SUMMARY
Coronary artery disease (CAD) is a major determinant of the long-term prognosis among patients with diabetes mellitus (DM). DM is associated with a 2 to 4-fold increased mortality risk from heart disease. Furthermore, in patients with DM there is an increased mortality after MI, and worse overall prognosis with CAD. Near-normal glycemic control for a median of 3.5 to 5 years does not reduce cardiovascular events. Thus, the general goal of HbA1c <7% appears reasonable for the majority of patients. Iatrogenic hypoglycemia is the limiting factor in the glycemic management of diabetes, and is an independent cause of excess morbidity and mortality. Statins are effective in reducing major coronary events, stroke, and the need for coronary revascularization.
Selection of the optimal myocardial revascularization strategy for patients with DM and multivessel coronary artery disease is crucial and requires a multidisciplinary team approach (‘heart team’). Large scale clinical trials have shown that for many patients with 1- or 2-vessel coronary artery disease, there is little prognostic benefit from any intervention over optimal medical therapy (OMT). PCI with drug-eluting or bare metal stents is appropriate for patients who remain symptomatic with OMT. Randomized trials comparing multivessel PCI to coronary artery bypass grafting (CABG) have consistently demonstrated the superiority of CABG in reducing mortality, myocardial infarctions and need for repeat revascularizations.
Keywords: Blood Glucose, Coronary Disease, Diabetes mellitus, Hypoglycemic Agents, Revascularization, Statins
Introduction
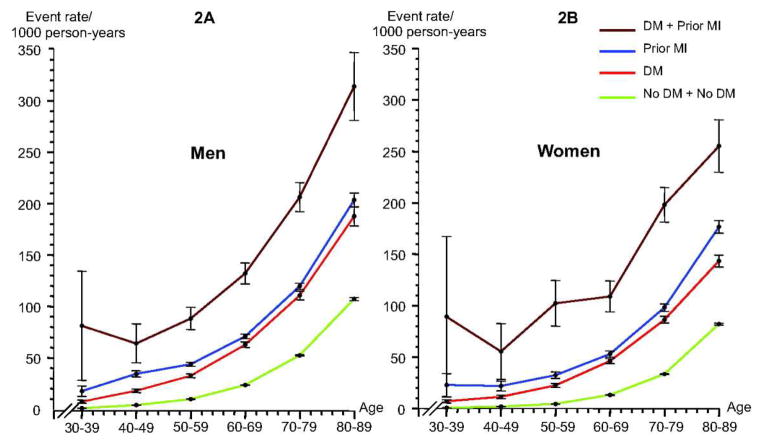
Diabetes mellitus (DM) has reached epidemic proportions worldwide, and its prevalence is rising.1,2 The implications of a diagnosis of DM are as severe as a diagnosis of coronary artery disease (CAD). Cardiovascular mortality in all age groups and for both sexes rises equivalently with DM or a history of myocardial infarction (MI) and the two are profoundly synergistic3 (Figure 1). In addition, DM (especially type 2 DM), is associated with clustered risk factors for cardiovascular disease (CVD). Amongst adults with DM there is a prevalence of 75% to 85% of hypertension, 70% to 80% for elevated LDL, and 60% to 70% for obesity.4 CAD is the main cause of death in both type 1 and type 2 DM,5 and DM is associated with a 2 to 4-fold increased mortality risk from heart disease. Over 70% of people >65 years of age with DM will die from some form of heart disease or stroke.2 Furthermore, in patients with DM there is an increased mortality after MI, and worse overall long-term prognosis with CAD.6,7
Figure 1.
Event rates for the composite endpoint of MI (nonfatal), stroke (nonfatal), and cardiovascular death in men (A) and women (B) stratified by age in relation to diabetes mellitus (DM) and a prior MI. From Schramm TK, Gislason GH, Kober L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117:1945–1954; with permission.
In the United States, approximately one third of all percutaneous coronary intervention (PCI) procedures are performed on patients with DM and ~25% of patients undergoing coronary artery bypass graft (CABG) surgery have DM5; the outcomes of these procedures is less effective than in those without DM. DM modifies the response to arterial injury, with profound clinical consequences in terms of risk for restenosis8 and stent thrombosis.9 Although there has been considerable improvement in the management of patients with CAD, coronary event rates remain heightened among patients with DM.2,10–12 Therefore, optimal medical therapy (OMT) and appropriate selection of myocardial revascularization strategy is critical for patients with DM. The following review summarizes the current evidence regarding the effectiveness of various medical therapies and revascularization strategies in patients with DM.
Glycemic control and cardiovascular outcomes
DM is a fascinating disease in that while it has been known since antiquity the disease we refer to can only be dated to the era after the wide-spread use of insulin. Prior to the introduction of insulin replacement DM was an almost universally fatal disease that primarily struck children. The DM of today, with all of its chronic manifestations, is the associated consequence of lifesaving and life-prolonging effects of insulin and naturally many have wondered how “tight” control of blood sugar with precise insulin dosing would affect cardiovascular risk. The results have been sobering; in general, tight glycemic control is associated with an increased risk for hypoglycemia but minimal to no benefit on mortality. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was designed to test whether treatment targeting nearly normal glycemic control reduces the risk of cardiovascular events in type 2 DM. More than ten thousand patients were randomized to either a standard treatment strategy that targeted HbA1c levels between 7% and 8% or an intensive strategy that sought to attain an hemoglobin (Hb) A1c <6.0%. The median HbA1c with the standard strategy was 7.5%; the intensive strategy achieved a median HbA1c of 6.4%.13 Yet, the intensive strategy was associated with 22% increase in all-cause mortality and the study was stopped after a median follow-up of 3.4 years.
The Action in Diabetes and Vascular Disease: A Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial randomized 11,140 participants to a strategy of intensive glycemic control (with primary therapy being the sulfonylurea gliclizide and additional medications as needed to achieve a target HbA1c of <6.5%) or to standard therapy, with the glycemic target set according to “local guidelines”. The median HbA1c levels achieved in the intensive and standard arms were 6.3% and 7.0%, respectively. Intensive treatment produced a relative reduction of 10% in the primary composite outcome of major macrovascular and microvascular events (HR, 0.90; 95% CI, 0.82 to 0.98; P=0.01), primarily as a consequence of a reduction in nephropathy (a microvascular complication). However, when major macrovascular events were considered separately (MI, stroke, and cardiovascular death), there was no observed significant reduction (HR, 0.94; 95% CI, 0.84 to 1.06; P=0.32).13
The Veterans Affairs Diabetes Trial (VADT) randomized 1791 participants with type 2 DM uncontrolled on insulin or maximal-dose oral agents (median entry HbA1c, 9.4%) to intensive glycemic control (goal HbA1c, <6.0%) or standard glycemic control, with a planned HbA1c separation of at least 1.5%.14 Over a 5.6 year follow up period there was no significant difference in the primary outcome of a composite of MI, stroke, cardiovascular death, revascularization, hospitalization for heart failure, and amputation for ischemia.
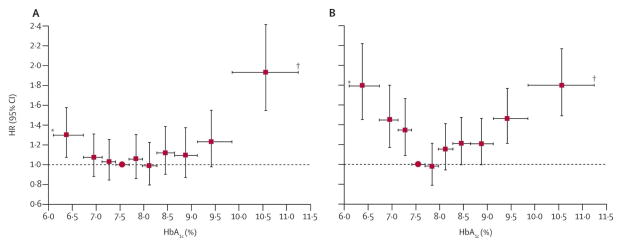
A large retrospective cohort study from the UK General Practice Research Database showed a U-shaped pattern of risk association between HbA1c and all-cause mortality and progression to large-vessel disease events among patients with type 2 DM.15 A HbA1c of approximately 7·5% was associated with the lowest risk and an increase or decrease from this mean HbA1c value was associated with a heightened risk of adverse outcomes (Figure 2).
Figure 2.
Adjusted hazard ratios for all-cause mortality by HbA1c deciles in people given oral combination and insulin-based therapies. Vertical error bars show 95% CIs, horizontal bars show HbA1c range. Red circle=reference decile. *Truncated at lower quartile. †Truncated at upper quartile. Metformin plus sulphonylureas (A); and insulin-based regimens (B). From Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–489; with permission.
The most compelling message from these studies is that near-normal glycemic control for a median of 3.5 to 5 years does not reduce cardiovascular events within that period.16 The contribution of glucose lowering to the reduction of macrovascular events in the ADVANCE and ACCORD trials appears to be minimal. It may very well be that even 90 years after insulin’s introduction as a therapeutic modality we are still unclear on the drivers of cardiovascular morbidity as a chronic manifestation of DM.
For the prevention of macrovascular disease, the general goal of HbA1c <7% appears reasonable (ACC/AHA, Class IIb recommendation; Level of Evidence: A).17 For selected individual patients, lower HbA1c goals may be reasonable in an attempt to reduce microvascular complications (low risk of hypoglycemia short duration of diabetes, long life expectancy, and no significant cardiovascular disease). Yet, it has also become clear that the potential risks of intensive glycemic control may outweigh its benefits in patients with a long duration of diabetes, known history of severe hypoglycemia, advanced atherosclerosis, and advanced age/frailty. Here less stringent HbA1c goals may be appropriate (7.5–8.0% or possibly even slightly higher).17,18
Antidiabetic drug safety
Until recently, insulin and then oral agents based on metformin and sulfonylureas dominated the therapy of DM. There are now several additional classes of drugs approved for diabetes management: α-glucosidase inhibitors, thiazolidinediones, meglitinides, glucagon-like peptide analogues, amylin analogues, dipeptidyl peptidase IV inhibitors and sodium-glucose cotransporter 2 (SGLT-2) inhibitors.19 Most oral diabetes medications reduce HbA1c levels by a similar amount, by approximately 1 absolute percentage point.20 Glycated hemoglobin, however, may not be a valid surrogate for assessing either the cardiovascular risks or benefits of diabetes therapy,21 and the long-term safety of these newer drugs with respect to cardiovascular disease (the leading cause of illness and death among patients with diabetes) remains poorly characterized. In addition, antidiabetic agents may have multiple, additional potential effects on risk factors for CAD and on cardiac function (Table 1).
Table 1.
Effect of antidiabetic agents on the cardiovascular system
| Therapeutic classes | Effects on CVD Risk Factors | Other direct and indirect effects on the heart |
|---|---|---|
| Biguanides (Metformin) | Reduction in macrovascular endpoints110
Improved lipid profile |
— |
| Sulfonylureas | Weight gain | Hypoglycemia Impaired ischemic pre- conditioning111 |
| Prandial glucose regulators (meglitinides) | Weight gain | Hypoglycemia |
| Thiazolidinediones / Glitazones | Increase LDL levels20,112
Reduced restenosis after coronary stenting113,114 |
Heart failure115,116
Excess ischemic cardiovascular risk with rosiglitazone (?)23,24 |
| Alpha-glucosidase inhibitors | Reduced inflammatory markers112
Possible decrease in risk of cardiovascular disease event117 |
— |
| DPP-4 inhibitors (gliptins) | Heart failure30,116 | |
| Amylin analogues (Pramlintide) | Weight loss | — |
| Incretin mimetics (GLP-1 analogues) | Weight loss | — |
| Insulin | Weight gain118 | Hypoglycemia |
Concerns have been raised that some antidiabetes agents may impart greater cardiovascular risk. The University Group Diabetes Project study suggested increased CV risk in patients treated with tolbutamide, a first-generation sulfonylurea. These results have been widely criticized based on study design flaws.22 A meta-analysis of clinical trials of rosiglitazone (Avandia), a thiazolidinedione, pointed to an increased risk of MI,23 although the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) study did show an increased risk of major adverse cardiovascular events (MACE).24
The initial concern with rosiglitazone led the FDA to issue an updated Guidance for Industry in 2008 requiring preapproval and post approval studies for all new antidiabetic drugs to rule out excess cardiovascular risk. In a postmarketing trial, the two-sided 95% CI for the estimated increased risk (risk ratio) should be less than 1.3.25
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial assessed therapeutic strategies rather than any specific drug. No safety concerns were seen for the insulin-sensitizing group, in which >60% received thiazolidinediones, predominantly rosiglitazone (55%).26 Notwithstanding, given that other options are now available, the use of rosiglitazone is not recommended.27 Newer antidiabetic agents, such as, agonists of the glucagon-like peptide-1 (GLP1) receptor or dipeptidyl peptidase 4 (DPP4) inhibitors, which prevent the breakdown of endogenous GLP1, have shown beneficial effects in patients undergoing angioplasty and CABG in small studies.28,29 However, in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR), the DPP-4 inhibitor saxagliptin did not change the primary composite endpoint of cardiovascular death, myocardial infarction, or ischemic stroke, when added to the standard of care in patients at high risk for cardiovascular events.30 Although saxagliptin clearly met the FDA guidance for cardiovascular safety, therapy with the drug was associated with an unexpected increased risk of hospitalization for heart failure (especially in patients with high baseline BNP levels) and a higher frequency of hypoglycemia.30 Similarly, in the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial, the DPP-4 inhibitor alogliptin was neutral with regard to major cardiovascular events that in patients with type 2 diabetes and cardiovascular disease or high cardiovascular risk, but met the FDA guidance for cardiovascular safety.31 Currently, there is no sufficient evidence to definitively support one drug or combination of drugs over another for long-term clinical outcomes of mortality and macrovascular and microvascular complications of diabetes.20,32
Hypoglycemia and Mortality
Iatrogenic hypoglycemia is the limiting factor in the glycemic management of DM, and classically arises from the interplay of mild-to-moderate absolute or even relative therapeutic hyperinsulinemia and compromised physiological and behavioral defenses against falling plasma glucose concentrations.33 Hypoglycemia is a substantial, independent cause of excess morbidity and mortality. In the ACCORD trial, severe hypoglycemia was associated with increased mortality by 2 and 4-fold in the intensive and conventional groups, respectively. However, hypoglycemia was not identified as the cause for this excess mortality in the intensive group.34
In the ADVANCE trial, severe hypoglycemia was associated with excess cardiovascular events and total mortality.35 However, neither a close temporal relationship nor a dose–response relationship was observed between hypoglycemic events and subsequent cardiovascular or fatal events. Therefore, hypoglycemia might be a marker for patient-related disorders or complications, which could predispose patients to an excess risk of coronary heart disease.34,35
Plausible mechanisms by which hypoglycemia might cause cardiovascular events or lead to death from cardiovascular disease include sympathoadrenal activation, abnormal cardiac repolarization, increased thrombogenesis, inflammation, and vasoconstriction leading to cardiac ischemia or fatal arrhythmia during recognized or unrecognized episodes of hypoglycemia.36,37 Although the relationship in hospitalized patients with acute MI between hypoglycemia and cardiovascular outcomes is complex,38 there was a clear J-shaped relationship between glucose values and adverse outcomes, including increased mortality.39,40 In this context, hypoglycemia has been shown to lower myocardial blood flow reserve41 and increase experimental infarct size.42 Although it is reasonable to suspect that iatrogenic hypoglycemia contributes directly to the excess mortality during intensive glycemic therapy, especially during acute ischemia, the association may represent increased vulnerability of people who are prone to hypoglycemia to other serious health outcomes due to the coexistence of other risk factors35,38 or hypoglycemia may be a marker for more critical illness.43 Notwithstanding, these data provide compelling reasons to minimize the risk of hypoglycemia in patients with diabetes and CAD.
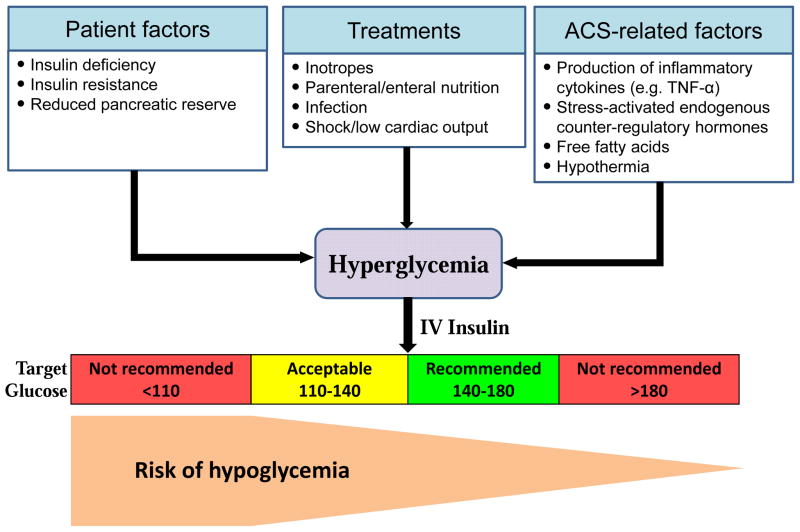
The use of insulin infusions to control hyperglycemia or to provide metabolic support in face of stress is falling off as severe hypoglycemia (<2.2 mmol/L [40 mg/dL]) occurs frequently (5%–17%)44,45 and is associated with higher mortality.46 There is no additional benefit from the lowering of blood glucose levels below the range of approximately 140 to 180 mg/dL.47–49 For the majority of critically ill patients, the American Diabetes Association recommends initiating insulin therapy for treatment of persistent hyperglycemia, starting at a threshold of no greater than 180 mg/dl (10.0 mmol/l), and aiming for glucose range of 140 –180 mg/dl (7.8–10.0 mmol/l).47 Similar recommendations were provided by the American College of Physicians48 and the American Heart Association (Figure 3).49
Figure 3.
Management of hyperglycemia in patients with acute coronary syndromes. Adapted from Aronson D, Edelman ER. Role of CABG in the management of obstructive coronary arterial disease in patients with diabetes mellitus. Current opinion in pharmacology. 2012;12:134–141; with permission.
Management of hyperlipidemia in patients with DM
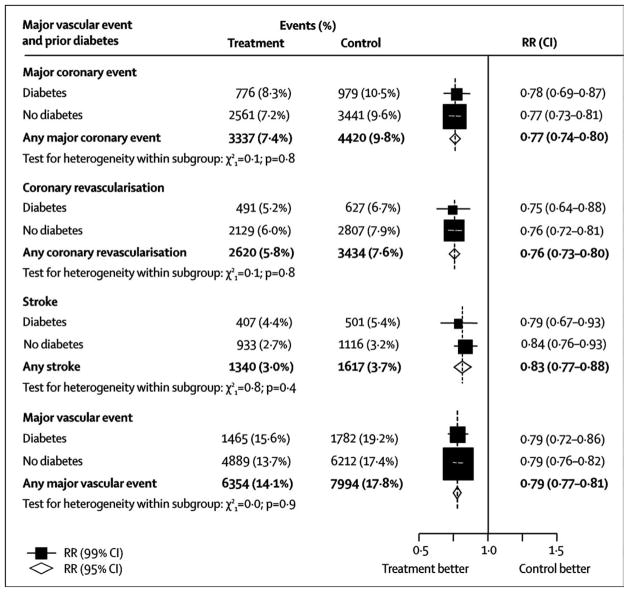
Hydroxymethyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are the cornerstone of lipid-associated cardiovascular-risk reduction, with established benefits in reducing clinical events in patients with diabetes shown in a multitude of trials. The Cholesterol Treatment Trialists’ (CTT) Collaboration analysis of data from 18686 individuals with diabetes (1466 with type 1 and 17 220 with type 2) in the context of 14 randomized trials of statin therapy, showed that statin therapy reduced the 5-year incidence of major vascular events by about a fifth per mmol/L reduction in LDL cholesterol, with similar proportional reductions in major coronary events, stroke, and the need for coronary revascularization.50
For primary prevention, patients with DM ages 40 to 75 years with an LDL–C 70 to189 mg/dL and without clinical cardiovascular disease, are one of the major statin benefit groups. Among the four randomized controlled trials focused exclusively on primary prevention, the highest rate of cardiovascular events occurred in Collaborative Atorvastatin Diabetes Study (CARDS),51 which exclusively enrolled a primary prevention population with diabetes. The CTT meta-analyses also supports the use of statins to reduce the risk of cardiovascular disease in individuals with type 1or 2 diabetes50,52 (Figure 4). Thus, a high level of evidence supports the use of moderate-intensity statin therapy in all primary-prevention-eligible adults age 40 to 75 years with DM (Table 2). For primary prevention in individuals with DM (both type-1 and type-2), the estimated 10-year cardiovscular risk can also be used to guide the intensity of statin therapy; such that when the estimated 10-y CVD risk is ≥7.5%, high-intensity statin can be used. The percent reduction in LDL–C can be used as an indication of response and adherence to therapy, but is not in itself a treatment goal.53 For secondary prevention, high-intensity statin therapy should be initiated for adults ≤75 years of age who are not receiving statin therapy or the intensity should be increased in those receiving a low- or moderate-intensity statin.53 Combination therapy does not provide additional cardiovascular benefit above statin therapy alone and is not generally recommended.54
Figure 4.
Cholesterol Treatment Trialists’ (CTT) Collaboration meta-analysis showing proportional effects of statins on major vascular events per mmol/L reduction in LDL cholesterol in participants presenting with or without diabetes. From Cholesterol Treatment Trialists C, Kearney PM, Blackwell L, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125; with permission.
Table 2.
Recommendations for Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults with Diabetes—Statin Treatment
| Recommendations | NHLBI Grade | ACC/AHA COR | ACC/AHA LOE |
|---|---|---|---|
| Moderate-intensity statin† therapy should be initiated or continued for adults 40 to 75 years of age with diabetes mellitus. | A (Strong) | I | A |
| High-intensity statin‡ therapy is reasonable for adults 40 to 75 years of age with diabetes mellitus with a ≥7.5% estimated 10-year CVD|| risk unless contraindicated. | E (Expert Opinion) | IIa | B |
| In adults with diabetes mellitus, who are <40 or >75 years of age, it is reasonable to evaluate the potential for CVD benefits and for adverse effects, for drug-drug interactions, and to consider patient preferences when deciding to initiate, continue, or intensify statin therapy | E (Expert Opinion) | IIa | C |
Daily dose lowers LDL–C on average, by approximately 30% to <50% (e.g. atorvastatin 10 mg, rosuvastatin 10 mg, simvastatin 20–40 mg and pravastatin 40 mg.
Daily dose lowers LDL–C on average, by approximately ≥50% (e. g. atorvastatin 40–80 mg or rosuvastatin 20–40 mg.
Adapted from Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; with permission.
Statin use and risk of diabetes
There has recently emerged a concern that statin use may be associated with the development of DM. A collaborative meta-analysis of 13 randomized placebo-controlled trials with more than 90,000 participants found a small, 9% increased risk for incident diabetes after four years of statin treatment, particularly in older people.55 For 255 patients (95% CI 150–852) treated for 4 years with a statin, one additional patient would develop diabetes. Another meta-analysis showed that intensive-dose statin therapy is associated with a somewhat higher risk than moderate dose therapy.56 In higher risk secondary prevention patients with established CAD, the diabetes risk associated with statin therapy is low in absolute terms when compared with the reduction in cardiovascular events. Based on these studies, the FDA added a warning regarding diabetes risk to the labeling of statins 57
In the randomized, placebo controlled Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, there was a modest risk of developing diabetes on statin therapy that was limited to patients who had preexisting biochemical evidence of impaired fasting glucose or multiple components of metabolic syndrome.58 However, even in the setting of this primary prevention trial, the cardiovascular and mortality benefits of statin therapy exceeded the diabetes hazard in the trial population as a whole as well as among those at higher risk for developing diabetes.
Overall, the risk of DM is ~1 excess case per 1000 individuals treated with a moderate-intensity statin for 1 year and ~3 excess cases per 1000 individuals treated with a high-intensity statin treated patients for 1 year.53 Individuals receiving statin therapy should be evaluated for new-onset diabetes mellitus. Those who develop diabetes mellitus during statin therapy should continue statin therapy to reduce their risk of CVD events.53 To date, a potential mechanism to explain the findings of a higher incidence of DM with statin therapy compared with placebo, and intensive-dose therapy compared with moderate dose therapy, has not been clearly identified.
Coronary revascularization in patients with DM
Revascularization versus Medical Therapy
Patients with DM and CAD are at high risk of cardiovascular events regardless of symptoms.59 Whether such patients with stable CAD should undergo prompt revascularization is an important clinical question with broad implications for risk stratification and treatment.60 As such, the BARI 2D trial tested the hypothesis that in patients with DM and stable CAD, prompt revascularization with either CABG or PCI, would reduce long-term rates of death and cardiovascular events as compared with OMT alone. BARI-2D randomized patients with demonstrated ischemia who were asymptomatic or who had mild to moderate symptoms, and documented CAD by angiography. The appropriate method of revascularization for each patient (PCI or CABG) was determined a priori by the responsible physician, resulting in a population of patients with a much greater atherosclerotic burden in the CABG stratum. The 5-year survival rate was 88.3% among patients in the revascularization group and not statistically different in the medical-therapy group (87.8%). Similarly, the major cardiovascular event rate did not differ significantly between the revascularization and the OMT groups. As compared with OMT, patients who underwent CABG (but not PCI) had significantly fewer major cardiac events, mainly a reduction in nonfatal MIs.26
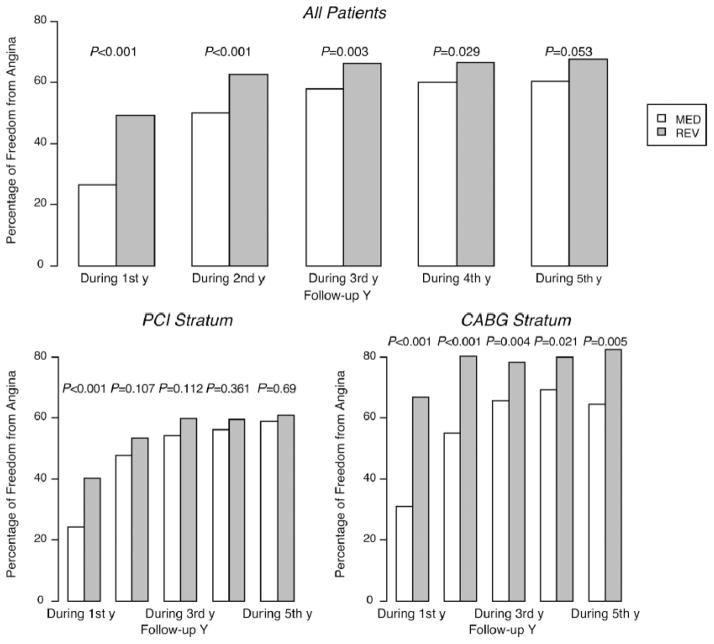
The benefits of revascularization were documented mostly during the year after the intervention, and most importantly were noticeably greater in patients undergoing CABG than PCI (Figure 5).26,61 Similarly, in COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation), the addition of early PCI to optimal medical therapy did not significantly reduce the risk of death or MI regardless of DM status.62
Figure 5.
Annual occurrence of freedom from angina in 1434 patients with angina at entry in BARI-2D. In the PCI stratum, the increase in freedom from angina was documented only at the first year of the follow-up. In the CABG stratum, the freedom from angina was increased during the 5-year follow-up. From Dagenais GR, Lu J, Faxon DP, et al. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation. 2011;123:1492–1500; with permission.
Use of Drug Eluting Stents in Patients With DM
Although the magnitude of restenosis reduction achieved with drug-eluting stents (DES) is impressive, it is important to recognize that these trials mandated an angiographic follow-up. Revascularization was therefore driven not only by clinical necessity but also by the angiographic appearance of narrowing within the treated segment even in patients who did not have documented ischemia.63 In real world practice, the benefit of DESs in patients with DM appears to be less impressive.64 For example, in the Swedish Coronary Angiography and Angioplasty Registry, the numbers needed to treat a diabetic patient with DES to avoid one additional restenosis per year with BMS ranged from 21 to 47 lesions in patients treated with one stent and 11 to 27 in patients with multiple stents.65 DES significantly reduced restenosis to half the rate seen with BMS. However, there was no difference in the combined outcome of death or MIs in diabetic patients treated with DES or BMS with up to 4 years of follow-up.65
Recent studies evaluated the comparative effectiveness of second-generation DES among diabetic patients.60 A post hoc subgroup analysis from 4 pooled randomized trials with 27.6% diabetic patients compared the Xience V everolimus eluting stent (EES) with a first generation DES, Taxus PES (paclitaxol eluting stent, Express and Liberté)66 In these 1,869 patients with diabetes, there were no differences in clinical outcomes after 2 years between the first- and newer generation DES.66
Data regarding the latest-generation Resolute Zotarolimus Eluting Stent (ZES; with controlled drug release over a longer time period) was analyzed using pooled patient-level data from the 5,130 patients (1,535 with DM). Target vessel failure (TVF) was defined as a composite of cardiac death, target vessel myocardial infarction, and ischemia-driven target vessel revascularization. The rate of TVF in a pre-specified analysis of patients with diabetes at 12 months was 7.8%, considerably less than the predefined DES performance goal of 14.5% (p<0.001).67 After 2 years, the cumulative incidence of TLF in patients with noninsulin-treated diabetes was comparable to that of patients without diabetes (8.0% vs. 7.1%). The higher risk insulin-treated DM demonstrated a significantly higher TLF (13.7%). Rates of ST were not significantly different between patients with and without diabetes (1.2% vs. 0.8%). Based on this analysis, the FDA approved the Resolute ZES with specific labeling indication for patients with DM.
Although both of the newer generation DES (i.e., the Resolute ZES and the Xience V) have improved outcomes compared with first-generation DES, there is still an opportunity to improve the treatment of CAD in patients DM, particularly those treated with insulin. Overall, the results emphasize the lack of mechanistic understanding with regard to the antiproliferative drugs eluted by the stent and adverse events after PCI.60
Stent Thrombosis after Drug Eluting Stent implantation
The possibility of increased rates of stent thrombosis (ST) after DES has been a matter of concern and can be particularly pertinent to diabetic patients. ST is classified based on the time of the adverse event relative to the index procedure. Early ST refers to the first 30 days after stent implantation and is further stratified into acute (<24 hours) and subacute (24 hours to 30 days). Late ST defines the time interval between 1 month and 1 year after stent implantation; very late ST includes any event beyond 1 year.68 After DES implantation, very late ST occurs at a relatively constant rate over time up to at least 5 years after stent implantation.69
ST is a multifactorial problem related to patient, lesion, and procedural factors and to the coagulation system and response to anti-platelet therapy.68 Delayed healing and impaired endothelialization (i.e., incomplete endothelial coverage of stent struts associated with persistence of fibrin deposits) is a common features of most cases of late and very late ST, which either alone or in combination with chronic inflammation and hypersensitivity reactions, and outward remodeling promote ST.70 All of these pathologic processes are amplified in DM and it therefore not surprising that several studies demonstrated higher ST rates in diabetic patients, particularly in insulin-treated patients.9,69,71,72
In the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial, insulin-requiring DM was a significant independent predictor of definite or probable ST occurring within 30 days (odds ratio, 2.35).73 In the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38), patients with DM and acute coronary syndrome (n = 3146) had twice the rate of stent thrombosis than those without DM (2.8% versus 1.4%, P < 0.0001), with highest rates among subjects treated with insulin (3.7%, P <0.0001).74 in the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, insulin-treated DM was an independent predictor of acute, subacute, late and very late ST.75
In a large 2-Institutional Cohort Study of 8,146 patients who underwent PCI with a sirolimus-eluting stents (SES) (n = 3,823) or paclitaxel-eluting stents (PES) (n = 4,323) and were followed up to 4 years after stent implantation, DM was an independent predictor of overall, early, and late definite ST.76 Similarly, in the Swedish Coronary Angiography and Angioplasty Registry of almost 74,000 DES and BMS, insulin-treated DM was an independent predictor of ST (RR 1.77).77 Finally, in the e-Cypher registry (n = 15157), insulin-dependent DM was an independent predictor of ST at 1-year (2.8-fold risk increase).71
The increased risk of diabetic patients for ST might be related to the more diffuse nature of atherosclerosis, accompanied by longer lesion lengths, smaller vessel size, and greater plaque burden, which might impose less optimal procedural results. Additionally, the detrimental effects of DM on endothelial function78 and platelet function79 may also promote the development of ST. However, further studies are needed to elucidate the mechanisms leading to increased ST risk among diabetic patients.
CABG versus PCI in Multivessel CAD
The Bypass Angioplasty Revascularization Investigation (BARI) study compared multivessel angioplasty to CABG in patients with medically treated DM and found a near doubling of mortality at 5-years with PCI (35% vs. 19%, P=0.003) 80. The survival benefit of CABG in patients with diabetes persisted at 10 years (PTCA 45.5% vs. CABG 57.8%, P=0.025) 81. In an analysis based on pooled individual patient data from 10 randomized trials comparing CABG with PCI (median follow-up of 5.9 years), mortality among patients with DM was 30% lower in the CABG group than in the PCI group.82 These large differences in mortality underscore the importance of appropriate decisions regarding the mode of revascularization in DM.
However, many cardiologists dismissed the results of these earlier randomized studies as outdated because the advent of DES technology.83 Two contemporary trials comparing PCI with DES to CABG in patients with DM attempted to address this renewed controversy. The pre-specified DM-subgroup analysis (n=452) of SYNTAX (SYNergy Between PCI With TAXus and Cardiac Surgery)84 showed that the 5-year composite MACCE rate was significantly higher in patients with DM after PCI compared with CABG (PCI: 46.5% vs CABG: 29.0%; P< 0.001) mainly due to an increased risk of repeat revascularization (PCI: 35.3% vs CABG: 14.6%; P< 0.001).85 However, the difference between PCI and CABG is larger for patients with DM than for those without.
The CARDia (Coronary Artery Revascularization in Diabetes) trial enrolled patients with DM (n=510) with either multivessel CAD or complex single-vessel CAD (ostial or proximal left anterior descending artery disease) in whom coronary revascularization was recommended on clinical grounds 86. The primary end point was a composite of death, MI, and stroke with a major secondary end point of the composite of the primary outcome and repeat revascularization using a noninferiority design. SES were used in 69% of patients in the PCI arm. The study could not demonstrate noninferiority of PCI for the primary endpoint (10.5% with CABG compared with 13.0% with PCI).
The long-term Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial, enrolled 1900 patients with diabetes and multivessel CAD (about as many patients with diabetes as in all previous trials combined) who were randomly assigned to undergo either CABG or PCI with drug-eluting stents (primarily first-generation PES or SES).87 After 5 years of follow-up, the 947 patients assigned to undergo CABG had significantly lower mortality (10.9% vs. 16.3%) and fewer myocardial infarctions (6.0% vs. 13.9%) than the 953 patients assigned to undergo PCI. However, patients in the CABG group had significantly more strokes (5.2% vs. 2.4%), mostly those that occurred within 30 days after revascularization. In the CABG group, the primary composite outcome of death, myocardial infarction, or stroke over 5 years was reduced by 7.9 percentage points, or a relative decrease of 30%, as compared with PCI (18.7% vs. 26.6%, P = 0.005).87 There was no interaction between SYNTAX score and outcomes among the overall population, suggesting that the increased event rate among patients randomized to PCI was not related to the anatomic complexity of disease at the time of revascularization.
Ongoing randomized studies of second-generation DES address important questions about revascularization strategies in patients severe CAD and DM. Currently, however, the results of the FREEDOM and other trials suggest that the comparative effectiveness of CABG and PCI on hard outcomes remains similar whether PCI is performed without stents, with bare-metal stents, or with drug-eluting stents,83 albeit at the price of an increased risk of nonfatal stroke.
Explaining the mortality benefit of CABG
The protective effects of CABG may be related to the increased restenosis rates following angioplasty in DM and incomplete revascularization associated with multivessel angioplasty.88,89 In the BARI study population, 3.1 grafts were placed per patient undergoing CABG, whereas the mean number of successfully treated lesions in the PTCA group was 2.0.80 Together with the greater degree of baseline atherosclerosis and the high restenosis rate among diabetic patients, it is likely that a higher proportion of the myocardium remains unprotected and unrevascularized in patients with DM, and a greater proportion of the myocardium becomes ischemic during an acute spontaneous myocardial infarction. The impact of incomplete revascularization may be even more severe because after PCI, progression of diffuse disease in diabetic patients forms new lesions that may cause ischemia and/or symptoms.
The amount of jeopardized myocardium decreases initially following revascularization and increases subsequently with target lesion restenosis, graft failure, or the development of new narrowings in native vessels. Follow-up angiographic analysis of the BARI patients at years 1 and 5 revealed that the total percentage of jeopardized myocardium, defined as the overall percentage of the coronary perfusion territory compromised by stenoses ≥50%, was higher in diabetic patients.90 The mean percentage increase in total jeopardized myocardium was significantly greater in diabetic compared with non-diabetic patients at 1-year protocol-directed angiography (42% versus 24%, P = 0.05) and on the first clinically performed (unscheduled) angiogram within 30 months (63% versus 50%, P = 0.01) but not at 5-year protocol-directed angiography (34% versus 26%, P = 0.33). In contrast, among CABG patients, DM was not associated with an increase in jeopardized myocardium at any angiographic follow-up interval. In this context, DM does not seem to affect the patency of internal thoracic artery (ITA) grafts, or the accelerated atherosclerotic process that characterizes vein grafts.91,92 The lower rate of nonfatal MI with surgical revascularization observed in BARI-2D26 is consistent with the hypothesis that, bypass grafts to the mid-coronary vessel treat the culprit lesion and prophylaxes against new proximal disease, progression of proximal narrowing or plaque rupture occurring proximal to a patent graft insertion. Proximal coronary arterial stents however, bare metal and drug-eluting, cannot protect against new disease.93
Graft Selection and Patency in DM patients
DM does not appear to adversely affect patency of ITA grafts.91,94 Nonrandomized analyses indicate that bilateral internal thoracic artery (BITA) grafting appears to be particularly important in the diabetic population.95 However, the use of BITA results in greater sternal wound complications in patients with DM (especially insulin-treated).96,97 Harvesting skeletonized ITA may reduce the risk of sternal wound complications associated with BITA by minimizing the risk of devascularization of the sternum as compared with removal with an attached muscle pedicle.95,98 Therefore, some surgeons believe that DM is not a contraindication for skeletonized BITA.99 Notwithstanding, presently, it is unclear whether selective referral of patients with DM for skeletonized BITA grafting despite higher risks of sternal infection is justified.100
The radial artery (RA) conduits obtained from patients with DM has greater tendency to spasm compared with RAs from patients without DM, and exhibit impaired endothelial function.101 In a randomized trial comparing angiographic RA graft patency vs. SVG patency at 1-year after CABG, there was a significant interaction between graft type and DM; RA grafts had lower patency rate than SVGs in patients with DM and the reverse was true in patients without DM.102
Approach to coronary revascularization in patients with DM
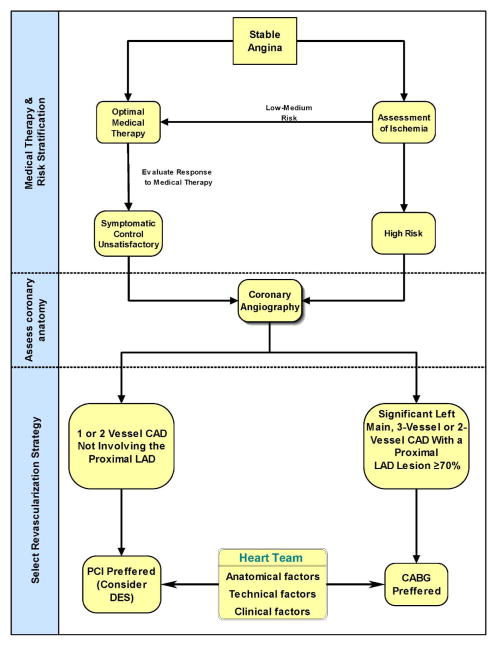
While revascularization therapies mechanically address specific local lesions, they all have limited longevity. As discussed, DM is a systemic disease with a vast array of metabolic effects that often escalate with time. It goes without saying then that the optimal therapy for vascular disease in diabetes is optimization of control of diabetes, and there is significant overlap in that drugs and approaches that control DM also regulate atherosclerotic and cardiovascular disease. Yet, almost a century after insulin transformed DM we still do not fully comprehend the breadth and depth of this disease and as such there is both promise of new therapies yet to be appreciated and the challenge of balancing the harm and benefit of tight glucose control. Careful medical therapy is an excellent first-line strategy for coronary disease in patients with DM who are asymptomatic 103 or with mild symptoms (CCS Class I or II) and less severe CAD (single-vessel or two-vessel CAD not involving the proximal left anterior descending artery).26,104 For these patients, it is unlikely that an initial revascularization strategy is better than medical therapy and may even be worse 105. Revascularization can reduce anginal symptoms 61 and may be applied later if medical therapy does not adequately control symptoms (
Figure 5: Annual occurrence of freedom from angina in 1434 patients with angina at entry in BARI-2D. In the PCI stratum, the increase in freedom from angina was documented only at the first year of the follow-up. In the CABG stratum, the freedom from angina was increased during the 5-year follow-up. From Dagenais GR, Lu J, Faxon DP, et al. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation. 2011;123:1492–1500; with permission.
Figure 6). In patients who require intervention after optimization of medical therapy there is a significantly higher risk of repeat revascularizations with PCI. DES, albeit, is superior to BMS in ischemia-driven repeat revascularization procedures (target lesion revascularization)100,106 and is a reasonable approach in these patients (
Figure 6.
Revascularization strategy in patients with DM and stable angina.
* may be useful to exclude significant CAD
Figure 5: Annual occurrence of freedom from angina in 1434 patients with angina at entry in BARI-2D. In the PCI stratum, the increase in freedom from angina was documented only at the first year of the follow-up. In the CABG stratum, the freedom from angina was increased during the 5-year follow-up. From Dagenais GR, Lu J, Faxon DP, et al. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation. 2011;123:1492–1500; with permission.
Figure 6). But there remains consistently higher repeat revascularization rates after PCI compared with surgical revascularization in patients with DM.86,107,108 The surgical approach has better survival, fewer recurrent infarctions and greater freedom from re-intervention for patients with treated DM, moderate to severe symptoms and multivessel CAD 107,109, or significant involvement of the proximal left anterior descending or left main coronary artery (Figure 6).26,104,109
Ultimately, no single treatment approach can be applied to all patients given the confluence of chronic obstructive atherosclerosis and the profound divergent metabolic derangements of DM. Decisions regarding potential revascularization must take into account multiple factors and as such require a multidisciplinary team approach (‘heart team’). The heart team approach guarantees that all therapeutic options (i.e. OMT, PCI, and CABG) are transparently discussed, and the individual patient preferences are considered in the decision-making process.109
KEY POINTS.
Large clinical trials have shown that a near-normal glycemic control does not reduce cardiovascular events in patients with diabetes mellitus.
Recent studies indicate that statin use may be associated with the development of diabetes mellitus; however, the overall excess risk is low.
There is a concern that some antidiabetes agents may impart greater cardiovascular risk but there is no sufficient evidence to support one drug or combination of drugs over another for the reduction of cardiovascular events.
Optimal medical therapy is an appropriate initial strategy in patients with diabetes mellitus, mild symptoms and moderate coronary artery disease.
Bypass surgery is superior to percutaneous intervention in most diabetic patients with multivessel coronary disease; however, selection of the optimal myocardial revascularization strategy must take into account multiple factors and requires a multidisciplinary team approach (‘heart team’).
Trial acronyms
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ACUITY
Acute Catheterization and Urgent Intervention Triage Strategy
- ADVANCE
A Preterax and Diamicron Modified Release Controlled Evaluation
- BARI
Bypass Angioplasty Revascularization Investigation
- BARI-2D
Bypass Angioplasty Revascularization Investigation 2 Diabetes
- CARDia
Coronary Artery Revascularization in Diabetes
- CARDS
Collaborative Atorvastatin Diabetes Study
- COURAGE
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation
- EXAMINE
Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care
- FREEDOM
Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease
- HORIZONS-AMI
Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction
- JUPITER
Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin
- RECORD
Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes
- SAVOR
Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus
- SYNTAX
SYNergy Between PCI With TAXus and Cardiac Surgery
- TRITON-TIMI 38
Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38
- VADT
Veterans Affairs Diabetes Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schramm TK, Gislason GH, Kober L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117:1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 4.Preis SR, Pencina MJ, Hwang SJ, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009;120:212–220. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part II: recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49:643–656. doi: 10.1016/j.jacc.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 6.Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:296–306. doi: 10.7326/0003-4819-126-4-199702150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 8.Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol. 1996;27:528–535. doi: 10.1016/0735-1097(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 9.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Advance Collaborative Group. Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 14.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 15.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 16.Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–2633. doi: 10.1056/NEJMe0804182. [DOI] [PubMed] [Google Scholar]
- 17.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 18.Sue Kirkman M, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. Journal of the American Geriatrics Society. 2012;60:2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs--insights from the rosiglitazone experience. N Engl J Med. 2013;369:1285–1287. doi: 10.1056/NEJMp1309610. [DOI] [PubMed] [Google Scholar]
- 22.Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830. [PubMed] [Google Scholar]
- 23.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 24.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 25.Food and Drug Administration. Guidance for Industry: diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008 www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf.
- 26.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 29.Sokos GG, Bolukoglu H, German J, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 31.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi C, Miccoli R, Daniele G, Penno G, Del Prato S. Is there evidence that oral hypoglycemic agents reduce cardiovascular morbidity/mortality? Yes. Diabetes Care. 2009;32(Suppl 2):S342–348. doi: 10.2337/dc09-S336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care. 2012;35:1814–1816. doi: 10.2337/dc12-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 36.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34(Suppl 2):S132–137. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485–1489. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 38.Yakubovich N, Gerstein HC. Serious cardiovascular outcomes in diabetes: the role of hypoglycemia. Circulation. 2011;123:342–348. doi: 10.1161/CIRCULATIONAHA.110.948489. [DOI] [PubMed] [Google Scholar]
- 39.Pinto DS, Skolnick AH, Kirtane AJ, et al. U-shaped relationship of blood glucose with adverse outcomes among patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2005;46:178–180. doi: 10.1016/j.jacc.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 40.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111:3078–3086. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 41.Rana O, Byrne CD, Kerr D, et al. Acute hypoglycemia decreases myocardial blood flow reserve in patients with type 1 diabetes mellitus and in healthy humans. Circulation. 2011;124:1548–1556. doi: 10.1161/CIRCULATIONAHA.110.992297. [DOI] [PubMed] [Google Scholar]
- 42.Libby P, Maroko PR, Braunwald E. The effect of hypoglycemia on myocardial ischemic injury during acute experimental coronary artery occlusion. Circulation. 1975;51:621–626. doi: 10.1161/01.cir.51.4.621. [DOI] [PubMed] [Google Scholar]
- 43.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301:1556–1564. doi: 10.1001/jama.2009.496. [DOI] [PubMed] [Google Scholar]
- 44.Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146:233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 45.Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827. doi: 10.1503/cmaj.090206. discussion 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Ancona G, Bertuzzi F, Sacchi L, et al. Iatrogenic hypoglycemia secondary to tight glucose control is an independent determinant for mortality and cardiac morbidity. Eur J Cardiothorac Surg. 2011;40:360–366. doi: 10.1016/j.ejcts.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 47.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Annals of internal medicine. 2011;154:260–267. doi: 10.7326/0003-4819-154-4-201102150-00007. [DOI] [PubMed] [Google Scholar]
- 49.Deedwania P, Kosiborod M, Barrett E, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117:1610–1619. doi: 10.1161/CIRCULATIONAHA.107.188629. [DOI] [PubMed] [Google Scholar]
- 50.Cholesterol Treatment Trialists C. Kearney PM, Blackwell L, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 51.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. The Lancet. 364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 52.Cholesterol Treatment Trialists C. Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 54.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 55.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 56.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 57.United States Food and Drug Administration. DA Drug Safety Communication: Important safety label changes to cholesterol lowering statin drugs. 2012 http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm.
- 58.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dagenais GR, Lu J, Faxon DP, et al. Prognostic impact of the presence and absence of angina on mortality and cardiovascular outcomes in patients with type 2 diabetes and stable coronary artery disease: results from the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial. J Am Coll Cardiol. 2013;61:702–711. doi: 10.1016/j.jacc.2012.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armstrong EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes mellitus. Circulation. 2013;128:1675–1685. doi: 10.1161/CIRCULATIONAHA.113.002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dagenais GR, Lu J, Faxon DP, et al. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation. 2011;123:1492–1500. doi: 10.1161/CIRCULATIONAHA.110.978247. [DOI] [PubMed] [Google Scholar]
- 62.Maron DJ, Boden WE, Spertus JA, et al. Impact of Metabolic Syndrome and Diabetes on Prognosis and Outcomes With Early Percutaneous Coronary Intervention in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) Trial. J Am Coll Cardiol. 2011;58:131–137. doi: 10.1016/j.jacc.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 63.King SB., 3rd Is surgery preferred for the diabetic with multivessel disease? Surgery is preferred for the diabetic with multivessel disease. Circulation. 2005;112:1500–1507. doi: 10.1161/CIRCULATIONAHA.104.483339. discussion 1514–1505. [DOI] [PubMed] [Google Scholar]
- 64.Legrand V. Therapy insight: diabetes and drug-eluting stents. Nat Clin Pract Cardiovasc Med. 2007;4:143–150. doi: 10.1038/ncpcardio0804. [DOI] [PubMed] [Google Scholar]
- 65.Stenestrand U, James SK, Lindback J, et al. Safety and efficacy of drug-eluting vs. bare metal stents in patients with diabetes mellitus: long-term of follow-up in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp424. [DOI] [PubMed] [Google Scholar]
- 66.Stone GW, Kedhi E, Kereiakes DJ, et al. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. [DOI] [PubMed] [Google Scholar]
- 67.Silber S, Serruys PW, Leon MB, et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global RESOLUTE program. JACC Cardiovasc Interv. 2013;6:357–368. doi: 10.1016/j.jcin.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Windecker S, Meier B. Late coronary stent thrombosis. Circulation. 2007;116:1952–1965. doi: 10.1161/CIRCULATIONAHA.106.683995. [DOI] [PubMed] [Google Scholar]
- 69.Kimura T, Morimoto T, Nakagawa Y, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012;125:584–591. doi: 10.1161/CIRCULATIONAHA.111.046599. [DOI] [PubMed] [Google Scholar]
- 70.Pfisterer ME. Late stent thrombosis after drug-eluting stent implantation for acute myocardial infarction: a new red flag is raised. Circulation. 2008;118:1117–1119. doi: 10.1161/CIRCULATIONAHA.108.803627. [DOI] [PubMed] [Google Scholar]
- 71.Urban P, Gershlick AH, Guagliumi G, et al. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation. 2006;113:1434–1441. doi: 10.1161/CIRCULATIONAHA.104.532242. [DOI] [PubMed] [Google Scholar]
- 72.Machecourt J, Danchin N, Lablanche JM, et al. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: the EVASTENT Matched-Cohort Registry. J Am Coll Cardiol. 2007;50:501–508. doi: 10.1016/j.jacc.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 73.Aoki J, Lansky AJ, Mehran R, et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents: the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation. 2009;119:687–698. doi: 10.1161/CIRCULATIONAHA.108.804203. [DOI] [PubMed] [Google Scholar]
- 74.Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626–1636. doi: 10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
- 75.Dangas GD, Caixeta A, Mehran R, et al. Frequency and predictors of stent thrombosis after percutaneous coronary intervention in acute myocardial infarction. Circulation. 2011;123:1745–1756. doi: 10.1161/CIRCULATIONAHA.110.981688. [DOI] [PubMed] [Google Scholar]
- 76.Wenaweser P, Daemen J, Zwahlen M, et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008;52:1134–1140. doi: 10.1016/j.jacc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Lagerqvist B, Carlsson J, Frobert O, et al. Stent thrombosis in sweden: a report from the Swedish coronary angiography and angioplasty registry. Circ Cardiovasc Interv. 2009;2:401–408. doi: 10.1161/CIRCINTERVENTIONS.108.844985. [DOI] [PubMed] [Google Scholar]
- 78.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 79.Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care. 2003;26:2181–2188. doi: 10.2337/diacare.26.7.2181. [DOI] [PubMed] [Google Scholar]
- 80.The Bypass Angioplasty Revascularization Investigation (BARI) Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease [see comments] Circulation. 1997;96:1761–1769. doi: 10.1161/01.cir.96.6.1761. [DOI] [PubMed] [Google Scholar]
- 81.The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol. 2007;49:1600–1606. doi: 10.1016/j.jacc.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 82.Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–1197. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 83.Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med. 2012;367:2437–2438. doi: 10.1056/NEJMe1212278. [DOI] [PubMed] [Google Scholar]
- 84.Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 85.Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013;43:1006–1013. doi: 10.1093/ejcts/ezt017. [DOI] [PubMed] [Google Scholar]
- 86.Kapur A, Hall R, Malik I, et al. Randomized Comparison of Percutaneous Coronary Intervention With Coronary Artery Bypass Grafting in Diabetic Patients: 1-Year Results of the CARDia (Coronary Artery Revascularization in Diabetes) Trial. J Am Coll Cardiol. 2010;55:432–440. doi: 10.1016/j.jacc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 87.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 88.Gum P, O'Keefe JJ, Borkon A, et al. Bypass surgery versus coronary angioplasty for revascularization of treated diabetic patients. Circulation. 1997;96:II–II710. [PubMed] [Google Scholar]
- 89.Detre KM, Lombardero MS, Brooks MM, et al. The effect of previous coronary-artery bypass surgery on the prognosis of patients with diabetes who have acute myocardial infarction. Bypass Angioplasty Revascularization Investigation Investigators. N Engl J Med. 2000;342:989–997. doi: 10.1056/NEJM200004063421401. [DOI] [PubMed] [Google Scholar]
- 90.Kip KE, Alderman EL, Bourassa MG, et al. Differential influence of diabetes mellitus on increased jeopardized myocardium after initial angioplasty or bypass surgery: bypass angioplasty revascularization investigation. Circulation. 2002;105:1914–1920. doi: 10.1161/01.cir.0000014967.78190.bb. [DOI] [PubMed] [Google Scholar]
- 91.Schwartz L, Kip KE, Frye RL, Alderman EL, Schaff HV, Detre KM. Coronary bypass graft patency in patients with diabetes in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2652–2658. doi: 10.1161/01.cir.0000038885.94771.43. [DOI] [PubMed] [Google Scholar]
- 92.Hoogwerf BJ, Waness A, Cressman M, et al. Effects of aggressive cholesterol lowering and low-dose anticoagulation on clinical and angiographic outcomes in patients with diabetes: the Post Coronary Artery Bypass Graft Trial. Diabetes. 1999;48:1289–1294. doi: 10.2337/diabetes.48.6.1289. [DOI] [PubMed] [Google Scholar]
- 93.Aronson D, Edelman ER. Role of CABG in the management of obstructive coronary arterial disease in patients with diabetes mellitus. Current opinion in pharmacology. 2012;12:134–141. doi: 10.1016/j.coph.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hwang HY, Choi JS, Kim KB. Diabetes does not affect long-term results after total arterial off-pump coronary revascularization. Ann Thorac Surg. 2010;90:1180–1186. doi: 10.1016/j.athoracsur.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 95.Kinoshita T, Asai T, Nishimura O, Suzuki T, Kambara A, Matsubayashi K. Off-pump bilateral versus single skeletonized internal thoracic artery grafting in patients with diabetes. Ann Thorac Surg. 2010;90:1173–1179. doi: 10.1016/j.athoracsur.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 96.Pevni D, Uretzky G, Mohr A, et al. Routine use of bilateral skeletonized internal thoracic artery grafting: long-term results. Circulation. 2008;118:705–712. doi: 10.1161/CIRCULATIONAHA.107.756676. [DOI] [PubMed] [Google Scholar]
- 97.Nakano J, Okabayashi H, Hanyu M, et al. Risk factors for wound infection after off-pump coronary artery bypass grafting: should bilateral internal thoracic arteries be harvested in patients with diabetes? J Thorac Cardiovasc Surg. 2008;135:540–545. doi: 10.1016/j.jtcvs.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 98.Saso S, James D, Vecht JA, et al. Effect of skeletonization of the internal thoracic artery for coronary revascularization on the incidence of sternal wound infection. Ann Thorac Surg. 2010;89:661–670. doi: 10.1016/j.athoracsur.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 99.Kinoshita T, Asai T, Suzuki T, Kambara A, Matsubayashi K. Off-pump bilateral versus single skeletonized internal thoracic artery grafting in high-risk patients. Circulation. 2011;124:S130–134. doi: 10.1161/CIRCULATIONAHA.110.010892. [DOI] [PubMed] [Google Scholar]
- 100.Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J. 2011;32:2748–2757. doi: 10.1093/eurheartj/ehr305. [DOI] [PubMed] [Google Scholar]
- 101.Choudhary BP, Antoniades C, Brading AF, Galione A, Channon K, Taggart DP. Diabetes mellitus as a predictor for radial artery vasoreactivity in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2007;50:1047–1053. doi: 10.1016/j.jacc.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Goldman S, Sethi GK, Holman W, et al. Radial artery grafts vs saphenous vein grafts in coronary artery bypass surgery: a randomized trial. JAMA. 2011;305:167–174. doi: 10.1001/jama.2010.1976. [DOI] [PubMed] [Google Scholar]
- 103.Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaitman BR, Hardison RM, Adler D, et al. The bypass angioplasty revascularization investigation 2 diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529–2540. doi: 10.1161/CIRCULATIONAHA.109.913111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 106.Aronson D, Edelman ER. Revascularization for coronary artery disease in diabetes mellitus: angioplasty, stents and coronary artery bypass grafting. Reviews in endocrine & metabolic disorders. 2010;11:75–86. doi: 10.1007/s11154-010-9135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol. 2010;55:1067–1075. doi: 10.1016/j.jacc.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 108.Onuma Y, Wykrzykowska JJ, Garg S, Vranckx P, Serruys PW. 5-Year follow-up of coronary revascularization in diabetic patients with multivessel coronary artery disease: insights from ARTS (arterial revascularization therapy study)-II and ARTS-I trials. JACC Cardiovasc Interv. 2011;4:317–323. doi: 10.1016/j.jcin.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 109.Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 110.Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–625. doi: 10.1001/archinternmed.2009.20. [DOI] [PubMed] [Google Scholar]
- 111.Engler RL, Yellon DM. Sulfonylurea KATP blockade in type II diabetes and preconditioning in cardiovascular disease. Time for reconsideration [see comments] Circulation. 1996;94:2297–2301. doi: 10.1161/01.cir.94.9.2297. [DOI] [PubMed] [Google Scholar]
- 112.Singh S, Bhat J, Wang PH. Cardiovascular effects of anti-diabetic medications in type 2 diabetes mellitus. Current cardiology reports. 2013;15:327. doi: 10.1007/s11886-012-0327-1. [DOI] [PubMed] [Google Scholar]
- 113.Marx N, Wohrle J, Nusser T, et al. Pioglitazone reduces neointima volume after coronary stent implantation: a randomized, placebo-controlled, double-blind trial in nondiabetic patients. Circulation. 2005;112:2792–2798. doi: 10.1161/CIRCULATIONAHA.105.535484. [DOI] [PubMed] [Google Scholar]
- 114.Takagi T, Akasaka T, Yamamuro A, et al. Troglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with non-insulin dependent diabetes mellitus: a serial intravascular ultrasound study. J Am Coll Cardiol. 2000;36:1529–1535. doi: 10.1016/s0735-1097(00)00895-0. [DOI] [PubMed] [Google Scholar]
- 115.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 116.McMurray JJV, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. The Lancet Diabetes & Endocrinology. 2014 doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 117.Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 118.Fox CS. Weighty matters: balancing weight gain with cardiovascular risk among patients with type 1 diabetes mellitus on intensive insulin therapy. Circulation. 2013;127:157–159. doi: 10.1161/CIRCULATIONAHA.112.152777. [DOI] [PubMed] [Google Scholar]
- 119.Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366:1319–1327. doi: 10.1056/NEJMcp1013127. [DOI] [PubMed] [Google Scholar]