Abstract
We discover and examine within a wide phylogenetic perspective spatial neophobia, avoidance of untrodden terrain, in fruit flies, in an experimental setup that reduces the gap between the field and the laboratory. In our setup, fruit flies use a natal fruit as their origin, freely exploring for days their surroundings, which consists of a mixture of trodden and untrodden terrain. The interface between trodden and untrodden is, however, reduced in our setup to a wide doorway, opened within a surrounding wall. Crossing this doorway, characterized by a sharp contrast interface between trodden and untrodden, generates a behavior whose dynamics betrays the flies' space neophobia. The moment-by-moment dynamics of crossing is remarkably similar to that reported in mouse models of anxiety. This means that neophobic behavior is either homologous across arthropods and vertebrates or, not less interesting, convergent, whereby the same behavior is mediated in the two phyla by two completely different schemata.
Introduction
The remarkable cognitive skills of insects have attracted the attention of naturalists for decades, including the complex routines exhibited by wasps attending to their offspring [1], the intricate navigational capacities exposed in ants [2], and the use of landmarks during return flights to a food source in honey bees [3]. Several cognitive abilities in Drosophila melanogaster behavior have been demonstrated in the laboratory. In a y-maze test, flies exhibited memory after being trained to avoid an arm coupled with electric shock, and were subsequently shown to identify and remember visual features such as odor and colored light [4], as well as size, color, and contour orientation [5] In a thermal-visual arena inspired by the Morris Water Maze [6] and the Heat Maze [7, 8], flies had to find and then exhibit memory of the location of a cool tile, based on remote spatial cues, in an otherwise hot environment [9]; male fly courtship behavior was shown to be modified by prior sexual experience [10]; and in a flight simulator tethered flies controlling the horizontal rotations of an arena exhibited memory templates [11], and discrimination and generalization learning [12].
Our aim here was to determine whether D. melanogaster distinguishes between visited and not visited (or less visited), areas of an environment; whether it behaves in a neophobic fashion and, if so, to describe the dynamics of the process and to quantify it. To achieve these aims it was necessary i) to design an experimental environment that would clearly distinguish between trodden and untrodden (unforeseen) parts of that environment: a gradual increase in activity in the untrodden part of the environment, and a concurrent gradual relative decrease in activity in the well-trodden part would suggest a change in relative preference for each of the two parts, differential recognition of each of them, spatial orientation, and choice between them; ii) to minimize coercion in order not to confound spontaneous and reactive behavior [13]; iii) to determine the time frame that would correspond to the phenomenon’s intrinsic time scale; iv) to represent behavior as a product of a dynamic interaction between the animal and the environment; and v) to allow the operational significance of the behavior to emerge.
The experimental environment consisted of a small (45mm) arena with a piece of fruit in it, connected to a large (130mm) empty arena by a wide (5mm), permanently open doorway. In nature, male fruit-flies often eclose near or on fermenting fruit, feed on the yeast that grows on it [14, 15] fight with other males, and court and copulate with females, which then complete a life cycle by oviposition on the fruit. While the fruit serves as a naturalistic attractor and center around which flies are expected to perform ethologically relevant behavior, flies are clearly not likely to encounter doorways in their natural habitat. Confronting wild organisms with supernormal stimuli that enhance their species-specific behavior to an exaggerated degree is, however, a standard methodology used by ethologists [16]. In our setup the doorway enhances the contrast between trodden and untrodden terrain, amplifying the response to the contrast. Two other ethological features of our experiment are sparing the fly a forced introduction into the setup by allowing it to eclose in the vicinity of the fruit, and perform at its own pace, without stress, for the time frame of three days found by us in preliminary experiments to be necessary for the phenomenon to unfold. The wide open doorway leading to a large empty arena provides a necessary distinction between two connected spaces. A quantification of the two-way doorway-crossing reveals the gradually changing functional permeability of the doorway and the concurrent change in the behavior of the fly in each of the two arenas. This is brought about by the fly's use of what appears to be an operation that changes the doorway's functional permeability.
Results
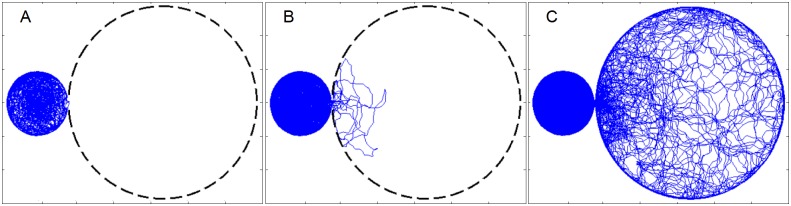
The smoothed output of the fly’s location time-series contains an average of more than 5.5 million data points that depict ca. 200 meters of walking distance covered during a single 3-day session. Fig 1 illustrates the paths traced by a selected fly in the course of 30 hours, just prior to the first entry into the large arena (1a); just after the first holding back (1b); and over the course of 60 hours (1c). To study the path’s developmental dynamics we performed low level partitioning of the path into progression and lingering segments [17] and higher level partitioning into entries (into the large arena) and departures (back to the small arena; see Methods).
Fig 1. The path traced by a fly in an experimental setup consisting of a small and a large arena connected by a 5 mm width doorway in the course of a session.
(a) after 29 hours, just before the first entry into the large arena, (b) after 36 hours, just before the first holding back, and (c) after 60 hours.
The developmental dynamics of doorway crossing in fruit flies
The flies' experimental setup comprised two arenas: a small one, in which the fly ecloses and where the fruit is located; and a large empty one connected to it by a doorway 5mm in width, about 5 times wider than the fly. We correspondingly divided the fly’s path into "entries" into the large arena and "departures" back into the small arena: an entry was defined as a segment that starts when the fly enters the large arena and ends when it leaves it; a departure was defined as a segment that starts when the fly enters the small arena and ends when it leaves it.
Our preliminary observations showed that the flies did not tend to cross into the large arena and, when they did, the first entries were short while the later ones became longer over time. Therefore, we plotted the entries successively in order to obtain a view of the developmental dynamics of entries across a session (Video A in S1 File).
Unlike the clear buildup in the extent and complexity of paths illustrated in Video A in S1 File, in the behavior of a selected fly, observation alone does not show a clear developmental trend in each of the flies; analysis of the data is necessary in order to determine whether trends exist.
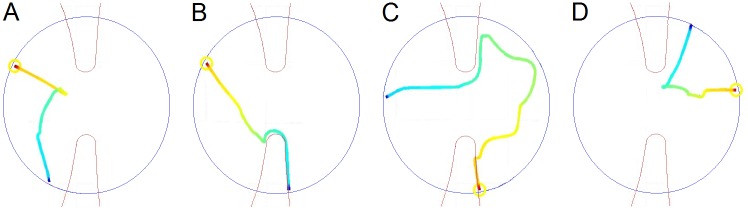
Another phenomenon that we observed in preliminary examination of the behavior was that the fly frequently arrived at the doorway but did not cross it although it could have readily done so. However, as time passed, the fly appeared more likely to cross the doorway upon approaching it. In order to test this hypothesis quantitatively, we examined what happened each time the fly approached the doorway while still in the small arena. Visits to the doorway were defined by a circular area centered at the doorway (see Methods). Once the fly had visited the defined circular area we established whether it i) began to cross the doorway into the large arena (Fig 2b); or ii) exited the circle without crossing the doorway (Fig 2a).
Fig 2. The four behavioral options exhibited by a fly upon approaching the doorway.
A distance of 8.5mm or less from the doorway’s center defines proximity to it. Path segments traced by the fly are colored from yellow (the beginning of the segment) to light blue (the end of the segment). The fly approaches the doorway (a) from the small arena but does not cross to the large arena, (b) from the small arena crossing into the large arena (cutting through), (c) from the large arena crossing into the small arena (d), from the large arena but does not cross to the small arena (holding back).
One approach to the sequence of visits was to partition it into a specific number of bins, e.g., 20, and to plot the percentage of crosses per bin (Fig 3). As illustrated, the probability of crossing the doorway, reflected by the percentage of crosses per bin, tended to increase across the sequence of visits bins.
Fig 3. The percentage of doorway crossing per bins of 20 visits to doorway area with a logistic regression plotted on top of it, in a selected fly.
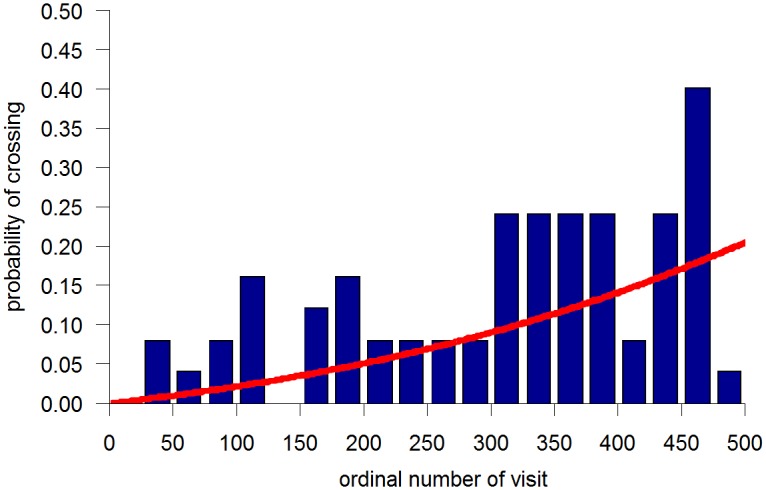
Note the increase in the likelihood of cutting through across the session.
Moreover, a logistic curve superimposed on the bin plot displayed in Fig 3, indicates that a reasonable model for the probability of crossing the doorway can be the logistic one (see Methods).
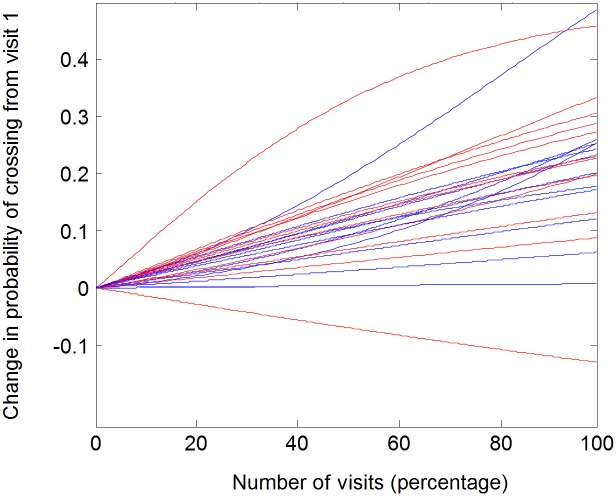
To avoid the arbitrariness associated with the number of bins and number per bin, as seen in the Fig, we directly quantify the probability of crossing the doorway as a function of the visit number, separately for each fly. Using the fitted logistic curves for each of the 24 flies, Fig 4 presents the change from its initial value of the probability of crossing from the trodden to the less trodden arena (note that the initial probability of crossing is not necessarily the same for all flies). As shown, 22 out of 24 flies showed an increase in crossing probability.
Fig 4. Dependency of odds to cross from the well-trodden to the less trodden arena on visit number, in individual flies.
The X-axis represents the ordinal number of visit (percentage out of total amount of visits). The Y- axis represents the difference in the likelihood of crossing, across the session. Black lines signify males and red lines females.
The fitted logistic model per fly allows us to quantify the rate of the increase of probability of crossing the doorway. In particular, the logarithm of the odds of crossing the doorway (i.e., logarithm of p/(1-p)) is linear in visit number with the estimated slope denoted by β: the higher the positive beta is, the faster is the increase, and obviously a negative beta indicates decrease.
Twenty two of the 24 flies exhibited positive slopes, indicating increasing probability of door crossing with visit number at doorway (p ≤ 0.0001, using sign test).
The most significant trend (p<0.0001) for the flies as a group was demonstrated for a radius of 8.5 mm (Fig 4; see Methods).
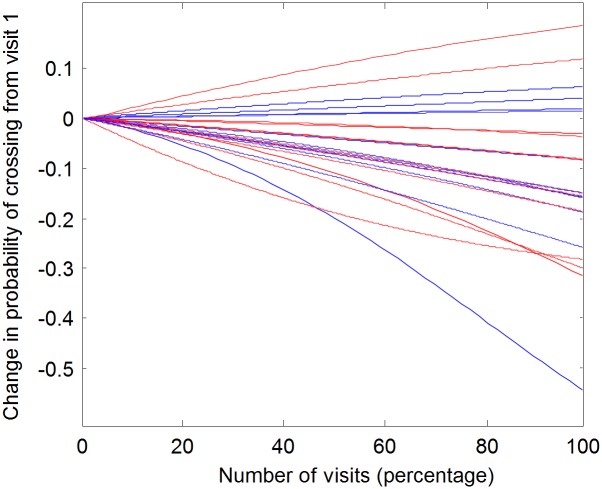
In contrast, the probability of crossing in the opposite direction—from the large to the small arena (Fig 2c and 2d) tends to decrease, with 18 of the 24 flies having negative slopes ((p = 0.0066, using the sign test). In summary, the more times a fly entered the pre-defined circular area, the higher its probability of crossing the doorway into the large arena and the lower chance of crossing the doorway back into the small arena (Fig 5).
Fig 5. Dependency of odds to cross from the less trodden to the well-trodden arena on visit number, in individual flies.
The X-axis represents the ordinal number of visit (percentage out of total amount of visits). The Y- axis represents the difference in the likelihood of crossing across the session. As shown, 18 out of 24 flies show a decrease in crossing likelihood. Black lines signify males and red lines females.
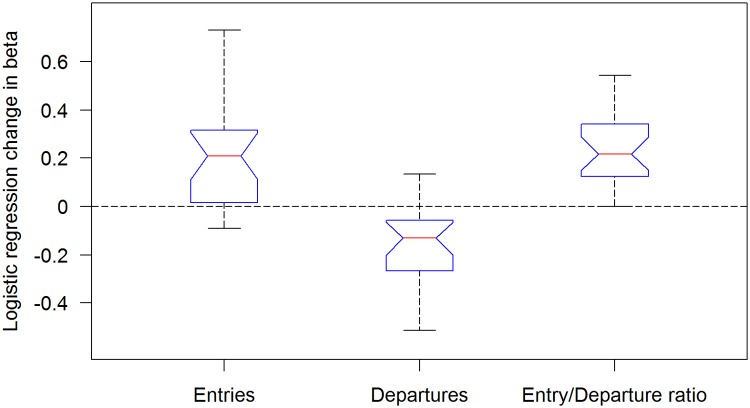
Box plot summaries show an increase in likelihood of crossing to the large arena and a decrease in likelihood of crossing to the small arena in the fly population (Fig 6).
Fig 6. Comparison between the developmental dynamics of doorway crossing from small to large arena (start of entries) and from large to small arena (Start of departures).
The comparison is between trends in the Beta of the logistic regression—the flies show an increase in the likelihood of crossing into the large arena (p<0.0001), and a decrease in the likelihood of crossing into the small arena (p = 0.0066).
The difference in slopes from small to large arena between males and females was p<0.126 and from large to small arena p<0.355 with 95% confidence interval (using Wilcoxon-Mann-Whitney). The difference is small and not statistically significant at 5%.
We have shown so far that the trends in doorway-crossing from small to large and from large to small arena were opposite, reducing the flies' bias towards the small arena. Our next question was whether the bias would also be expressed in a change in the relationship between the distances traveled and times spent in each of the two arenas. To answer these questions we divided the distance traveled in each entry by the distance traveled in the departure that preceded it, and examined whether there was a change across the sequence of ratios of distances, and whether this was negative or positive.
Plotting the sequence of logarithm of ratios of distances in a selected fly indicated a positive trend across most flies, implying a progressive increase in distance traveled in the large arena relative to distance traveled in the small arena (Fig 7). The trend was summarized per each fly by the Spearman correlation of the series with the visit number, and 24 of the 24 flies exhibited positive slopes (p ≤ 0.0001, using sign test).
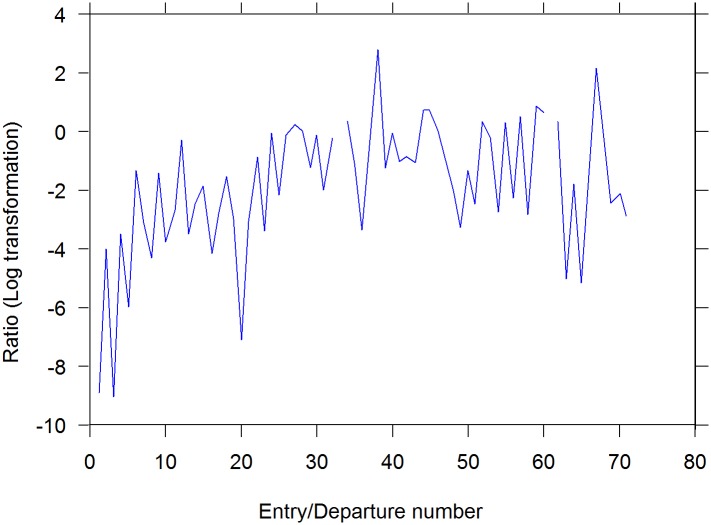
Fig 7. The developmental dynamics of the ratio between distance traveled per entry and distance traveled in its successive departure.
As shown, in this specific fly, there is a gradual increase in the relative distance traveled in the large arena.
A significant positive change in the entry/departure distance travelled ratio is evident in the fly population as a whole (Fig 8; n = 24; p<0.0001).
Fig 8. The developmental dynamics of distance traveled per entry, per departure and per the ratio between successive entry/departure pairs.
As seen by the Spearman RHO the distance traveled per entry increases across the sequence of entries (p = 0.0015) while the distance traveled per departure decreases across the sequence of departures (p = 0.00028), resulting in an increase across the sequence of entry/departure distance traveled ratios (p<0.0001).
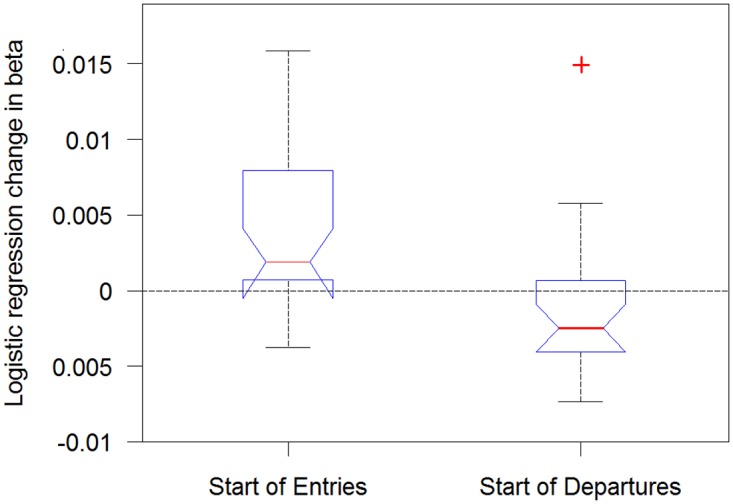
As illustrated in Fig A in S1 File and summarized in Fig B in S1 File, similar trends are seen in the entry/departure duration ratios.
The gradual reduction in the preference for the small arena compared to that of the large arena is thus exhibited in the increased probability of crossing from the small to the large arena, in the decreased probability of crossing from the large to the small arena, and in the increasing distances traveled and time spent in the large compared to the small arena.
The initial reluctance to enter the large arena is illustrated in Fig 1, where before the performance of its first entry, the fly covered ca. 11m during the first 29 hours of the session. Following the first entry into the large arena the fly covered, for another 460 minutes (almost 8 hours), ca. 43 meters (punctuated into entries), before the performance of its first doorway skip (holding back). This two-way phenomenon is illustrated in the animation of the sequence of paths that approach the doorway in the small arena (virtual circle centered on doorway, with radius of 8.5 mm), until the performance of the first substantial entry (Video B in S1 File); and by the sequence of paths in the large arena that approach the doorway and return to the small arena, until performance of the first doorway skipping (Video C in S1 File).
Discussion
In what sense is the reported behavior neophobic? The main objective of this study was to uncover, characterize, and quantify additional novel constraints on fruit fly exploratory behavior. In our experiment a natal fruit was placed in an arena that then became progressively trodden by a freshly eclosed fly, in addition to an untrodden arena, and a distinct, sharp interface between the two arenas, marked by an interruption (doorway) in the wall. The dynamics of behavior exhibited around the interface can be characterized as upstream from well-trodden to untrodden area (relative functional impermeability followed by increased permeability; Fig 6 left boxplot), and downstream from untrodden to well-trodden area (almost obligatory crossing followed by optional crossing; Fig 6 right boxplot). At the beginning of the experiment we observed relatively predictable behavior (stereotyped responses), while by the end we observed less predictable responses on both sides of the interface. Finally, there was a progressive increase in relative distance traveled and in relative time spent in the less trodden arena (Figs 7 and 8, Fig A in S1 File, Fig B in S1 File). These three dynamic processes imply that the fly's behavior is coupled to how well-trodden the large empty arena is. If the relative time spent in that arena reflects preference, and if the dynamics of crossing reflect a change in preference or, rather, management of the contrast between the two arenas, then the behavior can be described as neophobic. The developmental dynamics of doorway-crossing is remarkably similar to that reported in neophobic mice exploring a similar setup [18–20]. In the fly, coping with neophobia is quantified by the slope depicting the trend in probability of doorway-crossing in the outbound and inbound directions (Figs 4 and 5). In the mouse, in a similar setup, doorway-crossing is similarly progressive, unfolding gradually during a specific stage of free exploration—the "peep and hide" stage. In the mouse, the buildup was quantified by calculating the portion of the body area (in pixels) extending out of the doorway during peeping as well as by the frequency of peeping, while holding back was quantified by the frequency of cage skips [18, 19]). Finally, there is a respective expansion across the session of repertoire size in flies (Figs 3–7) and in mice (Fig 1 in [19]). Cutting through from the trodden to the untrodden and resisting the attraction of the well-trodden “beaten track” when away from it, are challenging endeavors in both flies and mice. Doorways seem to accentuate the phenomenon by discretizing, and in this way fettering the transition.
System identification and specification of the system’s demand on the CNS
To study the sensory channels and mechanisms that mediate a behavior it is useful to identify and isolate the behavioral system that is to be studied and to specify the demand it makes on the CNS. We therefore suspended judgment at this stage regarding the sensorimotor mechanisms that mediate the behavior. The operations that appeared to be complex, involving the management of one or more perceptual inputs: visual, olfactory, tactile, and proprioceptive, based on (internal) memory and/or (external) marking behavior. Our present lack of knowledge regarding the actual sensory channels that are used by the fly, and the question of whether the fly's memory is stored internally or deposited externally, does not detract from the demonstration of the very existence of this sensorimotor scheme. For example, staying in the large arena might be mediated by visual memory, by olfactory cues emanating from excretions deposited previously by the fly, by the management of proprioceptive input used for navigation on the basis of self-motion cues [21], or by a combination of several of these sensory inputs. Both mice and flies change the functionality of the doorway by using a remarkably similar behavior, but this does not imply that they use the same sensorimotor operation. For example, it has been taken for granted that rodents' space neophobia reflects a response to novelty (which implies memory and, therefore, cognition; [18, 19, 22, 23]. If we accept the claim that in the present study fly neophobia has not been proven beyond any doubt to be cognitive, then the same benefit of the doubt should be applied to rodent neophobia. In other words, the space neophobia of either flies or rodents, or of both, might or might not involve cognition. Recently, it has been shown that in a virtual reality setup head fixed fruit flies walking on a ball combine landmark based orientation and angular path integration. When both visual and self-motion cues are absent, a representation of the animal's orientation is maintained in the animal's neural network through persistent activity, a potential substrate for short term memory [21]. It has also been claimed recently that a multitude of similarities in network connectivity, embryonic lineages, and genetic programming "suggest deep homology of arthropod central complex and vertebrate basal ganglia circuitries underlying the selection and maintenance of behavioral actions" [24], and evidence that the central complex is involved in navigation in allocentric space has been reported in several arthropod species [21, 25–27].
Why do we use a piece of fruit in our setup? In order to demonstrate exploratory abilities, we measured the fruit-fly's unconditioned behavior in a setup that included a strong naturalistic attractor. Such an attractor was provided by a piece of fruit, which is the food substrate for yeast, on which the fly feeds [14, 15]. Fruits induce in Drosophila site fidelity, influencing perching locations and heights, foraging distance, and pupation distance from natal fruit, distances moved after disturbance at natal fruit [28], and defense against intruding males [29]. Letting the fly eclose in the vicinity of a piece of fruit was implemented in order to eliminate experimental coercion [13], reduce variability in growing conditions, induce site fidelity, and obtain ethologically relevant exploratory behavior that would be performed in reference to that fruit in the adjacent environment.
Setup essentials inducing neophobic behavior
The fruit and the pupa were surrounded by a wall with a wide doorway in it in order to obtain a clear cut interface between natal fruit vicinity and an adjacent, first untrodden, and later relatively untrodden, environment. The natal fruit induced directional polarity across the two arenas. The doorway appeared to both attract fly visits and hinder smooth crossing because of the sharp interface it provided between the well-trodden and untrodden spaces. It may have acted as a one-way perceptual cliff face: while the terrain surrounding the fruit offered a mixture of trodden and untrodden substrate, creating, as it were, a mild gradual increase in how untrodden it was, at the doorway there was a sharp juxtaposition of trodden and untrodden, requiring a discrete outbound "leap" upstream while facilitating the inbound downstream return. The doorway thus functioned as a supernormal stimulus, used in classical ethology for enhancing species-specific behavior to an exaggerated degree, much like the exaggerated brooding response to eggs elicited in oystercatchers by supersize eggs [16]. The doorway also appeared to be a vantage point facing the untrodden, from where the untrodden can be experienced piecemeal (either management of novelty or, for example, management of deposited scent). Repeated visits increased cumulative time spent in the vicinity of the doorway. This was associated with an increase in the versatility of responses to the open doorway, augmenting the probability of either cutting through or holding back (amount of exposure determines repertoire size). The process involved a progressive increase in the relative occupancy of the less trodden arena (Video A in S1 File).
Other operations on the environment
Examples of invertebrate locomotor operations are walking [30], turning [30], negotiation of barriers and adaptation to slippery ground [31], fixating objects in the face of expanding optic flow [32], and prey capture [33, 34]. Some of the cognitive operations described to date include selecting a specific arm in a Y-Maze [4], selecting a place with reinforcing properties [9, 35, 36], discrimination and generalization learning exhibited by tethered flies [11, 12], habituation in relation to specific stimuli delivered in several sensory modalities in specific behavioral contexts [37], and avoidance of rare unfamiliar flowers in bumble bee pollinators[38]. In vertebrates, operations on the environment include locomotor schemas like that of the mobility gradient [39], and the construction of allocentric space [18, 19, 40]. In allocentric space, a doorway connecting the mouse's home cage with an untrodden arena is used as the origin from which the mouse performs increasingly longer excursions, managing untrodden terrain in a piecemeal, neophobic way.
Operational schemas like the ones described in this study provide a high level vocabulary for describing operations on the environment. The operation "maintain a certain level of novelty" [18, 19, 41] could, for example, account for the progressive gradual increase in exposure to the previously untrodden environment brought about by the repeated probing in "cutting through" and in the progressive increase in staying on the less trodden side in "holding back".
Concluding remarks
The question "What is different in the lab and field?" raised in a feature article on drosophila [42], leads to the next challenge, of designing practical measures that will bridge the gap between the lab and the field. In the current study we define the differences between lab and field, and design an ethologically relevant paradigm, which on the one hand reduces the differences and on the other hand takes advantage of them: in the wild, fruit flies use a natal rotten fruit as their origin, freely exploring its surroundings, which consists of a mixture of trodden and untrodden terrain, for days. These features are employed in our ethologically relevant setup except that the interface between trodden and untrodden is reduced to a widely open doorway within a surrounding wall. Crossing this doorway, characterized by a sharp contrast interface, amounts to a dynamics that betrays the flies' space neophobia. The doorway's asymmetric functional permeability shapes the gradual systematic occupancy of the surrounding untrodden space, and together they define fruit fly space neophobia, a feature of fly behavior that could reflect spatial cognition. Most important, the setup, involving origin-related exploration is universal, allowing cross phylum comparisons where origin can be a natal fruit for a fly, a home base for a mouse, or even a mother for a human baby, offering a universal, fully translational, animal model involving same setup, same measures and same kinematic variables across phyla.
Materials and Methods
Ethics statement
Since the study was based on mere observation and videotaping of free fruit fly behavior no permit was necessary.
Animals
We used 24 wild type D. melanogaster, 12 males and 12 females of the Oregon strain, raised on a 16:8 light/dark cycle at 25°C. Pupae that were expected to eclose shortly (within a day) were selected and placed in the experimental setup; a single pupa per experiment.
Experimental protocol
Each pupa was placed next to a piece of apple piece (cone shaped, 20mm base diameter and 4mm height) and the fly was then tracked in seclusion, for 72 hours, under constant light and 25°C. We excluded any data collected prior to eclosure.
Experimental setup
The experimental setup consisted of a small (45mm diameter) and a large (130mm) circular arena connected by a 5mm wide open doorway and covered by an 8mm high transparent Perspex ceiling. The flies in our experiment eclosed at their own pace. This dictated long experiments, as it usually took the flies between 10 and 20 hours to eclose and another 16 hours on average to walk the first few meters. [13].
Videotaping and tracking
Each fly was videotaped with a 720X576 pixel resolution camera, 25 frames per second, for a period of 72 hours. The videos were tracked with a MATLAB function called "FTrack", which was developed by Dan Valente [43]. The output received from the "FTrack" function (X and Y coordinates representing the Fly's center of mass) was smoothed using "SEE", a software supported Strategy for the Exploration of Exploration (http://www.tau.ac.il/~ilan99/see/).
Defining and scoring the fly's approaches to the doorway
Using the fly's tracking output, we could easily define the boundaries of the two circular arenas as two ellipses. The midway between the two closest points (where the two ellipses almost "touch" each other) was defined as the doorway's center. Around this center we traced four virtual circles with a radius of 10, 20, 30 and 40 pixels (~3,6,9 and 12 millimeters). The smallest circle had a radius slightly longer than a fly's length and the largest almost reached the fruit, which was placed in the center of the small arena. These circles were used to determine and score the approaches of the fly to the doorway. Approaches or visits to doorway proximity were then scored as either followed by crossing or by leaving the circle without crossing to the other side (see Fig 2). Having quantified the number of crossings per fixed number of visits for each virtual circle separately we selected the circle and scoring that represented the phenomenon most clearly. Having scored several options we corrected for multiple comparisons using the FDR (False Discovery Rate) to ascertain that we did not have false positives.
Data analysis using logistic regression
Logistic regression is used to model the probability of crossing the doorway (or not) once at the doorway, as a function of the visit number at doorway. With p i being the probability of fly i to cross, p i /(1- p i ) its odds to cross and t the visit number, the model is log(p i /(1-p i )) = β 0 +β t.
The parameters in the models were estimated using the Matlab function mnrfit (multinomial logistic regression).
For the change in distance traveled the ratios were log-transformed an then the Spearman correlation between these and the number of visit were calculated (no testing was done per fly at this stage because of the obvious serial correlation).
Testing of the coefficient for both for the group of 24 flies was done using the sign test.
Fine tuning of arena sizes and fruit location
In seeking the optimal parameters that would highlight cognitive behavior we performed preliminary experiments. With two large arenas of the same size and a piece of fruit placed in one of them, the fly did not enter the empty arena a sufficiently large, statistically significant, number of times in the course of three days (a small enough arena increased the likelihood of doorway encounters, which led to entries to the untroden arena). Placing the fruit and the pupa in the small arena or in the large arena yielded an initial preference for the arena where the fruit was placed and a subsequent gradual tendency to cross to the empty arena, thus reducing the asymmetry in the behavior exhibited in the two arenas. Flies that eclosed near a piece of fruit placed in the large arena gradually expanded the maximal distance reached, during successive roundtrips performed from that fruit. We therefore settled on placing the fruit in a small arena connected by a doorway to the large arena.
Supporting Information
Animation of the paths traced during successive entries by a specific fly in the course of a 3 days session (Video A). The paths traced by a specific fly in the vicinity of the doorway before and up until the first entry (Video B). The paths traced by a specific fly in the untrodden arena in the vicinity of the doorway before and up until the first time it resists crossing to the trodden arena (Video C). The sequence of ratios between the duration of each entry and the duration of the departure that preceded it in a selected fly (Fig A). The developmental dynamics of entry duration, departure duration and the ratio between them (Fig B).
(DOCX)
Acknowledgments
This study was supported by a grant from the Israel Science Foundation (ISF 1351). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Prof. Daniel Segal of the department of Microbiology and Biotechnology, Tel Aviv University, Tel Aviv, Israel, for supplying the experimental animals, and Prof. Segal and Prof. John Ringo of the University of Maine, Orono, ME USA for their advice along the way. Dr. Alex Gomez Marin of Champalimaud Neuroscience Lisbon and Dr. Eyal Gruntman of Janelia Farm HHMI provided useful comments on the manuscript. Ms Naomi Paz edited and proofread the manuscript. We thank Yair Wexler of Tel Aviv University for his help with the Figs.
Data Availability
All data of this study are available at: (http://figshare.com/articles/Flies_coordinates_1st_set/1594814).
Funding Statement
This study was supported by a grant from the Israel Science Foundation (ISF 1351). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Baerends GP. Fortpflanzungsverhalten und Orientierung der Grabwespe Ammophila campestris Jur: Ponsen & Looijen; 1941. [Google Scholar]
- 2. Wehner R, Srinivasan M. Searching behaviour of desert ants, genusCataglyphis (Formicidae, Hymenoptera). J Comp Physiol. 1981. 1981/09/01;142(3):315–38. English. [Google Scholar]
- 3. Cartwright B, Collett T. How honey bees use landmarks to guide their return to a food source. Nature. 1982. [Google Scholar]
- 4. Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1974. March;71(3):708–12. . Pubmed Central PMCID: 388082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439(7076):551–6. [DOI] [PubMed] [Google Scholar]
- 6. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods. 1984;11(1):47–60. [DOI] [PubMed] [Google Scholar]
- 7. Mizunami M, Weibrecht JM, Strausfeld NJ. Mushroom bodies of the cockroach: their participation in place memory. Journal of Comparative Neurology. 1998;402(4):520–37. [PubMed] [Google Scholar]
- 8. Wessnitzer J, Mangan M, Webb B. Place memory in crickets. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1637):915–21. 10.1098/rspb.2007.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011. June 9;474(7350):204–7. Pubmed Central PMCID: 3169673. 10.1038/nature10131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proceedings of the National Academy of Sciences. 1979;76(7):3430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ernst R, Heisenberg M. The memory template in Drosophila pattern vision at the flight simulator. Vision research. 1999. November;39(23):3920–33. . [DOI] [PubMed] [Google Scholar]
- 12. Brembs B, Hempel de Ibarra N. Different parameters support generalization and discrimination learning in Drosophila at the flight simulator. Learning & memory. 2006. Sep-Oct;13(5):629–37. . Pubmed Central PMCID: 1783617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Connolly K. Locomotor activity in Drosophila. 3. A distinction between activity and reactivity. Animal behaviour. 1967. January;15(1):149–52. . [DOI] [PubMed] [Google Scholar]
- 14. Barrows WM. The reactions of the pomace fly, Drosophila ampelophila Loew, to odorous substances. Journal of Experimental Zoology. 1907;4(4):515–37. [Google Scholar]
- 15. Da Cunha AB, Dobzhansky T, Sokoloff A. On food preferences of sympatric species of Drosophila. Evolution. 1951:97–101. [Google Scholar]
- 16. Tinbergen N. Wat prikkelt een scholekster tot broeden. De Levende Natuur. 1948;51:65–9. [Google Scholar]
- 17. Drai D, Benjamini Y, Golani I. Statistical discrimination of natural modes of motion in rat exploratory behavior. Journal of neuroscience methods. 2000;96(2):119–31. [DOI] [PubMed] [Google Scholar]
- 18. Benjamini Y, Fonio E, Galili T, Havkin GZ, Golani I. Quantifying the buildup in extent and complexity of free exploration in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011. September 13;108 Suppl 3:15580–7. Pubmed Central PMCID: 3176604. 10.1073/pnas.1014837108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fonio E, Benjamini Y, Golani I. Freedom of movement and the stability of its unfolding in free exploration of mice. Proceedings of the National Academy of Sciences of the United States of America. 2009. December 15;106(50):21335–40. Pubmed Central PMCID: 2795555. 10.1073/pnas.0812513106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hager T, Jansen RF, Pieneman AW, Manivannan SN, Golani I, van der Sluis S, et al. Display of individuality in avoidance behavior and risk assessment of inbred mice. Frontiers in behavioral neuroscience. 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seelig JD, Jayaraman V. Neural dynamics for landmark orientation and angular path integration. Nature. 2015;521(7551):186–91. 10.1038/nature14446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cools A, Ellenbroek B, Heeren D, Lubbers L. Use of high and low responders to novelty in rat studies on the role of the ventral striatum in radial maze performance: effects of intra-accumbens injections of sulpiride. Canadian journal of physiology and pharmacology. 1993;71(5–6):335–42. [DOI] [PubMed] [Google Scholar]
- 23. Cools AR, Gingras MA. Nijmegen high and low responders to novelty: a new tool in the search after the neurobiology of drug abuse liability. Pharmacology Biochemistry and Behavior. 1998;60(1):151–9. [DOI] [PubMed] [Google Scholar]
- 24. Strausfeld NJ, Hirth F. Deep homology of arthropod central complex and vertebrate basal ganglia. Science. 2013;340(6129):157–61. 10.1126/science.1231828 [DOI] [PubMed] [Google Scholar]
- 25. Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315(5814):995–7. [DOI] [PubMed] [Google Scholar]
- 26. Pfeiffer K, Homberg U. Coding of azimuthal directions via time-compensated combination of celestial compass cues. Current biology. 2007;17(11):960–5. [DOI] [PubMed] [Google Scholar]
- 27. Vitzthum H, Müller M, Homberg U. Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. The Journal of neuroscience. 2002;22(3):1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stamps J, Buechner M, Alexander K, Davis J, Zuniga N. Genotypic differences in space use and movement patterns in< i> Drosophila melanogaster. Animal behaviour. 2005;70(3):609–18. [Google Scholar]
- 29. Hoffmann AA, Cacoyianni Z. Selection for territoriality in< i> Drosophila melanogaster: correlated responses in mating success and other fitness components. Animal behaviour. 1989;38(1):23–34. [Google Scholar]
- 30. Strauss R, Heisenberg M. Coordination of legs during straight walking and turning in Drosophila melanogaster. Journal of comparative physiology A, Sensory, neural, and behavioral physiology. 1990. August;167(3):403–12. . [DOI] [PubMed] [Google Scholar]
- 31. Ritzmann RE, Büschges A. 10 Insect Walking: From Reduced Preparations to Natural Terrain. Cold Spring Harbor Monograph Archive. 2007;49:229–50. [Google Scholar]
- 32. Reiser MB, Dickinson MH. Drosophila fly straight by fixating objects in the face of expanding optic flow. The Journal of experimental biology. 2010. May;213(Pt 10):1771–81. Pubmed Central PMCID: 2861965. 10.1242/jeb.035147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mittelstaedt H. Prey capture in mantids. Recent advances in invertebrate physiology. 1957:51–71. [Google Scholar]
- 34. Olberg RM. Visual control of prey-capture flight in dragonflies. Current opinion in neurobiology. 2012. April;22(2):267–71. 10.1016/j.conb.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 35. Mizunami M, Weibrecht JM, Strausfeld NJ. Mushroom bodies of the cockroach: their participation in place memory. The Journal of comparative neurology. 1998. December 28;402(4):520–37. . [PubMed] [Google Scholar]
- 36. Wessnitzer J, Mangan M, Webb B. Place memory in crickets. Proceedings Biological sciences / The Royal Society. 2008. April 22;275(1637):915–21. Pubmed Central PMCID: 2599942. 10.1098/rspb.2007.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological review. 1966;73(1):16 [DOI] [PubMed] [Google Scholar]
- 38. Forrest J, Thomson JD. Pollinator experience, neophobia and the evolution of flowering time. Proceedings of the Royal Society of London B: Biological Sciences. 2009;276(1658):935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Golani I. A mobility gradient in the organization of vertebrate movement: the perception of movement through symbolic language. Behavioral and Brain Sciences. 1992;15(02):249–66. [Google Scholar]
- 40. Golani I. The developmental dynamics of behavioral growth processes in rodent egocentric and allocentric space. Behavioural brain research. 2012. June 1;231(2):309–16. 10.1016/j.bbr.2012.01.039 [DOI] [PubMed] [Google Scholar]
- 41. Gordon G, Fonio E, Ahissar E. Learning and control of exploration primitives. Journal of computational neuroscience. 2014:1–22. [DOI] [PubMed] [Google Scholar]
- 42. Markow TA. The secret lives of Drosophila flies. eLife. 2015;4:e06793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Valente D, Golani I, Mitra PP. Analysis of the trajectory of Drosophila melanogaster in a circular open field arena. PloS one. 2007;2(10):e1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animation of the paths traced during successive entries by a specific fly in the course of a 3 days session (Video A). The paths traced by a specific fly in the vicinity of the doorway before and up until the first entry (Video B). The paths traced by a specific fly in the untrodden arena in the vicinity of the doorway before and up until the first time it resists crossing to the trodden arena (Video C). The sequence of ratios between the duration of each entry and the duration of the departure that preceded it in a selected fly (Fig A). The developmental dynamics of entry duration, departure duration and the ratio between them (Fig B).
(DOCX)
Data Availability Statement
All data of this study are available at: (http://figshare.com/articles/Flies_coordinates_1st_set/1594814).