Abstract
Purpose
To evaluate candidate FDG-PET/CT imaging biomarkers for head and neck chemoradiotherapy outcomes in the cooperative group trial setting.
Methods
RTOG 0522 patients consenting to a secondary FDG-PET/CT sub-study were serially imaged at baseline and 8 weeks following radiation. Maximum standardized uptake value (SUVmax), SUV peak (mean SUV within a 1 cm sphere centered on SUVmax), and metabolic tumor volume (MTV) using 40% of SUVmax as threshold were obtained from primary tumor and involved nodes.
Results
Out of 940 patients entered onto RTOG 0522, 74 were analyzable for this sub-study. Neither high baseline SUVmax nor SUVpeak from primary or nodal disease were associated with poor treatment outcomes. However, primary tumor MTV above the cohort median was associated with worse LRC (HR 4.01, 95% CI [1.28, 12.52], p = 0.02) and PFS (HR 2.34, 95% CI [1.02, 5.37], p = 0.05). Although MTV and T stage appeared to correlate (mean MTV 6.4, 13.2, 26.8 for T2, T3, and T4 tumors, respectively), MTV remained a strong independent prognostic factor for PFS in bivariate analysis that included T stage. Primary MTV remained prognostic in p16-associated oropharyngeal cancer cases, although sample size was limited.
Conclusion
High baseline primary tumor MTV was associated with worse treatment outcomes in this limited patient subset of RTOG 0522. Additional confirmatory work will be required to validate primary tumor MTV as a prognostic imaging biomarker for patient stratification in future trials.
INTRODUCTION
Effective patient selection drives successful clinical cancer trial design. Tissue-based biomarkers have been used towards this end, with a promise to increase study power and reduce treatment development costs. However, tumor tissue collection is expensive and burdensome. Tumor imaging provides an alternative means to non-invasively define disease phenotype and treatment response.
Functional imaging delivers quantitative characterization of tumor and host tissue physiology. FDG-PET, with or without co-registered CT, serves as the traditional workhorse for functional head and neck imaging. Published experience with FDG-PET- defined staging is mature, and has been summarized by meta-analyses (1), expert consensus reports (2, 3), and comparative effectiveness studies (4). FDG-PET incrementally improves staging accuracy and treatment response assessment over anatomic imaging (5–8), particularly if guided by complementary clinical features (9, 10). Some institutional series suggest that certain FDG-PET parameters, such as the maximum or peak standardized uptake values (SUV), may serve as quantifiable imaging biomarkers for radiotherapy outcomes (11–13). However, conflicting reports refute the predictive value of SUV (14), and quantitative head and neck FDG-PET outcome measures remain untested in the cooperative group trial setting.
RTOG 0522 recently completed enrollment of 940 patients diagnosed with locally advanced head and neck cancer. Study subjects received concurrent radiation therapy and cisplatin, with or without the addition of cetuximab. All RTOG 0522 subjects with N2-3 disease (with the exception of N2c with both sides ≤ N1) were eligible for baseline and post-treatment co-registered PET/CT imaging analysis. Predefined study objectives included correlation of pre- and post-treatment PET/CT scan findings with histologic findings of neck dissection specimens and treatment outcomes. Given interval publication of encouraging institutional pilot findings for use of FDG-PET-defined metabolic tumor volume (MTV) as an imaging biomarker for radiotherapy treatment response and tumor control outcomes (15–17), MTV was included as a secondary post-hoc study objective in this current report.
METHODS
Study Population
Patients enrolled to RTOG 0522 with N2a, N2b, N2c (with right and/or left side N2a-N2b), or N3 disease, who agreed to participate in the PET/CT study, and for whom at least one PET image set was available for central review were included in this analysis.
PET/CT Image and Scanner Compatibility Requirements
All centers participating in this imaging study had to provide one test case to the ACRIN PET Core Lab prior to start of enrollment to credential their file transfer capabilities and image quality. The PET Core Lab provided software for imaging facilities to collect, de-identify, and submit image sets either from a PET/CT scanner or a PACS system to the ACRIN image archive. All imaging had to be performed on a combined PET/CT instrument with full ring PET and four-slice or greater multi-detector CT operating in high-sensitivity 2D mode, if available. To simplify multi-institutional participation, centers were not required to use uniform PET-CT software. 3D mode was permissible for patients imaged on combined PET/CT scanners without a 2D mode.
Patient Preparation and FDG Injection
Participating centers were instructed to record patient height and weight prior to each PET scan, to have patients observe a four to six-hour fasting period prior to FDG injection, and to measure serum glucose concentration prior to scanning. A serum glucose value less than 200 mg/dL was necessary to proceed to imaging. Centers were instructed to inject a dose of 10–20 mCi of FDG intravenously and to begin imaging within 50–70 minutes following tracer administration.
Baseline and Post-Treatment PET/CT Imaging
Imaging was required to encompass the vertex of the head down through the entire pelvis. The recommended imaging protocol incorporated two discrete phases: during the first phase, head and neck scanning was performed with arms resting at sides and full neck extension using a 120 keV/300 mA, 0.5-second detector rotation time (“high mA”) CT scan with intravenous contrast (100 cc contrast bolus administered at 1.5 cc/second, with a 50 second scan delay and with the scan started inferiorly, moving cranially), followed by a 120 keV/80 mA, 0.8-second detector rotation time (“low mA”) CT scan for PET attenuation correction, followed lastly by PET scanning. Standard manufacturer recommendations for specific low and high mA CT scanning parameters could be substituted. Two fields of view (approximately 15 cm) were required for full head and neck PET imaging from manubrium to vertex. Centers were instructed to allow patients to rest their neck for 1–2 minutes. For the second phase of imaging, the neck was shifted into neutral position, and the remainder of the body was surveyed per routine local institutional protocol with arms raised above the head to allow for thoracic and abdominal imaging. At least four to five PET fields of view were to be used for this phase. PET data were corrected for dead time, scatter, random coincidence events and attenuation, and ultimately reconstructed into images via the filtering algorithm provided by the scanner manufacturer. A post-treatment FDG-PET/CT scan was recommended 8–9 weeks following completion of treatment (in addition to mandatory CT or MR post-treatment imaging). Centers were requested to perform post-treatment PET/CT imaging in this time frame and on the same scanner, if possible.
Maximum Standardized Uptake Value (SUVmax), Peak SUV (SUVpeak), and Metabolic Tumor Volume (MTV)
SUV normalized by specific injected dose and patient weight was calculated on centralized review by two head and neck radiation oncologists with specific expertise in PET-CT interpretation (DLS and MY) employing commercial image analysis software (MIM Software, v.5.2, Cleveland, OH). Detection of primary and nodal disease by FDG-PET/CT was determined qualitatively as FDG uptake greater than surrounding normal soft tissue within a CT-delineated anatomic (primary disease or nodal) abnormality. SUVmax was defined as (tissue activity) (μCi/ml)/(injected dose (mCi)/(patient weight [kg]) within the voxel having the highest activity within a given region of interest (ROI). These values were determined separately for ROIs within primary tumor and involved cervical lymph nodes. Each ROI had to encompass the entire FDG-avid lesion of interest, with boundaries guided by CT delineation. SUVpeak for primary and nodal disease was measured from a 1 cubic centimeter sphere centered on the voxel with the highest mean SUV value. The location of this sphere was manually checked to assure reasonable position within disease. Primary tumor MTV contours were generated from voxels that were equal to or greater than 40% of primary SUVmax. A specific tumor can have a much higher SUV than another tumor, but MTV calculations and volume generation will remain consistent across tumors since the analysis tool performs identical calculations of proportionality regardless of baseline SUVmax value. Nodal MTV was measured separately for each side of the neck, inclusive of all metabolically active nodes (including cystic regions within these nodes), and defined as the nodal tumor volume above 40% of the nodal SUVmax. For bilateral neck disease, the maximum of right and left sides was used for SUV and the sum for MTV. Total MTV, defined as primary MTV plus nodal MTV, was calculated for patients with both measures.
Data Analysis and Statistics
The following pre- and post-treatment PET values were obtained from central review: SUVmax, SUVpeak, and MTV of the primary tumor, right nodal disease, and left nodal disease. The protocol analysis plan pre-specified the analysis of SUV measures. Evaluation of MTV represents post-hoc secondary analysis, and must be formally considered exploratory in nature. The study cohort was dichotomized into equal size comparator groups by median PET values to maximize statistical power for analysis. Patients with at least one readable value from either reader were included. Consistency between readers was measured by intraclass correlation coefficient.
Clinical endpoints specified by the trial protocol were local relapse (including salvage surgery for primary site with tumor present/unknown), regional relapse (including neck dissection > 15 weeks after the end of radiation therapy with tumor present/unknown), local-regional relapse, distant metastasis, progression-free survival, and overall survival. Only death was considered a competing risk for local relapse, regional relapse, local-regional relapse, or distant metastasis. Progression-free survival events were defined as local or regional relapse, distant metastasis, or death due to any cause. All endpoints were measured from the date of randomization. Rates for local, regional, and local-regional relapse, and distant metastasis were estimated by the cumulative incidence method (18), and rates for progression-free and overall survival were estimated by the Kaplan-Meier method (19). Hazard ratios were estimated by Cox proportional hazards models (20). To address whether negative (SUVmax < 3, per Yao, et. al. (21)) post-treatment PET/CT in patients who achieve a clinical complete nodal response (CR, defined as no evidence of nodal disease on physical exam and/or imaging) predicts for a low nodal relapse rate (< 10%) at 2 years, the 2-year regional relapse rates in 4 groups were calculated: (1) clinical CR, negative PET; (2) clinical <CR, negative PET; (3) clinical CR, positive PET; (4) clinical <CR, positive PET.
Exploratory post-hoc subgroup analysis was performed to selectively evaluate PET measures in patients with p16-positive oropharyngeal cancer. Missing p16 values were imputed 20 times using conditional model specification for multivariate imputation via Gibbs sampling (22), and the resulting datasets were combined using Rubin’s formula (23), and sensitivity analyses were conducted to validate the robustness of the imputation procedure. Akaike information criterion (AIC) (24) was used to measure the relative quality of our statistical models.
RESULTS
Imaging Study Cohort
One-hundred sixteen patients agreed to participate in the PET/CT study. Forty-two subjects did not follow through with post-treatment imaging or had unreadable image files, yielding 74 patients from 19 centers with both pre- and post-treatment PET/CT imaging available for this analysis. In addition, 535 patients were eligible for the PET/CT study based on N stage but chose not to participate, for a total of 577 patients not included in this analysis. Analyzed patients enjoyed better performance status and shorter pack-year tobacco exposure, but presented at a more advanced T stage than excluded patients (Table 1). At the time of analysis, median follow-up for surviving patients (58 of 74) was 4.2 years (range, 3.1 to 6.2).
Table 1.
Patient Characteristics
| Included in PET/CT study (n=74) | Eligible for PET/CT study but excluded (n=577) | |
|---|---|---|
| Assigned treatment, p=0.26 [1] | ||
| RT + cisplatin | 42 (56.8%) | 287 (49.7%) |
| RT + cisplatin + cetuximab | 32 (43.2%) | 290 (50.3%) |
| Age (years), p=1.00 [2] | ||
| Mean (standard deviation) | 56.8 (6.67) | 56.7 (8.22) |
| Median (range) | 56 (42–73) | 57 (34–79) |
| Gender, p=0.68 [1] | ||
| Male | 65 (87.8%) | 516 (89.4%) |
| Female | 9 (12.2%) | 61 (10.6%) |
| Zubrod performance status, p=0.03 [1] | ||
| 0 | 58 (78.4%) | 380 (65.9%) |
| 1 | 16 (21.6%) | 197 (34.1%) |
| Smoking history: pack-years, p=0.03 [2] | (n=54) | (n=512) |
| Mean (standard deviation) | 20.8 (29.88) | 26.1 (26.96) |
| Median (range) | 8.75 (0–135) | 21 (0–162) |
| Primary site, p=0.72 [1] | ||
| Oropharynx | 58 (78.4%) | 449 (77.8%) |
| Hypopharynx | 7 (9.5%) | 43 (7.5%) |
| Larynx | 9 (12.2%) | 85 (14.7%) |
| p16 status, oropharynx only, p=0.98 [1] | (n=33) | (n=229) |
| Negative | 8 (24.2%) | 55 (24.0%) |
| Positive | 25 (75.8%) | 174 (76.0%) |
| T stage, p=0.04 [2] | ||
| T2 | 29 (39.2%) | 303 (52.5%) |
| T3 | 26 (35.1%) | 157 (27.2%) |
| T4 | 19 (25.7%) | 117 (20.3%) |
| N stage, p=0.23 [2] | ||
| N2a | 5 (6.8%) | 73 (12.7%) |
| N2b | 34 (45.9%) | 259 (44.9%) |
| N2c | 29 (39.2%) | 207 (35.9%) |
| N3 | 6 (8.1%) | 38 (6.6%) |
Pearson chi-square test.
Wilcoxon rank-sum test.
PET Summary and Reviewer Agreement
Summary FDG-PET/CT outcome measures are listed in Table 2. Reader agreement was excellent (intraclass correlation coefficient ≥0.80) for all measurements, except for post-treatment nodal SUVpeak values (0.51–0.59). Low post-treatment SUV values made thresholding for MTV calculation difficult to reproduce; thus, post-treatment MTVs were not tabulated.
Table 2.
FDG-PET/CT Outcome Measures
| Measurement | Intraclass Correlation Coefficient (95% CI) | |
|---|---|---|
| Pre-treatment primary SUVmax [1] | (n=68) | 1.00 (N/A) |
| Mean (standard deviation) | 15.84 (6.68) | |
| Median (range) | 15.07 (3.45 – 40.02) | |
| Post-treatment primary SUVmax [1,2] | (n=67) | 0.93 (0.89, 0.96) |
| Mean (standard deviation) | 4.65 (2.06) | |
| Median (range) | 4.31 (1.75 – 14.48) | |
| Pre-treatment primary SUVpeak [1] | (n=68) | 0.99 (0.98, 0.99) |
| Mean (standard deviation) | 12.68 (6.19) | |
| Median (range) | 11.23 (2.54 – 37.36) | |
| Post-treatment primary SUVpeak [1,2] | (n=67) | 0.83 (0.73, 0.89) |
| Mean (standard deviation) | 3.23 (1.53) | |
| Median (range) | 3.18 (1.28 – 11.78) | |
| Pre-treatment primary MTV [1] | (n=68) | 0.99 (0.98, 0.99) |
| Mean (standard deviation) | 13.80 (12.55) | |
| Median (range) | 8.76 (2.22 – 64.45) | |
| Pre-treatment nodal SUVmax [1,3] | (n=65) | R: 0.99 (0.98, 0.99) |
| Mean (standard deviation) | 11.62 (6.81) | L: 1.00 (N/A) |
| Median (range) | 10.55 (2.08 – 42.96) | |
| Post-treatment nodal SUVmax [1,2,3] | (n=62) | R: 0.82 (0.69, 0.90) |
| Mean (standard deviation) | 2.93 (1.20) | L: 0.87 (0.77, 0.93) |
| Median (range) | 2.66 (1.03 – 6.91) | |
| Pre-treatment nodal SUVpeak [1,3] | (n=65) | R: 0.99 (0.98, 0.99) |
| Mean (standard deviation) | 8.47 (5.00) | L: 1.00 (N/A) |
| Median (range) | 7.73 (1.34 – 27.93) | |
| Post-treatment nodal SUVpeak [1,2,3] | (n=62) | R: 0.59 (0.34, 0.76) |
| Mean (standard deviation) | 1.97 (0.56) | L: 0.51 (0.23, 0.71) |
| Median (range) | 1.95 (0.73 – 3.90) | |
| Pre-treatment nodal MTV [1,4] | (n=61) | R: 0.99 (0.98, 0.99) |
| Mean (standard deviation) | 14.38 (18.25) | L: 0.95 (0.91, 0.97) |
| Median (range) | 10.10 (1.46 – 141.01) | |
| Pre-treatment total MTV [1,5] | (n=60) | |
| Mean (standard deviation) | 27.56 (21.89) | |
| Median (range) | 21.77 (3.85 – 149.64) |
CI: confidence interval; R: right neck; L: left neck; N/A: not applicable.
If both reader values available, mean was used.
Patients that progressed prior to post-treatment PET were excluded.
If bilateral, maximum of right and left sides was used.
If bilateral, sum of right and left sides was used.
Sum of primary and nodal MTV.
Correlation of Baseline SUV Values with Treatment Outcomes
Elevated baseline primary/nodal FDG SUVmax or SUVpeak values above median value of the study cohort were not associated with poor clinical outcomes (Table 3). This also held true when either value was alternatively analyzed as a continuous variable (data not shown).
Table 3.
Correlation of Baseline SUV Values with Treatment Outcomes
| Variable | Endpoint | Events/total | Hazard ratio (95% CI) | p-value |
|---|---|---|---|---|
| Primary SUVmax (> vs. ≤ median) | Local relapse | 0/34 vs. 8/34 | Cannot estimate | |
| Local-regional relapse | 4/34 vs. 12/34 | 0.31 (0.10, 0.97) | 0.04 | |
| Distant metastasis | 2/34 vs. 6/34 | 0.32 (0.06, 1.57) | 0.16 | |
| Progression-free survival | 6/34 vs. 18/34 | 0.30 (0.12, 0.75) | 0.01 | |
| Overall survival | 4/34 vs. 11/34 | 0.38 (0.12, 1.20) | 0.10 | |
| Nodal SUVmax (> vs. ≤ median) | Regional relapse | 6/32 vs. 7/33 | 0.89 (0.30, 2.66) | 0.84 |
| Local-regional relapse | 6/32 vs. 9/33 | 0.72 (0.26, 2.05) | 0.54 | |
| Distant metastasis | 2/32 vs. 5/33 | 0.40 (0.08, 2.05) | 0.27 | |
| Progression-free survival | 8/32 vs. 15/33 | 0.56 (0.24, 1.33) | 0.19 | |
| Overall survival | 4/32 vs. 10/33 | 0.44 (0.14, 1.39) | 0.16 | |
| Primary SUVpeak (> vs. ≤ median) | Local relapse | 1/34 vs. 7/34 | 0.15 (0.02, 1.23) | 0.08 |
| Local-regional relapse | 6/34 vs. 10/34 | 0.60 (0.22, 1.65) | 0.32 | |
| Distant metastasis | 3/34 vs. 5/34 | 0.62 (0.15, 2.62) | 0.52 | |
| Progression-free survival | 9/34 vs. 15/34 | 0.62 (0.27, 1.42) | 0.25 | |
| Overall survival | 5/34 vs. 10/34 | 0.55 (0.19, 1.62) | 0.28 | |
| Nodal SUVpeak (> vs. ≤ median) | Regional relapse | 6/32 vs. 7/33 | 0.93 (0.31, 2.77) | 0.90 |
| Local-regional relapse | 6/32 vs. 9/33 | 0.75 (0.27, 2.13) | 0.59 | |
| Distant metastasis | 3/32 vs. 4/33 | 0.81 (0.18, 3.63) | 0.79 | |
| Progression-free survival | 9/32 vs. 14/33 | 0.74 (0.32, 1.72) | 0.49 | |
| Overall survival | 5/32 vs. 9/33 | 0.61 (0.20, 1.82) | 0.37 |
CI = confidence interval.
Hazard ratios estimated from Cox models.
Correlation of Post-Treatment SUV Values with Nodal Response
The 2-year nodal relapse rates were: 3.9% (95% CI: 0.0, 11.4) in patients with clinical CR, negative PET; 33.3% (95% CI: 8.4, 58.2) in clinical <CR, negative PET; 16.7% (95% CI: 0.0, 38.8) in clinical CR, positive PET; and 11.1% (95% CI: 0.0, 33.1) in clinical <CR, positive PET. There were only 10 nodal relapses across these 4 groups, and differences were not statistically significant (p=0.15).
Correlation of Baseline MTV with Treatment Outcomes
Pretreatment primary MTV above the study cohort median was associated with local-regional relapse (hazard ratio 4.01, 95% CI [1.28, 12.52], p=0.02) and poorer progression-free survival (hazard ratio 2.34, 95% CI [1.02, 5.37], p=0.05) (Table 4, Figure 1). Elevated baseline nodal MTV values, on the other hand, were non-prognostic. Results for combined primary and nodal MTV were similar to primary MTV alone (hazard ratio for progression-free survival 2.37, 95% CI [0.99, 5.69], p=0.05). Primary MTV and total MTV were highly correlated (Spearman correlation 0.78 on the raw values; phi coefficient 0.57 for measures dichotomized by group median).
Table 4.
Correlation of Baseline Primary MTV with Treatment Outcomes
| Variable | Endpoint | Events/total | Hazard ratio (95% CI) | p-value |
|---|---|---|---|---|
| Primary MTV (continuous) | Local relapse | 8/68 | 1.05 (1.00, 1.09) | 0.06 |
| Local-regional relapse | 16/68 | 1.05 (1.02, 1.08) | <.01 | |
| Distant metastasis | 8/68 | 1.04 (1.01, 1.08) | 0.02 | |
| Progression-free survival | 24/68 | 1.05 (1.02, 1.07) | <.01 | |
| Overall survival | 15/68 | 1.03 (1.00, 1.06) | 0.08 | |
| Primary MTV (> vs. ≤ median) | Local relapse | 5/33 vs. 3/35 | 1.96 (0.47, 8.23) | 0.36 |
| Local-regional relapse | 12/33 vs. 4/35 | 4.01 (1.28, 12.52) | 0.02 | |
| Distant metastasis | 6/33 vs. 2/35 | 3.62 (0.73, 18.04) | 0.12 | |
| Progression-free survival | 15/33 vs. 9/35 | 2.34 (1.02, 5.37) | 0.05 | |
| Overall survival | 8/33 vs. 7/35 | 1.40 (0.51, 3.86) | 0.52 |
CI = confidence interval.
Hazard ratios estimated from Cox models.
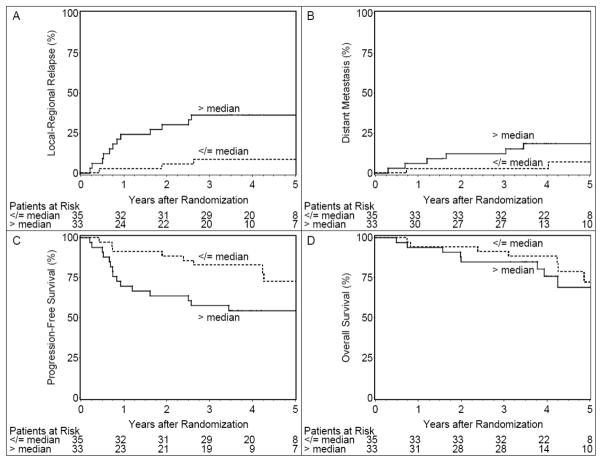
Figure 1.
Cumulative incidence estimates of local-regional relapse (Panel A) and distant metastasis (Panel B) and Kaplan-Meier estimates of progression-free survival (Panel C) and overall survival (Panel D) for patients with baseline primary MTV ≤ median or > median. Two-year local-regional relapse rates were 5.7% (95% CI: 0, 13.5) for patients with MTV ≤ median and 30.3% (95% CI: 14.3, 46.3) for patients with MTV > median. Two-year distant metastases rates were 2.9% (95% CI: 0, 8.5) for patients with MTV ≤ median and 12.1% (95% CI: 0.8, 23.4) for patients with MTV > median. Two-year progression-free survival rates were 88.6% (95% CI: 78.0, 99.1) for patients with MTV ≤ median and 63.6% (95% CI: 47.2, 80.0) for patients with MTV > median. Two-year overall survival rates were 94.3% (95% CI: 86.6, 100) for patients with MTV ≤ median and 84.9% (95% CI: 72.6, 97.1) for patients with MTV > median.
Supplementary Table 1 presents summary statistics for primary MTV by T stage. The mean primary MTV roughly doubled with each upstage: T2 6.39, T3 13.24, T4, 26.79. Primary MTV was a stronger prognostic factor than T stage for local-regional relapse and progression-free survival (Table 5). The hazard ratios for primary MTV changed little when T stage was added to a bivariate model, but T stage lost much of its prognostic value for local-regional relapse, and all for progression-free survival, when primary MTV was added to the model. Akaike information criterion (AIC) is lowest when primary MTV remained as a single variable in the model. Collinearity was not an issue, with the variance inflation factor limited to 1.27.
Table 5.
Primary MTV vs. T Stage
| Model | AIC [1] | Covariate(s) | Hazard ratio (95% CI) | p-value |
|---|---|---|---|---|
| Local-regional relapse | ||||
| 1 | 120.28 | Primary MTV (> vs. ≤ median) | 4.01 (1.28, 12.52) | 0.02 |
| 2 | 124.63 | T stage (T4 vs. T2–3) | 2.34 (0.83, 6.59) | 0.11 |
| 3 | 121.98 | Primary MTV (> vs. ≤ median) T stage (T4 vs. T2–3) |
3.59 (1.07, 12.11) 1.36 (0.45, 4.11) |
0.04 0.58 |
| Progression-free survival | ||||
| 1 | 183.20 | Primary MTV (> vs. ≤ median) | 2.34 (1.02, 5.37) | 0.05 |
| 2 | 186.52 | T stage (T4 vs. T2–3) | 1.54 (0.63, 3.74) | 0.34 |
| 3 | 185.19 | Primary MTV (> vs. ≤ median) T stage (T4 vs. T2–3) |
2.31 (0.94, 5.70) 1.03 (0.39, 2.71) |
0.07 0.95 |
CI = confidence interval.
Hazard ratios estimated from Cox models.
Akaike information criterion.
Subgroup Analysis for p16-Positive Oropharynx Cases
Primary MTV and total MTV remained prognostic in patients with p16-associated oropharyngeal cancer (Supplementary Table 2). This ability was associated exclusively with MTV; SUVmax and SUVpeak remained non-prognostic.
DISCUSSION
A mixed picture has emerged regarding utility of FDG-PET-derived imaging biomarkers in head and neck cancer. Although older series suggest poor outcomes in patients presenting with highly elevated SUV values (11–13), prospective institutional data (14) suggest that baseline SUVmax and SUVpeak values are not prognostic, either in unselected patients or in patients with HPV-associated oropharyngeal disease. In fact, our current results from RTOG 0522 alternatively suggest better locoregional control and progression-free survival in patients presenting with primary SUVmax above the median value for the study cohort (Table 3). Whether this reflects statistical noise from a small sample or true limitations of SUV-based measures, it is clear that validation of a more robust FDG-PET- defined biomarker could be of considerable clinical value, complementing the incrementally improved staging accuracy of PET-CT.
Metabolic tumor volume is a novel PET-specific measure, which incorporates complementary information relevant to both disease burden (akin to clinical stage and CT-derived tumor volume, which are prognostic for outcomes (25, 26)) and tumor metabolic activity. Although the size of our dataset is limited, our findings add to an emerging literature, which suggests that high primary metabolic tumor volume is associated with local-regional relapse and reduced progression-free survival following radiotherapy.
Despite expected correlation of primary MTV with T stage (15), earlier reports suggest that MTV dominates univariate and multivariate models of treatment outcome, independent of T stage or HPV infection status (16, 17). Interestingly, these same reports also suggest lack of utility for SUV values and nodal MTV. Although formal multivariate analysis was not deemed appropriate due to limited events, the effect of primary MTV was independent of age, gender, Zubrod performance status, pack-years, T stage, and N stage in bivariate analysis. And while elevated primary MTV was strongly associated with regional and distant disease failure in our series, nodal MTV remained non-prognostic. Although tempting to speculate that biology and tissue architecture specific to nodal disease (such as cystic adenopathy seen in HPV-associated disease) confounds productive use of MTV in regional disease sites, this requires follow-up study for definitive characterization. Along this vein, closer correlation of primary MTV with locoregional/distant disease failure than with local failure (Table 4) is in keeping with prior reported data [17], and suggests need for complementary work to mechanistically explain the exact biological significance of primary MTV.
Pretreatment imaging biomarkers can potentially direct personalized treatment intensity. Our findings also suggest that intraobserver PET reading consistency is best in the pretreatment setting. Both issues are of relevance to HPV-associated oropharyngeal disease, which typically responds briskly to treatment and may benefit from pretreatment PET-guided de-intensification strategies to limit toxicity. Seven of 8 oropharyngeal cancer relapses in the PET imaging analysis cohort were p16-positive. These 7 patients suffered recurrences in varied locations: 1 tumor recurred in the primary site, 4 recurred in the neck, 1 recurred locoregionally, and 1 recurred both locoregionally and distantly. Six of these 7 patients had discordant above-median baseline primary MTV values in the face of below-median baseline SUVmax. This spotlights the candidacy of MTV as an imaging-based identifier of “intermediate-risk” p16-positive disease (27, 28) (i.e. HPV-associated oropharynx patients with bulky primary tumor and/or traditional risk-factor exposure history who suffer inferior treatment outcomes and are not appropriate for de-intensified therapy). Nonetheless, this remains an early hypothesis-generating result subject to the potential biases and study power limitations of unplanned subgroup analysis. Formal testing in future multicenter trials is mandatory for validation.
Presence of a strong prognostic signal for MTV in our small, heterogeneous study cohort is encouraging. Nonetheless, MTV has limitations as a candidate imaging biomarker. No standard definition for MTV currently exists. Earlier series have used variable, arbitrarily defined % primary and nodal SUVmax cutoffs to delineate MTV. Accordingly, the relative prognostic performance of specific MTV cutoff values is difficult to compare across published series. In addition, practical use of MTV following treatment is challenging given return of responding tumor sites to background tracer uptake levels. Although prior pilot work supports the use of a post-treatment MTV threshold of SUV = 2.0 (29), this could not be feasibly reproduced in this study with commercially available software. Future confirmatory work remains necessary.
Another key limitation of our findings is that formal participation of patients enrolled onto the RTOG 0522 trial was limited to 116 patients, and that only 74 of these patients had a full complement of serial imaging available for the analysis specified by protocol. While reasons for low participation remain speculative, the resulting small sample size suffers from limited statistical power and potential provider-dependent selection biases. Fortunately, the clinical characteristics of the subgroup match reasonably with those of the eligible population as a whole (Table 1), suggesting broader applicability of our findings to the general head and neck radiotherapy patient population. Similar study power issues also impact our secondary subgroup analysis of p16+ patients.
To conclude, we found a unique prognostic signal for primary tumor FDG-PET-derived MTV in patients with locally advanced head and neck cancer. This finding remains preliminary, and requires technical refinement and clinical confirmation. There is growing need to better match resource-intensive treatment to individual patient risk. Validation of MTV, a straightforward, non-invasive measure derived from routine workup imaging, could be an important step towards accomplishing this goal.
Supplementary Material
Supplementary Table 1. Primary MTV by T Stage
Supplementary Table 2. Correlation of Baseline Primary MTV with Treatment Outcomes—Subgroup Analysis for p16-Positive Oropharynx Cases
SUMMARY.
There is need to better match cancer treatment to individual patient risk. Tumor imaging provides a means to define disease phenotype and treatment response. Predefined study objectives of the recently concluded RTOG 0522 trial included correlation of PET/CT findings with treatment outcomes. In this report, we describe a potential prognostic signal for primary tumor FDG-PET-derived MTV in patients with locally advanced head and neck cancer. This finding remains preliminary, and requires further confirmation.
Acknowledgments
Supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI) and Bristol-Myers Squibb. This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflicts of Interest: None
Conflicts of Interest: Dr. Robert Foote reports grant support from RTOG, personal fees from ASTRO for editorial services, and personal fees from UpToDate, all outside the scope of the submitted work. Dr. David Rosenthal reports personal fees from Bristol Myers during the conduct of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. 2008;100:712–20. doi: 10.1093/jnci/djn125. [DOI] [PubMed] [Google Scholar]
- 2.Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, et al. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol. 2004;22:4357–68. doi: 10.1200/JCO.2004.08.120. [DOI] [PubMed] [Google Scholar]
- 3.Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med. 2007;48 (Suppl 1):78S–88S. [PubMed] [Google Scholar]
- 4.Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess. 2007;11:iii–iv. xi–267. doi: 10.3310/hta11440. [DOI] [PubMed] [Google Scholar]
- 5.Syed R, Bomanji JB, Nagabhushan N, Hughes S, Kayani I, Groves A, et al. Impact of combined (18)F-FDG PET/CT in head and neck tumours. Br J Cancer. 2005;92:1046–50. doi: 10.1038/sj.bjc.6602464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoder H, Yeung HW, Gonen M, Kraus D, Larson SM. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology. 2004;231:65–72. doi: 10.1148/radiol.2311030271. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DL, Ford E, Rajendran J, Yueh B, Coltrera MD, Virgin J, et al. FDG-PET/ CT imaging for preradiotherapy staging of head-and-neck squamous cell carcinoma. International journal of radiation oncology, biology, physics. 2005;61:129–36. doi: 10.1016/j.ijrobp.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Veit-Haibach P, Luczak C, Wanke I, Fischer M, Egelhof T, Beyer T, et al. TNM staging with FDG-PET/CT in patients with primary head and neck cancer. Eur J Nucl Med Mol Imaging. 2007;34:1953–62. doi: 10.1007/s00259-007-0564-5. [DOI] [PubMed] [Google Scholar]
- 9.Gregoire V. Is there any future in radiotherapy planning without the use of PET: unraveling the myth. Radiother Oncol. 2004;73:261–3. doi: 10.1016/j.radonc.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Moeller BJ, Rana V, Cannon BA, Williams MD, Sturgis EM, Ginsberg LE, et al. Prospective imaging assessment of mortality risk after head-and-neck radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:667–74. doi: 10.1016/j.ijrobp.2009.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halfpenny W, Hain SF, Biassoni L, Maisey MN, Sherman JA, McGurk M. FDG-PET. A possible prognostic factor in head and neck cancer. Br J Cancer. 2002;86:512–6. doi: 10.1038/sj.bjc.6600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allal AS, Dulguerov P, Allaoua M, Haenggeli CA, El-Ghazi el A, Lehmann W, et al. Standardized uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol. 2002;20:1398–404. doi: 10.1200/JCO.2002.20.5.1398. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz DL, Rajendran J, Yueh B, Coltrera MD, Leblanc M, Eary J, et al. FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg. 2004;130:1361–7. doi: 10.1001/archotol.130.12.1361. [DOI] [PubMed] [Google Scholar]
- 14.Moeller BJ, Rana V, Cannon BA, Williams MD, Sturgis EM, Ginsberg LE, et al. Prospective risk-adjusted [18F]Fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol. 2009;27:2509–15. doi: 10.1200/JCO.2008.19.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–8. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 16.La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–41. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83:1514–20. doi: 10.1016/j.ijrobp.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalbfleish J, Prentice R. The statistical analysis of failure time data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–81. [Google Scholar]
- 20.Cox DR. Regression models and life tables. J Royal Statist Soc. 1972;34:187–229. [Google Scholar]
- 21.Yao M, Graham MM, Hoffman HT, Smith RB, Funk GF, Graham SM, et al. The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. International journal of radiation oncology, biology, physics. 2004;59:1001–10. doi: 10.1016/j.ijrobp.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, et al. Fully conditional specification in multivariate imputation. J Stat Comput Sim. 2006;76:1049–64. [Google Scholar]
- 23.Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 24.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- 25.Hermans R, Op de beeck K, Van den Bogaert W, Rijnders A, Staelens L, Feron M, et al. The relation of CT-determined tumor parameters and local and regional outcome of tonsillar cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2001;50:37–45. doi: 10.1016/s0360-3016(00)01559-5. [DOI] [PubMed] [Google Scholar]
- 26.Lodder WL, Pameijer FA, Rasch CR, van den Brekel MW, Balm AJ. Prognostic significance of radiologically determined neck node volume in head and neck cancer: a systematic review. Oral Oncol. 2012;48:298–302. doi: 10.1016/j.oraloncology.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–47. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 28.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy JD, La TH, Chu K, Quon A, Fischbein NJ, Maxim PG, et al. Postradiation metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:514–21. doi: 10.1016/j.ijrobp.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Primary MTV by T Stage
Supplementary Table 2. Correlation of Baseline Primary MTV with Treatment Outcomes—Subgroup Analysis for p16-Positive Oropharynx Cases