Abstract
As cord blood (CB) lacks memory T- and B-cells, and recent decreases in herd immunity to vaccine preventable diseases in many developed countries have been documented, vaccine responses in CB transplantation (CBT) survivors are of great interest. We analyzed vaccine responses in double-unit CBT recipients transplanted for hematologic malignancies. In 103 vaccine eligible patients, graft-versus-host disease (GVHD) most commonly precluded vaccination. Sixty-five (63%) patients (engrafting units median HLA-allele match 5/8, range 2–7/8) received protein-conjugated vaccines, and 63 (median age 34 years, range 0.9–64) were evaluated for responses. Median vaccination time was 17 months (range 7–45) post-CBT. GVHD (n = 42) and prior rituximab (n = 13) delayed vaccination. Responses to Prevnar 7 and/or 13 vaccines (serotypes 14, 19f, 23f) were seen in children and adults (60% versus 49%, p = 0.555). Responses to tetanus, diphtheria, pertussis, H. influenzae, and polio were observed in children (86–100%) and adults (53–89%) even if patients had prior GVHD or rituximab. CD4+CD45RA+ and CD19+ cell recovery significantly influenced tetanus and polio responses. In a smaller cohort, responses were seen to measles (65%), mumps (50%), and rubella (100%) vaccines. No vaccine side-effects were identified and all vaccinated patients survive (median follow-up 57 months). While GVHD and rituximab can delay vaccination, CBT recipients (including adults and those with prior GVHD) have similar vaccine response rates to adult donor allograft recipients supporting vaccination in CBT recipients.
Keywords: Cord blood transplantation, immune reconstitution, vaccine response, pneumococcal vaccines
Introduction
Disease-free survival after cord blood (CB) transplantation (CBT) is comparable to that of adult donor allografts in many series1–8. With increasing survivorship and the loss of pre-transplant immunity, vaccine responses in CBT recipients are of great clinical relevance. Moreover, vaccine responses may differ from those observed after adult donor unmodified hematopoietic stem cell (HSC) transplantation due to the lack of transfer of memory T and B cells capable of peripheral expansion. As in all allograft recipients, responses could be altered by the development of graft-versus-host disease (GVHD). Vaccine responses are also of scientific interest in CBT survivors given they are an excellent test of immune competence. Although several groups have evaluated the kinetics of immune recovery after single and double-unit CBT9–16, no data exist examining vaccine responses in CBT recipients. Many centers vaccinate at fixed time-points without formal evaluation of responses17,18 precluding evaluation of vaccine efficacy. Documentation of vaccine efficacy is especially important in older patients, those with clinically significant GVHD, and patients who have received peri- or post-CBT rituximab who are at particular risk of negligible or inadequate responses.
Memorial Sloan Kettering Cancer Center (MSKCC) practice has increasingly been to initiate vaccines once patients have achieved immunological milestones with measurement of pre- and post-titers to evaluate responses. This is appropriate in CBT recipients given the lack of data concerning vaccine responses. We examined the incidence and time to vaccination, the incidence of severe vaccine side-effects, responses to protein-conjugated vaccines, and survival after vaccination in double-unit CBT recipients transplanted for hematological malignancies at our center. In a smaller cohort of patients, we also evaluated responses to live vaccines. Our hypothesis was that while GVHD and/or the administration of rituximab could prevent or delay vaccination, CBT recipients could otherwise respond to non-live vaccines similar to adult donor allograft recipients.
Methods
Patients
All consecutive CBT recipients treated for hematological malignancies at MSKCC from October 2005 to February 2012 were reviewed for study inclusion. Patients eligible for this retrospective analysis included recipients of a first allograft for the treatment of high-risk or advanced hematologic malignancies who were engrafted and disease-free at 6 months post-CBT (the earliest time point that vaccines were considered), and had pre- and post-vaccine titers. All patients received double-unit grafts that were 4–6/6 HLA-A,-B antigen, -DRB1 allele matched to the recipient as previously published19. High-resolution typing for Class I HLA-alleles was also performed. Patients predominantly received fludarabine and total body irradiation-based conditioning although a small cohort received chemotherapy-only preparative regimens5,20,21. Immunosuppression was given as previously described with a calcineurin-inhibitor and mycophenolate mofetil without anti-thymocyte globulin5,14. Permission to use clinical and laboratory information was obtained from the Institutional Review/Privacy Board.
Vaccines and definitions of response
The primary series included protein conjugated vaccines as follows: pneumococcal vaccine (Prevnar 7 which was replaced by Prevnar 13 in 2010), tetanus, diphtheria, pertussis (usually given as Boostrix with 8 ug/dose), Hemophilus influenza b (HiB), and inactivated polio vaccine. Patients were also offered hepatitis B vaccine given as Twinrix (hepatitis A and B combination), hepatitis B recombinant vaccine, or Pediarix (which also contains tetanus, diphtheria, and polio). Most clinicians vaccinated patients after 6 months (usually one year in adults) and achievement of basic immune milestones (CD4+ count > 200 cells/microL and an IgG level > 500 mg/dL independent of intravenous immunoglobulin replacement) who were on no or minimal immune suppression. Some clinicians also considered phytohemaglutinin responses14 and more recently, a CD19+ count > 50 cells/microL has also been included in the guidelines for vaccine initiation.
Pre-vaccine titers were measured by ELISA and were drawn prior to vaccine initiation. Three doses of Prevnar 7 or 13, Tdap, HiB (± hepatitis B vaccines) were recommended at time points zero, +1 month and +2 months but only 2 doses of Polio (time point 0 and +1 month) with titers 1–3 months later. A response was defined as seroconversion in a seronegative individual, a 3-fold geometric mean fold rise (GMRF) of the IgG geometric mean concentration (GMC), or 2-fold for pertussis. The ABBOTT Architect i2000R instrument was used for Hepatitis B titers with the manufacturer cutoff of < 8.0 mIU/mL for negative and ≥12.00 mIU/mL for positive. For the purposes of analysis, patients were categorized as having a pneumococcal vaccine response if they had a > 3-fold rise in titers against the clinically significant serotypes 14, 19F and 23F found in both Prevnar 7 and Prevnar 13 as these serotypes can cause resistant invasive infection post-HCT22,23. Polio response was defined as response to all 3 serotypes. Boosters were recommended in patients without a response to the primary series although subsequent assessment of vaccine efficacy was influenced by frequency of patient follow-up.
Patients off immune suppression, without active GVHD, and with documented responses to ≥ 2 primary series vaccines who were seronegative were offered live vaccines (measles, mumps, and rubella, MMR). Patients received 1–2 doses of the MMR vaccine. Response was defined as seroconversion in a seronegative individual or a 3-fold GMFR of the IgG GMC.
Statistical analysis
Children were defined as aged < 16 years of age. Acute and chronic GVHD were graded according to IBMTR24 and NIH25 criteria, respectively. Clinically significant GVHD was defined as grade 2–4 (B–D) acute GVHD and/or moderate-severe chronic GVHD. Low dose immunosuppression was defined as calcineurin-inhibitor level less than half the lower limit of therapeutic range, less than 0.2 mg/kg per day of prednisone, and/or other immunosuppressive agent prescribed at less than half the therapeutic dose. Comparisons of time to vaccination used the Mann-Whitney U test whereas the assessment of factors associated with vaccine responses used the Fisher’s exact test (2-sided). A p value < 0.05 was considered statistically significant.
Results
Eligibility for vaccination
Of 143 patients transplanted during the study period, 103 (72%) were disease-free at 6 months post-CBT and potentially eligible for vaccines. Of them, 65/103 (63%) patients were vaccinated with the primary series protein conjugated vaccines. Sixty-three of these patients were evaluable for vaccine responses as they had long-term follow-up with both pre- and post-vaccine titers whereas the 2 patients who left the New York area and did not have post-vaccine titers were excluded. The remaining 38/103 (37%) patients were not vaccinated at the time of analysis primarily due to GVHD ± administration of rituximab (n = 28 including 12 rituximab). Other reasons for lack of vaccination included relapse (n = 6), corticosteroids for pulmonary disease (n = 2), or loss to follow-up (n = 2).
Characteristics of patients evaluated for vaccine responses
The patient and graft demographics of the 63 evaluable vaccinated patients are listed in Table 1. Patients (median age 34 years) predominantly had acute leukemia (67%) and received high-dose (51%) or intermediate intensity but functionally myeloablative (32%) conditioning. Engrafting units had a high degree of donor-recipient HLA-mismatch. Thirteen (21%) patients received rituximab as part of the preparative regimen (n = 6), treatment of CD20+ relapse (n = 1), as both part of the conditioning and treatment of relapse (n = 1), as treatment for Epstein-Barr viremia (n = 3), treatment of red cell aplasia (n = 1) or autoimmune hemolysis (n = 1). The last dose of rituximab was given a median of 15 months (range 3.0–35) prior to the commencement of vaccination.
Table 1.
Patient and graft demographics of 63 vaccinated double unit CBT recipients.
| Characteristic | Value |
|---|---|
|
| |
| Age (median, range) | 34 years (0.9–64) |
|
| |
| Diagnosis (N, %) | |
| AML (CR1–3) | 31 (49%) |
| ALL (CR1–4) | 11 (17%) |
| MDS/CML | 3 (5%) |
| Lymphoma | 18 (29%) |
|
| |
| Conditioning (N) | |
| High dose: | |
| Cy/Flu/TBI 1320–1375 | 26 |
| Thio/Flu/TBI 1320 | 1 |
| Clo/Mel/Thio | 5 |
| Intermediate: | |
| Cy/Flu/Thio/TBI 400 | 17 |
| Reduced intensity: | |
| Mel/Flu | 3 |
| Non-myeloablative: | |
| Cy/Flu/TBI 200 | 11 |
|
| |
| Engrafting units (median, range) | |
| 8 HLA-allele match* | 5/8 (2–7) |
| Infused TNC × 107/kg | 2.1 (1.2–11.3) |
| Infused CD34+ × 105/kg | 0.8 (0.1–4.1) |
|
| |
| Pre-vaccination rituximab, (N, %) | 13 (21%) |
|
| |
| GVHD (N, %) | |
| Grade II–IV acute (grade) | 38 (60%)** |
| Chronic | 10 (16%)*** |
|
| |
| Vaccinated on immunosuppression (N, %) | |
| No | 45 (71%) |
| Yes | 18 (29%) |
|
| |
| Pre-vaccine immune recovery Median (Range) | |
| CD4+ | 577/μl (range 190–2,296) |
| CD4+ CD45RA+ | 127/μl (range 14–1,856) |
| CD19+ | 793/μl (range 0–5,533) |
| IgG | 786 mg/dl (range 502–2,965) |
Donor recipient HLA-match;
27 II, 10 III, 1 IV;
9 mild, 1 severe (Prior acute: 6 yes, 4 no)
AML, Acute Myeloid Leukemia; ALL, Acute Lymphoblastic Leukemia; MDS, Myelodysplastic Syndrome; CML, Chronic Myelogenous Leukemia; Cy, Cyclophosphamide; Flu, Fludarabine; TBI, Total Body Irradiation; Thio, Thiotepa; Clo, Clofarabine; Mel, Melphalan; HLA; Human Leukocyte Antigen; TNC, Total Nucleated Cell Count; GVHD, Graft-verus-Host Disease
The majority of vaccinated patients (n = 38, 60%) had grade II–IV acute GVHD prior to day 180 post-CBT. Ten patients had previously had chronic GVHD (6 with prior acute GVHD and 4 with de novo disease). Thus, 42/63 (67%) of patients had acute and/or chronic GVHD before vaccination. Eighteen of 63 (29%) of patients were vaccinated while on low dose immune suppression whereas it had been stopped in the remainder. CD4+ and CD19+ cell counts and IgG levels were adequate in the majority of patients although there was a wide range of immune recovery immediately prior to vaccine initiation.
Time to vaccination initiation
The 63 evaluable patients were vaccinated at a median of 17 months post-CBT (range 7–45). Within this group, vaccination was delayed in the rituximab recipients [19 months (range 12–45)] compared to the patients who did not receive anti-B-cell therapy [15 months (range 7–41)], p = 0.034. Vaccination was also delayed in 42 patients with prior grade 2–4 acute and/or moderate-severe chronic GVHD with a median onset of 17 months (range 9–45) post-CBT as compared with 14 months (range 7–33) in those without clinically significant prior GVHD, p = 0.029. The median time from GVHD onset to vaccination was 15.7 mos (range 6–44).
Responses to pneumococcal vaccines
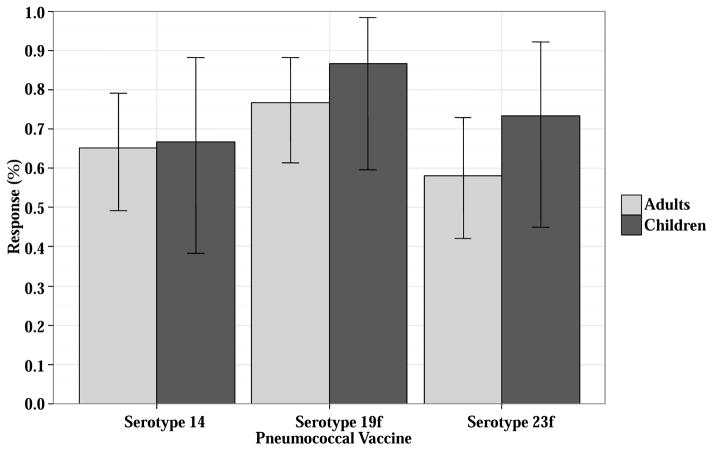
Response to 3 doses of the pneumococcal vaccine was evaluable in 58 patients. Of the 5 other patients, 3 received only 2 vaccines, 1 with prior pneumococcal infection was not vaccinated, and one did not have post-vaccine pneumococcal titers. The 58 evaluable patients received Prevnar 7 (n = 24), Prevnar 13 (n = 33), or both (n = 1). Thirty (53%) responded to all 3 of the clinically critical pneumococcal serotypes (14, 19F and 23F). Response rates by clinically significant serotype (Figure 1) did not differ between children (9/15, 60%) and adults (21/43, 49%), p = 0.555. Among the 28 non-responders, 19 patients (33%) responded to 1–2 critical serotypes, and 9 patients (16%) did not respond to any.
Figure 1. Prevnar response for 3 clinically significant pneumococcal serotypes (14, 19f, and 23f) in 58 double unit CBT recipients.
Responses are split by children (n = 15) and adults (n = 43).
There were no significant differences between pneumococcal vaccine responses and exposure to prior rituximab, prior clinically significant GVHD, or vaccination while on low dose immunosuppression. There was also no association between vaccine response and basic measures of immune recovery (CD4+, CD4+CD45RA+, CD19+ cell counts, IgG levels and phytohemaglutinin responses14 above versus below the median or in tertiles, data not shown).
Responses to tetanus, diphtheria, pertussis, HiB and polio vaccines
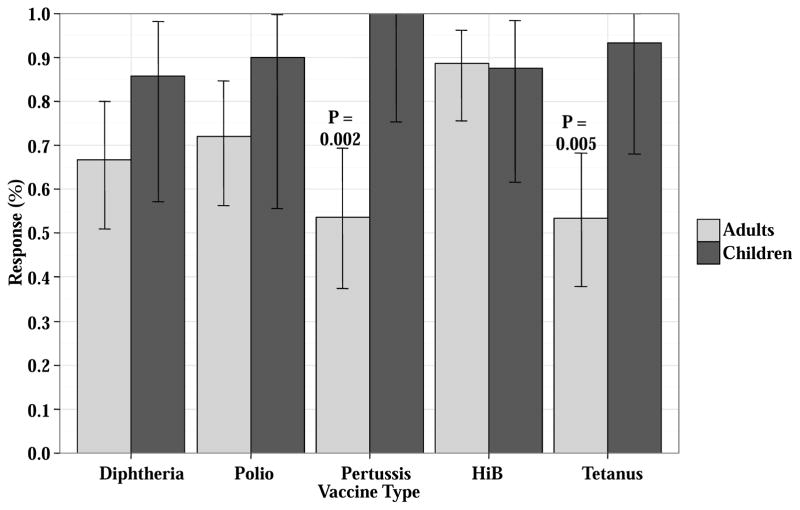
Responses to tetanus, diphtheria, pertussis, HiB, and polio were evaluated in all 63 patients. However, only patients who received ≥ 2 primary series vaccines were included in the response evaluation (N ranged 53–60 patients, Figure 2, Table 2). Overall response rates ranged from 86–100% in children and 53–89% in adults. Children had better responses to tetanus (93% versus 53%, p = 0.005) and pertussis (100% versus 54%, p = 0.002) vaccines, respectively. There were no differences in response rates according to prior rituximab (p values ranged 0.256–1.00), or prior clinically significant GVHD (p values ranged 0.155–1.00), and no adverse effects of low dose immunosuppression were identified.
Figure 2. Primary series responses for Tdap, HiB and polio in double unit CBT recipients who received ≥ 2 vaccines in the primary series.
Patients are split by age (children versus adults, 2A) and according to prior clinically significant GVHD (2B). Three children received either Pediarix or Infanrix whereas all other patients received Boostrix.
Table 2.
Response rates for primary series protein conjugated vaccines including tetanus, diphtheria, pertussis, HiB, and polio.
| Responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Tetanus | p | Diphtheria | p | Pertussis | p | Polio | p | HiB | p |
| Age (years) < 16 | 14/15 (93%) | 0.005 | 12/14 (86%) | 0.310 | 13/13 (100%) | 0.002 | 9/10 (90%) | 0.419 | 14/16 (88%) | 0.99 |
| Age (years) ≥ 16 | 24/45 (53%) | 30/45 (68%) | 22/41 (54%) | 31/43 (72%) | 39/44 (89%) | |||||
| No Prior GVHD | 14/18 (78%) | 0.155 | 14/17 (82%) | 0.344 | 9/15 (60%) | 0.753 | 12/15 (80%) | 0.736 | 17/19 (90%) | 0.99 |
| Prior GVHD | 24/42 (57%) | 28/42 (68%) | 26/39 (68%) | 28/38 (74%) | 36/41 (88%) | |||||
| No Prior Rituximab | 31/48 (65%) | 0.744 | 33/47 (70%) | 0.99 | 29/44 (66%) | 0.728 | 32/40 (80%) | 0.265 | 42/47 (89%) | 0.639 |
| Prior Rituximab | 7/12 (58%) | 9/12 (75%) | 6/10 (60%) | 8/13 (62%) | 11/13 (85%) | |||||
| IS: No | 24/42 (57%) | 0.155 | 26/42 (62%) | 0.013 | 22/38 (58%) | 0.128 | 29/38 (76%) | 0.99 | 37/42 (88%) | 0.99 |
| IS: Yes | 14/18 (78%) | 16/17 (94%) | 13/16 (81%) | 11/15 (73%) | 16/18 (89%) | |||||
| Total | 60 | 59 | 54 | 53 | 60 | |||||
HiB, Hemophilus influenza B; GVHD, Graft-verus-Host Disease; IS, Immunosuppression
There was a significant association between tetanus and polio responses and measurements of immune recovery. Patients with CD+4CD45RA+ counts above the median had better response rates to tetanus vaccine (79% versus 50%, p = 0.032), and patients with CD19+ cells above the median had higher responses to tetanus (86% versus 44%, p = 0.001) and polio (92% versus 62%, p = 0.023). Other parameters of immune reconstitution including CD4+ cell count, phytohemagglutinin responses14, and IgG levels were not significantly associated with response.
Hepatitis B vaccine responses
Forty-two patients were evaluable for Hepatitis B response (2–3 vaccines administered). However, 10 of these patients had positive pre-vaccine titers so their responses were considered indeterminate. Of 32 remaining patients with negative pre-transplant titers, one of 4 (25%) responded to 2 vaccines, and 12/28 (43%) recipients of 3 vaccines responded. Two of the non-responders received a second series and responded.
Response to pneumococcal boosters
Of 9 patients who did not respond to one or more of the pneumococcal serotypes 14, 19f, and 23f after 3 vaccines in the primary series, 6 were re-vaccinated with 1–2 Prevnar boosters. Of these, one was not evaluated due to disease relapse, and the remaining 5 patients all responded (3 patients to one and 2 patients to 2 boosters).
Measles, mumps and rubella vaccine responses
To date, 59 patients have had measles titers. Nineteen had positive titers. Twenty-three sero-negative patients have been vaccinated with measles to date with post-vaccine titers and 15/23 (65%) responded (median age 16 years, range 1–51). Similarly, of 57 patients with pre-vaccine mumps titers, 30% were positive; 10/20 (50%) patients with negative titers responded to mumps (median 23 years, range 1–51). Finally, of 59 patients, 23 (39%) had positive rubella titers; 21/21 (100%) of negative titer patients vaccinated to date have responded to rubella vaccine.
Vaccine safety and survival in vaccine recipients
There were no severe adverse side-effects reported or identified after vaccination. With a median follow-up of 57 months (range 24–100), all vaccinated patients survive.
Discussion
Similar to the young and the elderly, HSC transplantation recipients rely on herd immunity until they have sufficient immune reconstitution to facilitate successful vaccination26. In the community, such protection is accomplished through mandatory vaccination in school aged children27, but this has been compromised by an increase in non-medical vaccination exemptions28,29. Such exemptions have been linked to outbreaks of vaccine-preventable illnesses30,31. Specifically, outbreaks of pneumococcus32,33, pertussis34–37, meningococcus38–40, polio41–43, measles44,45, and mumps46–48 have been documented as detailed by the lay press49–52 and reviewed in recent publications by Omer et al31 and Orenstein et al53. Moreover, Brazilian allograft recipients succumbed to measles during a national epidemic54, and a recent measles outbreak in New York involved exposure in medical facilities50. Also of concern is the recent measles outbreak at Disneyland55 as patients often choose such destinations once they can travel post-transplant and increasingly patients are also traveling internationally. Decreased vaccination rates in under-developed countries combined with increased cross-continental travel could further enhance disease transmission such as polio53,56, and a risk associated with biological warfare has been proposed57. These factors make vaccination of both the community and allograft recipients of paramount importance.
We investigated vaccine responses in adult and pediatric CBT survivors. Our results have practical clinical importance and are an important addition to the literature concerning immune reconstitution. The quality of immune recovery after CBT is controversial with some series suggesting delayed immune reconstitution58 and others showing better recovery especially in those transplanted without ATG11,12,14,15,59,60. We demonstrate that CBT recipients can respond to protein conjugated vaccines similar to adult donor allograft recipients. GVHD and rituximab delayed vaccination but did not preclude responses to primary series vaccines post-CBT in many patients, and responses to live vaccines were also observed. Vaccination of CBT recipients was safe and vaccinated patients had excellent survival. This is the first major analysis of vaccine responses in CBT especially in a cohort of predominantly (80%) adult patients. It must be acknowledged that only 63% of potentially eligible CBT recipients were vaccinated at the time of analysis, and GVHD was the major contributor to the delay or inability to vaccinate some patients to date. Measures to abrogate GVHD61,62,63,64 could improve GVHD control and thereby potentially enhance the ability to proceed with vaccination provided they did not unduly delay immune reconstitution.
Invasive disease caused by Pneumococcus occurs with increased frequency and higher morbidity and mortality in allograft recipients than in healthy individuals. Implementation of routine childhood pneumococcal vaccination has been associated with a reduction in invasive disease. We focused on the 3 most clinically significant serotypes responsible for pneumococcal infections65, and showed no differences in response rates between pediatric and adult CBT recipients. While there was no association between basic measures of immune recovery and vaccine response, this is likely due to most patients having achieved at least minimal immune recovery prior to vaccination. In a small number of non-responders, pneumococcal boosters were successful emphasizing the possibility of achieving a response if one is not initially obtained. The recent Centers for Disease Control recommendations for older adults include the addition of a 23-valent pneumococcal polysaccharide vaccine (PPV23) following PCV-1366,67, and this has been recently shown to be safe and able to illicit responses in the transplant recipients upon completion of the Prevnar series68.
Response rates of 86–100% in children and 53–89% in adults were also achieved with other protein conjugated vaccines. The responses differed by vaccine type likely due to their varying immunogenicity and tetanus and pertussis responses were better in children than adults. An inferior result was demonstrated in patients with low CD4+CD45RA+ cell counts for tetanus and with a low CD19+ cell count for polio. In a small cohort, responses to Hepatitis B were observed although overall this vaccine appeared less effective. From the standpoint of live vaccines, our preliminary data suggests that in patients off immunosuppression in whom responses to protein conjugated vaccines were documented, live vaccines are safe and can be effective. Administration was delayed in adults as compared to children likely due to differences in clinical practice, especially given the need for revaccination prior to returning to school. No CBT recipients have been evaluated for varicella zoster vaccine responses, and further investigation into MMR and varicella vaccine responses in a larger cohort is required18,69.
Our study has several limitations. The sample size was relatively small, although it did have a significant number of adult patients. Also, while we focused on the pneumococcal serotypes known to be clinically relevant, we acknowledge that the response to other subtypes could be valuable. Finally, adverse events were based on chart review and not recorded prospectively which may have minimized the identification of minor side-effects. Nonetheless, despite these limitations, the vaccine responses after CBT demonstrated in this report are similar to other allo-transplant groups for protein conjugated vaccines70. For example, the adult CBT response rate to pneumococcal vaccine of 53% is similar to the 44% rate in adult donor allograft recipients reported by Pao et al.65 Less information is available for other vaccines especially in adults, but responses for protein conjugated vaccines have been reported as 36–58% for Tdap using 8 micrograms of pertussis toxoid71,72, 47–92% for HiB73,74, 48–95% for polio74,75, 40–64% for hepatitis B76,77, and 64–77% for MMR78–80, and these rates are similar to those in our series. Only a prospective study with an intention to treat design with concurrent measurements of immune recovery could accurately compare response rates in defined patient populations stratified by HSC source.
In summary, similar to individuals in the community, CBT recipients can benefit from immunization against vaccine-preventable diseases, and while GVHD is the major cause of vaccination delay, prior GVHD or rituximab, and administration of low dose immunosuppression are not prohibitive in many patients. Our strategy to vaccinate according to immune milestones and document response by pre- and post-vaccination titers is in contrast to the Infectious Diseases Society of America and the American Society for Blood and Marrow Transplant guidelines17,18,81,82 which recommend vaccination at 6 months to 1 year without testing immune function or titers. The optimal immunization schedule in CBT recipients, however, has not been determined. In this study the median time to vaccination was 17 months. It is possible that patients may benefit from earlier administration of some vaccines. The most appropriate timing may also vary by vaccine. Multicenter studies evaluating pre- and post-vaccination titers to determine optimal vaccination schedule and the determinants of vaccine efficacy are needed. Moreover, while our findings strongly support vaccination in CBT recipients, multiple unanswered questions remain that require future prospective evaluation including the immunologic determinants of response (e.g. the presence of CD19+CD27+IgD+ memory B cells), the role of boosters to obtain, augment and maintain responses, the role of vaccination in patients with positive post-transplant serologies, and the responses to live vaccines in a larger patient cohort.
Highlights.
CBT recipients respond to protein conjugated vaccines similar to adult donor allograft recipients.
GVHD and rituximab do not preclude responses to primary series vaccines after CBT.
Vaccines in CBT recipients are safe and vaccinated patients have a high post-transplant survival.
Acknowledgments
The authors wish to acknowledge the life, work and dedication of Dr. Trudy Small who greatly contributed to the understanding of vaccine responses after allogeneic transplantation. This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research (J.N.B.), the MSKCC (J.N.B.), the Translational and Integrative Medicine Research Grant (J.N.B.), and P01 CA23766 from the National Cancer Institute, National Institutes of Health (J.N.B.).
Footnotes
Author Contributions: G.L.S., L.S., D.P., S.D., and J.N.B. analyzed and interpreted the data and wrote the manuscript. E.L. and M.L. analyzed the data. V.B., N.A.K., A.S., S.G., M.A.P., D.M.P. J.W.Y., MS, and GP wrote the manuscript.
Conflict of Interest: The authors have no relevant conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eapen M, Rubinstein P, Zhang M, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 2.Hwang W, Samuel M, Tan D, Koh L, Lim W, Linn Y. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. BBMT. 2007;13(4):444–53. doi: 10.1016/j.bbmt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein C, Gutman J, Weisdorf D, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–60. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponce D, Zheng J, Gonzales A, et al. Reduced late mortality risk contributes to similar survival after double-unit cord blood transplantation compared with related and unrelated donor hematopoietic stem cell transplantation. BBMT. 2011;17(9):1316–26. doi: 10.1016/j.bbmt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Aldridge J, Kim H, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. BBMT. 2012;18(5):805–12. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Barker J, Fei M, Karanes C, et al. Results of a prospective multicentre myeloablative double-unit cord blood transplantation trial in adult patients with acute leukaemia and myelodysplasia. Br J Haematol. 2014 doi: 10.1111/bjh.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks D, Woo K, Zhong X, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2014;99(2):322–8. doi: 10.3324/haematol.2013.094193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartelink I, Belitser S, Knibbe C, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. BBMT. 2013;19(2):305–13. doi: 10.1016/j.bbmt.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Danby R, Rocha V. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front Immunol. 2014;5(68) doi: 10.3389/fimmu.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson C, Turki A, McDonough S, et al. Immune Reconstitution after Double Umbilical Cord Blood Stem Cell Transplantation: Comparison with Unrelated Peripheral Blood Stem Cell Transplantation. BBMT. 2012;18(4):565–74. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanda J, Chiou L-W, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. BBMT. 2012;18(11):1664–1676e1. doi: 10.1016/j.bbmt.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oshrine B, Li Y, Teachey D, Heimall J, Barrett D, Bunin N. Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies: comparison of recipients of partially T cell-depleted peripheral blood stem cells and umbilical cord blood. BBMT. 2013;19(11):1581–9. doi: 10.1016/j.bbmt.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauter C, Abboud M, Jia X, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. BBMT. 2011;17(10):1460–71. doi: 10.1016/j.bbmt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Heijst J, Ceberio I, Lipuma L, et al. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat Med. 2013;19(3):372–7. doi: 10.1038/nm.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindemans C, Chiesa R, Amrolia P, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123(1):126–32. doi: 10.1182/blood-2013-05-50238. [DOI] [PubMed] [Google Scholar]
- 17.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. BBMT. 2009;15(10):1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):e44–100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 19.Barker J, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117(8):2332–9. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponce D, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. BBMT. 2013;19(5):799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponce D, Gonzales A, Lubin M, et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. BBMT. 2013;19(6):904–11. doi: 10.1016/j.bbmt.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J. Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J. 2005;24(1):17–23. doi: 10.1097/01.inf.0000148891.32134.36. [DOI] [PubMed] [Google Scholar]
- 24.Rowlings P, Przepiorka D, Klein J, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 25.Filipovich A, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. BBMT. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Von Gottberg A, de Gouveia L, Tempia S, et al. Effects of Vaccination on Invasive Pneumococcal Disease in South Africa. NEJM. 2014;371(20):1889–99. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- 27.Advisory Committee on Immunization Practices. CDC; 2014. Recommended Immunization Schedule for Persons Age 0 Through 18 Years. Available at http://www.cdc.gov/vaccines/schedules. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. [Google Scholar]
- 28.Elam-Evans L, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years--United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(29):625–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Seither R, Masalovich S, Knighton C, Mellerson J, Singleton J, Greby S. Vaccination coverage among children in kindergarten - United States, 2013–14 school year. MMWR Morb Mortal Wkly Rep. 2014;63(41):913–20. [PMC free article] [PubMed] [Google Scholar]
- 30.Omer S, Enger K, Moulton L, Halsey N, Stokley S, Salmon D. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168(12):1389–96. doi: 10.1093/aje/kwn263. [DOI] [PubMed] [Google Scholar]
- 31.Omer SB, Salmon DA, Orenstein WA, Halsey N. Vaccine Refusal, Mandatory Immunization, and the Risks of Vaccine-Preventable Diseases. NEJM. 2009;360(19):1981–8. doi: 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 32.Tan C, Ostrawski S, Bresnitz E. A preventable outbreak of pneumococcal pneumonia among unvaccinated nursing home residents in New Jersey during 2001. Infect Control Hosp Epidemiol. 2003;24(11):848–852. doi: 10.1086/502148. [DOI] [PubMed] [Google Scholar]
- 33.Tyrrell G, Lovgren M, Ibrahim Q, et al. Epidemic of Invasive Pneumococcal Disease, Western Canada, 2005–2009. Emerg Infect Dis. 2012;18(5):733–740. doi: 10.3201/eid1805.110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atwell J, Van Otterloo J, Zipprich J, et al. Nonmedical vaccine exemptions and pertussis in California, 2010. Pediatrics. 2013;132(4):624–630. doi: 10.1542/peds.2013-0878. [DOI] [PubMed] [Google Scholar]
- 35.Theofiles A, Cunningham S, Chia N, et al. Pertussis outbreak, southeastern Minnesota, 2012. Mayo Clin Proc. 2014;89(10):1378–88. doi: 10.1016/j.mayocp.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.California Department of Public Health. [Accessed December 23, 2014];Pertussis Report. http://www.cdph.ca.gov/programs/immunize/Documents/Pertussis_report11-26-2014.pdf. Available at: http://www.cdph.ca.gov/programs/immunize/Documents/Pertussis_report11-26-2014.pdf.
- 37.Centers for Disease Control and Prevention. [Accessed December 23, 2014];Pertussis Outbreak Trends. http://www.cdc.gov/pertussis/outbreaks/trends.html. Available at: http://www.cdc.gov/pertussis/outbreaks/trends.html.
- 38.Centers for Disease Control and Prevention. Outbreak of meningococcal disease associated with an elementary school -- Oklahoma, March 2010. MMWR Morb Mortal Wkly Rep. 2012;61(13):217–21. [PubMed] [Google Scholar]
- 39.World Health Organization. [Accessed January 14, 2015];Global Alert and Response: Meningococcal Disease. http://www.who.int/csr/don/archive/disease/meningococcal_disease/en/ Available at: http://www.who.int/csr/don/archive/disease/meningococcal_disease/en/
- 40.Centers for Disease Control and Prevention. [Accessed December 23, 2014];Serogroup B Meningococcal Vaccine & Outbreaks. http://www.cdc.gov/meningococcal/outbreaks/vaccine-serogroupb.html. Available at: http://www.cdc.gov/meningococcal/outbreaks/vaccine-serogroupb.html.
- 41.Centers for Disease Control and Prevention. Follow-up on poliomyelitis--United States, Canada, Netherlands 1979. MMWR Morb Mortal Wkly Rep. 1979;46(50):1195–9. [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Poliovirus infections in four unvaccinated children--Minnesota, August–October 2005. MMWR Morb Mortal Wkly Rep. 2005;54(41):1053–5. [PubMed] [Google Scholar]
- 43.Andre M, Wolff C, Tangermann R, et al. Assessing and mitigating the risks for polio outbreaks in polio-free countries - Africa, 2013–2014. MMWR Morb Mortal Wkly Rep. 2014;63(34):756–61. [PMC free article] [PubMed] [Google Scholar]
- 44.Arciuolo R, Brantley T, Asfaw M, et al. Notes from the field: measles outbreak among members of a religious community - Brooklyn, New York, March–June 2013. MMWR Morb Mortal Wkly Rep. 2013;62(36):752–3. [PMC free article] [PubMed] [Google Scholar]
- 45.Gastañaduy P, Redd S, Fiebelkorn A, et al. Measles - United States, January 1–May 23, 2014. MMWR Morb Mortal Wkly Rep. 2014;63(22):496–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Peltola H, Kulkarni P, Kapre S, Paunio M, Jadhav S, Dhere R. Mumps outbreaks in Canada and the United States: time for new thinking on mumps vaccines. Clin Infect Dis. 2007;45(4):459–66. doi: 10.1086/520028. [DOI] [PubMed] [Google Scholar]
- 47.Barskey A, Schulte C, Rosen J, et al. Mumps outbreak in Orthodox Jewish communities in the United States. NEJM. 2012;367(18):1704–13. doi: 10.1056/NEJMoa1202865. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. [Accessed December 23, 2014];Mumps Cases and Outbreaks. http://www.cdc.gov/mumps/outbreaks.html#outbreaks. Available at: http://www.cdc.gov/mumps/outbreaks.html#outbreaks.
- 49.Fischetti M. Too Many Children Go Unvaccinated. Sci Am. 2013;308(6) doi: 10.1038/scientificamerican0613-58. Available at http://www.scientificamerican.com/art. Available at: http://www.scientificamerican.com/article/too-many-children-go-unvaccinated/ [DOI] [PubMed] [Google Scholar]
- 50.Hartocollis A. Measles Outbreak May Have Spread in Medical Facilities, a City Official Says. The New York Times. 2014 Mar 19; Available at http://www.nytimes.com/2014/03/1.
- 51.Offit P. [Accessed December 23, 2014];The Anti-Vaccine Epidemic. http://online.wsj.com/articles/paul-a-offit-the-anti-vaccination-epidemic-1411598408. Available at: http://online.wsj.com/articles/paul-a-offit-the-anti-vaccination-epidemic-1411598408.
- 52.CHILD Inc. [Accessed December 12, 2014];Some Outbreaks of Vaccine-Preventable Disease in Groups with Religious or Philosophical Exemptions to Vaccination. http://childrenshealthcare.org/?page_id=200. Available at: http://childrenshealthcare.org/?page_id=200.
- 53.Orenstein W, Seib K. Mounting a good offense against measles. NEJM. 2014;371(18):1661–1663. doi: 10.1056/NEJMp1408696. [DOI] [PubMed] [Google Scholar]
- 54.Machado C. Measles in bone marrow transplant recipients during an outbreak in Sao Paulo, Brazil. Blood. 2002;99(1):83–87. doi: 10.1182/blood.V99.1.83. [DOI] [PubMed] [Google Scholar]
- 55.California Department of Public Health. [Accessed January 14, 2015];California Department of Public Health Confirms Measles Cases. http://www.cdph.ca.gov/Pages/NR15-002.aspx. Available at: http://www.cdph.ca.gov/Pages/NR15-002.aspx.
- 56.CDC. [Accessed December 23, 2014];Measles Cases and Outbreaks. http://www.cdc.gov/measles/cases-outbreaks.html. Available at: http://www.cdc.gov/measles/cases-outbreaks.html.
- 57.Abimbola S, Malik A, Mansoor G. The final push for polio eradication: addressing the challenge of violence in Afghanistan, Pakistan, and Nigeria. PLoS Med. 2013;10(10):e1001529. doi: 10.1371/journal.pmed.1001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komanduri K, St John L, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110(13):4543–51. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiesa R, Gilmour K, Qasim W, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naïve CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. 2012;156(5):656–66. doi: 10.1111/j.1365-2141.2011.08994.x. [DOI] [PubMed] [Google Scholar]
- 60.Lindemans C, Chiesa R, Amrolia P, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123(1):126–32. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 61.Rogosheske J, Fargen A, DeFor T, et al. Higher therapeutic CsA levels early post transplantation reduce risk of acute GVHD and improves survival. BBMT. 2014;49(1):122–5. doi: 10.1038/bmt.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bejanyan N, Rogosheske J, Defor T, et al. Less Acute Graft-Versus-Host Disease with Higher Mycophenolate Dose in. BBMT. 2014;20(2)(suppl):S28–S29. [Google Scholar]
- 63.Harnicar S, Ponce D, Mathew S, et al. Higher Mycophenolic Acid (MPA) Trough Levels Result in Lower Day 100 Severe Acute Graft-Versus-Host Disease (aGVHD) without Increased Toxicity in Double-Unit Cord Blood Transplantation (CBT) Recipients. BBMT. 2014;20(2)(suppl):S52–S53. doi: 10.1016/j.bbmt.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ponce D, Hilden P, Mumaw C, et al. High Day 28 ST2 Biomarker Levels Predict Severe Day 100 Acute Graft-Versus-Host Disease and Day 180 Transplant-Related Mortality after Double-Unit Cord Blood Transplantation. BBMT. 2014;20(2)(suppl):S278–S279. doi: 10.1182/blood-2014-06-584789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pao M, Papadopoulos EB, Chou J, et al. Response to pneumococcal (PNCRM7) and haemophilus influenzae conjugate vaccines (HIB) in pediatric and adult recipients of an allogeneic hematopoietic cell transplantation (alloHCT) BBMT. 2008;14(9):1022–30. doi: 10.1016/j.bbmt.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomczyk S, Bennett N, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63(37):822–5. [PMC free article] [PubMed] [Google Scholar]
- 67.Bonten M, Bolkenbaas M, Huijts S, et al. Community Aquired Pneumonia Immunisation Trial in Adults (CAPITA) Pneumonia. 2014;3:95. Abst 0541. [Google Scholar]
- 68.Cordonnier C, Ljungman P, Juergens C, et al. Immunogenicity, Safety, and Tolerability of 13-Valent Pneumococcal Conjugate Vaccine Followed by 23-Valent Pneumococcal Polysaccharide Vaccine in Recipients of Allogeneic Hematopoietic Stem Cell Transplant Aged >=2 Years: An Open-Label Study. Clin Infect Dis. 2015:1–11. doi: 10.1093/cid/civ287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. BBMT. 2014;20(2):285–7. doi: 10.1016/j.bbmt.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 70.Small T, Cowan M. Immunization of hematopoietic stem cell transplant recipients against vaccine-preventable diseases. Expert Rev Clin Immunol. 2011;7(2):193–203. doi: 10.1586/eci.10.103. [DOI] [PubMed] [Google Scholar]
- 71.Papadopoulos E, Iovino CS, Knowles M, et al. Pertussis Response to Tdap (8 mcg/dose of the Pertussis Toxoid (PT) In Recipients of Allogeneic Hematopoietic Stem Cell Transplants (Allo HSCT). 52nd Annual Meeting of the American-Society-of-Hematology (ASH); Orlando, FL, USA. 2010; p. Abstract 1282. [Google Scholar]
- 72.Boles E, Chiuzan C, Ragucci D, Hudspeth M. Analysis of factors affecting immune recovery and initial response to tetanus after DTaP vaccination in pediatric allogeneic HSCT patients. Pediatr Transpl. 2014 doi: 10.1111/petr.12361. [DOI] [PubMed] [Google Scholar]
- 73.Molrine D, Guinan E, Antin J, et al. Donor immunization with Haemophilus influenzae type b (HIB)-conjugate vaccine in allogeneic bone marrow transplantation. Blood. 1996;87(7):3012–3018. [PubMed] [Google Scholar]
- 74.Parkkali T, Käyhty H, Hovi T, et al. A randomized study on donor immunization with tetanus-diphtheria, Haemophilus influenzae type b and inactivated poliovirus vaccines to improve the recipient responses to the same vaccines after allogeneic bone marrow transplantation. BMT. 2007;39(3):179–188. doi: 10.1038/sj.bmt.1705562. [DOI] [PubMed] [Google Scholar]
- 75.Ljungman P, Duraj V, Magnius L. Response to immunization against polio after allogeneic marrow transplantation. BMT. 1991;7(2):89–93. [PubMed] [Google Scholar]
- 76.Machado C, Rocha I, Diomede B, et al. Effectiveness of hepatitis B vaccination and persistence of immunity after BMT (Abstract). The Ninth International Symposium on Infections in the Immunocompromised Host Assisi; Italy. 1996; Abstract. [Google Scholar]
- 77.Jaffe D, Papadopoulos E, Young J, et al. Immunogenicity of recombinant hepatitis B vaccine (rHBV) in recipients of unrelated or related allogeneic hematopoietic cell (HC) transplants. Blood. 2006;108(7):2470–2475. doi: 10.1182/blood-2006-04-006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ljungman P, Fridell E, Lönnqvist B, et al. Efficacy and safety of vaccination of marrow transplant recipients with a live attenuated measles, mumps, and rubella vaccine. J Infect Dis. 1989;159(4):610–615. doi: 10.1093/infdis/159.4.610. [DOI] [PubMed] [Google Scholar]
- 79.Pauksen K, Linde A, Ljungman P, et al. Specific T and B cell immunity to measles after allogeneic and autologous bone marrow transplantation. BMT. 1995;16(6):807–813. [PubMed] [Google Scholar]
- 80.King S, Saunders E, Petric M, Gold R. Response to measles, mumps and rubella vaccine in paediatric bone marrow transplant recipients. BMT. 1996;17(4):633–636. [PubMed] [Google Scholar]
- 81.Ljungman P, Cordonnier C, Einsele H, et al. Vaccination of hematopoietic cell transplant recipients. BMT. 2009;44(8):521–6. doi: 10.1038/bmt.2009.263. [DOI] [PubMed] [Google Scholar]
- 82.Ljungman P, Small TN. Update to vaccination guidelines. BBMT. 2010;16(11):1608–9. doi: 10.1016/j.bbmt.2010.08.009. [DOI] [PubMed] [Google Scholar]