Abstract
Objective
The transition from childhood to teenaged years is associated with increased testosterone and a decreased iron status. It is not clear whether higher testosterone levels cause the decreased iron status, and to what extent, obesity-related inflammation influences the iron-testosterone relationship. The aim of the present study was to examine relationships of testosterone, iron status, and anti-/proinflammatory cytokines in relation to nutritional status in boys and young adolescent Taiwanese males.
Methods
In total, 137 boys aged 7~13 yr were included. Parameters for obesity, the iron status, testosterone, and inflammatory markers were evaluated.
Results
Overweight and obese (ow/obese) boys had higher mean serum testosterone, interleukin (IL)-1β, and nitric oxide (NO) levels compared to their normal-weight counterparts (all p<0.05). Mean serum ferritin was slightly higher in ow/obese boys compared to normal-weight boys, but this did not reach statistical significance. A multiple linear regression showed that serum ferritin (β = -0.7470, p = 0.003) was inversely correlated with testosterone, while serum IL-10 (β = 0.3475, p = 0.009) was positively associated with testosterone after adjusting for covariates. When normal-weight boys were separately assessed from ow/obesity boys, the association between testosterone and serum ferritin became stronger (β = -0.9628, p<0.0001), but the association between testosterone and IL-10 became non-significant (β = 0.1140, p = 0.4065) after adjusting for covariates. In ow/obese boys, only IL-10 was weakly associated with serum testosterone (β = 0.6444, p = 0.051) after adjusting for age.
Conclusions
Testosterone and serum ferritin are intrinsically interrelated but this relationship is weaker in ow/obese boys after adjusting for age.
Introduction
Associations between androgens and erythropoiesis have been known for more than half a century [1]. Low testosterone levels are a potential risk factor for anemia in older men and women [2]. In particular, hypogonadal men have a 5-fold (1.41~21.8) higher risk of anemia compared to eugonadal men [2]. Testosterone administration to hypogonadal men induces erythropoiesis via increased erythropoietin (EPO) and inhibited hepcidin levels [3,4]. Low hepcidin, a key regulator of iron metabolism, leads to a higher iron absorption rate in the small intestine. EPO can increase iron incorporation into red blood cells (RBCs) in the bone marrow [5]. It is also recognized that iron may exert specific effects on androgen. For example, a pituitary iron overload predicts hypogonadism in thalassemia patients with transfusional iron overload [6]. Liver iron overload is associated with increased sex hormone-binding globulin (SHBG) and moderate hypogonadotropic hypogonadism in men with non-genetically dysmetabolic iron overload syndrome (DIOS) [7]. Eugonadal men with iron-deficiency anemia (IDA) who received intravenous iron therapy (800~1200 mg elemental iron) for 12 weeks exhibited increased levels of testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and sperm parameters [8].
Obesity is frequently associated with low testosterone [9] and high serum ferritin levels [10]. Both testosterone and iron may interact with inflammatory responses. Testosterone can suppress proinflammatory responses but upregulates immunomodulatory cytokines such as interleukin (IL)-10 [11,12]. Proinflammatory cytokines are potent regulators of serum ferritin and hepcidin. Hepcidin plays a key role in the innate and adaptive immunities [13]. Elevated serum ferritin can function as a proinflammatory modulator by upregulating IL-1β, tumor necrosis factor (TNF)-α, and nitric oxide (NO) transcriptional activity [14,15].
The transition from childhood to teenaged years is associated with increased testosterone and a decreased iron status. Currently, it is not clear whether higher testosterone levels cause the decreased iron status, and to what degree obesity-related inflammation influences the iron-testosterone relationship in young boys. The broad aims of this study were: 1) to investigate the relationship between testosterone and the iron status in terms of the nutritional status; and 2) to evaluate the effects of anti-/proinflammatory cytokines on testosterone levels in boys and young adolescent males.
Materials and Methods
Study participants
In total, 137 (71 normal-weight and 66 overweight and obese (ow/obese)) boys were included in the analysis: 36 boys were aged 7.43±0.56 yr (20 normal weight and 16 ow/obese), 46 boys were aged 10.68±0.51 yr (27 normal weight and 19 ow/obese), and 56 young adolescents were aged 13.11±1.08 yr (23 normal weight and 33 ow/obese). The study was approved by the Research Ethics Committee of Taipei Medical University (201204011). Informed parental written consent was obtained before enrollment in the study.
Data collection
Details of data collection were previously described elsewhere [16]. Age- and sex-specific cutoff points for the body-mass index (BMI) were used to define overweight and obesity in boys and adolescent males according to guidelines of the Department of Health, Taiwan (Table 1) [17,18]. The BMI was calculated as the mass (kg)/[height (m)]2.
Table 1. Age- and gender-specific cutoff points for the body-mass index (BMI) for overweight and obese boys and young adolescents according to guidelines of the Department of Health, Taiwan.
| BMI (kg/m2) | |||
|---|---|---|---|
| Age (years) | Normal | Overweight | Obese |
| 7 | 14.7~18.5 | ≥18.6 | ≥21.2 |
| 8 | 15.0~19.2 | ≥19.3 | ≥22.0 |
| 9 | 15.2~19.6 | ≥19.7 | ≥22.5 |
| 10 | 15.4~20.2 | ≥20.3 | ≥22.9 |
| 11 | 15.8~20.9 | ≥21.0 | ≥23.5 |
| 12 | 16.4~21.4 | ≥21.5 | ≥24.2 |
| 13 | 17.0~22.1 | ≥22.2 | ≥24.8 |
Blood biochemical assessment
Fasting blood samples were collected in vacuum tubes containing EDTA. All blood samples were separated into RBCs and serum, and stored at -80°C until being analyzed. Serum IL-1β, interferon (IFN)-γ, and IL-10 levels were determined by enzyme-linked immunosorbent assay (ELISA) kits (Procarta Cytokine Assay Kit; Affymetrix, Santa Clara, CA, USA) according to the manufacturer’s instructions. As an indicator of NO production, the nitrite concentration in the serum was determined with the Griess reagent (Sigma-Aldrich, St. Louis, MO, USA). Serum hepcidin was assessed by an ELISA (DRG International, Marburg, Germany). Serum ferritin was measured using a commercially available electrochemiluminescence immunoassay and was quantitated with a Roche Modular P800 analyzer (Mannheim, Germany). Serum iron and the total iron-binding capacity (TIBC) were measured by a ferrozine-based colorimetric method. The percent of transferrin saturation (%TS) was calculated by [serum iron/TIBC] x 100%. Serum testosterone was measured by an electrochemiluminescence immunoassay and was quantitated by a Modular analytics cobas E601 analyzer (Roche).
Statistical analysis
Statistical analyses were performed using the Statistical Analysis Systems software (SAS vers. 9.22; SAS Institute, Cary, NC, USA). Continuous data are presented as the mean±standard deviation (SD) and were assessed by an unpaired Student’s t-test. Variables not normally distributed were natural log-transformed to achieve a normal distribution and to allow the use of parametric tests. Associations between the serum testosterone concentration and other laboratory parameters were assessed using Pearson’s rank correlation coefficients. A multivariate linear regression model was used to examine relationships between the dependent variable (serum testosterone) and potential variables including age, BMI, iron parameters, and inflammatory cytokines. p<0.05 was considered statistically significant.
Results
Baseline characteristics
In total, 137 boys participated in this study. The mean age was 10.48±0.26 yr and the mean BMI was 20.2±4.1 kg/m2. The mean serum testosterone was 4.1±5.9 nmol/L, and mean serum ferritin was 151.9±130.3 pmol/L. Ow/obese boys had higher serum testosterone concentrations compared to their normal-weight counterparts (Table 2). The mean serum ferritin was slightly higher in ow/obese boys compared to normal-weight boys, but this did not reach statistical significance (Table 2). There were no significant differences in age, serum iron, TIBC, %TS, hepcidin, IFN-γ, or IL-10 between normal weight and ow/obese boys (Table 2). Compared to their normal-weight counterparts, ow/obese boys had higher levels of IL-1β and NO (both p<0.05; Table 2).
Table 2. Clinical and biochemical data according to the nutritional status (N = 137).
| Variable a | Boys (N = 137) | ||
|---|---|---|---|
| Normal (n = 71) | Ow/obese (n = 66) | p value b | |
| Age (yr) | 10.13 (0.28) | 10.82 (0.30) | 0.093 |
| Body-mass index (kg/m2) | 17.20 (2.10) | 24.30 (5.60) | <0.0001 |
| Log serum iron (μmol/L) | 0.79 (0.01) | 0.80 (0.01) | 0.694 |
| Log serum TIBC (μmol/L) c | 1.04 (0.00) | 1.04 (0.00) | 0.960 |
| Log serum ferritin (pmol/L) | 8.89 (0.18) | 9.32 (0.13) | 0.068 |
| Log transferrin saturation (%) | 3.24 (0.05) | 3.27 (0.05) | 0.701 |
| Log hepcidin (ng/ml) | 4.43 (0.08) | 4.29 (0.10) | 0.291 |
| Log interleukin-1β (pg/ml) | 0.11 (0.08) | 0.49 (0.46) | 0.044 |
| Log interferon-γ (pg/ml) | 1.55 (0.09) | 1.46 (0.78) | 0.554 |
| Log nitric oxide (μM) | 1.40 (0.11) | 1.97 (0.09) | 0.031 |
| Log interleukin-10 (pg/ml) | 1.44 (10.24) | 1.07 (10.19) | 0.401 |
| Log testosterone (nmol/L) | 0.10 (0.02) | 0.50 (0.08) | 0.005 |
a Mean (standard deviation).
b According to an unpaired Student’s t-test.
c TIBC, total iron-binding capacity.
Ow, overweight.
Distributions of testosterone, iron parameters, and cytokines in relation to age and the nutritional status
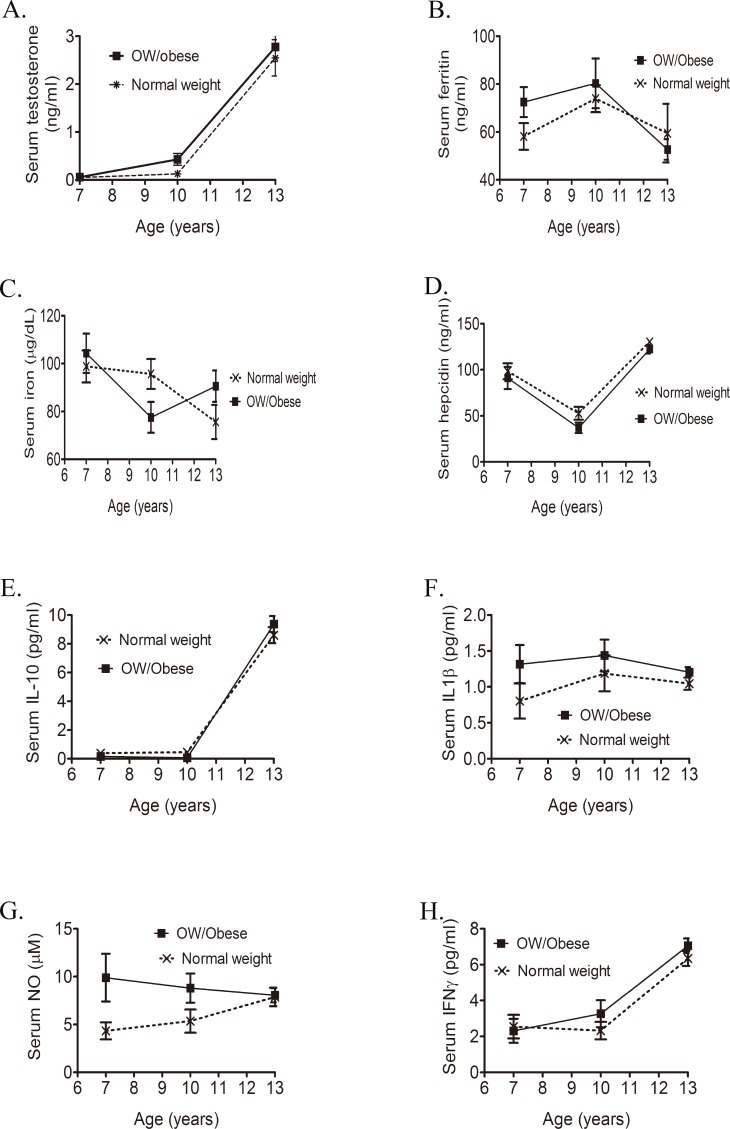
We next evaluated distributions of testosterone, iron parameters, and inflammatory cytokines stratified by age and BMI (Table 3). Distributions of serum testosterone (A), IL-10 (E), and IFN-γ (H) were positively associated with age and, to a lesser extent, BMI (Fig 1). In contrast, serum ferritin and serum iron concentrations sharply decreased in those aged 13 yr (Fig 1B and 1C). A V-shaped hepcidin curve was found in both normal-weight and ow/obese boys (Fig 1D). Distributions of serum IL-1β (F) and NO (G) remained stable during the transition from childhood to teenaged years (Fig 1).
Table 3. Biochemical characteristics of study participants according to their age and nutritional status.
| Variable a | Boys | Age (years) | ||
| 7.4 (0.6) | 10.7 (0.5) | 13.1 (1.1) | ||
| BMI (kg/m2) | normal | 15.5 (1.9) | 17.2 (2.2) | 18.3 (3.5) |
| ow/obese b | 20.6 (3.1)*** | 23.1 (3.8)*** | 25.0 (4.6)*** | |
| Serum iron (μmol/L) | normal | 16.3 (1.2) | 17.2 (1.2) | 13.5(1.3) |
| ow/obese | 18.7 (1.5) | 14.4 (1.1) | 16.2 (1.2) | |
| Serum ferritin (pmol/L) | normal | 130.8 (12.6) | 166.1 (13.3) | 133.7 (27.6) |
| ow/obese | 162.9 (14.2) | 186.1 (24.0) | 118.4 (9.7) | |
| Transferrin saturation (%) | normal | 29.9 (1.8) | 31.0 (2.0) | 22.1 (2.3) |
| ow/obese | 33.3 (2.7) | 25.6 (2.1) | 27.5 (2.1) | |
| Hepcidin (ng/ml) | normal | 98.3 (8.6) | 56.7 (6.6) | 130.5 (4.0) |
| ow/obese | 91.1 (12.0) | 46.0 (6.1) | 122.5 (4.8) | |
| Testosterone (nmol/L) | normal | 0.2 (0.0) | 0.4 (0.1) | 8.8 (1.3) |
| ow/obese b | 0.2 (0.1) | 1.4 (0.5)* | 9.6 (1.1) | |
| NO (μM) | normal | 4.3 (0.9) | 5.6 (1.2) | 7.9 (1.0) |
| ow/obese | 9.9 (2.5) | 7.8 (1.2) | 8.1 (0.7) | |
| IL1β (pg/ml) | normal | 0.8 (0.2) | 1.1 (0.2) | 1.0 (0.1) |
| ow/obese | 1.3 (0.3) | 1.4 (0.2) | 1.2 (0.1) | |
| IFNγ (pg/ml) | normal | 1.2 (0.4) | 3.1 (0.7) | 17.9 (1.5) |
| ow/obese b | 1.1 (0.4) | 2.3 (0.6) | 25.6 (1.7)** | |
| IL10 (pg/ml) | normal | 0.39 (0.12) | 0.43 (0.13) | 8.6 (0.6) |
| ow/obese b | 0.23 (0.13) | 0.08 (0.05)* | 9.4 (0.6) | |
a Mean (standard deviation).
b Unpaired student’s t-test for comparing normal and overweight (ow)/obese boys in the same age group
* p<0.05
** p<0.01
*** p<0.001.
Fig 1. Distributions of serum testosterone (A), ferritin (B), iron (C) hepcidin (D), interleukin (IL)-10 (E), IL-1β (F), nitric oxide (NO) (G), and interferon (IFN)-γ (H) stratified by age and the body-mass index (BMI) (n = 137).
Serum ferritin is independently associated with testosterone in normal-weight boys
Pearson’s rank correlations analysis showed a strong positive correlation between serum testosterone and IL-10 (r = 0.3082), and a significant inverse relationship between serum testosterone and serum ferritin (r = -0.2821) after adjusting for age and the BMI (Table 4, adjusted; both p<0.01). We next performed a multiple linear regression analysis to predict variants that were independently associated with testosterone concentrations. After adjusting for covariates, serum ferritin (β = -0.7470, p = 0.0003) was inversely correlated with testosterone, while serum IL-10 (β = 0.3475, p = 0.009) was positively associated with testosterone (Table 5, pooled, multivariant). When normal-weight boys were assessed separately from ow/obese boys, the association between testosterone and serum ferritin (β = -0.9628, p<0.0001) became stronger after adjusting for covariates (Table 5, normal weight, multivariant). However, the association between testosterone and IL-10 (β = 0.1140, p = 0.4065) became non-significant after adjusting for age and serum ferritin. In ow/obese boys, only IL-10 was weakly associated with serum testosterone (β = 0.6444, p = 0.051) after adjusting for age (Table 5, ow/obese).
Table 4. Pearson’s rank correlation coefficient and partial r of log-transformed serum testosterone with selected iron statuses and inflammatory cytokines in 137 boys.
| Variable | Boys (log testosterone) | |||
|---|---|---|---|---|
| Crude | Adjusted* | |||
| r | p value | r | p value | |
| Age | 0.7789 | <0.0001 | - | - |
| Log serum iron (μmol/L) | -0.0912 | 0.289 | 0.1403 | 0.103 |
| Log serum TIBC (μmol/L) | 0.1981 | 0.020 | 0.0749 | 0.385 |
| Log serum ferritin (pmol/L) | -0.3458 | <0.0001 | -0.2821 | 0.001 |
| Log transferrin saturation (%) | 0.1054 | 0.221 | 0.1054 | 0.221 |
| Log hepcidin (ng/ml) | 0.3399 | 0.0001 | 0.1547 | 0.092 |
| Log interleukin-1β (pg/ml) | -0.1080 | 0.252 | -0.035 | 0.712 |
| Log interferon-γ (pg/ml) | 0.3881 | <0.0001 | -0.076 | 0.413 |
| Log nitric oxide (μM) | 0.0560 | 0.533 | -0.1074 | 0.233 |
| Log interleukin-10 (pg/ml) | 0.7501 | <0.0001 | 0.3082 | 0.003 |
*Adjusted for age and the body-mass index.
TIBC, total iron-binding capacity.
Table 5. Multivariate regression coefficients for log-transformed serum testosterone in relation to the nutritional status in 137 boys.
| Pooled | Crude | Age-adjusted | Multivariant * | |||
| β | p value | β | p value | β | p value | |
| Log serum iron (μmol/L) | -0.4765 | 0.289 | 0.4725 | 0.103 | ||
| Log serum TIBC (μmol/L) | 2.7230 | 0.020 | 0.6588 | 0.385 | ||
| Log serum ferritin (pmol/L) | -1.0990 | <0.0001 | -0.5700 | 0.001 | -0.7470 | 0.0003 |
| Log transferrin saturation | -0.7537 | 0.071 | 0.3351 | 0.221 | ||
| Log hepcidin (ng/ml) | 0.9321 | 0.0001 | 0.2767 | 0.092 | ||
| Log interleukin-1β (pg/ml) | -0.4033 | 0.252 | -0.0863 | 0.712 | ||
| Log interferon-γ (pg/ml) | 1.02901 | <0.0001 | -0.1507 | 0.712 | ||
| Log nitric oxide (μM) | 0.1311 | 0.533 | -0.1584 | 0.233 | ||
| Log interleukin-10 (pg/ml) | 0.9667 | <0.0001 | 0.4215 | 0.003 | 0.3475 | 0.009 |
| Normal weight | Crude | Age-adjusted | Multivariant * | |||
| β | p value | β | p value | β | p value | |
| Log serum iron (μmol/L) | -0.8392 | 0.133 | 0.31849 | 0.391 | ||
| Log serum TIBC (μmol/L) | 3.5131 | 0.029 | 1.4981 | 0.151 | ||
| Log serum ferritin (pmol/L) | -1.2020 | <0.0001 | -0.8419 | <0.0001 | -0.9628 | <0.0001 |
| Log transferrin saturation | -1.1557 | 0.029 | 0.1259 | 0.729 | ||
| Log hepcidin (ng/ml) | 1.3455 | 0.003 | 0.5173 | 0.079 | ||
| Log interleukin-1β (pg/ml) | -0.7039 | 0.131 | -0.4281 | 0.166 | ||
| Log interferon-γ (pg/ml) | 0.6818 | 0.058 | -0.2466 | 0.316 | ||
| Log nitric oxide (μM) | 0.3145 | 0.268 | -0.2466 | 0.316 | ||
| Log interleukin-10 (pg/ml) | 0.8792 | <0.0001 | 0.3249 | 0.042 | 0.1140 | 0.4065 |
| Overweight and obese | Crude | Age-adjusted | Multivariant | |||
| β | p value | β | p value | β | p value | |
| Log serum iron (μmol/L) | -0.0432 | 0.951 | 0.6006 | 0.189 | ||
| Log serum TIBC (μmol/L) | 2.0027 | 0.220 | -0.0153 | 0.988 | ||
| Log serum ferritin (pmol/L) | -1.0795 | 0.0272 | -0.0899 | 0.790 | ||
| Log transferrin saturation | -0.3287 | 0.601 | 0.4893 | 0.236 | ||
| Log hepcidin (ng/ml) | 0.8427 | 0.002 | 0.2598 | 0.195 | ||
| Log interleukin-1β (pg/ml) | -0.1064 | 0.843 | 0.2813 | 0.430 | ||
| Log interferon-γ (pg/ml) | 1.3770 | <0.0001 | 0.1391 | 0.623 | ||
| Log nitric oxide (μM) | -0.2278 | 0.470 | -0.3408 | 0.091 | ||
| Log interleukin-10 (pg/ml) | 1.0900 | <0.0001 | 0.6444 | 0.051 | ||
# Overweight and obese: body-mass index of ≥85th percentile of the age- and Sex-specific value.
* Multivariate model adding age, serum ferritin, and interleukin-10.
Discussion
Our study indicated that testosterone and serum ferritin are intrinsically interrelated, but this relationship became weaker in ow/obese boys after adjusting for age. It has long been speculated that sex hormones may interact with iron at the systemic level, but the effects of obesity on this relationship are not clear. Obesity is associated with decreased serum testosterone but increased serum ferritin levels [7]. Elevated serum ferritin, an acute-phase reactant, is strongly associated with central obesity and metabolic syndrome [10,19–21]. A recent study involving 1999 healthy Chinese adult men showed that serum ferritin levels were inversely correlated with testosterone, free testosterone, and SHBG levels [22]. Our study in normal-weight boys and adolescent males in Taiwan confirmed this relationship. Other studies showed that serum ferritin levels significantly decreased in elderly obese hypogonadal men who received testosterone therapy [3,4]. These data suggest that testosterone exerts a direct regulatory function on ferritin synthesis, and decreased testosterone may lead to higher serum ferritin levels in obese men. Whether elevated serum ferritin further downregulates testosterone synthesis remains unclear. Overall, our study, together with others, suggests that the testosterone-ferritin axis may play an important role in maintaining physiological androgen function in boys.
Our study is in agreement with results reported in elderly men in whom testosterone and iron levels are closely associated [3]. Aging may affect this relationship, but the mechanisms underlying age-related differences in the erythropoietic response to testosterone are unknown [23,24]. Elderly men experience a decline in testosterone and iron levels and pathophysiological changes that may accompany this decline. The presence of chronic inflammation leads to elevated serum hepcidin levels and anemia of chronic inflammation in the elderly [25]. In addition, aging also affects hemopoietic stem cell production and the endocrine milieu (e.g., EPO secretion) [25]. Coviello and colleagues compared the effects of testosterone therapy on erythropoiesis in young and older men and reported that testosterone-induced increases in the hemoglobin (Hb) and hematocrit levels are more pronounced in older men [23]. However, the greater increase in the Hb level observed in older men during testosterone therapy was not explained by changes in EPO [23]. Interestingly, Bachman et al. showed that greater increases in Hb and hematocrit levels in older men during 20 weeks of testosterone therapy were related to greater suppression of serum hepcidin levels in older men than in young men [24]. In our study, the crude analysis of pooled samples showed a significant positive association between testosterone and hepcidin levels, but this relationship became non-significant after adjusting for age. When elementary school boys (aged 7 and 10 yrs) were separately from junior high school (aged 13 yrs), a significant inverse relationship between testosterone and hepcidin was found in elementary school boys (r = -0.405; p = 0.0027), which remained significant after adjusting for age and BMI (r = -0.376; p = 0.048) (data not shown). No significant difference was found in junior high school boys (r = 0.126; p = 0.623). Overall, these data suggest that the relationship between testosterone and hepcidin is age-related, and biological changes that occur during puberty may transiently alter this relationship.
Our study found a positive relationship between IL-10 and testosterone. We hypothesized that the effect of IL-10 on testosterone might not be direct, but rather, indirect via interacting with serum ferritin. The literature suggests that the interaction between serum ferritin and IL-10 is bidirectional. The ferritin H chain was shown to inhibit the immune response of lymphocytes through inducing IL-10 production [26]. However, excess IL-10 may also cause hyperferritinemia. An in vitro study showed that recombinant IL-10 treatment directly stimulated ferritin translation in human monocytic cells [27]. A human study reported that IL-10 supplementation is associated with increased risks of hyperferritinemia and anemia in Crohn’s disease patients [27]. On the other hand, sickle cell anemia patients with iron overload, defined by elevated serum ferritin of >2247 pmol/L, had lower serum IL-10 levels compared to non-iron-overloaded patients [28]. Future studies investigating the interactive effects of IL-10 and serum ferritin on testosterone are needed in order to understand how a shift in the anti-/proinflammatory balance contributes to testosterone levels in boys and adult men.
Measuring hepcidin in biological fluids has been difficult [29]. In addition, differences in methodology and the lack of normal reference ranges for serum hepcidin hamper the use of hepcidin as a diagnostic tool and therapeutic target [30]. Mass spectrometry (MS) [31] and immunological-based assays such as ELISA [32] are two of the most-often used methods to analyze serum hepcidin levels. The circulating bioactive form of hepcidin is a small 25-amino-acid (aa) peptide. Being a small peptide, it is difficult to raise antibodies against it. The advantage of MS-based platforms is that they are able to discriminate between the bioactive 25-aa form and other smaller bioinactive isoforms (e.g., 22- and 20-aa peptides) [33]. However, MS-based assays require expensive equipment that is not widely available. According to literature reports [30,34,35], MS- and ELISA-based detecting methods yield similar results in terms of analytical variations and between-sample variations. However, some authors also observed that immunological assays tend to yield higher concentrations of hepcidin than do MS methods. This can be due to either (1) differences in the internal and external standards used by the different methods or (2) the concomitant detection of both the bioactive form of hepcidin-25 and bioinactive isoforms of hepcidin-20 and -22 by the ELISA assay. Our study used a commercially available hepcidin ELISA kit from DRG International, which is based on the principle of competitive binding. Therefore, our assay excluded prohepcidin (the 60-aa premature form of hepcidin), but may also detect isoforms hepcidin-20 and -22 in addition to hepcidin-25. The immunological assay offers a simple, accurate, and reproducible method for detecting serum hepcidin levels. Future studies on large subsets from general populations are recommended in order to establish reliable reference ranges of serum hepcidin concentrations for clinical diagnoses.
Data on obesity and androgen levels in children and adolescent boys are scarce and inconsistent [36]. Hence, causal relationships between obesity and androgen levels remain undefined. Some studies showed that obese boys had lower SHBG and total testosterone compared to normal-weight boys [36,37], but another study revealed elevated testosterone in obese children [9]. In our study, ow/obese boys had higher total testosterone levels than normal-weight boys. Testosterone is an important regulator of the body composition, particularly muscle mass and fat mass [38]. Elderly men with a low to normal gonadal status that received testosterone supplementation for 1 yr showed increased muscle mass and decreased fat mass compared to those who received a placebo [38]. Wabitsch and colleagues first demonstrated that the testosterone level is negatively associated with serum leptin in boys, and the addition of testosterone to human primary adipocytes reduced leptin secretion by up to 62% compared to a control [39]. Later, Soderberg et al. further suggested that the negative influence of testosterone on leptin production is lost with increasing adiposity [40]. These data suggest that testosterone is an important regulator of central adiposity, and decreased testosterone may increase adiposity in obese individuals.
There are several limitations to our study which need to be taken into account when interpreting the results. The small sample size and the cross-sectional nature of the study are two limitations. In order to understand the causal relationship between androgen and the iron status, a longitudinal study is needed to determine if changes in serum ferritin concentrations over time predict testosterone levels in boys. A follow-up study will also help clarify the interactive effect of serum ferritin and obesity-related inflammation (e.g., IL-10) on testosterone expression in boys. Our study did not assess the pubertal status and only measured total testosterone due to time and budget constraints. The pubertal status is known to affect testosterone levels and the iron status. Despite the relative small sample size and the lack of information on pubertal development and other sex steroid hormones, we still observed a significant inverse relationship between testosterone and serum ferritin in boys. This suggests there are strong cross-talk signals between sex hormones and ferritin at the systemic level.
Conclusions
Overall, our study results suggest that serum ferritin independently predicted testosterone levels but this relationship became weaker in ow/obese boys after adjusting for age. Understanding the interactive relationship between serum ferritin and testosterone may help clarify the etiology of obesity-related hypogonadism.
Acknowledgments
We express our sincere appreciation to the study participants. We also wish to thank all of the staff at Taipei Medical Hospital for their support.
Abbreviations
- BMI
body-mass index
- DIOS
dysmetabolic iron overload syndrome
- EPO
erythropoietin
- FSH
follicle-stimulating hormone
- IDA
iron-deficiency anemia
- IFN-γ
interferon gamma
- IL-1β
interleukin-1β
- IL-10
interleukin-10
- LH
luteinizing hormone
- NO
nitric oxide
- ow/obese
overweight and obese
- RBCs
red blood cells
- SF
serum ferritin
- SHBG
sex hormone-binding globulin
- TIBC
total iron-binding capacity
- TNF-α
tumor necrosis factor-α
- %TS
percent transferring saturation
Data Availability
All relevant data are within the paper.
Funding Statement
Dr. Jung-Su Chang was supported by grants from Taipei Medical University Hospital (103TMU-TMUH-11 and 104TMU-TMUH-18) and the Ministry of Science and Technology, Taiwan (MOST 103-2320-B-038-015 and MOST 104-2311-B-038-005).
References
- 1. Hawkins WW, Speck E, Leonard VG. Variation of the hemoglobin level with age and sex. Blood 1954, 9:999–1007. [PubMed] [Google Scholar]
- 2. Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med 2006, 166:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachman E, Travison TG, Basaria S, Davda MN, Guo W, Li M, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014, 69:725–735. 10.1093/gerona/glt154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beggs LA, Yarrow JF, Conover CF, Meuleman JR, Beck DT, Morrow M, et al. Testosterone alters iron metabolism and stimulates red blood cell production independently of dihydrotestosterone. Am J Physiol Endocrinol Metab 2014, 307:E456–461. 10.1152/ajpendo.00184.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo W, Bachman E, Li M, Roy CN, Blusztajn J, Wong S, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell 2013, 12:280–291. 10.1111/acel.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noetzli LJ, Panigrahy A, Mittelman SD, Hyderi A, Dongelyan A, Coates TD, et al. Pituitary iron and volume predict hypogonadism in transfusional iron overload. Am J Hematol 2012, 87:167–171. 10.1002/ajh.22247 [DOI] [PubMed] [Google Scholar]
- 7. Gautier A, Laine F, Massart C, Sandret L, Piguel X, Brissot P, et al. Liver iron overload is associated with elevated SHBG concentration and moderate hypogonadotrophic hypogonadism in dysmetabolic men without genetic haemochromatosis. Eur J Endocrinol 2011, 165:339–343. 10.1530/EJE-11-0215 [DOI] [PubMed] [Google Scholar]
- 8. Soliman A, Yassin M, De Sanctis V. Intravenous iron replacement therapy in eugonadal males with iron-deficiency anemia: Effects on pituitary gonadal axis and sperm parameters; A pilot study. Indian J Endocrinol Metab 2014, 18:310–316. 10.4103/2230-8210.131158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab 2005, 90:5588–5595. [DOI] [PubMed] [Google Scholar]
- 10. Chang JS, Lin SM, Huang TC, Chao JC, Chen YC, Pan WH, et al. Serum ferritin and risk of the metabolic syndrome: a population-based study. Asia Pac J Clin Nutr 2013, 22:400–407. 10.6133/apjcn.2013.22.3.07 [DOI] [PubMed] [Google Scholar]
- 11. Ansar Ahmed S, Karpuzoglu E, Khan D. Effects of Sex Steroids on Innate and Adaptive Immunity. In Sex Hormones and Immunity to Infection. Edited by Klein SL, Roberts C: Springer Berlin; Heidelberg; 2010: 19–51 [Google Scholar]
- 12. Zhang YZ, Xing XW, He B, Wang LX. Effects of testosterone on cytokines and left ventricular remodeling following heart failure. Cell Physiol Biochem 2007, 20:847–852. [DOI] [PubMed] [Google Scholar]
- 13. Vyoral D, Petrak J. Hepcidin: a direct link between iron metabolism and immunity. Int J Biochem Cell Biol 2005, 37:1768–1773. [DOI] [PubMed] [Google Scholar]
- 14. Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, et al. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009, 49:887–900. 10.1002/hep.22716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang CM, Li SJ, Wu CH, Hu CM, Cheng HW, Chang JS. Transient knock down of Grp78 reveals roles in serum ferritin mediated pro-inflammatory cytokine secretion in rat primary activated hepatic stellate cells. Asian Pac J Cancer Prev 2014, 15:605–610. [DOI] [PubMed] [Google Scholar]
- 16. Chang JS, Chang CC, Chien EY, Lin SS, Cheng-Shiuan T, Bai CH, et al. Association between interleukin 1beta and interleukin 10 concentrations: a cross-sectional study in young adolescents in Taiwan. BMC Pediatr 2013, 13:123 10.1186/1471-2431-13-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Exceutive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 18. Tan CE, Ma S, Wai D, Chew SK, Tai ES. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004, 27:1182–1186. [DOI] [PubMed] [Google Scholar]
- 19. Chang JS, Lin SM, Chao JC, Chen YC, Wang CM, Chou NH, et al. Serum ferritin contributes to racial or geographic disparities in metabolic syndrome in Taiwan. Public Health Nutr 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord 2001, 25:639–645. [DOI] [PubMed] [Google Scholar]
- 21. Wu H, Qi Q, Yu Z, Sun L, Li H, Lin X. Opposite associations of trunk and leg fat depots with plasma ferritin levels in middle-aged and older Chinese men and women. PLOS ONE 2010, 5:e13316 10.1371/journal.pone.0013316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Z, Ye F, Zhang H, Gao Y, Tan A, Zhang S, et al. The association between the levels of serum ferritin and sex hormones in a large scale of Chinese male population. PLOS ONE 2013, 8:e75908 10.1371/journal.pone.0075908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008, 93:914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bachman E, Feng R, Travison T, Li M, Olbina G, Ostland V, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab 2010, 95:4743–4747. 10.1210/jc.2010-0864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price EA. Aging and erythropoiesis: current state of knowledge. Blood Cells Mol Dis 2008, 41:158–165. 10.1016/j.bcmd.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 26. Gray CP, Franco AV, Arosio P, Hersey P. Immunosuppressive effects of melanoma-derived heavy-chain ferritin are dependent on stimulation of IL-10 production. Int J Cancer 2001, 92:843–850. [DOI] [PubMed] [Google Scholar]
- 27. Tilg H, Ulmer H, Kaser A, Weiss G. Role of IL-10 for induction of anemia during inflammation. J Immunol 2002, 169:2204–2209. [DOI] [PubMed] [Google Scholar]
- 28. Barbosa MC, Dos Santos TE, de Souza GF, de Assis LC, Freitas MV, Goncalves RP. Impact of iron overload on interleukin-10 levels, biochemical parameters and oxidative stress in patients with sickle cell anemia. Rev Bras Hematol Hemoter 2013, 35:29–34. 10.5581/1516-8484.20130011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kroot JJ, Tjalsma H, Fleming RE, Swinkels DW. Hepcidin in human iron disorders: diagnostic implications. Clin Chem 2011, 57:1650–1669. 10.1373/clinchem.2009.140053 [DOI] [PubMed] [Google Scholar]
- 30. Kroot JJ, Kemna EH, Bansal SS, Busbridge M, Campostrini N, Girelli D, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica 2009, 94:1748–1752. 10.3324/haematol.2009.010322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood 2008, 112:4292–4297. 10.1182/blood-2008-02-139915 [DOI] [PubMed] [Google Scholar]
- 32. Macdougall IC, Malyszko J, Hider RC, Bansal SS. Current status of the measurement of blood hepcidin levels in chronic kidney disease. Clin J Am Soc Nephrol 2010, 5:1681–1689. 10.2215/CJN.05990809 [DOI] [PubMed] [Google Scholar]
- 33. Laarakkers CM, Wiegerinck ET, Klaver S, Kolodziejczyk M, Gille H, Hohlbaum AM, et al. Improved mass spectrometry assay for plasma hepcidin: detection and characterization of a novel hepcidin isoform. PLOS ONE 2013, 8:e75518 10.1371/journal.pone.0075518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van Tienoven D, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 2011, 117:e218–225. 10.1182/blood-2011-02-337907 [DOI] [PubMed] [Google Scholar]
- 35. Uijterschout L, Swinkels DW, Domellof M, Lagerqvist C, Hudig C, Tjalsma H, et al. Serum hepcidin measured by immunochemical and mass-spectrometric methods and their correlation with iron status indicators in healthy children aged 0.5–3 y. Pediatr Res 2014, 76:409–414. 10.1038/pr.2014.109 [DOI] [PubMed] [Google Scholar]
- 36. Vandewalle S, De Schepper J, Kaufman JM. Androgens and obesity in male adolescents. Curr Opin Endocrinol Diabetes Obes 2015, 22:230–237. 10.1097/MED.0000000000000160 [DOI] [PubMed] [Google Scholar]
- 37. Mogri M, Dhindsa S, Quattrin T, Ghanim H, Dandona P. Testosterone concentrations in young pubertal and post-pubertal obese males. Clin Endocrinol (Oxf) 2013, 78:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 2003, 58:618–625. [DOI] [PubMed] [Google Scholar]
- 39. Wabitsch M, Blum WF, Muche R, Braun M, Hube F, Rascher W, et al. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J Clin Invest 1997, 100:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soderberg S, Olsson T, Eliasson M, Johnson O, Brismar K, Carlstrom K, et al. A strong association between biologically active testosterone and leptin in non-obese men and women is lost with increasing (central) adiposity. Int J Obes Relat Metab Disord 2001, 25:98–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.