluate the ability of this adaptive electrical-stimulation-assisted therapy to promote neural plasticity after SCI or brain injury.
Acknowledgments
This work was supported in part by the National Institutes of Health under Grant R01-HD040335 and Grant R01-HD049773.
Biographies

Seung-Jae Kim was born in Korea in 1971. He received the B.S. degree in mechanical engineering from Han-Yang University, Seoul, Korea, in 1993, the M.S. degree from Pohang University Science and Technology, Pohang, Korea, in 1995, and the M.S. and Ph.D. degrees in bioengineering from the University of Utah, Salt Lake City, in 2001 and 2006, respectively.
From 1995 to 1997, he was a Korean Overseas Volunteer in The Philippines. He is currently a Research Scientist in the Center for Adaptive Neural Systems, Arizona State University, Tempe. His biomedical research has focused on neural interfaces, centered on exploring a direct intraneural nerve stimulation and neural signal recording from the cortex. His past research included development of prosthetic arm, myoelectric control techniques, and tendon transfer biomechanics. His current research interests include rehabilitation engineering, neural interface technologies, neuroprostheses, functional electrical stimulation, and investigations to improve urinary functions and movement in the lower extremity for people with spinal cord injury.

Mallika D. Fairchild (S’06) received the B.E. degree (with honors) in electrical engineering from Goa University, Taleigao Plateau, India, in 1997, and the M.S. degree in bioengineering, in 2003, from Arizona State University, Tempe, where she is currently working toward the Ph.D. degree at the Harrington Department of Bioengineering.
She is a Graduate Research Associate at the Center for Adaptive Neural Systems, Arizona State University. Her current research interests include application of functional neuromuscular stimulation therapy after spinal cord injury and understanding the underlying mechanisms of improvement in sensorimotor function.

Alexandre Iarkov (Yarkov) received the M.Sc. degree in neurophysiology and the Ph.D. degree from the Department of High Nervous Activity, Biology Faculty, Lomonosov State University, Moscow, Russia, in 1981 and 1994, respectively.
During 1991–1994, he was a Postdoctoral Fellow at the Institute of Experimental and Theoretical Biophysics, Moscow. From 1998 to 2001, he was a Research Professor at the Laboratory of Neuroendocrinology, Centro Universitario de Investigasiones Biomedicas, University of Colima, Mexico. Since 2004, he has been a Research Scientist at the Center for Adaptive Neural Systems, Arizona State University, Tempe. His current research interests include understanding the principles of functioning of the neurotransmitter systems involved in control of motor systems.

James J. Abbas (S’87–M’92) received the B.S. degree in bioelectrical engineering from Brown University, Providence, RI, and the M.S. and Ph.D. degrees in biomedical engineering from Case Western Reserve University, Cleveland, OH.
He is currently an Associate Professor in the Harrington Department of Bioengineering, Fulton School of Engineering, Arizona State University, Tempe, where he is also the Co-Director of the Center for Adaptive Neural Systems. His current research interests include applications of neural engineering techniques and technology in the area of medical rehabilitation. He is also involved in several projects including the development and assessment of systems that use electrical stimulation for therapy after spinal cord injury, systems to improve neuromotor control in children with cerebral palsy, systems to restore sensory capabilities to amputees, and techniques to improve sensorimotor function in people with Parkinson’s disease.
Mr. Abbas was an Officer of the International Functional Electrical Stimulation Society. He is on the Editorial Board of the Journal of Neuroengineering and Rehabilitation.

Ranu Jung (S’88–M’90–SM’06) received the B.Tech. degree (with distinction) in electronics and communications engineering from the National Institute of Technology, Warangal, India, and the M.S. and Ph.D. degrees in biomedical engineering from Case Western Reserve University, Cleveland, OH.
She is currently an Associate Professor in the Harrington Department of Bioengineering, Arizona State University, Tempe, where she is also the Co-Director of the Center for Adaptive Neural Systems. Her current research interests include understanding and promoting adaption in neural systems, particularly after neurotrauma, computational neuroscience, and the dynamical interaction between the brain and spinal neural circuits for sensorimotor integration. The projects she leads include computational modeling of the rodent neuromusculoskeletal system, development and assessment of electrical stimulation therapy systems after spinal cord injury in the rodent, and development and implementation of technology to interface neural-enabled prosthetic hands to transradial amputees.
Dr. Jung is an Associate Editor of the IEEE Transactions on Biomedical Engineering. She is also the President of the Organization for Computational Neurosciences, Inc. She was a National Institutes of Health Individual National Research Service Award Fellow. She was a recipient of the 2002 Science and Engineering Award, and the Governors Certificate of Recognition, Commonwealth of Kentucky.
Contributor Information
Seung-Jae Kim, Center for Adaptive Neural Systems, Arizona State University, Tempe, AZ 85287 USA.
Mallika D. Fairchild, Center for Adaptive Neural Systems and Department of Bioengineering, Ira A. Fulton School of Engineering, Arizona State University, Tempe, AZ 85287-4404 USA.
Alexandre Iarkov (Yarkov), Center for Adaptive Neural Systems, Arizona State University, Tempe, AZ 85287 USA.
James J. Abbas, Center for Adaptive Neural Systems and Department of Bioengineering, Ira A. Fulton School of Engineering, Arizona State University, Tempe, AZ 85287-4404 USA.
Ranu Jung, Email: ranu.jung@asu.edu, Center for Adaptive Neural Systems and Department of Bioengineering, Arizona State University, Tempe, AZ 85287 USA.
References
- 1.Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physiol. 2004 May;96(5):1954–1960. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- 2.Goldshmit Y, Lythgo N, Galea MP, Turnley AM. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008 Mar;25(5):449–465. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- 3.Fouad K, Pearson K. Restoring walking after spinal cord injury. Prog Neurobiol. 2004 Jun;73:107–126. doi: 10.1016/j.pneurobio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Lynskey JV, Belanger A, Jung R. Activity dependent plasticity in spinal cord injury. J Rehabil Res Dev. 2008;45:229–240. doi: 10.1682/JRRD.2007.03.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plummer P, Behrman AL, Duncan PW, Spigel P, Saracino D, Martin J, Fox E, Thigpen M, Kautz SA. Effects of stroke severity and training duration on locomotor recovery after stroke: A pilot study. Neurorehabil Neural Repair. 2007 Mar;21(2):137–151. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 6.Seif-Naraghi AH, Herman RM. A novel method for locomotion training. J Head Trauma Rehabil. 1999 Apr;14:146–162. doi: 10.1097/00001199-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Stein RB, Chong SL, James KB, Kido A, Bell GJ, Tubman LA, Belanger M. Electrical stimulation for therapy and mobility after spinal cord injury. Prog Brain Res. 2002;137:27–34. doi: 10.1016/s0079-6123(02)37005-5. [DOI] [PubMed] [Google Scholar]
- 8.Thrasher TA, Flett HM, Popovic MR. Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord. 2006 Jun;44(6):357–361. doi: 10.1038/sj.sc.3101864. [DOI] [PubMed] [Google Scholar]
- 9.Postans NJ, Hasler JP, Granat MH, Maxwell DJ. Functional electric stimulation to augment partial weight-bearing supported treadmill training for patients with acute incomplete spinal cord injury: A pilot study. Arch Phys Med Rehabil. 2004 Apr;85:604–610. doi: 10.1016/j.apmr.2003.08.083. [DOI] [PubMed] [Google Scholar]
- 10.Field-Fote EC. Electrical stimulation modifies spinal and cortical neural circuitry. Exerc Sports Sci Rev. 2004 Oct;32(4):155–160. doi: 10.1097/00003677-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: A comparative study of human spinal cord injury. J Neurotrauma. 2000 Jan;17(1):1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001 Mar;13(6):1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 13.Thota A, Carlson S, Jung R. Recovery of locomotor function after treadmill training of incomplete spinal cord injured rats. Biomed Sci Instrum. 2001;37:63–67. [PubMed] [Google Scholar]
- 14.Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol. 2004 Aug;476:130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- 15.Ichihara K, Venkatasubramanian G, LaBelle A, Ashton E, Abbas JJ, Jung R. Muscle stimulation in a rodent model: Electrode design, implantation and assessment. Proc. 9th Annu. Conf. Int. Funct. Electr. Stimul. Soc. (IFESS); Bournemouth, U.K. 2004; pp. 404–406. [Google Scholar]
- 16.Jung R, Belanger A, Kanchiku T, Lynskey J, Mukherjee M, Hagner D, Abbas JJ. Hindlimb neuromuscular stimulation therapy after thoracic contusion injury promotes locomotor recovery. Proc. 11th Annu. Conf. Int. Funct. Electr. Stimul. Soc; Miyagi-Zao, Japan. 2006; pp. 118–120. [Google Scholar]
- 17.Abbas JJ, Chizeck HJ. Neural network control of functional neuromuscular stimulation systems: Computer simulation studies. IEEE Trans Biomed Eng. 1995 Nov;42(11):1117–1127. doi: 10.1109/10.469379. [DOI] [PubMed] [Google Scholar]
- 18.Riess J, Abbas JJ. Adaptive neural network control of cyclic movements using functional neuromuscular stimulation. IEEE Trans Rehabil Eng. 2000 Mar;8(1):42–52. doi: 10.1109/86.830948. [DOI] [PubMed] [Google Scholar]
- 19.Riess J, Abbas JJ. Adaptive control of cyclic movements as muscles fatigue using functional neuromuscular stimulation. IEEE Trans Neural Syst Rehabil Eng. 2001 Sep;9(3):326–330. doi: 10.1109/7333.948462. [DOI] [PubMed] [Google Scholar]
- 20.Ginz HF, Zorzato F, Iaizzo PA, Urwyler A. Effect of three anaesthetic techniques on isometric skeletal muscle strength. Br J Anaesth. 2004 Mar;92(3):367–372. doi: 10.1093/bja/aeh080. [DOI] [PubMed] [Google Scholar]
- 21.Kanchiku T, Lynskey JV, Protas D, Abbas JJ, Jung R. Neuromuscular electrical stimulation induced forelimb movement in a rodent model. J Neurosci Methods. 2008 Jan;167(2):317–326. doi: 10.1016/j.jneumeth.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stites EC, Abbas JJ. Sensitivity and versatility of an adaptive system for controlling cyclic movements using functional neuromuscular stimulation. IEEE Trans Biomed Eng. 2000 Sep;47(3):1287–1292. doi: 10.1109/10.867965. [DOI] [PubMed] [Google Scholar]
- 23.Abbas JJ, Triolo RJ. Experimental evaluation of an adaptive feedforward controller for use in functional neuromuscular stimulation systems. IEEE Trans Rehabil Eng. 1997 Mar;5(1):12–22. doi: 10.1109/86.559345. [DOI] [PubMed] [Google Scholar]
- 24.Thota AK, Carlson Watson S, Knapp EJ, Thompson BT, Jung R. Neuromechanical control of locomotion in the rat. J Neurotrauma. 2005 Apr;22(4):442–465. doi: 10.1089/neu.2005.22.442. [DOI] [PubMed] [Google Scholar]
- 25.Thrasher A, Graham GM, Popovic MR. Reducing muscle fatigue due to functional electrical stimulation using random modulation of stimulation parameters. Artif Organs. 2005 Jun;29(6):453–458. doi: 10.1111/j.1525-1594.2005.29076.x. [DOI] [PubMed] [Google Scholar]
- 26.McNeal DR. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng. 1976 Jul;BME-23(4):329–337. doi: 10.1109/tbme.1976.324593. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Amer J Anatomy. 1984;171:256–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- 28.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil. 2001 Jun;82(2):818–824. doi: 10.1053/apmr.2001.23752. [DOI] [PubMed] [Google Scholar]
- 29.Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain Res Rev. 2008 Jan;57(1):241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang GC, Luh JJ, Liao GD, Lai JS, Cheng CK, Kuo BL, Kuo TS. A neuro-control system for the knee joint position control with quadriceps stimulation. IEEE Trans Rehabil Eng. 1997 Mar;5(1):2–11. [PubMed] [Google Scholar]
- 31.Kurosawa K, Futami R, Watanabe T, Hoshimiya N. Joint angle control by FES using a feedback error learning controller. IEEE Trans Neural Syst Rehabil Eng. 2005 Sep;13(3):359–371. doi: 10.1109/TNSRE.2005.847355. [DOI] [PubMed] [Google Scholar]
- 32.Hook MA, Grau JW. An animal model of functional electrical stimulation: Evidence that the central nervous system modulates the consequences of training. Spinal Cord. 2007 Nov;45(11):702–712. doi: 10.1038/sj.sc.3102096. [DOI] [PMC free article] [PubMed] [Google Scholar]