Abstract
Mammalian whole embryo culture (WEC) is a widely used technique for examining pharmacological toxicity in developing mouse and rat embryos and for investigating the mechanisms of developmental processes. Immediately centrifuged (IC) rat serum is commonly used for WEC and is essential for the growth and development of cultured mouse and rat embryos ex vivo. For the culture of midgestation embryos (i.e., E8.0-12.5 for the mouse, and E10.0-14.5 for the rat), 100% rat serum is the best media for supporting the growth of the embryo ex vivo. To prepare rat serum suitable for WEC, the collected blood should be centrifuged immediately to separate the blood cells from the plasma fraction. After centrifugation, the fibrin clot forms in the upper layer; this clot should be squeezed gently using a pair of sterile forceps and subsequently centrifuged to completely separate the blood cells from the serum. In this video article, we demonstrate our standard protocol for the preparation of optimal IC rat serum, including blood collection from the abdominal aorta of male rats and extraction of the serum by centrifugation.
Keywords: Physiology, Issue 90, WEC, male rats, abdominal aorta, rat serum, fibrin clot, hemolysis
Introduction
A variety of model animals are used in developmental biology to investigate developmental mechanisms at the molecular and cellular levels. For example, amphibian and avian species have been widely used as classical model animals that are suitable for direct manipulation of embryos because these embryos develop outside of the mother. In contrast to these animals, mammalian embryos grow in the uterus of the mother, and growth at later stages is crucially dependent on the function of the uterus. Therefore, it is typically difficult to directly manipulate mammalian embryos such as those from the mouse and rat at early stages. In the 1960s, Denis New established a mammalian whole embryo culture (WEC) technique using WEC apparatuses with a continuous oxygen supply and heat control1. In WEC, mouse and rat embryos can grow ex vivo, (i.e., outside of the uterus). Although the WEC technique was often used in teratology by adding various chemical compounds into the culture medium, this technique has also been used in various developmental biology studies to examine unique developmental mechanisms in mammals2-4. For example, WEC is combined with other techniques, such as cell labeling, in wild-type and mutant embryos by using fluorescent dye5, cell transplantation6, and gene introduction via lipofection7 and electroporation8-13.
Recently, in utero manipulation has been used to analyze developmental processes in rodent embryos at later stages and has been combined with electroporation techniques14-16. However, these techniques are not suitable for the manipulation of postimplantation and midgestation embryos due to difficulties achieving accurate local injection of the DNA solution into embryos at the early stages. Although ultrasound-guided cell transplantation and injection of viral vectors into early embryos (i.e., E8.5-E-9.5 in the mouse) in utero have been reported previously17,18, excellent skills are required to perform these experiments with a high success rate. Therefore, WEC with high accessibility ex vivo has advantages with respect to the manipulation of mouse and rat embryos.
Immediately centrifuged (IC) rat serum prepared from male rats is often used for WEC medium. When embryos are cultured at the postimplantation stage (i.e., earlier than E8.0 in the mouse or E10.0 in the rat), a mixture of synthetic medium and IC rat serum is often used as a medium for WEC19. However, to culture embryos at mid-gestation (i.e., E8.0-12.5 in mouse embryos or E10.0-E14.5 in rat embryos), 100% serum should be used as a medium because no currently available alternative media allows embryos to grow normally in vitro for more than 2 days.
The preparation of high-quality rat serum is a critical step for achieving reproducibility in WEC experiments. Compared to delayed centrifugation, IC has the benefit of reducing hemolysis when the serum is collected by squeezing the fibrin clot because most of the red blood cells have already been separated from the fibrin clot. As hemolytic rat serum fails to support the normal growth of rat and mouse embryos, the preparation of serum using immediate centrifugation is preferable to preparation using delayed centrifugation. Our protocol contains two additional steps compared to other protocols18-20 (i.e., storing the collected blood on ice prior to the first centrifugation and maintaining the collected blood samples for 2 hr at 4 °C after the first centrifugation). The former step can delay the formation of the blood clot, and the latter step promotes the solidification of the fibrin clot for easy squeezing. Therefore, our protocol can be used by beginners. However, reproducing blood collection and serum extraction accurately by simply referring to protocol books is extremely difficult19-21. In this video article, we demonstrate our standard protocol for the preparation of optimal IC rat serum, which includes blood collection from the abdominal aorta of male rats and extraction of the serum by centrifugation.
Protocol
NOTE: Animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Committee for Animal Experimentation of the Tohoku University School of Medicine approved the experimental procedures described herein.
1. Anesthesia and Laparotomy
For blood collection, use specific pathogen-free male Sprague-Dawley rats. Fast the rats for at least 18 hr while providing water. Use retired male breeder rats at 6-8 months of age (550-650 g) for collecting blood.
- Flow 2.5-4.0% isoflurane, which is an inhalational anesthetic, into a box connected to an anesthesia apparatus to introduce anesthesia at 2.0-3.5 L/min for 10 min. Transfer rats into the box to introduce anesthesia.
- Confirm anesthetization by checking for the loss of the response to stimulation on the paws with forceps. Cover the nose of the rats with a mask connected to an anesthesia apparatus to deeply anesthetize the rats during blood collection. The concentration and flow are maintained at 2.5-4.0% and 2.0-3.5 L/min, respectively. NOTE: Halothane should not be used for inhalation anesthesia because embryos cultured in the serum that is collected from rats anesthetized with this reagent exhibit a delay in embryonic development compared to embryos cultured in the serum obtained from rats anesthetized with isoflurane22.
To avoid contamination of the fur, disinfect the skin by pouring 70% ethanol onto the abdomen of the anesthetized rat. Pick up the disinfected skin and abdominal wall at the lower region with a pair of forceps and cut these layers simultaneously toward the thorax region with a pair of large scissors to expose the internal organs. Reflect the gut outside of the abdominal cavity with a pair of forceps.
Cut the skin and abdominal wall toward the hind limbs with a pair of large scissors to further expose the posterior abdominal region. Reflect the extra fat outside of the abdominal cavity with a pair of forceps.
Pick up the visceral fat at the midline using two pairs of forceps and split the fat carefully in a parallel direction to expose the abdominal aorta. Repeat this procedure to split the visceral fat in a longitudinal direction to easily identify the bifurcation of the abdominal aorta. In addition, the aorta is pulsatile and can be distinguished from the adjacent vena cava using this characteristic.
Pick up the fat around the abdominal aorta and the bifurcation carefully with two pairs of forceps and separate the fat on the abdominal aorta.
2. Blood Collection
Carefully insert the tip of a 21 G needle connected to a 20 ml syringe immediately cranial to the bifurcation of the aorta, with the bevel in a downward direction. Hold the syringe with one hand, and pull the syringe slowly to collect 15 ml of blood from a male rat.
Remove the needle from the syringe, and pour the blood into two ice-cold 10-ml sterile test tubes (Figure 1A). Keep the tubes on ice until centrifugation to delay the formation of the blood clot. Reduce the centrifugation time by centrifuging several tubes at one time.
Euthanize the anesthetized rat by thoracotomy and cutting the heart or decapitation.
3. Rat Serum Preparation
Centrifuge the collected blood for 5 min at 1,200 x g and room temperature (RT) to separate the blood into upper and lower layers (Figure 1C).
Keep the tubes at 4 °C for 1.5-2 hr to fix the fibrin clot.
Pick up the fibrin clot in the upper layer using a pair of curved forceps, and squeeze the fibrin clot to separate the serum. Then, press the fibrin clot with the curved forceps facing downwards near the interface between the two layers (Figure 1D).
Repeat step 3.3 to further detach the fibrin clot.
Centrifuge the test tubes for 5 min at 1,200 x g and RT (Figure 1E).
Carefully transfer the serum into a 15 ml sterile tube using a sterile pipette in a laminar air-flow cabinet.
Centrifuge the collected rat serum for 5 min at 1,200 x g and RT to remove any remaining red cells.
Pool the serum carefully into a new 15 ml sterilized tube using a sterile pipette to avoid contamination by residual red cells at the bottom of the tube (Figure 1F).
Incubate the serum in a water bath at 56 °C for 30 min to inactivate the complement system.
Cool the tubes of rat serum to RT in the laminar air-flow cabinet. The serum should be aliquoted to 10 ml in individual sterile tubes. Store the tubes at -20 °C until use (Figure 1H).
Representative Results
Figure 1 shows representative results of the described procedures for the separation of blood cells and serum. We typically obtain 15 ml of blood from a retired male rat (Figure 1A). By centrifugation, the collected blood can be separated into an upper layer containing the serum and the fibrin clot, which is indicated with an arrow, and a lower layer containing the blood cells (Figure 1C). Importantly, hemolytic blood samples cannot be separated into serum and blood layers (Figure 1B). After leaving the tube at 4 °C for 2 hr, the fibrin clot becomes solid. This solidification is convenient for the separation of the serum from the fibrin clot using a pair of curved forceps (Figure 1D). The test tube was subsequently centrifuged. The fibrin clot is visible at the interface between the serum and blood cell layers (Figure 1E). The serum from the two test tubes was gathered into a new tube using a disposable pipette. We can obtain approximately 6 ml of serum from a male rat if the entire procedure is successful. To further remove red cells, the collected serum was centrifuged again. Any remaining red cells precipitated at the bottom of the tube (Figure 1F). We transferred the supernatant to a new tube and checked the color of the purified serum to judge the degree of hemolysis even though we are able to collect the serum from the upper layer after centrifugation. The ideal rat serum for WEC typically exhibits a light yellow color (left tube in Figure 1G), while rat serum with mild hemolysis exhibits a pinkish color (right tube in Figure 1G). IC rat serum can be stored for at least 6-12 months at -20 °C (left in Figure 1H). The serum with mild hemolysis exhibited a deeper color than the ideal serum when frozen (left in Figure 1H).
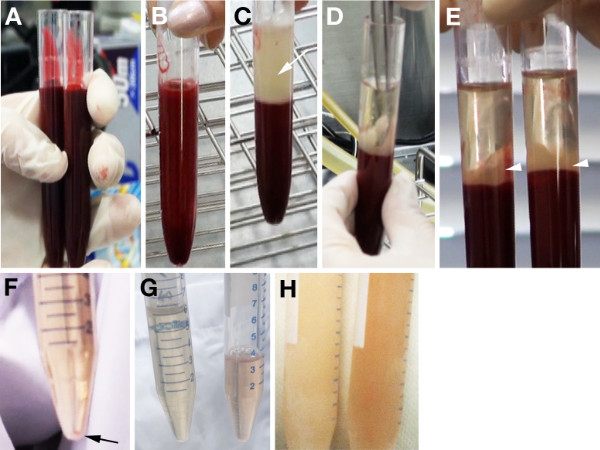 Figure 1. Procedures for blood centrifugation and preparation of serum from male rats. A) The blood samples collected from a male rat are divided equally into two test tubes for centrifugation. B, C) Non-separated sample (B) and ideal separation into the upper layer containing the serum and the fibrin clot (arrow in C) and the lower layer containing the blood cells after the first centrifugation. D) The fibrin clot is squeezed using a pair of forceps. E) The serum and blood cell layers after the second centrifugation. The fibrin clot is observed at the interface (arrowheads). F) Precipitation of blood cells after the third centrifugation (arrow). G) The left tube shows ideal rat serum with a light yellowish color. The right tube shows rat serum with mild hemolysis. H) Frozen optimal (left) and mildly hemolyzed (right) rat serum.
Figure 1. Procedures for blood centrifugation and preparation of serum from male rats. A) The blood samples collected from a male rat are divided equally into two test tubes for centrifugation. B, C) Non-separated sample (B) and ideal separation into the upper layer containing the serum and the fibrin clot (arrow in C) and the lower layer containing the blood cells after the first centrifugation. D) The fibrin clot is squeezed using a pair of forceps. E) The serum and blood cell layers after the second centrifugation. The fibrin clot is observed at the interface (arrowheads). F) Precipitation of blood cells after the third centrifugation (arrow). G) The left tube shows ideal rat serum with a light yellowish color. The right tube shows rat serum with mild hemolysis. H) Frozen optimal (left) and mildly hemolyzed (right) rat serum.
Discussion
Reproducible results in WEC experiments are dependent on the use of high quality IC rat serum and accurate embryo dissection technique3. Do not shorten the period of fasting before collecting blood because fasting is necessary to standardize glucose concentrations in the blood. Female rats should not be used for the preparation of serum because hormone levels change in female animals according to the estrous cycle, and such changes in estradiol hormones are unsuitable for embryo cultures. In addition, extremely fatty male rats should not be used for serum preparation to avoid a large amount of lipid contamination in the blood.
When collecting the blood, first ensure that the color of the arterial blood is a bright red color, reflecting normal breathing under anesthesia. Decreased breathing leads to a reduction of the amount of collected blood. To recover in such cases, temporarily reduce the speed at which the syringe is pulled. The most critical problem in serum preparation is hemolysis. If the collected blood samples exhibit severe hemolysis, the blood fails to be separated into two layers by centrifugation. Because mildly hemolyzed serum may support sub-optimal development, such serum should be discarded. As hemolysis tends to be induced by forced pressure, the syringe should be pulled slowly when collecting the blood. It is important to avoid bubbling the blood when the collected blood samples are poured into test tubes, which increases hemolysis.
To culture mouse embryos, IC serum collected from male mice may be ideal; however, this requirement is not practical because we cannot obtain a large enough volume of blood from a mouse. When we prepare rat serum, we prepare at least 10 retired breeding male rats. In our lab, blood collection is routinely performed by at least two individuals: the individual collecting the blood and the individual anesthetizing and performing the first centrifugation.
To date, several studies using commercial rat serum in WEC by combining chemically defined medium have been reported23-25. For example, a mixture of 50% commercially available rat serum and 50% DMEM supports the normal growth of E8.5 mouse embryos for 36 hr23 and the normal growth of E9.75 mouse embryos for 40 hr24. In another protocol, a mixture composed of 50% rat serum available from another company and 50% F12 medium supplemented with N-2 also supports the proper growth of E7.5 and E8.5 mouse embryos for 24 hr25. However, it remains unclear whether these combinatorial media are suitable for the culture of rat and mouse embryos at later stages, such as E11.5-E12.5 in mice and E13.5-E14.5 in rats. WEC studies using various alternative media instead of rat serum have also been reported26-28. For example, a combination of DMEM/F12 and 20% fetal bovine serum (FBS) supports the development of rat embryos from E11.5 for 28 hr26. However, this medium is not suitable for rat embryo culture from E10.5 for 28 hr26. Therefore, we believe that the normal growth of embryos in medium containing FBS is dependent on the embryonic stage of the embryos.
WEC using serum-free culture media comprised only of defined reagents, including commercially available embryonic stem cell media, bovine serum albumin, and N-2 supplement have been reported. This medium supports the culture of E10.5 mouse embryos for 16-40 hr27, 28. Morre-Scott, et al. demonstrated that the crown-to-rump size of mouse embryos cultured for 24 hr is significantly smaller than that of E11.5 embryos grown in utero. However, the principal structures, such as somites and dorsal root ganglia, appear normal in these cultured embryos. On the other hand, Kalaskar and Lauderdale reported that 60% of embryos tested in this medium exhibited normal development 18 hr later, but the rate of good development in embryos cultured for an additional 20-22 hr dropped to 30-40%. In contrast to these studies, we can successfully reproduce good development of mouse and rat embryos cultured in 100% IC rat serum produced using our protocol for two days (i.e., approximately 48 hr) from E12.5 in rats6 or E10.5 in mice8 by changing the medium once at 24 hr. These results suggest that our method for preparing IC rat serum is useful for a variety of experiments applying mammalian WECat different embryonic stages. Therefore, we hope that our video protocol will help new researchers introduce experiments using WEC to their laboratories.
Disclosures
No conflicts of interest are declared.
Acknowledgments
We thank Mr. Hajime Ichijo for video recording and helpful advice concerning editing the video. We also thank the Osumi lab members for animal care. This work is supported by a Grant-in-Aid for Scientific Research on Innovative Area, Neural Diversity and Neocortical Organization, from MEXT of Japan (to N. O.). T. K. was supported by a Research Fellowship of JSPS for Young Scientists.
References
- New DAT. Introduction. In: Copp AJ, Cockroft DL, editors. Mammalian Postimplantation Embryos. A Practical Approach. Oxford, UK: IRL Press; 1990. pp. 1–14. [Google Scholar]
- Eto K, Takakubo F. Improved development of rat embryos in culture during the period of craniofacial morphogenesis. J Craniofac Genet Dev Biol. 1985;5:351–355. [PubMed] [Google Scholar]
- Takahashi M, Osumi N. The method of rodent whole embryo culture using the rotator-type bottle culture system. J Vis Exp. 2010. p. e2170. [DOI] [PMC free article] [PubMed]
- New DA. Whole embryo culture, teratogenesis, and the estimation of teratologic risk. Teratology. 1990;42:635–642. doi: 10.1002/tera.1420420608. [DOI] [PubMed] [Google Scholar]
- Matsuo T, et al. A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain crest cells. Nat Genet. 1993;3:299–304. doi: 10.1038/ng0493-299. [DOI] [PubMed] [Google Scholar]
- Nomura T, Holmberg J, Frisen J, Osumi N. Pax6-dependent boundary defines alignment of migrating olfactory cortex neurons via the repulsive activity of ephrin A5. Development. 2006;133:1335–1345. doi: 10.1242/dev.02290. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, et al. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- Inoue T, et al. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development. 2001;128:561–569. doi: 10.1242/dev.128.4.561. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Osumi N. Pax6 regulates specification of ventral neurone subtypes in the hindbrain by establishing progenitor domains. Development. 2002;129:1327–1338. doi: 10.1242/dev.129.6.1327. [DOI] [PubMed] [Google Scholar]
- Calegari F, Haubensak W, Yang D, Huttner WB, Buchholz F. Tissue-specific RNA interference in postimplantation mouse embryos with endoribonuclease-prepared short interfering RNA. Proc Natl Acad Sci U S A. 2002;99:14236–14240. doi: 10.1073/pnas.192559699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Nomura T, Osumi N. Transferring genes into cultured mammalian embryos by electroporation. Dev Growth Differ. 2008;50:485–497. doi: 10.1111/j.1440-169X.2008.01046.x. [DOI] [PubMed] [Google Scholar]
- Pryor SE, Massa V, Savery D, Greene ND, Copp AJ. Convergent extension analysis in mouse whole embryo culture. Methods Mol Biol. 2012;839:133–146. doi: 10.1007/978-1-61779-510-7_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa T, et al. Dmrta1 regulates proneural gene expression downstream of Pax6 in the mammalian telencephalon. Genes Cells. 2013;18:636–649. doi: 10.1111/gtc.12061. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Turnbull DH. Ultrasound backscatter microscopy of mouse embryos. Methods Mol Biol. 2000;135:235–243. doi: 10.1385/1-59259-685-1:235. [DOI] [PubMed] [Google Scholar]
- Pierfelice TJ, Gaiano N. Ultrasound-guided microinjection into the mouse forebrain in utero at E9.5. J Vis Exp. 2010. p. e2047. [DOI] [PMC free article] [PubMed]
- Quinlan GA, Khoo PL, Wong N, Trainor PA, Tam PP. Cell grafting and labeling in postimplantation mouse embryos. Methods Mol Biol. 2008;461:47–70. doi: 10.1007/978-1-60327-483-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vinterstein K, Behringer R. Roller culture of postimplantation embryos. In: Nagy A, Gertsenstein M, Vinterstein K, Behringer R, editors. Manipulating the Mouse Embryo: A Laboratory Manual. 3rd ed. New York, New York: CSH Press; 2003. pp. 237–241. [Google Scholar]
- Garcia MD, Udan RS, Hadjantonakis AK, Dickinson ME. Preparation of rat serum for culturing mouse embryos. Cold Spring Harb Protoc. 2011;2011:391–393. doi: 10.1101/pdb.prot5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Woodman PD, Ritchie HE, Korabelnikoff A, Emmanuel C. Replacement of ether with alternate volatile anesthetics for collection of rat serum used in embryo culture. Toxicol In Vitro. 2004;18:719–724. doi: 10.1016/j.tiv.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Gray J, Ross ME. Neural tube closure in mouse whole embryo culture. J Vis Exp. 2011. p. e3132. [DOI] [PMC free article] [PubMed]
- Machado CB, et al. Reconstruction of phrenic neuron identity in embryonic stem cell-derived motor neurons. Development. 2014;141:784–794. doi: 10.1242/dev.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville-Jones HC, Woo N, Arkell RM. Successful whole embryo culture with commercially available reagents. Int J Dev Biol. 2013;57:61–67. doi: 10.1387/ijdb.120098ra. [DOI] [PubMed] [Google Scholar]
- Ornoy A, Yacobi S, Yaffee P. A simple method of culture of 11.5-day-old rat embryos in DMEM/F12 and 20% fetal bovine serum. J Anat. 2003;203:419–423. doi: 10.1046/j.1469-7580.2003.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Scott BA, Gordon J, Blackburn CC, Condie BG, Manley NR. New serum-free in vitro culture technique for midgestation mouse embryos. Genesis. 2003;35:164–168. doi: 10.1002/gene.10179. [DOI] [PubMed] [Google Scholar]
- Kalaskar VK, Lauderdale JD. Mouse Embryonic Development in a Serum-free Whole Embryo Culture System. J Vis Exp. 2014. p. e50308. [DOI] [PMC free article] [PubMed]


