Abstract
Cyst enlargement in autosomal dominant polycystic kidney disease (ADPKD) requires the transepithelial secretion of fluid into the cyst lumen. We previously showed that physiological amounts of ouabain enhance cAMP-dependent fluid secretion and cyst growth of human ADPKD cyst epithelial cells in culture and formation of cyst-like dilations in metanephric kidneys from Pkd1 mutant mice. Here, we investigated the mechanisms by which ouabain promotes cAMP-dependent fluid secretion and cystogenesis. Ouabain (3 nM) enhanced cAMP-induced cyst-like dilations in embryonic kidneys from Pkd1m1Bei mice, but had no effect on metanephroi from Pkd1m1Bei mice that lack expression of the cystic fibrosis transmembrane conductance regulator (CFTR). Similarly, ouabain stimulation of cAMP-induced fluid secretion and in vitro cyst growth of ADPKD cells were abrogated by CFTR inhibition, showing that CFTR is required for ouabain effects on ADPKD fluid secretion. Moreover, ouabain directly enhanced the cAMP-dependent Cl− efflux mediated by CFTR in ADPKD monolayers. Ouabain increased the trafficking of CFTR to the plasma membrane and upregulated the expression of the CFTR activator PDZK1. Finally, ouabain decreased plasma membrane expression and activity of the Na,K-ATPase in ADPKD cells. Altogether, these results show that ouabain enhances net fluid secretion and cyst formation by activating apical anion secretion via CFTR and decreasing basolateral Na+ transport via Na,K-ATPase. These results provide new information on the mechanisms by which ouabain affects ADPKD cells and further highlight the importance of ouabain as a non-genomic stimulator of cystogenesis in ADPKD.
Keywords: Polycystic kidney disease, Fluid secretion, Cystogenesis, Signaling
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited disease of the kidney, characterized by the growth of fluid-filled renal cysts that relentlessly disrupt the renal architecture, leading to a decline in kidney function and commonly end-stage renal disease (Grantham 2008; Paul and Vanden Heuvel 2014). Growth of renal cysts results from the combined capacity of cystic epithelial cells to proliferate and secrete fluid into the lumen of the cyst (Sullivan et al. 1998). While ADPKD is caused by mutations of the Pkd1 or Pkd2 genes that encode for polycystin-1 (PC-1) and polycystin-2 (PC-2), respectively, progression of the disease is highly influenced by non-genetic factors (Fedeles et al. 2014; Harris and Rossetti 2010; Pei 2011). Several agents circulating in blood are thought to accelerate ADPKD cyst growth, including hormones, such as arginine vasopressin (AVP), epidermal growth factor (EGF), prostaglandins, insulin growth factor (IGF), catecholamines, and endogenous forskolin, as well as ingested substances such as caffeine and dietary forskolin, reviewed in Wallace (2011).
Ouabain has traditionally been viewed as a toxin produced by plants; however, more recently, it was found to be a hormone that is synthesized by the adrenal glands and circulates in the blood of mammals at nanomolar concentrations (Bagrov et al. 2009; Schoner and Scheiner-Bobis 2005; Silva and Soares-da-Silva 2012). Ouabain exerts its action by binding to its plasma membrane receptor, Na,K-ATPase (Pierre and Xie 2006; Xie and Cai 2003). While relatively high concentrations (mM) of ouabain are toxic due to complete inhibition of Na,K-ATPase activity, low (nM) concentrations have been shown to elicit a variety of cell-type specific effects, including regulation of cell proliferation, hypertrophy, apoptosis, mobility, and metabolism (Chueh et al. 2001; Dmitrieva and Doris 2003; Kometiani et al. 1998; Riganti et al. 2011; Yan et al. 2012; Zhang et al. 2012). These physiological effects require the binding of ouabain to a specific population of Na,K-ATPase located within the cholesterol-rich domains of the cell membrane caveolae (Liu et al. 2003). This subset of Na,K-ATPase molecules functions as a cell signal transducer that mediates the effects of ouabain by triggering a cascade of intracellular phosphorylation events (Xie and Cai 2003).
Ouabain has been shown to regulate cell growth, apoptosis, and Na+ reabsorption in normal tubular epithelial cells and kidneys (Blaustein and Hamlyn 2010; Dmitrieva and Doris 2003; Khundmiri et al. 2006; Li et al. 2010). Recently, we found that ouabain at concentrations normally found in the circulation enhances the proliferation of ADPKD cyst cells (Blanco and Wallace 2013; Nguyen et al. 2007). Moreover, ouabain augments cAMP-dependent fluid secretion across ADPKD monolayers, growth of cysts of ADPKD cells cultured within a collagen matrix, and cyst-like tubule dilations in embryonic kidneys from Pkd1 mutant mice (Jansson et al. 2012). The proliferative and secretory effects of ouabain in ADPKD are mediated by activation of the epidermal growth factor receptor (EGFR), the kinase Src, and the downstream mitogen-activated protein kinase ERK pathway (Nguyen et al. 2011). The response is specific to ADPKD cells, since ouabain does not significantly affect proliferation, fluid secretion, and the EGFR-Src-ERK pathway in normal human kidney cells.
The present study was carried out to determine the mechanisms by which ouabain promotes fluid secretion and cyst growth in ADPKD epithelial cells. Our data indicate that ouabain, applied on the basolateral side of the cells, activates signaling pathways that affect anion transport at the apical membrane of ADPKD cells. Ouabain increases the movement of CFTR to the plasma membrane and upregulates the expression of PDZK1, an activator of CFTR sorting and function, leading to increased cAMP-induced anion secretion. In addition, ouabain directly reduces the activity of Na,K-ATPase and increases cytosolic Na+ concentration. We propose that ouabain enhances fluid secretion in ADPKD cells by increasing the apical Cl− efflux pathway via activation of CFTR and by decreasing Na+ absorption through inhibition of Na,K-ATPase.
Methods
Mice
All experimental protocols involving mice were approved by the KUMC Institutional Animal Care and Use Committee. Pkd1m1Bei mice were originally obtained from the Mutant Mouse Regional Resource Center (University of North Carolina, Chapel Hill, NC) (Herron et al. 2002) and were stabilized onto a C57BL/6 background (Magenheimer et al. 2006). Cftrm1UNC (S489X) mice, which exhibit a null CFTR phenotype due to unstable CFTR expression at the mRNA and protein level (Takacs-Jarrett et al. 2001), were also used in this study. Double heterozygous mice (Pkd1+/−; Cftr+/−) were generated as previously described (Magenheimer et al. 2006).
Mouse Metanephric Organ Cultures
Embryonic kidneys were isolated at E15.5 from pups obtained by crossing mice heterozygous for both Pkd1 and Cftr. Metanephroi were cultured on Transwell filters (24 mm, 0.4 μm pore size; Corning Incorporated, Corning, NY), in 6-well culture plates as previously described (Jansson et al. 2012). The culture medium was a 50:50 mixture of DME/F12 supplemented with 2 mM L-glutamine, 10 mM HEPES, ITS, 25 ng/mL prostaglandin E1, 32 pg/mL triiodothyronine, and penicillin/streptomycin. Medium in the lower chamber of the culture inserts was supplemented with 100 μM 8-Br-cAMP in the absence or presence of 30 nM ouabain. The higher amounts of ouabain used for the organ culture, compared to ADPKD cultures, correspond to the known differences in ouabain affinity of the Na,K-ATPase of rodents and humans (Blanco and Mercer 1998). Metanephric tissue was maintained at 37 °C in a humidified chamber containing 5 % CO2 and 95 % air, and images of each sample were captured daily for 4 days under light microscopy. Dilated tubule area was quantified from the images using analySIS software, and fractional cyst area was calculated as the ratio of total dilated cystic area divided by the entire area of the kidney section, as described previously (Magenheimer et al. 2006).
ADPKD Cell Cultures
Primary cell cultures were derived from renal cysts of ADPKD kidneys by the PKD Biomaterials Core at the University of Kansas Medical Center. A protocol for the use of discarded human kidney tissues was approved by the Institutional Review Board at the University of Kansas Medical Center. Cells were cultured in DME/F12 supplemented with 5 % heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 0.1 mg/mL streptomycin, 5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL sodium selenite (ITS) as described (Wallace et al. 1996). For microcyst cultures, ADPKD cells were suspended in ice-cold type I collagen (PureCol®, Advanced Biomatrix, San Diego, CA) in DME-F12 medium containing 100 U/mL penicillin, 0.1 mg/mL streptomycin, ITS, 5 × 10−8 M hydrocortisone, and 5 × 10−12 M triiodothyronine (defined medium). The cell suspensions were plated into the wells of a 96-well culture plate at an seeding density of 4 × 103 cells/well as described previously (Jansson et al. 2012). Plates were incubated at 37 °C for 45 min to allow polymerization of the collagen. Cultures were then bathed in defined medium with the addition of 5 μM forskolin and 5 ng/mL EGF for 2–4 days to allow for the induction of microcyst growth. Once the cysts began forming, agonists were removed and cultures were treated with defined medium, in the absence and presence of 3 nM ouabain or 5 μM forskolin, alone or together. Some experiments were performed in the absence or presence of 10 μM of the specific CFTR inhibitor, CFTR(inh)-172 (Sigma-Aldrich, St. Louis, MO). Cysts were allowed to grow for 5–7 days and were fixed with 0.5 % buffered formalin in PBS. Cultures were photographed and the diameters of individual cysts were measured to calculate a total surface area using analySIS software (Lakewood, CO) as previously described (Reif et al. 2011). Data were expressed as average total surface area ± SEM for each culture condition.
Fluid Secretion Assay
Confluent monolayers of ADPKD cells were established on permeable SnapwellTM inserts (12 mm, 0.4 μm pore size; Corning Incorporated, Corning, NY) in 6-well tissue culture plates as previously described (Nguyen et al. 2007). Cells were cultured for 1 week, until a tight epithelium was formed, as determined by the establishment of a transepithelial electrical resistance across the cell monolayer (Nguyen et al. 2007). Then, cells were serum starved in basal DME/F12 media for 24 h, and the basolateral side of the cell monolayers was treated with 3 nM ouabain or 5 μM forskolin alone and in combination. Control monolayers were incubated in basal media alone. Fresh medium (150 μL) was placed on the apical surface of the cells, and mineral oil was layered over the top of the medium to prevent evaporation. Treated monolayers were incubated at 37 °C for an additional 24 h, and the apical medium was collected and measured as previously described (Wallace et al. 2004). To determine the involvement of CFTR in fluid secretion, CFTR(inh)-172 (10 μM) was added to the apical media applied to some of the monolayers. Fluid secretion data were expressed as μl/h/cm2.
Chloride Efflux Assay
Chloride efflux was assessed using a chloride-sensitive fluorescent indicator, N-[ethoxycarbonylmethyl]-6-methoxyquinolinium bromide (MQAE) (Life Technologies, Grand Island, NY), as previously described (West and Molloy 1996). Briefly, ADPKD cells were plated with 1 % FBS in a 96-well plate (1 × 104 cells/well). After 24 h, cells were serum starved overnight in media containing 0.002 % FBS. Cells were then treated with and without ouabain for 48 h. At 16 h prior to the end of the treatment period, cells were loaded with MQAE (7 mM) in a chloride-containing Loading Buffer (5.4 mM KCl, 122.6 mM NaCl, 1.0 mM CaCl2, 1.0 mM Na2SO4, 0.6 mM NaH2PO4, 2.4 mM Na2HPO4, 1.0 mM MgSO4, 10 mM HEPES, 10 mM D-glucose, pH 7.4)± ouabain. Chloride efflux was induced by switching cells to Efflux Buffer (5.4 mM KNO3, 122.6 mM NaNO3, 1.0 mM Ca(NO3)2, 1.0 mM Na2SO4, 0.6 mM NaH2PO4, 2.4 mM Na2HPO4, 1.0 mM MgSO4, 10 mM HEPES, 10 mM D-glucose, pH 7.4) in which chloride was exchanged for nitrate. Cells were washed three times in Loading Buffer with and without CFTR(inh)-172. After the last wash, forskolin (5 μM) was added for 10 min. Media were then quickly switched to Efflux Buffer maintaining the presence of experimental treatments. Fluorescence was measured at an excitation/emission ratio of 360 nm/460 nm every minute for 10 min using a microplate reader (HTS Synergy, Biotek, VT). Chloride efflux was calculated as the difference between the measured fluorescence in each well at a given time point (Ft) and the initial fluorescence of the sample just before switching to Efflux Buffer (F0), expressed in arbitrary fluorescence units. For each experimental condition, CFTR-dependent efflux was determined as the difference in fluorescence change (Ft − F0) between samples treated with and without CFTR(inh)-172.
Biotinylation and Immunoblot Analysis
ADPKD cells (2 × 105 cells) were grown to confluence in 5 % FBS on Transwell filters (24 mm, 0.4 μm pore size; Corning Incorporated, Corning, NY) in a 6-well culture plate. Once confluent, monolayers were serum starved overnight and then treated without and with ouabain (3 nM) for 24 or 48 h. Monolayers were then washed 3× with ice-cold PBS and then incubated with 0.75 mg/mL Biotin in 1 mL of PBS, pH 8.0 for 50 min at 4 °C, refreshing the biotin solution after 30 min. After washing twice with ice-cold PBS and once with 100 mM glycine, cells were lysed in 500 μl Lysis Buffer (1 %NP-40, 0.25 % sodium deoxycholate, 1 mM Na3VO4, 1 mM NaF, 1 mM EDTA (pH 8.0), 150 mM NaCl, 50 mM Tris (pH 7.4), 50 μM phenylarsine oxide, 1× protease, and 1× phosphatase inhibitors (both from Roche Diagnostics Corporation, Indianapolis, IN). The soluble supernatants were isolated after centrifugation at 10,000×g for 10 min. Protein concentration was determined by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Then lysates with equal amounts of protein were added to streptavidin-coated magnetic beads and rotated overnight at 4 °C. Samples were washed twice in PBS, resuspended in loading buffer (100 mM Tris–HCl, pH 6.8, 2 % SDS, 33 % glycerol, 100 mM DTT), subjected to SDS-PAGE on a 7.5 % gel, and transferred to nitrocellulose. Membranes were immunoblotted with different specific primary antibodies, depending on the experiment. Species-specific secondary antibodies conjugated to horse-radish peroxidase and enhanced chemiluminescence were used for protein detection. In addition to biotinylation, membrane fractions from the cell were prepared for the study of surface expression of the basolaterally located Na/K/Cl co-transporter NKCC1, following previously published protocols (Grindstaff et al. 1995). Antibodies used for protein detection included anti-human-CFTR (clone 24-1, R&D Systems, Minneapolis, MN), anti-NKCC1 (Cell Signaling, Danvers, MA), anti-PDZK1 (Novus Biologicals, Littleton, CO), anti-NHERF1 (Cell Signaling, Danvers, MA), anti-Na,K-ATPase α1 subunit (α6F, Developmental Studies Hybridoma Bank, University of Iowa), anti-human-β1-Na,K-ATPase (ABR Affinity Bioreagents, Golden, CO), and anti-α-tubulin (Sigma-Aldrich, St. Louis, MO). Protein expression levels were determined by densitometry, expressed relative to the corresponding controls, and normalized to the total amount of protein present in the sample as determined by staining with Ponceau S.
Rubidium (86Rb) Uptake
Na,K-ATPase-mediated ion transport was determined by measuring the ability of the cells to uptake 86Rb as previously described (Wagoner et al. 2005). ADPKD cells (2 × 105 cells) were seeded in 5 % FBS on Transwell filters (24 mm, 0.4 μm pore size; Corning Incorporated, Corning, NY) in a 6-well culture plate and grown to confluence. Following overnight starvation, monolayers were treated with and without ouabain (3 nM) for 30 min. Monolayers were then placed into 6-well plates containing flux medium (Hank’s Balanced Salt Solution + 10 mM HEPES) containing 5 μCi 86Rb. Uptake was allowed to proceed for 5 min and was then terminated by immersing the monolayers in ice-cold, isotonic buffer containing 100 mM MgSO4 and 137 mM sucrose. Cells were lysed and radioactivity was measured by scintillation counting. 86Rb uptake was calculated as nmol 86Rb/mg protein in the cell lysate.
Immunofluorescence
Cells were grown to confluency on SnapwellTM inserts (12 mm, 0.4 μm pore size; Corning Incorporated, Corning, NY) in a 6-well tissue culture plate. Following overnight serum starvation, cells were treated with and without ouabain (3 nM) for 24 h, fixed in 100 % methanol for 45 min at −20 °C, and analyzed by immunocytochemistry as previously described. Expression of Na,K-ATPase was detected using anti-Na,K-ATPase α1 subunit (6F), followed by exposure to an Alexa Flour 594 secondary antibody (Life Technologies, Grand Island, NY). Cells were mounted with Slow Fade ® Gold anti-fade reagent with DAPI (Life Technologies, Grand Island, NY). Samples were analyzed using a Zeiss LSM510 confocal microscope. Images were acquired in Multitrack channel mode with LSM510 (v3.2) software, and a Plan-Apochromat 63x/1.4 Oil DIC objective with a frame size of 1024 × 1024 pixels and a zoom factor of 2. z-line views was obtained by averaging 10 sections over a line at each z position in 1.0 μm steps.
Intracellular Sodium Measurement
Changes in intracellular Na+ were determined using the fluorescent dye Sodium Green tetra acetate (Life Technologies, Grand Island, NY) as described (Hernandez-Gonzalez et al. 2006). ADPKD cells (2 × 104 cells) were grown in the wells of an 8-well chamber slide. Following overnight serum starvation, cells were treated with and without ouabain (3 nM) in media containing Sodium Green tetra acetate for 30, 60, and 90 min. Then images of individual cells or groups of cells were analyzed using the excitation/emission pair 470/490 nm on an inverted microscope attached to a digital CCD camera. Mean fluorescence intensity was expressed as arbitrary units, relative to mean fluorescence of untreated control cells. During analysis, samples were maintained at 37 °C by use of a heating chamber regulated on-line with the system acquisition control.
Data Analysis
Statistical significance of the differences between means was determined by one-way ANOVA with a Student Newman–Keuls post-test for multiple comparisons or unpaired t test for single comparisons. Statistical significance was defined as P <0.05.
Results
Ouabain-Induced Cyst Growth Requires the Presence and Activity of CFTR
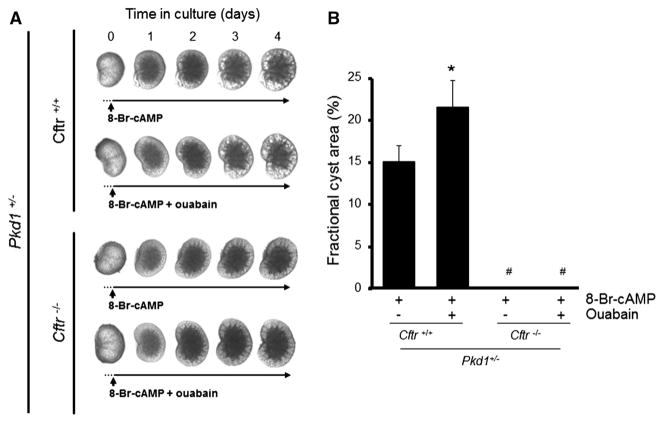
Work from our laboratory has shown that ouabain promotes cAMP-mediated fluid secretion and in vitro cyst formation of human ADPKD cells and cystic dilations in metanephric organ cultures from Pkd1m1Bei mouse embryos (Jansson et al. 2012). In contrast, ouabain had no effect on fluid secretion and cystogenesis of normal human kidney cells, and caused only slight cystic dilations in wild-type mouse kidneys (Jansson et al. 2012). Here, we further explored the mechanisms by which ouabain promotes fluid secretion and cyst formation in ADPKD. To determine whether the effect of ouabain on fluid secretion was mediated by CFTR Cl− channels, we tested the cystogenic effects of ouabain in metanephric organ cultures from Pkd1 mutant mouse embryos, in which CFTR was deleted (Magenheimer et al. 2006). Metanephric organ cultures were treated with 100 μM 8-Br-cAMP in the presence and absence of 30 nM ouabain and cystic area was measured after 4 days. Media containing 8-Br-cAMP stimulated the growth of cyst-like dilations in metanephric kidneys from Pkd1 mutant mice expressing wild-type CFTR (Pkd1m1Bei+/−; Cftr+/+). Ouabain further enhanced 8-Br-cAMP-dependent cyst dilations in these metanephric kidneys (Fig. 1a, b). In contrast, the lack of CFTR in Pkd1 mutant mice deficient in CFTR (Pkd1m1Bei+/−; Cftr−/−) prevented the development of 8-Br-cAMP-induced and ouabain-stimulated cyst dilations (Fig. 1a, b). These data suggest that ouabain requires the presence of CFTR to exacerbate the growth of 8-Br-cAMP-induced cyst expansions in the Pkd1m1Bei embryonic kidneys.
Fig. 1.
Effect of CFTR gene knockout on ouabain-enhanced cyst growth in embryonic kidneys. Embryonic kidneys (E15.5) from Pkd1m1Bei+/−; Cftr+/+ and Pkd1m1Bei+/−; Cftr−/− mice were isolated and placed into culture in media containing 100 μM 8-Br-cAMP in the absence and presence of 30 nM ouabain. a Representative images of metanephric kidneys in culture. b Average fractional cyst area was calculated after 4 days of treatment. Bars represent the mean ± SEM of 5–8 kidneys depending on the treatment. *P < 0.05 and #P < 0.01 versus 8-Br-cAMP treatment alone in the Cftr+/+ kidneys
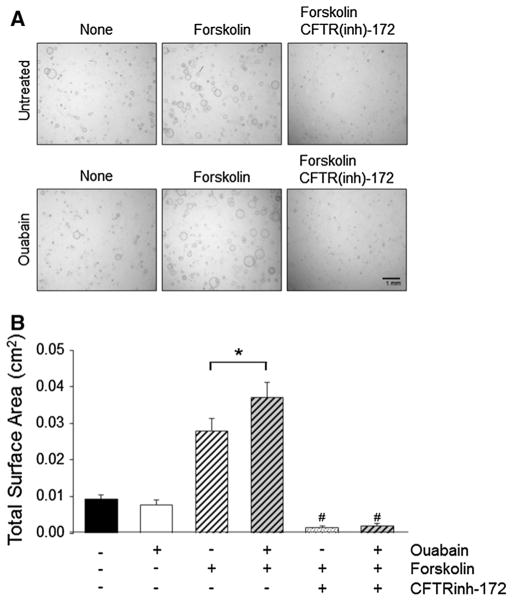
To further investigate the involvement of CFTR in ouabain-induced cyst growth, ADPKD cells were cultured in a three-dimensional matrix of polymerized collagen and stimulated to form microcysts (Jansson et al. 2012; Reif et al. 2011). Under these conditions, ADPKD cultures were treated without and with ouabain (3 nM) in the presence and absence of forskolin (5 μM) and in the absence and presence of 10 μM of the specific CFTR inhibitor, CFTR(inh)-172. As shown in Fig. 2a, b, forskolin stimulated ADPKD microcyst formation in agreement with previous results (Jansson et al. 2012). Ouabain alone had no effect on ADPKD microcysts; however, it significantly increased the total surface area of ADPKD microcysts when it was simultaneously applied with forskolin (Fig. 2a, b). The microcyst growth induced by forskolin alone or in combination with ouabain was abolished in the presence of CFTR(inh)-172. These data, similar to the results from the metanephric organ cultures, suggest that the potentiating effect of ouabain on cAMP-induced cyst growth requires CFTR.
Fig. 2.
Effect of CFTR inhibition on ouabain-enhanced ADPKD microcyst growth. Microcyst cultures were established by seeding ADPKD cells within a three-dimensional collagen matrix. Cultures were treated without and with 5 μM forskolin in the absence and presence of 3 nM ouabain. Additionally, some cultures were treated with 10 μM CFTR(inh)-172 and forskolin (5 μM) in the absence and presence of ouabain. Untreated, control cultures remained in defined media alone during the treatment period. After 5–7 days, total cyst surface area of the microcyst cultures was measured. a Representative images of microcyst cultures for each of the indicated treatments. b Average total surface area of microcyst cultures was measured for each treatment condition. Bars represent the mean ± SEM for microcyst cultures grown in sextuplicate with cells from different ADPKD kidneys. *P < 0.05 versus forskolin alone; #P < 0.01 versus forskolin + ouabain treatment
Ouabain-Enhanced Fluid Secretion Requires CFTR Activity
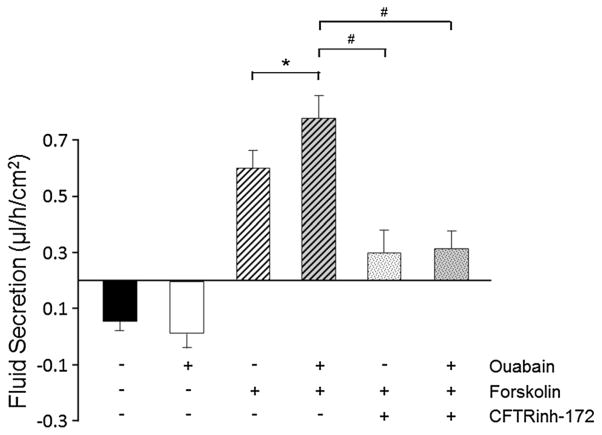
Physiological concentrations of ouabain can augment cAMP-induced fluid secretion across ADPKD monolayers (Jansson et al. 2012). Here, we determined the involvement of CFTR in the pro-secretory effect that ouabain exerts on ADPKD monolayers. To achieve this, ADPKD cell monolayers grown on permeable membrane supports were switched to media containing 0.002 % FBS and treated for 24 h with and without ouabain (3 nM). Monolayers were also treated in the absence and presence of 5 μM forskolin alone or in combination with ouabain. Additionally, cultures were treated in the absence and presence of CFTR(inh)-172. Following treatment, media on the apical side of each monolayer was collected and measured after 24 h. Addition of forskolin resulted in net basolateral to apical transepithelial fluid movement (Jansson et al. 2012). Ouabain alone had no significant effect on fluid transport; however, in the simultaneous presence of forskolin, it augmented the forskolin-induced transepithelial secretion of fluid (Fig. 3). The presence of CFTR(inh)-172 prevented the forskolin-stimulated increase in fluid secretion both in the absence and presence of ouabain (Fig. 3). These results indicate that ouabain enhanced fluid secretion by ADPKD monolayers is dependent on cAMP activation of CFTR.
Fig. 3.
Effect of CFTR inhibition on net fluid secretion in ADPKD monolayers. Confluent monolayers of ADPKD cells were treated without and with 5 μM forskolin in the absence and presence of 3 nM ouabain. Monolayers were also treated with 10 μM CFTR(inh)-172 and 5 μM forskolin in the absence and presence 3 nM ouabain. Untreated, control monolayers were incubated in media containing no experimental agents. After 24 h, the volume of fluid on the apical side of each monolayer was removed and measured. Data are expressed as fluid secretion rate, μl/h/cm2. Bars represent the fluid secretion rate mean ± SEM for monolayers grown in triplicate from different ADPKD kidneys. *P < 0.05 versus forskolin alone; #P < 0.01 versus forskolin + ouabain treatment
Ouabain Enhances Forskolin-Dependent Chloride Transport in ADPKD Cells
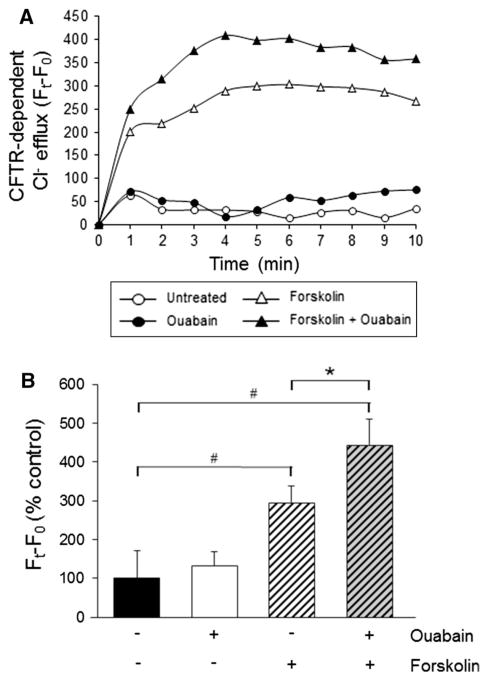
In a previous report, we have shown that physiologic concentrations of ouabain enhanced the forskolin-induced anion secretion, compatible with Cl− secretion via the apical CFTR (Jansson et al. 2012). To more directly examine whether ouabain is influencing the activity of CFTR, we performed chloride efflux assays in ADPKD cell monolayers. Cells plated in 96-well plates were placed overnight in media containing 0.002 % FBS and were treated with and without 3 nM ouabain for 48 h. Cells were then loaded with the fluorescent indicator dye, MQAE (7 mM), in a chloride-containing buffer, maintaining the cells with and without ouabain. After one wash with dye-free, chloride-containing buffer, forskolin was added for 10 min during the final wash to cells. Samples were also treated in the absence and presence of CFTR(inh)-172. To induce chloride efflux, cells were switched from a chloride-containing buffer to a chloride-free buffer maintaining the presence of experimental treatment. Fluorescence was monitored in a plate reader, every minute for 10 min. Chloride efflux was determined as the change in fluorescence of a sample at a given time point (Ft) from the initial fluorescence (F0) prior to inducing chloride efflux. The portion of fluorescence change (Ft − F0) sensitive to CFTR inhibition was calculated to determine the CFTR-dependent chloride efflux. Figure 4a shows representative traces of the CFTR-dependent fluorescence change. Average Ft − F0 values were determined to analyze the effect of the various treatments on the activation state of CFTR (Fig. 4b). As shown, CFTR-dependent chloride efflux was not affected by ouabain treatment alone. Forskolin caused a significant increase in CFTR-dependent chloride efflux, consistent with the role of forskolin as a cAMP-agonist in ADPKD cells. Interestingly, the simultaneous addition of ouabain and forskolin resulted in a synergistic effect on CFTR-dependent chloride efflux. These results support the hypothesis that the enhancement of fluid secretion produced by nanomolar concentrations of ouabain is mediated through cAMP-activated and CFTR-mediated Cl− efflux.
Fig. 4.
Ouabain effect on forskolin-induced-CFTR-dependent chloride efflux in ADPKD cells. ADPKD cells grown in 96-well plates were treated without and with 3 nM ouabain for 48 h. Cells were loaded with MQAE, and chloride efflux was induced by switching to a chloride-free buffer. Prior to chloride efflux induction, forskolin (5 μM) was added to cells with and without ouabain. Fluorescence measurements were taken every minute for 10 min. Chloride efflux was determined from the difference between the fluorescence of a sample at a given time (Ft) and the initial fluorescence of that sample prior to inducing chloride efflux (F0). The portion of the fluorescence change (Ft − F0) sensitive to CFTR(inh)-172 was determined to be CFTR-dependent chloride efflux. a Representative trace of CFTR-dependent chloride efflux for samples exposed to the indicated treatments. b Ft − F0 at the plateau of each efflux curve was calculated for each treatment condition and expressed relative to each control fluorescence change. Bars represent the mean ± SEM of six different experiments using ADPKD cells from different kidneys. *P < 0.01 versus forskolin alone; #P < 0.05 versus untreated control
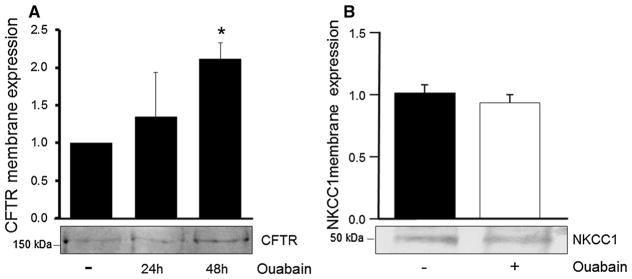
Ouabain Increases the Amount of CFTR but not NKCC in the Plasma Membrane of ADPKD Cells
A correlation between the expression level of CFTR and the extent of renal cyst growth has been reported in ADPKD (Davidow et al. 1996). We have previously shown that ouabain does not alter the total cellular expression of CFTR in ADPKD cells (Jansson et al. 2012). However, the possibility exists that ouabain may be inducing selective changes in CFTR localization, modifying its amount at the plasma membrane, where the function of the transporter is physiologically relevant. To determine this, we performed cell membrane biotinylation and streptavidin precipitation assays. ADPKD monolayers were established on permeable membrane supports, serum starved, and treated without or with 3 nM ouabain for 24–48 h. Cells were then incubated with biotin and lysed in RIPA buffer, and biotinylated proteins were immunoprecipitated overnight with streptavidin-conjugated magnetic beads. The immunoprecipitated proteins were subjected to SDS-PAGE (7.5 % gel), transferred to nitrocellulose membranes, and probed for CFTR. Ouabain caused a significant increase in the amounts of CFTR at the plasma membrane at 48 h (Fig. 5a). The Na/K/Cl transporter (NKCC1) in the basolateral membrane of ADPKD cells has also been implicated in the overall mechanism for Cl− transport in ADPKD cells. We have shown that total expression of NKCC1 remains unchanged in ADPKD cells after ouabain treatment (Jansson et al. 2012). Here, we determined if ouabain affects the plasma membrane levels and thus cell distribution of NKCC1. As shown in Fig. 5b, ouabain did not significantly influence the amount of NKCC1 at the cell surface. Altogether, these results suggest that exposure of ADPKD cells to ouabain causes a change in localization of CFTR, enhancing its trafficking and levels at the cell plasma membrane. In contrast, ouabain did not affect NKCC1 level at the cell surface. The effects of ouabain on CFTR would provide more CFTR molecules available for the efflux of Cl− across the apical side of the cells.
Fig. 5.
Ouabain effect on plasma membrane amounts of CFTR and NKCC1 in ADPKD monolayers. Confluent monolayers of ADPKD cells were treated without and with ouabain (3 nM). Following treatment, CFTR (a) and NKCC1 (b) levels at the plasma membrane were determined. Membrane proteins from ADPKD cell monolayers were isolated by biotinylation and streptavidin immunoprecipitation for CFTR or by isolating membrane fractions from the cells for NKCC1. Samples were analyzed by SDS-PAGE and immunoblot using antibodies specific for human CFTR and NKCC1, respectively. Bars represent membrane protein expression for each treatment relative to untreated control levels and normalized to total protein present in the sample, determined by Ponceau S. Values are expressed as the mean ± SEM of four determinations using cells from different ADPKD kidneys. Representative immunoblots are shown below the graph. *P < 0.05 versus untreated control
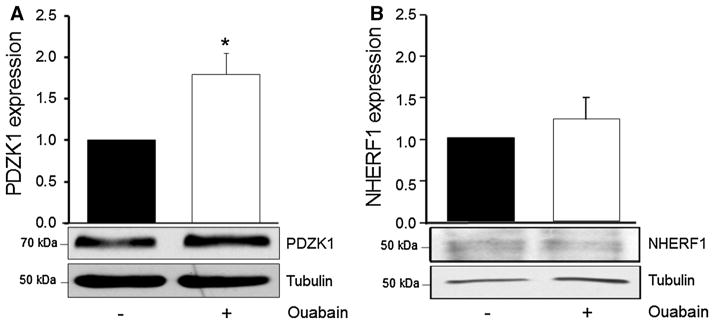
Ouabain Enhances Expression of PDZK1 in ADPKD Cells
Cellular trafficking of CFTR to and from the plasma membrane, its membrane retention, and dimerization are important mechanisms for CFTR modulation in different tissues. This is regulated by proteins that contain the protein–protein motifs (PDZ domains) of the postsynaptic density protein PSD95, the Drosophila homolog Disc-large, and the tight junction protein Zonula occludens-1 (Guggino and Stanton 2006; Singh et al. 2009). To explore whether ouabain modulates the levels of PDZ proteins, we determined the amounts of the PDZ domain-containing protein kidney 1 (PDZK1) and Na/H exchange regulatory factor-1 (NHERF-1) in ADPKD cells, in the absence and presence of ouabain. ADPKD cells were serum starved, treated with and without 3 nM ouabain for 24 h and lysed, and the solubilized proteins were analyzed by immunoblot with PDZK1- and NHERF-1-specific antibodies. Treatment of ADPKD cells with 3 nM ouabain significantly increased the expression levels of PDZK1 in ADPKD cells (Fig. 6a). In contrast, ouabain did not significantly affect the amounts of NHERF-1 (Fig. 6b). Altogether these results indicate that physiological amounts of ouabain induce up-regulation of PDZK1, a CFTR regulator involved in CFTR sorting to the surface of ADPKD cells. These mechanisms of CFTR activation are consistent with the ability of ouabain to augment cAMP-dependent anion transport across the apical side of ADPKD cells and fluid secretion.
Fig. 6.
Ouabain effects on the expression of the CFTR regulators PDZK1 and NHERF1 in ADPKD cells. Cells were treated without and with ouabain (3 nM) for 24 h. Following treatment, cells were lysed and equal amounts of cell lysate were separated by SDS-PAGE and then immunoblotted using antibodies specific for human PDZK1 and NHERF1. Protein expression was determined by densitometry. Bars represent protein expression relative to untreated control levels and normalized to tubulin. Data are expressed as the mean ± SEM of three determinations using cells from different ADPKD kidneys. A representative immunoblot is displayed below the graph. *P < 0.05 versus untreated control
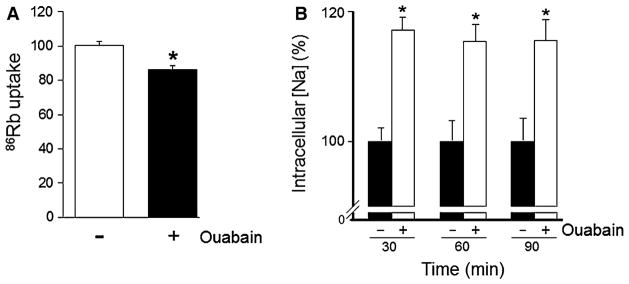
Ouabain Decreases Na,K-ATPase-Mediated Ion Transport in ADPKD Cells
Na,K-ATPase plays a primary role in salt and water reabsorption in the renal tubular epithelium, and changes in Na,K-ATPase function can alter the reabsorptive capacity of renal epithelial cells (Feraille and Doucet 2001). We previously reported that, in ADPKD cells, a fraction of the total Na,K-ATPase is more sensitive to inhibition by ouabain. It is therefore possible that physiological concentrations of ouabain, by reducing Na,K-ATPase activity, could affect Na+ reabsorption and alter the balance of fluid movement in favor of an accumulation of fluid in the cyst lumen. To determine the effect of physiologic concentrations of ouabain on Na,K-ATPase-dependent ion transport, the uptake of 86Rb (taken as a marker for K+) that depends on the activity of Na,K-ATPase was determined. ADPKD cells were grown to confluence on permeable membrane supports. Following serum starvation, cells were treated with and without 3 nM ouabain for 30 min, and the uptake of 86Rb by the cells was measured. Figure 7a shows that, compared to untreated monolayers, ouabain reduced the internalization of 86Rb into the ADPKD cells by approximately 15 %. This result shows that the relatively low amounts of ouabain used are capable of reducing the activity of the Na,K-ATPase of ADPKD cells.
Fig. 7.
Ouabain effect on Na,K-ATPase-mediated ion transport in ADPKD cells. a 86Rb uptake in ADPKD cells. Confluent monolayers of ADPKD cells were treated with and without ouabain (3 nM) for 24 h. 86Rb uptake was measured and data are expressed as 86Rb uptake relative to uptake in untreated control monolayers. Bars represent mean ± SEM of six determinations performed on cells obtained from different ADPKD kidneys. *P <0.05 versus untreated control. b Intracellular sodium, [Na]i, was determined using Sodium Green tetra acetate. ADPKD cells were incubated in media containing the fluorescent dye with and without ouabain (3 nM) for the indicated times. Mean fluorescence intensity of each sample was then analyzed and expressed relative to the untreated control for each time point. Bars represent the mean ± SEM for experiments performed on cells obtained from different ADPKD kidneys. *P <0.05 versus untreated control for each corresponding time point
Since Na,K-ATPase is the active ion transport mechanism responsible for tightly maintaining intracellular Na+ ([Na+]i) levels, we determined the effects of ouabain on cytosolic Na+ in ADPKD cells. ADPKD cells were incubated in media with and without 3 nM ouabain for 30, 60, and 90 min, and [Na+]i was measured using the fluorescent indicator Sodium Green tetra acetate. As shown in Fig. 7b, ouabain caused a significant increase in [Na+]i at 30 min and this was sustained along the time points studied. This increase in [Na+]i reflects the decreased efflux of Na+ due to reduced activity of Na,K-ATPase. Altogether, these data suggest that ouabain decreases the Na,K-ATPase-mediated internalization of 86Rb (K+) and the extrusion of Na+ out of ADPKD cells.
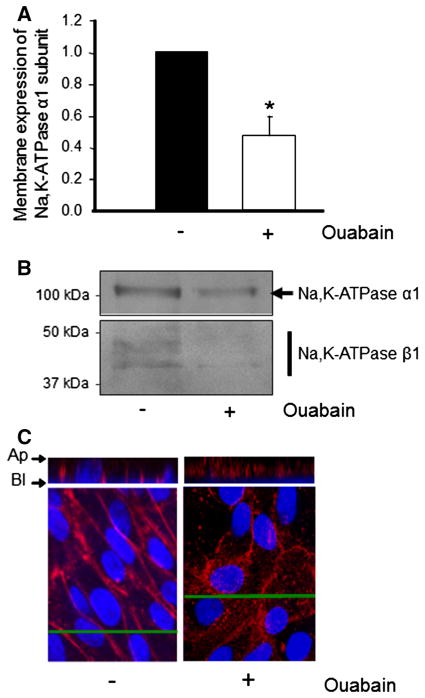
Ouabain Decreases Na,K-ATPase Amounts at the Plasma Membrane of ADPKD Cells
Ouabain has been shown to cause endocytosis of Na,K-ATPase in different cell types, reviewed in Blanco and Wallace (2013). To determine if ouabain affects the amount of Na,K-ATPase in the plasma membrane of ADPKD cells, we quantified the cell surface levels of the transporter using biotinylation and immunocytochemistry analysis. After treatment with and without 3 nM ouabain, ADPKD cell monolayers were biotinylated and streptavidin-precipitated proteins were separated in SDS-PAGE (7.5 % gel). Immunoblots were analyzed using antibodies specific for the α and β subunits of the Na,K-ATPase. Ouabain produced a significant decrease in membrane expression of the Na,K-ATPase α subunit (Fig. 8a), as well as of the β subunit (Fig. 8b). Also, the distribution of the Na,K-ATPase in the cells was followed by immunocytochemistry. As shown in Fig. 8c, ouabain caused a reduction in labeling of the Na,K-ATPase α subunit at the cell plasma membrane and increased the labeling in the cytoplasmic compartment. These results indicate that physiologic concentrations of ouabain decrease the plasma membrane levels of Na,K-ATPase in ADPKD cells.
Fig. 8.
Ouabain effects on membrane expression of Na,K-ATPase in ADPKD cells. a Immunoblot analysis of the α1 subunit of Na,K-ATPase in ADPKD plasma membrane samples, obtained after cell biotinylation and precipitation with streptavidin. Expression levels of Na,K-ATPase α1 was determined by densitometric analysis of immunoblots labeled with an anti-Na,K-ATPase α1-specific antibody. Bars represent the mean ± SEM of three determinations performed on cells obtained from different ADPKD kidneys, with (*) indicating P < 0.01 versus untreated control. b Immunoblot analysis of the plasma membrane expression levels of both Na,K-ATPase α1 and β1 subunits in ADPKD cells, either untreated or treated with 3 nM ouabain for 24 h. Proteins were determined on samples obtained from the cells after biotinylation and streptavidin precipitation. c Immunofluorescence microscopy of confluent cultures grown on filter supports treated in the absence or presence of ouabain for 24 h and labeled for the α1 subunit of Na,K-ATPase. Top panels show z-line views positioned at the lines indicated in the x–y views shown in the bottom panels. Ap apical, Bl basolateral side of the cells
Discussion
The hormone ouabain is an agent that promotes cystogenesis in ADPKD and one of its effects is to enhance the cAMP-dependent basolateral to apical movement of fluid in the renal cystic epithelium (Jansson et al. 2012). In contrast to the effects in ADPKD cells, ouabain does not affect fluid secretion and cystogenesis of normal human kidney epithelial cells and has minimal effect on promoting cystic dilations of wild-type mouse embryonic kidneys (Jansson et al. 2012). In this work, we further investigated the mechanisms by which ouabain specifically affects transepithelial fluid secretion in ADPKD cells. We established that, to exacerbate forskolin-induced cyst growth and fluid secretion, ouabain requires the presence of CFTR. This was apparent from the incapacity of ouabain to induce cystic dilations in metanephric organ cultures from Pkd1m1Bei mice in which the expression of CFTR was concomitantly abolished. Also, ouabain was unable to enhance the cAMP-induced microcyst development in ADPKD cell cultures grown in a matrix of polymerized collagen in the presence of the CFTR inhibitor, CFTR(inh)-172. Moreover, ouabain increased forskolin-induced efflux of Cl− mediated by CFTR. These results agree with our previous observations, which showed that ouabain enhanced cAMP-dependent Cl− secretion of ADPKD monolayers (Jansson et al. 2012). Augmentation in CFTR activity, via abnormally increased cAMP stimulation, has been described as the primary mechanism accounting for salt and water secretion in ADPKD (Wallace 2011). As such, the cystogenic effects of ouabain involve the targeting of the classical Cl− efflux pathway known to cause fluid accumulation in ADPKD cysts.
Ouabain activation of CFTR does not result from changes in total expression of this ion transporter, since the mRNA and protein levels remained constant in ADPKD cells after treatment with ouabain (Jansson et al. 2012). However, this work shows that the plasma membrane levels of CFTR are increased by ouabain. This suggests that ouabain produces changes in the cellular localization of CFTR, inducing its trafficking to the cell plasma membrane. Interestingly, a role for ouabain as a modulator of the localization and plasma membrane amounts of CFTR has also been reported in human bronchial epithelial cells containing the most common mutated form of CFTR, F508del-CFTR (Guggino and Stanton 2006). This mutation produces the abnormal retention of CFTR in the cell endoplasmic reticulum and is responsible for causing cystic fibrosis. In these cells, nanomolar concentrations of ouabain partially corrected the trafficking of F508del-CFTR to the plasma membrane, recovering Cl− secretion in the bronchial epithelium. These results agree with our observations in ADPKD cells, and show that ouabain influences the movement of CFTR to the cell surface, increasing its amounts at the plasma membrane.
Ouabain has no effect on the overall cell expression of NKCC1 in ADPKD cells (Jansson et al. 2012). Here, we observed that similarly, ouabain does not influence the plasma membrane levels of NKCC. This suggests that, different from CFTR, the action of ouabain on enhancement of basolateral to apical Cl− secretion does not require additional production of NKCC1 at the cell membrane and the proteins already present at the cell surface are able to sufficiently carry out Cl− transport at the basolateral membrane of the cells. Future experiments on NKCC1 and different Cl− channels will be required to further elucidate the role of ouabain in basolateral Cl− transport in ADPKD cells.
Regulation of vectorial Cl− transport in epithelia is complex and depends on the interaction of CFTR with a variety of chaperones and scaffolding proteins. PDZ domain-containing proteins, such as PDZ1, play an essential role in trafficking and retention of CFTR at the cell surface (Guggino and Stanton 2006). Our results show that ouabain treatment of ADPKD cells led to an overall increase in the expression of PDZK1, suggesting that PDZK1 is one of the mechanisms that facilitates the increase in CFTR membrane localization in ADPKD cells. In addition to regulating CFTR membrane trafficking, PDZK1 has been shown to induce dimerization of the CFTR present at the plasma membrane, an event that favors the activation of CFTR by protein kinase A (PKA) (Wang et al. 2000). Our results are consistent with PDZK1 being involved in the up-regulation of CFTR by ouabain. Thus, we find that ouabain alone increases CFTR trafficking to the cell surface, but by itself, ouabain is not sufficient to increase fluid secretion and anion efflux by the ADPKD epithelium, or cyst growth in metanephric organ cultures from Pkd1m1Bei mice, which requires the concomitant presence of cAMP activation. This suggests that ouabain may function as a permissive factor, which by increasing the plasma membrane levels of CFTR, enhances the responsiveness of ADPKD cells to cAMP-dependent signals.
Our previous results and the present observations show that the synergism between ouabain and cAMP takes place at several levels in the ADPKD cells. Thus, we have shown that ouabain mediates the mitogenic activity and fluid secretion of ADPKD cells by activating the mitogen kinase, B-Raf/MEK/ERK signaling pathway, which functions downstream of forskolin-stimulated increases in cAMP (Nguyen et al. 2011). Moreover, ouabain uses the Na,K-ATPase signalosome apparatus to exert its effects in ADPKD cells, including effects on the EGFR, Src, and B-Raf/MEK/ERK pathways (Nguyen et al. 2011). Other agents, including arginine vasopressin, prostaglandins, EGF, and Src activators also affect ADPKD cystogenesis via these pathways (Belibi et al. 2004; Reif et al. 2011; Torres and Harris 2007; Wallace 2011), indicating that these messengers are common mediators where several stimuli can promote ADPKD cyst growth. Our results here identify CFTR as a downstream effector where ouabain and cAMP-dependent signaling converge to potentiate ADPKD fluid secretion and cyst growth.
Normally, in the renal tubular epithelium, reabsorption of salt and fluid is the mechanism that predominates over secretion (Wallace et al. 2002). Fluid reabsorption largely depends on the activity of the basolaterally located Na,K-ATPase, which by maintaining [Na]i levels low, allows the movement of this cation from the tubular lumen to the cell cytosol (Feraille and Doucet 2001). Our results show that nanomolar amounts of ouabain affect the ion transport activity of Na,K-ATPase, to decrease the uptake of 86Rb into the cells. This agrees with our previous results, indicating that ouabain partially inhibits the enzymatic capacity of the Na,K-ATPase to hydrolyze ATP (Nguyen et al. 2007). Consistent with the idea that Na,K-ATPase is the main active regulator of [Na]i, we found that ouabain increases Na+ levels in the ADPKD cell cytoplasm. In addition, ouabain decreases the ADPKD plasma membrane levels of Na,K-ATPase. Similar to our results, studies conducted in normal porcine epithelial cells have demonstrated a decrease in Na,K-ATPase plasma membrane expression by nanomolar concentrations of ouabain (Liu et al. 2004, 2005; Yan et al. 2012). The reduction and partial inhibition of Na,K-ATPase by ouabain in ADPKD cells diminishes both the electrical and the chemical driving force for Na+ entry via the apical membrane. This effect in ADPKD cells agrees with the known natriuretic effect that ouabain exerts on several tubular segments in the normal kidney (Cai et al. 2008; Fedorova et al. 2000; Loreaux et al. 2008; Nesher et al. 2009). In ADPKD cells, a reduction of basolateral Na+ exit via inhibition of Na,K-ATPase will decrease salt and water reabsorption. The inward Na+ gradient is also used by the basolateral Cl− entry mechanism through NKCC1. This could potentially slow Cl− movement across the basolateral plasma membrane of ADPKD cells. However, the increase in Cl− efflux that ouabain causes in ADPKD cells suggests that the ouabain-induced reduction in the Na+ gradient is not limiting Cl− movement through NKCC1. This might depend on the fact that NKCC1 transport is electroneutral and, therefore, less sensitive to a relatively small degree of inhibition in pump activity. Alternatively, movement of Cl− through other transport mechanisms may be involved in the effects of ouabain at the basolateral aspect of the cells.
In this manner, the net actions of ouabain on ADPKD cell fluid secretion comprise effects at both the apical and basolateral side of the cells. Apically, ouabain has synergistic effects with other effectors in activating Cl− efflux via CFTR, to enhance the canonical route of ADPKD fluid secretion. Basolaterally, ouabain reduces Na,K-ATPase function, diminishing Na+ transport, which will further promote net transepithelial fluid secretion.
It is important to note that only a population of Na,K-ATPase in ADPKD cells is sensitive to nanomolar concentrations of ouabain (Nguyen et al. 2007). This population may correspond to the Na,K-ATPase that is localized in caveolae and is able to transmit ouabain effects into the cell through its association with other signaling molecules (Liu et al. 2003). Our data show that in ADPKD cells, the Na,K-ATPase involved in signaling also functions in ion transport. In agreement with this, preparations of caveolar fractions from rat cardiac myocytes and pig kidney outer medulla, which contain the signaling Na,K-ATPase, showed Na,K-ATPase hydrolytic activity (Ferrandi et al. 2004; Liu et al. 2011). This suggests that the Na,K-ATPase that signals ouabain effects retains its activity to hydrolyze ATP and can actively exchange ions. Therefore, ouabain affects ADPKD cells not only by activating the Na,K-ATPase signaling apparatus but also by modulating Na,K-ATPase activity.
In conclusion, this work has identified specific mechanisms by which ouabain increases ADPKD basolateral to apical vectorial movement of fluid, including the enhancement in the response of CFTR to cAMP-dependent secretory agonists and the reduction of Na,K-ATPase ion transport function. This provides new insights into the mechanisms by which ouabain influences ADPKD cells and further highlights the role of ouabain as an endogenous non-genomic factor that can enhance ADPKD cystogenesis.
Acknowledgments
This study was supported by a National Institutes of Health Grant DK081431 to G.B. We thank the Polycystic Kidney Disease Foundation and the PKD Research Biomaterials and Cellular Models Core at the University of Kansas Medical Center and hospitals participating in the Polycystic Kidney Research Retrieval Program for providing human ADPKD kidneys from which primary cells were obtained.
Footnotes
Compliance with Ethical Standards
Conflict of interest No conflict of interest, financial or otherwise related with this work, are declared by the authors.
References
- Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belibi FA, et al. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004;66:964–973. doi: 10.1111/j.1523-1755.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na,K-ATPase: heterogeneity in structure. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Blanco G, Wallace DP. Novel role of ouabain as a cystogenic factor in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2013;305:F797–F812. doi: 10.1152/ajprenal.00248.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Hamlyn JM. Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na(+) pump, the Na(+)/Ca(2+) exchanger and TRPC proteins. Biochim Biophys Acta. 2010;1802:1219–1229. doi: 10.1016/j.bbadis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Wu L, Qu W, Malhotra D, Xie Z, Shapiro JI, Liu J. Regulation of apical NHE3 trafficking by ouabain-induced activation of the basolateral Na,K-ATPase receptor complex. Am J Physiol Cell Physiol. 2008;294:C555–C563. doi: 10.1152/ajpcell.00475.2007. [DOI] [PubMed] [Google Scholar]
- Chueh SC, Guh JH, Chen J, Lai MK, Teng CM. Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol. 2001;166:347–353. [PubMed] [Google Scholar]
- Davidow CJ, Maser RL, Rome LA, Calvet JP, Grantham JJ. The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int. 1996;50:208–218. doi: 10.1038/ki.1996.304. [DOI] [PubMed] [Google Scholar]
- Dmitrieva RI, Doris PA. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J Biol Chem. 2003;278:28160–28166. doi: 10.1074/jbc.M303768200. [DOI] [PubMed] [Google Scholar]
- Fedeles SV, Gallagher AR, Somlo S. Polycystin-1: a master regulator of intersecting cystic pathways. Trends Mol Med. 2014;20:251–260. doi: 10.1016/j.molmed.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation. 2000;102:3009–3014. doi: 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev. 2001;81:345–418. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- Ferrandi M, Molinari I, Barassi P, Minotti E, Bianchi G, Ferrari P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem. 2004;279:33306–33314. doi: 10.1074/jbc.M402187200. [DOI] [PubMed] [Google Scholar]
- Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–1485. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- Grindstaff KK, Blanco G, Mercer RW. Characterization of Na,K-ATPase isoform expression and activity in MDCK and Caco-2 epithelial cells. Epithelial Cell Biol. 1995;4:17–24. [PubMed] [Google Scholar]
- Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6:197–206. doi: 10.1038/nrneph.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Gonzalez EO, et al. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem. 2006;281:5623–5633. doi: 10.1074/jbc.M508172200. [DOI] [PubMed] [Google Scholar]
- Herron BJ, et al. Efficient generation and mapping of recessive developmental mutations using ENU mutagenesis. Nat Genet. 2002;30:185–189. doi: 10.1038/ng812. [DOI] [PubMed] [Google Scholar]
- Jansson K, et al. Endogenous concentrations of ouabain act as a cofactor to stimulate fluid secretion and cyst growth of in vitro ADPKD models via cAMP and EGFR-Src-MEK pathways. Am J Physiol Renal Physiol. 2012;303:F982–F990. doi: 10.1152/ajprenal.00677.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol. 2006;291:C1247–C1257. doi: 10.1152/ajpcell.00593.2005. [DOI] [PubMed] [Google Scholar]
- Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem. 1998;273:15249–15256. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- Li J, et al. Ouabain protects against adverse developmental programming of the kidney. Nat Commun. 2010;1:42. doi: 10.1038/ncomms1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003;284:C1550–C1560. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66:227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Liang M, Liu L, Malhotra D, Xie Z, Shapiro JI. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005;67:1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Ivanov AV, Gable ME, Jolivel F, Morrill GA, Askari A. Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase. Biochemistry. 2011;50:8664–8673. doi: 10.1021/bi2009008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain-Sensitive alpha1 Na,K-ATPase enhances natriuretic response to saline load. J Am Soc Nephrol JASN. 2008;19:1947–1954. doi: 10.1681/ASN.2008020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenheimer BS, et al. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+), K(+),2Cl(−) Co-transporter-dependent cystic dilation. J Am Soc Nephrol. 2006;17:3424–3437. doi: 10.1681/ASN.2006030295. [DOI] [PubMed] [Google Scholar]
- Nesher M, Dvela M, Igbokwe VU, Rosen H, Lichtstein D. Physiological roles of endogenous ouabain in normal rats. Am J Physiol Heart Circ Physiol. 2009;297:H2026–H2034. doi: 10.1152/ajpheart.00734.2009. [DOI] [PubMed] [Google Scholar]
- Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol. 2007;18:46–57. doi: 10.1681/ASN.2006010086. [DOI] [PubMed] [Google Scholar]
- Nguyen AN, Jansson K, Sanchez G, Sharma M, Reif G, Wallace DP, Blanco G. Ouabain activates the Na,K-ATPase signalosome to induce autosomal dominant polycystic kidney disease cell proliferation. Am J Physiol Renal Physiol. 2011;301:F897–F906. doi: 10.1152/ajprenal.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BM, Vanden Heuvel GB. Kidney: polycystic kidney disease Wiley interdisciplinary reviews. Dev Biol. 2014;3:465–487. doi: 10.1002/wdev.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2011;118:c19–c30. doi: 10.1159/000320887. [DOI] [PubMed] [Google Scholar]
- Pierre SV, Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys. 2006;46:303–316. doi: 10.1385/cbb:46:3:303. [DOI] [PubMed] [Google Scholar]
- Reif G, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP. Tolvaptan inhibits ERK-dependent cell proliferation, Cl− secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol. 2011;301:F1005–F1013. doi: 10.1152/ajprenal.00243.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C, et al. Pleiotropic effects of cardioactive glycosides. Curr Med Chem. 2011;18:872–885. doi: 10.2174/092986711794927685. [DOI] [PubMed] [Google Scholar]
- Schoner W, Scheiner-Bobis G. Endogenous cardiac glycosides: hormones using the sodium pump as signal transducer. Semin Nephrol. 2005;25:343–351. doi: 10.1016/j.semnephrol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Silva E, Soares-da-Silva P. New insights into the regulation of Na+, K+-ATPase by ouabain. Int Rev Cell Mol Biol. 2012;294:99–132. doi: 10.1016/B978-0-12-394305-7.00002-1. [DOI] [PubMed] [Google Scholar]
- Singh AK, et al. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest. 2009;119:540–550. doi: 10.1172/JCI35541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev. 1998;78:1165–1191. doi: 10.1152/physrev.1998.78.4.1165. [DOI] [PubMed] [Google Scholar]
- Takacs-Jarrett M, Sweeney WE, Avner ED, Cotton CU. Generation and phenotype of cell lines derived from CF and non-CF mice that carry the H-2K(b)-tsA58 transgene. Am J Physiol Cell Physiol. 2001;280:C228–C236. doi: 10.1152/ajpcell.2001.280.1.C228. [DOI] [PubMed] [Google Scholar]
- Torres VE, Harris PC. Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J Intern Med. 2007;261:17–31. doi: 10.1111/j.1365-2796.2006.01743.x. [DOI] [PubMed] [Google Scholar]
- Wagoner K, Sanchez G, Nguyen AN, Enders GC, Blanco G. Different expression and activity of the alpha1 and alpha4 isoforms of the Na,K-ATPase during rat male germ cell ontogeny. Reproduction. 2005;130:627–641. doi: 10.1530/rep.1.00806. [DOI] [PubMed] [Google Scholar]
- Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta. 2011;1812:1291–1300. doi: 10.1016/j.bbadis.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DP, Grantham JJ, Sullivan LP. Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int. 1996;50:1327–1336. doi: 10.1038/ki.1996.445. [DOI] [PubMed] [Google Scholar]
- Wallace DP, Christensen M, Reif G, Belibi F, Thrasher JB, Herrell D, Grantham JJ. Electrolyte and fluid secretion by cultured human inner medullary collecting duct cells. Am J Physiol Renal Physiol. 2002;283:F1337–F1350. doi: 10.1152/ajprenal.00165.2002. [DOI] [PubMed] [Google Scholar]
- Wallace DP, Reif G, Hedge AM, Thrasher JB, Peitrow P. Adrenergic regulation of salt and fluid secretion in human medullary collecting duct cells. Am J Physiol Renal Physiol. 2004;287:F639–F648. doi: 10.1152/ajprenal.00448.2003. [DOI] [PubMed] [Google Scholar]
- Wang S, Yue H, Derin RB, Guggino WB, Li M. Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell. 2000;103:169–179. doi: 10.1016/s0092-8674(00)00096-9. [DOI] [PubMed] [Google Scholar]
- West MR, Molloy CR. A microplate assay measuring chloride ion channel activity. Anal Biochem. 1996;241:51–58. doi: 10.1006/abio.1996.0377. [DOI] [PubMed] [Google Scholar]
- Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- Yan Y, et al. Ouabain-stimulated trafficking regulation of the Na/K-ATPase and NHE3 in renal proximal tubule cells. Mol Cell Biochem. 2012;367:175–183. doi: 10.1007/s11010-012-1331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, et al. Ouabain mimics low temperature rescue of F508del-CFTR in cystic fibrosis epithelial cells. Front Pharmacol. 2012;3:176. doi: 10.3389/fphar.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]